A Descriptive, Retrospective Analysis of COVID-19 Passive Antibody Therapy and Its Effects on Morbidity and Mortality in Patients Receiving B-Cell-Depleting Therapies
Abstract
1. Introduction
2. Materials and Methods
2.1. Setting and Cohort
2.2. Outcomes
2.3. Covariates
2.4. Statistical Analysis
3. Results
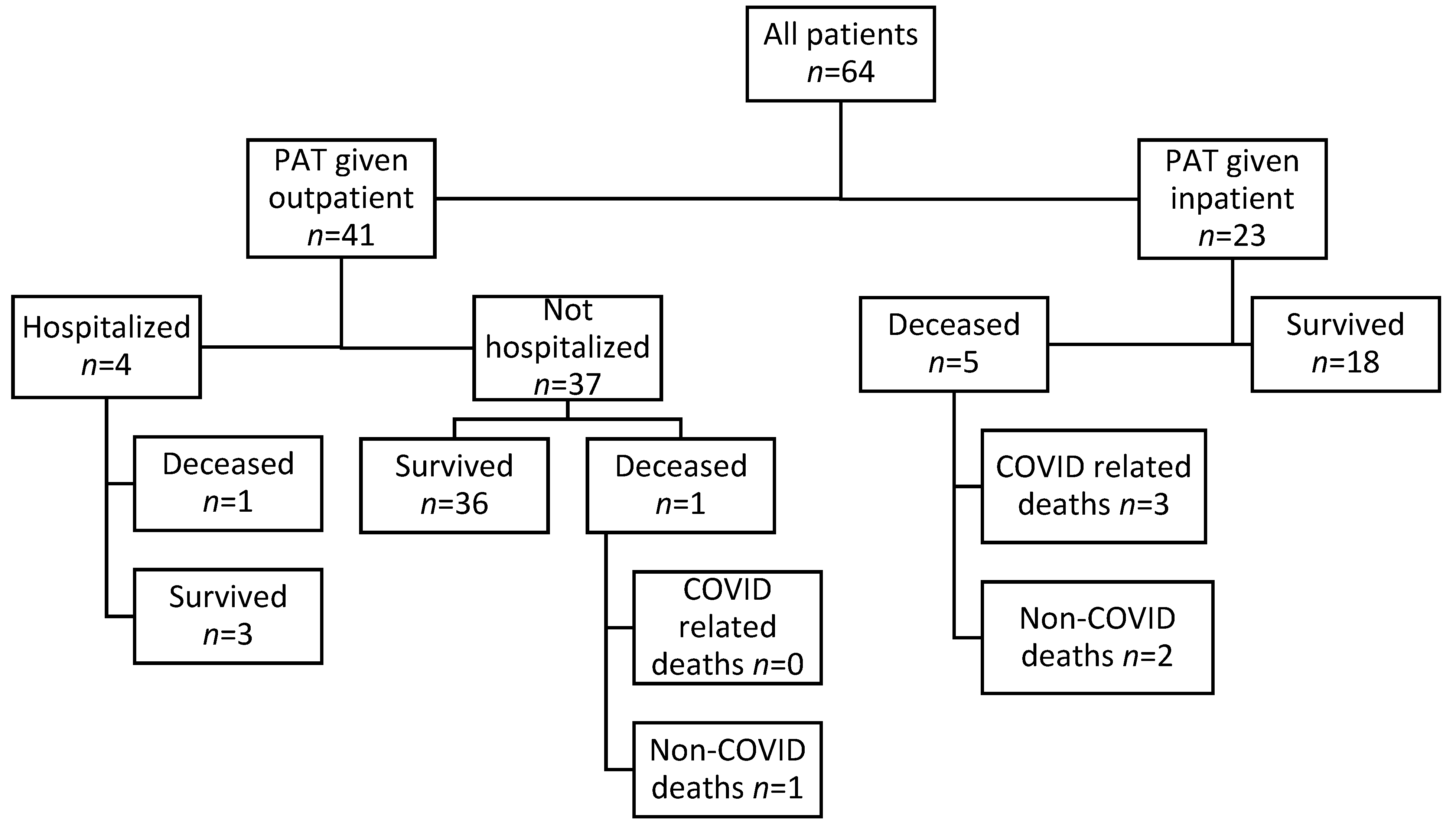
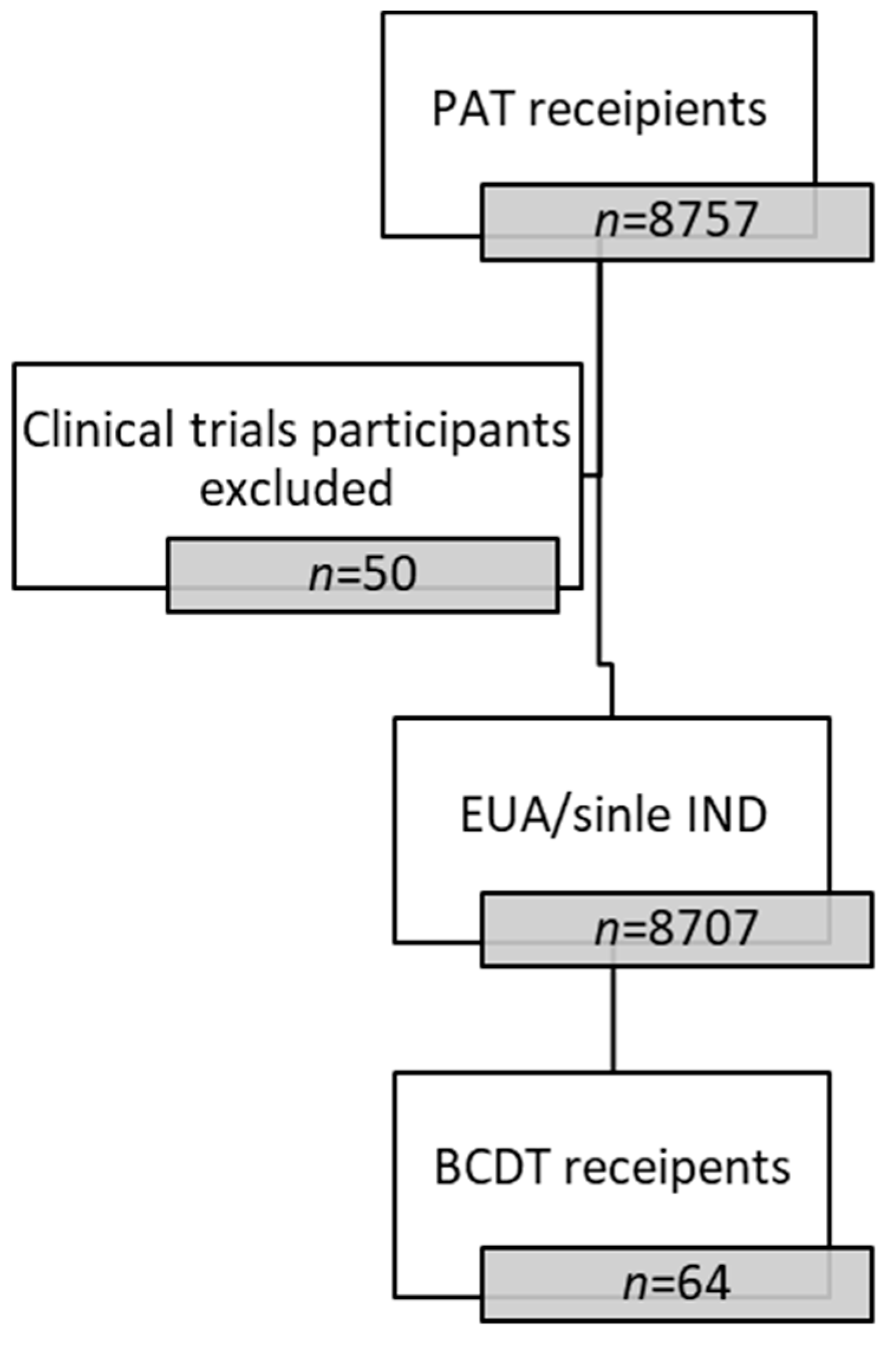
3.1. Cohort Description
3.2. Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B
| Age | Sex | BCDT | PAT | Duration between (days) | Cause of Death | |||
|---|---|---|---|---|---|---|---|---|
| Indication | Type | Type | Location | Symptom Onset and PAT | PAT and Death | |||
| 80 | M | CLL | Rituxan | CCP | IP | 4 | 9 | Respiratory failure |
| 71 | M | DLBCL | Rituxan | CCP | IP | 11 | 13 | Respiratory failure |
| 71 | M | DLBCL | Rituxan | mAb | IP | 8 | 13 | Respiratory failure |
| 71 | F | FL | Rituxan | CCP | IP | 21 | 65 | Cancer progression |
| 79 | F | DLBCL | Rituxan | mAb | OP | 1 | 66 | Cancer progression |
| 40 | M | CLL | Obinutuzumab | mAb | IP | 18 | 55 | Cancer progression |
| 81 | F | MCL | Rituxan | mAb | OP | 6 | 28 | Intracranial hemorrhage |
References
- Mathieu, E.; Ritchie, H.; Rodés-Guirao, L.; Appel, C.; Giattino, C.; Hasell, J.; Macdonald, B.; Dattani, S.-L.; Beltekian, D.; Ortiz-Ospina, E.; et al. Coronavirus Pandemic (COVID-19) 2020. Available online: https://ourworldindata.org/coronavirus (accessed on 8 December 2023).
- Rodríguez-Pinto, D. B cells as antigen presenting cells. Cell. Immunol. 2005, 238, 67–75. [Google Scholar] [CrossRef]
- McLaughlin, P.; Grillo-López, A.J.; Link, B.K.; Levy, R.; Czuczman, M.S.; Williams, M.E.; Heyman, M.R.; Bence-Bruckler, I.; White, C.A.; Cabanillas, F.; et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: Half of patients respond to a four-dose treatment program. J. Clin. Oncol. 1998, 16, 2825–2833. [Google Scholar] [CrossRef]
- Gaitzsch, E.; Passerini, V.; Khatamzas, E.; Strobl, C.D.; Muenchhoff, M.; Scherer, C.; Osterman, A.; Heide, M.; Reischer, A.; Subklewe, M.; et al. COVID-19 in Patients Receiving CD20-depleting Immunochemotherapy for B-cell Lymphoma. HemaSphere 2021, 5, e603. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Gavriatopolous, M.; Fotiou, D.; Giatra, C.; Asimakopoulos, I.; Dimou, M.; Sklirou, A.D.; Ntanasis-Stathopoulos, I.; Darmani, I.; Briasoulis, A.; et al. Poor neutralizing antibody responses in 132 patients with CLL, NHL and HL after vac-cination against SARS-CoV-2: A prospective study. Cancers 2021, 13, 4480. [Google Scholar] [CrossRef] [PubMed]
- Furlan, A.; Forner, G.; Cipriani, L.; Vian, E.; Rigoli, R.; Gherlinzoni, F.; Scotton, P. COVID-19 in B Cell-Depleted Patients After Rituximab: A Diagnostic and Therapeutic Challenge. Front. Immunol. 2021, 12, 763412. [Google Scholar] [CrossRef] [PubMed]
- Razonable, R.R.; Pawlowski, C.; O’Horo, J.C.; Arndt, L.L.; Arndt, R.; Bierle, D.M.; Borgen, M.D.; Hanson, S.N.; Hedin, M.C.; Lenehan, P.; et al. Casirivimab–Imdevimab treatment is associated with reduced rates of hospitalization among high-risk patients with mild to moderate coronavirus disease-19. EClinicalMedicine 2021, 40, 101102. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. Available online: https://www.covid19treatmentguidelines.nih.gov/ (accessed on 2 November 2023).
- Rubin, E.B.; Boiarsky, J.A.; Canha, L.A.; Giobbie-Hurder, A.; Liu, M.; Townsend, M.J.; Dougan, M. Bamlanivimab Efficacy in Older and High-BMI Outpatients With COVID-19 Selected for Treatment in a Lottery-Based Allocation Process. Open Forum Infect. Dis. 2021, 8, ofab546. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Ming, L.; Chen, L.; Zhu, X.; Shi, Y. The Effectiveness of Convalescent Plasma for the Treatment of Novel Corona Virus Disease 2019: A Systematic Review and Meta-Analysis. Front. Med. 2021, 8, 641429. [Google Scholar] [CrossRef] [PubMed]
- Klassen, S.A.; Senefeld, J.W.; Johnson, P.W.; Carter, R.E.; Wiggins, C.C.; Shoham, S.; Grossman, B.J.; Henderson, J.P.; Musser, J.; Salazar, E.; et al. The Effect of Convalescent Plasma Therapy on Mortality Among Patients With COVID-19: Systematic Review and Meta-analysis. Mayo Clin. Proc. 2021, 96, 1262–1275. [Google Scholar] [CrossRef]
- Senefeld, J.W.; Franchini, M.; Mengoli, C.; Cruciani, M.; Zani, M.; Gorman, E.K.; Focosi, D.; Casadevall, A.; Joyner, M.J. COVID-19 Convalescent Plasma for the Treatment of Immunocompromised Patients: A Systematic Review and Meta-analysis. JAMA Netw. Open 2023, 6, e2250647. [Google Scholar] [CrossRef]
- Malin, J.J.; Di Cristanziano, V.; Horn, C.; Pracht, E.; Borrega, J.G.; Heger, E.; Knops, E.; Kaiser, R.; Böll, B.; Lehmann, C.; et al. SARS-CoV-2–neutralizing antibody treatment in patients with COVID-19 and immunodeficiency due to B-cell non-Hodgkin lymphoma. Blood Adv. 2022, 6, 1580–1584. [Google Scholar] [CrossRef] [PubMed]
- Rabascall, C.X.; Lou, B.X.; Navetta-Modrov, B.; Hahn, S.S. Effective use of monoclonal antibodies for treatment of persistent COVID-19 infection in a patient on rituximab. BMJ Case Rep. 2021, 14, e243469. [Google Scholar] [CrossRef] [PubMed]
- D’abramo, A.; Vita, S.; Maffongelli, G.; Beccacece, A.; Agrati, C.; Cimini, E.; Colavita, F.; Giancola, M.L.; Cavasio, A.; Nicastri, E.; et al. Clinical Management of Patients With B-Cell Depletion Agents to Treat or Prevent Prolonged and Severe SARS-CoV-2 Infection: Defining a Treatment Pathway. Front. Immunol. 2022, 13, 911339. [Google Scholar] [CrossRef] [PubMed]
- Furie, N.; Mandelboim, M.; Zuckerman, N.; Belkin, A.; Seluk, L.; Shafran, I.; Mass, R.; Levy, L.; Chatterji, S.; Baltaxe, E.; et al. Persistent SARS-CoV-2 Pneumonia in Patients Treated with Anti-CD20 Monoclonal Antibodies. Open Forum Infect. Dis. 2023, 10, ofad464. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Fact Sheet for Healthcare Providers. Emergency Use Authorization (EUA) for Casirivmab and Imdevimab. Regeneron EUA HCP Fact Sheet 01242022 (fda.gov). Available online: https://www.regeneron.com/downloads/treatment-covid19-eua-fact-sheet-for-hcp.pdf (accessed on 10 December 2023).
- U.S. Food and Drug Administration. Fact Sheet for Healthcare Providers. Emergency Use Authorization (EUA) of Bamlanivimab and Etesevimab. Available online: https://www.fda.gov/media/145802/download (accessed on 10 December 2023).
- U.S. Food and Drug Administration. Fact Sheet for Healthcare Providers. Emergency Use Authorization (EUA) of Sotrovimab. Available online: https://www.fda.gov/media/149534/download (accessed on 10 December 2023).
- U.S. Food and Drug Administration. Fact Sheet for Healthcare Providers. Emergency Use Authorization (EUA) of COVID-19 Convalescent Plasma. Available online: https://www.fda.gov/media/141479/download (accessed on 10 December 2023).
- Patel, N.J.; D’Silva, K.M.; Hsu, T.Y.; DiIorio, M.; Fu, X.; Cook, C.; Prisco, L.; Martin, L.; Vanni, K.M.M.; Zaccardelli, A.; et al. Coronavirus Disease 2019 Outcomes Among Recipients of Anti-CD20 Monoclonal Antibodies for Immune-Mediated Diseases: A Comparative Cohort Study. ACR Open Rheumatol. 2022, 4, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Alhowaish, T.S.; Alhamadh, M.S.; Mathkour, A.; Alamoudi, M.; Alqahtani, H.A.; Alrashid, A. Clinical Course and Outcomes of COVID-19 Infection in Patients Treated with Rituximab: A Tertiary Care Center Experience. Open Access Rheumatol. Res. Rev. 2023, 15, 145–159. [Google Scholar] [CrossRef]
- Levavi, H.; Lancman, G.; Gabrilove, J. Impact of rituximab on COVID-19 outcomes. Ann. Hematol. 2021, 100, 2805–2812. [Google Scholar] [CrossRef]
- Gupta, A.; Gonzalez-Rojas, Y.; Juarez, E.; Casal, M.C.; Moya, J.; Falci, D.R.; Sarkis, E.; Solis, J.; Zheng, H.; Scott, N.; et al. Effect of Sotrovimab on Hospitalization or Death Among High-risk Patients with Mild to Moderate COVID-19: A Randomized Clinical Trial. JAMA 2022, 327, 1236–1246. [Google Scholar] [CrossRef]
- Weinreich, D.M.; Sivapalasingam, S.; Norton, T.; Ali, S.; Gao, H.; Bhore, R.; Xiao, J.; Hooper, A.T.; Hamilton, J.D.; Musser, B.J.; et al. REGEN-COV Antibody Combination and Outcomes in Outpatients with COVID-19. N. Engl. J. Med. 2021, 385, e81. [Google Scholar] [CrossRef]
- Dougan, M.; Nirula, A.; Azizad, M.; Mocherla, B.; Gottlieb, R.L.; Chen, P.; Hebert, C.; Perry, R.; Boscia, J.; Heller, B.; et al. Bamlanivimab plus Etesevimab in Mild or Moderate COVID-19. N. Engl. J. Med. 2021, 385, 1382–1392. [Google Scholar] [CrossRef]
- Simeunovic, G.; Polega, J.; Toor, S.; Andersen, N.J. Retrospective Analysis of Vaccinated and Unvaccinated COVID-19 Patients Treated with Monoclonal Antibodies (mAb) and Their Emergent Needs (RAVEN). Vaccines 2023, 11, 688. [Google Scholar] [CrossRef] [PubMed]
- Zitek, T.; Jodoin, K.; Kheradia, T.; Napolillo, R.; Dalley, M.T.; Quenzer, F.; Farcy, D.A. Vaccinated patients have reduced rates of hospitalization after receiving casirivimab and imdevimab for COVID-19. Am. J. Emerg. Med. 2021, 56, 370. [Google Scholar] [CrossRef] [PubMed]
- Self, W.H.; Sandkovsky, U.; Reilly, C.S.; Vock, D.M.; Gottlieb, R.L.; Mack, M.; Golden, K.; Dishner, E.; Vekstein, A.; Ko, E.R.; et al. Efficacy and safety of two neutralising monoclonal antibody therapies, sotrovimab and BRII-196 plus BRII-198, for adults hospitalised with COVID-19 (TICO): A randomised controlled trial. Lancet Infect. Dis. 2021, 22, 622–635. [Google Scholar] [CrossRef] [PubMed]
- RECOVERY Collaborative Group. Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet 2022, 399, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Somersan-Karakaya, S.; Mylonakis, E.; Menon, V.P.; Wells, J.C.; Ali, S.; Sivapalasingam, S.; Sun, Y.; Bhore, R.; Mei, J.; Miller, J.; et al. Casirivimab and Imdevimab for the Treatment of Hospitalized Patients with COVID-19. J. Infect. Dis. 2022, 227, 23–34. [Google Scholar] [CrossRef]
- Calabrese, C.M.; Kirchner, E.; Husni, E.M.; Moss, B.P.; Fernandez, A.P.; Jin, Y.; Calabrese, L.H. Breakthrough SARS-CoV-2 Infections in Patients with Immune-Mediated Disease Undergoing B Cell–Depleting Therapy: A Retrospective Cohort Analysis. Arthritis Rheumatol. 2022, 74, 1906–1915. [Google Scholar] [CrossRef]
- Andersen, K.M.; Bates, B.A.; Rashidi, E.S.; Olex, A.L.; Mannon, R.B.; Patel, R.C.; Singh, J.; Sun, J.; Auwaerter, P.G.; Ng, D.K.; et al. Long-term use of immunosuppressive medicines and in-hospital COVID-19 outcomes: A retrospective cohort study using data from the National COVID Cohort Collaborative. Lancet Rheumatol. 2021, 4, e33–e41. [Google Scholar] [CrossRef]
- Yusof, Y.M.; Arnold, J.; Saleem, B.; Vandevelde, C.; Dass, S.; Savic, S.; Vital, E.M.; Emery, P. Breakthrough SARS-CoV-2 infections and prediction of moderate-to-severe outcomes during rituximab therapy in patients with rheumatic and musculoskeletal diseases in the UK: A single-centre cohort study. Lancet Rheumatol. 2023, 5, e88–e98. [Google Scholar] [CrossRef]
- Simpson-Yap, S.; De Brouwer, E.; Kalincik, T.; Rijke, N.; Hillert, J.A.; Walton, C.; Edan, G.; Moreau, Y.; Spelman, T.; Geys, L.; et al. Associations of Disease-Modifying Therapies With COVID-19 Severity in Multiple Sclerosis. Neurology 2021, 97, e1870–e1885. [Google Scholar] [CrossRef]
- Tai, D.B.G.; Shah, A.; Doubeni, C.A.; Sia, I.G.; Wieland, M.L. The Disproportionate Impact of COVID-19 on Racial and Ethnic Minorities in the United States. Clin. Infect. Dis. 2021, 72, 703–706. [Google Scholar] [CrossRef]
- Yang, J.; Li, X.; He, T.; Ju, F.; Qiu, Y.; Tian, Z. Impact of Physical Activity on COVID-19. Int. J. Environ. Res. Public Health 2022, 19, 14108. [Google Scholar] [CrossRef] [PubMed]
| Total n = 64 (100%) | Outpatient n = 41 (64%) | Inpatient n = 23 (36%) | p-Value | |
|---|---|---|---|---|
| Age, years | 56.6 | 51.4 | 65.9 | |
| Sex, n (%) | 0.9738 | |||
| Male | 28 (44) | 18 (44) | 10 (43) | |
| Female | 36 (56) | 23 (56) | 13 (56) | |
| Race, n (%) | 0.0604 | |||
| Non-Hispanic Caucasian | 61 (95) | 41 (100) | 20 (87) | |
| Black or African-American | 2 (3) | 0(0) | 2 (9) | |
| Hispanic | 1 (2) | 0(0) | 1 (4) | |
| Vaccination status, n (%) | 0.0025 | |||
| Vaccinated | 43 (67) | 33 (80) | 10 (43) | |
| Unvaccinated | 21 (33) | 8 (20) | 13 (57) | |
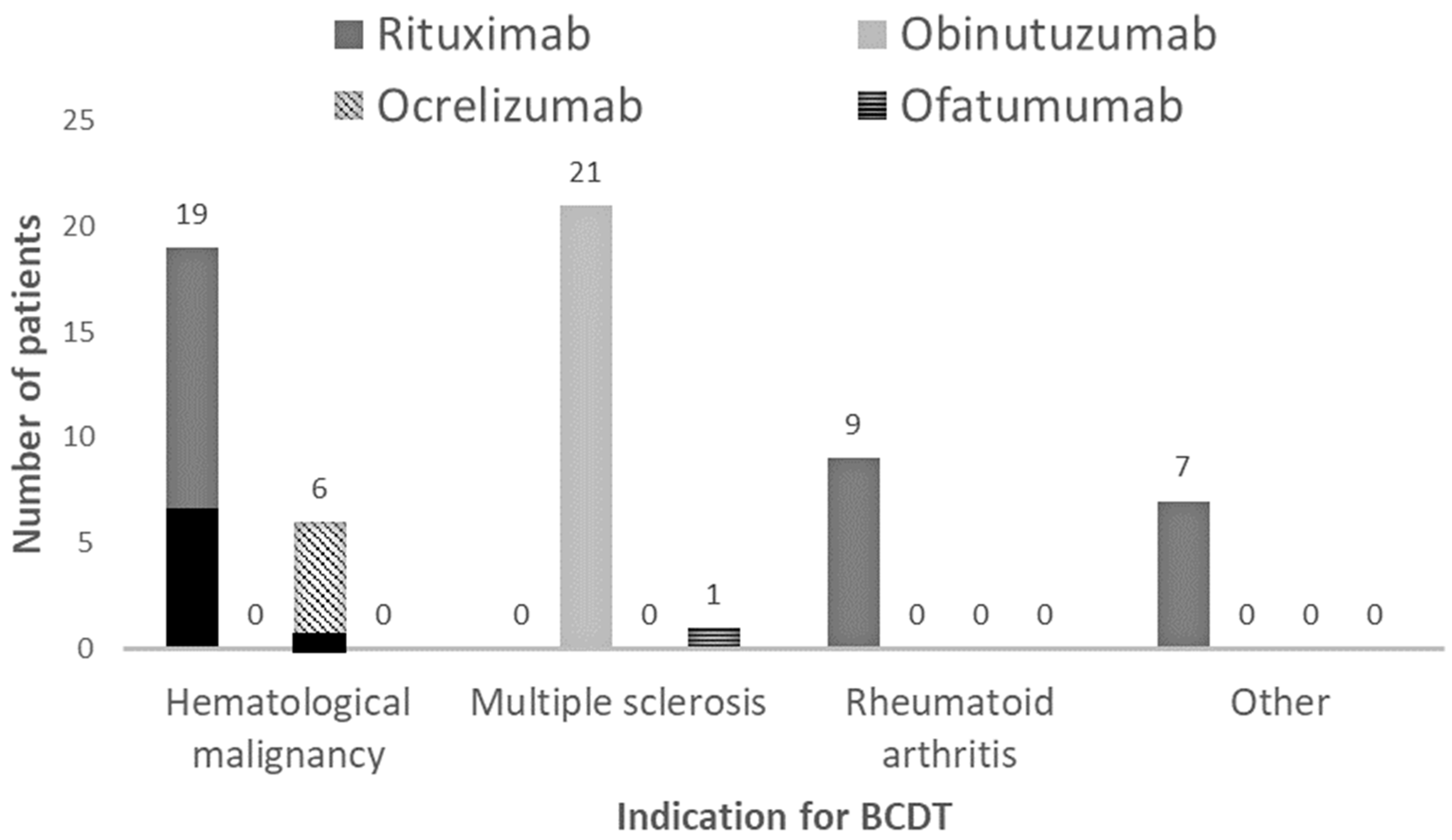
| Indication for BCDT, n (%) | 0.0002 | |||
| Hematological malignancy | 26 (40) | 9 (22) | 17 (74) | |
| Multiple sclerosis | 22 (34) | 21 (51) | 1 (4) | |
| Rheumatoid arthritis | 10 (16) | 7 (17) | 3 (13) | |
| Other * | 6 (10) | 4 (10) | 2 (9) | |
| Type of BCDT, n (%) | 0.0013 | |||
| Rituximab | 35 (55) | 18 (44) | 17 (74) | |
| Obinutuzumab | 21 (33) | 20 (49) | 1 (4) | |
| Ocrelizumab | 7 (11) | 2 (5) | 5 (22) | |
| Ofatumumab | 1 (1) | 1 (2) | 0 (0) | |
| Number of comorbidities, n (%) | 0.019 | |||
| Zero | 15 (23) | 13 (32) | 2 (9) | |
| One | 20 (31) | 14 (34) | 6 (3) | |
| Two | 10 (16) | 7 (17) | 3 (13) | |
| Three or more | 19 (30) | 7 (17) | 12 (52) | |
| Days from the symptom onset till PAT | <0.00001 | |||
| 0–7 | 40 (63) | 35 (85) | 5 (22) | |
| 8–14 | 8 (12) | 4 (10) | 4 (17) | |
| 14–21 | 3 (5) | 1 (2) | 2 (9) | |
| >21 | 13 (20) | 1 (2) | 12 (52) | |
| Type of PAT, n (%) | ||||
| mAb | 49 (77) | 41 (100) | 8 (35) | |
| Casirivimab–imdevimab | 31 (48) | 25 (4) | 8 (35) | |
| Sotrovimab | 13 (20) | 13 (32) | 0 | |
| Bamlanivimab–etesevimab | 4 (6) | 2 (5) | 0 | |
| Bebtelovimab | 1 (2) | 1 (2) | 0 | |
| CCP | 15 (23) | 0 | 15 (65) | |
| Additional COVID treatments | 0.1225 | |||
| Paxlovid | 1 (2) | 1 (2) | 0 | |
| Evusheld | 1 (2) | 1 (2) | 0 | |
| Remdesivir | 21 (33) | 4 (1) | 17 (74) | |
| Corticosteroids ** | 21 (33) | 5 (12) | 16 (69) | |
| Tocilizumab | 2 (4) | 0(0) | 2 (8) | |
| All-cause 90-day mortality | 7 (11) | 2 (4.88) | 5 (21.74) | 0.0381 |
| COVID-19-related mortality | 3 (4.69) | 0(0) | 3 (13.04) | 0.0178 |
| Total n = 64 (100%) | Survived n = 57 (89%) | Deceased n = 7 (11%) | p-Value | |
|---|---|---|---|---|
| Age, years | 56.6 | 54.9 | 70.4 | |
| Sex, n(%) | 0.4491 | |||
| Male | 28 (44) | 24 (42) | 4 (57) | |
| Female | 36 (56) | 34 (58) | 3 (43) | |
| Race, n (%) | 0.5039 | |||
| Non-Hispanic Caucasian | 61 (95) | 55 (96) | 6 (86) | |
| Black or African-American | 2 (3) | 1 (2) | 1 (14) | |
| Hispanic | 1 (2) | 1 (2) | 0 (0) | |
| Vaccination status, n (%) | 0.8001 | |||
| Vaccinated | 43 (67) | 38 (67) | 5 (71) | |
| Unvaccinated | 21 (33) | 19 (33) | 2 (29) | |
| Indication for BCDT, n (%) | 0.0093 | |||
| Hematological malignancy | 26 (40) | 19 (33) | 7 (100) | |
| Multiple sclerosis | 22 (34) | 22 (39) | 0 (0) | |
| Rheumatoid arthritis | 10 (16) | 10 (17) | 0 (0) | |
| Other | 6 (9) | 6 (11) | 0 (0) | |
| Type of BCDT, n (%) | 0.2441 | |||
| Rituximab | 35 (55) | 29 (51) | 6 (86) | |
| Obinutuzumab | 21 (33) | 21 (37) | 0 (0) | |
| Ocrelizumab | 7 (11) | 6 (11) | 1 (14) | |
| Ofatumumab | 1 (1) | 1 (1) | 0 (0) | |
| Number of comorbidities, n (%) | 0.0526 | |||
| Zero | 15 (23) | 15 (26) | 0 (0) | |
| One | 20 (31) | 18 (32) | 2 (29) | |
| Two | 10 (16) | 10 (17) | 0 (0) | |
| Three or more | 19 (30) | 14 (25) | 5 (71) | |
| Infusion location, n (%) | 0.0381 | |||
| Outpatient | 41 (64) | 39(68) | 2(29) | |
| Inpatient | 23 (36) | 18(32) | 5(71) | |
| Type of PAT, n (%) | ||||
| mAb | 49 (77) | 45 (79) | 4 (57) | 0.7708 |
| Casirivimab–imdevimab | 31 (48) | 28 (49) | 3 (43) | |
| Sotrovimab | 13 (20) | 12 (21) | 1 (4) | |
| Bamlanivimab–etesevimab | 4 (6) | 4 (7) | 0 (0) | |
| Bebtelovimab | 1 (2) | 1 (2) | 0 (0) | |
| CCP | 15 (23) | 12 (21) | 3 (43) | |
| Additional COVID treatments | 0.0940 | |||
| Paxlovid | 1 (2) | 1 (2) | 0 (0) | |
| Evusheld | 1 (2) | 1 (2) | 0 (0) | |
| Remdesivir | 21 (33) | 17 (30) | 4 (57) | |
| Corticosteroids | 21 (33) | 17 (30) | 4 (57) | |
| Tocilizumab | 2 (4) | 0 (0) | 2 (28) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gentile, S.; Sullivan, L.R.; Brooks, H.; Simeunovic, G. A Descriptive, Retrospective Analysis of COVID-19 Passive Antibody Therapy and Its Effects on Morbidity and Mortality in Patients Receiving B-Cell-Depleting Therapies. Diseases 2024, 12, 33. https://doi.org/10.3390/diseases12020033
Gentile S, Sullivan LR, Brooks H, Simeunovic G. A Descriptive, Retrospective Analysis of COVID-19 Passive Antibody Therapy and Its Effects on Morbidity and Mortality in Patients Receiving B-Cell-Depleting Therapies. Diseases. 2024; 12(2):33. https://doi.org/10.3390/diseases12020033
Chicago/Turabian StyleGentile, Sonia, Liam R. Sullivan, Heather Brooks, and Gordana Simeunovic. 2024. "A Descriptive, Retrospective Analysis of COVID-19 Passive Antibody Therapy and Its Effects on Morbidity and Mortality in Patients Receiving B-Cell-Depleting Therapies" Diseases 12, no. 2: 33. https://doi.org/10.3390/diseases12020033
APA StyleGentile, S., Sullivan, L. R., Brooks, H., & Simeunovic, G. (2024). A Descriptive, Retrospective Analysis of COVID-19 Passive Antibody Therapy and Its Effects on Morbidity and Mortality in Patients Receiving B-Cell-Depleting Therapies. Diseases, 12(2), 33. https://doi.org/10.3390/diseases12020033






