Abstract
Lean body mass is a significant component of survival from sepsis. Several equations can be used for calculating lean body mass based on age, sex, body weight, and height. We hypothesized that lean body mass is a better predictor of outcomes than the body mass index (BMI). This study used a multicenter cohort study database. The inclusion criteria were age ≥18 years and a diagnosis of sepsis or septic shock. BMI was classified into four categories: underweight (<18.5 kg/m2), normal (≥18.5–<25 kg/m2), overweight (≥25–<30 kg/m2), and obese (≥30 kg/m2). Four lean body mass equations were used and categorized on the basis of quartiles. The outcome was in-hospital mortality among different BMI and lean body mass groups. Among 85,558 patients, 3916 with sepsis were included in the analysis. Regarding BMI, in-hospital mortality was 36.9%, 29.8%, 26.7%, and 27.9% in patients who were underweight, normal weight, overweight, and obese, respectively (p < 0.01). High lean body mass did not show decreased mortality in all four equations. In critically ill patients with sepsis, BMI was a better predictor of in-hospital mortality than the lean body mass equation at intensive care unit (ICU) admission. To precisely predict in-hospital mortality, ICU-specific lean body mass equations are needed.
1. Introduction
Muscle is an important component of the body [1]. Muscle contractions enable physical functions, including movements and posture stabilization [2]. In addition to physical functions, muscles play a crucial role in the pumping of blood, temperature management, energy storage, immunological functions, and the production of various cytokines known as myokines [3]. The condition characterized by decreased muscle mass is termed sarcopenia [4]. Recognizing sarcopenia is important because it is associated with the risk of disease progression [5]. Therefore, muscle is considered a vital organ for survival [6,7].
In critically ill patients with sepsis, lean body mass, including muscle mass, is a significant predictor of outcomes [8,9]. A previous study reported that lean body mass at intensive care unit (ICU) admission was associated with survival and physical impairments at discharge [10,11]. Decreased lean body mass reflects a malnutrition status [12]. International clinical nutrition societies recommend the assessment of muscle mass, the main lean body mass component, using the Global Leadership Initiative on Malnutrition (GLIM) criteria for malnutrition assessment [13,14]. Screening is recommended within 24–48 h following ICU admission [15,16]. Therefore, lean body mass assessment is imperative during ICU admission.
Although lean body mass assessment is significant during ICU admission, the method for assessing lean body mass is unclear in critically ill patients. Bioelectrical impedance analysis or dual-energy X-ray absorptiometry can be used for lean body mass assessment [17]. However, in critically ill patients, dynamic fluid changes can affect these assessments [18]. Computed tomography is frequently used for the retrospective analysis of lean body mass assessment; however, it requires transfer to the examination room and exposes patients to radiation. Ultrasonography is an emerging tool for lean body mass assessment. Ultrasonography-based muscle mass assessment is applied in acute [19] or chronic diseases [20]; however, it is not widely used owing to a lack of technical skills. In a previous questionnaire survey, ultrasonography-based muscle mass assessment was conducted only in 14% of health care workers [21].
One type of lean body mass assessment is calculation using equations. As obesity is assessed by calculating the body mass index (BMI), calculating lean body mass from the equation is reasonable. Several studies have reported equations for lean body mass assessment [22,23,24,25]; these equations are significantly correlated with computed tomography-based lean body mass in critically ill patients [26]. As several studies have reported that BMI is a significant predictor of outcomes in critically ill patients, we hypothesized that these lean body mass equations could be more useful for assessing mortality in critically ill patients compared to BMI. In this study, we aimed to compare the calculated lean body mass and BMI for predicting the outcomes of critically ill adults using the Japanese intensive care database.
2. Materials and Methods
2.1. Study Design
This is a retrospective study using a multicenter cohort study database in the Japanese Intensive Care Patient Database (JIPAD). This study was approved by both the clinical research ethics committees of Tokushima University Hospital (approval number 3721) and the administration office of JIPAD [27]. This study was registered as a clinical trial (UMIN—Clinical Trials Registry: 000039754).
2.2. JIPAD
JIPAD is a database of critically ill patients in Japan, established by the Japanese Society of Intensive Care Medicine in 2014. We used the dataset from April 2014 to March 2018. The database included 46 ICUs as of 2018. The quality of the data was maintained by the certification system of the registerer, who passed registration training and quality tests. Furthermore, the quality of the database was periodically examined by the administration office. The dataset was anonymized for analysis.
2.3. Patients
Inclusion criteria included (1) age ≥18 years and (2) a diagnosis of sepsis in the primary or secondary disease name record, which included sepsis and septic shock from any infection source. The exclusion criteria involved the presence of missing data and apparently abnormal data for height and body weight required for BMI and lean body mass calculation.
2.4. BMI
BMI was calculated by dividing weight (kg) by height squared (m2). It was classified into the following four categories based on the World Health Organization: underweight (<18.5 kg/m2), normal (≥18.5–<25 kg/m2), overweight (≥25–<30 kg/m2), and obese (≥30 kg/m2) [28].
2.5. Lean Body Mass Calculation
Lean body mass was calculated for each patient using the following four separate equations and classified into four categories on the basis of quartiles (lowest, low, high, and highest quartiles).
Equation (1) by Kulkarni et al. [22]:
Males: lean body mass (kg) = −15.605 − (0.032 × age [y]) + (0.192 × height [cm]) + (0.502 × weight [kg])
Females: lean body mass (kg) = −15.034 − (0.018 × age [y]) + (0.165 × height [cm]) + (0.409 × weight [kg])
Females: lean body mass (kg) = −15.034 − (0.018 × age [y]) + (0.165 × height [cm]) + (0.409 × weight [kg])
Equation (2) by Weijs et al. [23]:
Lean body mass (kg) = weight (kg) × 0.01 × (100 − [64.5 − 848 × height {m}2/weight {kg} + 0.079 × age {y} − 16.4 × sex (1 [male], 0 [female]) + 0.05 × sex (1 [male], 0 [female]) × age (y) + 39.0 × sex (1 [male], 0 [female]) × height [m]2/weight [kg])
Equation (3) by Janmahasatian et al. [24]:
Males: lean body mass (kg) = (9.27 × 103 × weight [kg])/(6.68 × 103 + 216 × BMI [kg/m2])
Females: lean body mass (kg) = (9.27 × 103 × weight [kg])/(8.78 × 103 + 244 × BMI [kg/m2])
Females: lean body mass (kg) = (9.27 × 103 × weight [kg])/(8.78 × 103 + 244 × BMI [kg/m2])
Equation (4) by Hume et al. [25]:
Lean body mass (kg) = 0.32810 × weight (kg) + 0.33929 × height (cm) − 29.5336
2.6. Outcome
The outcome was in-hospital mortality. Mortality differences were compared among individuals with underweight, normal weight, overweight, and obesity in BMI, as well as across four quartile categories of lean body mass using the Kulkarni, Weijs, Janmahasatian, and Hume et al. formulas.
2.7. Variables
JIPAD data used in the analysis included the following: age; sex; weight; height; comorbidities; date of ICU admission/discharge; ICU admission route, including (1) transfer from the ward, (2) through the emergency room, (3) following elective surgery, and (4) following urgent surgery; primary diagnosis code; secondary diagnosis code; mechanical ventilation; Acute Physiology and Chronic Health Evaluation (APACHE) II and III scores; Simplified Acute Physiology Score (SAPS) II score; and Sequential Organ Failure Assessment (SOFA) score.
2.8. Statistical Analysis
Continuous variables were presented as means ± standard deviations or medians (interquartile ranges). Categorical variables were expressed as numbers and percentages. Categorical variables were compared using the χ2 test. Bonferroni correction was performed for multiple tests of secondary outcomes. Two-sided p values < 0.05 were considered statistically significant. The sample size was not calculated beforehand owing to the exploratory nature of this study. All statistical analyses were performed using Statistical Package for the Social Sciences (version 27, IBM, Armonk, NY, USA).
3. Results
3.1. Patient Characteristics
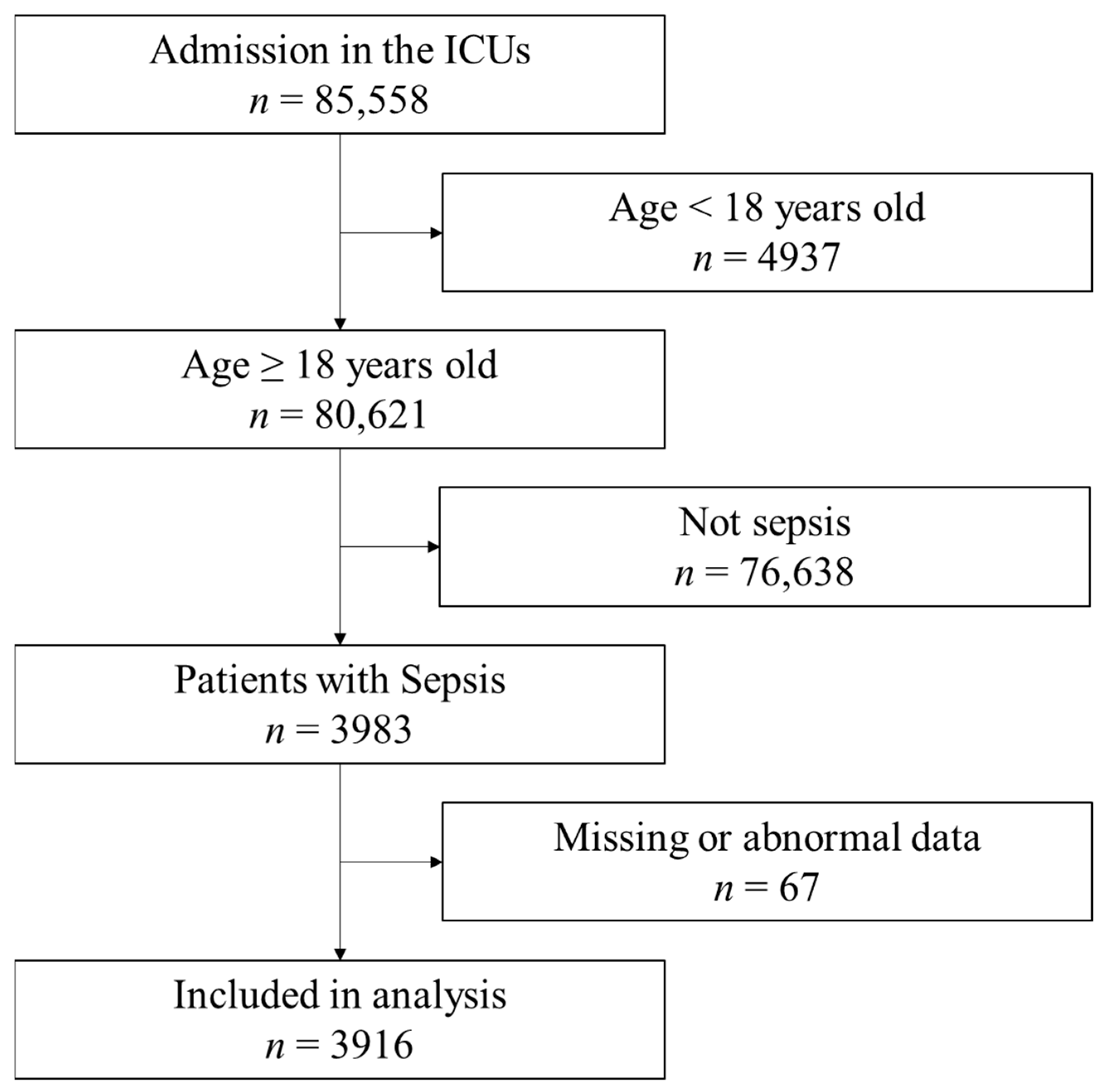
During the study period, 85,558 patients were admitted to the ICUs (Figure 1). Among them, 3983 patients met the inclusion criteria. Sixty-seven patients had missing or abnormal data and were excluded. Finally, 3916 samples were included in the analysis. The characteristics of patients are shown in Table 1. The median age of patients was 73 (64–81) years, and 2399 (61.3%) patients were males. Sepsis without and with urinary tract infection were 24.7% and 4.2%, respectively. Septic shock without and with urinary tract infection were 58.9% and 12.1%, respectively. The mortality among the included patients was 1230 (30.7%). The median BMI was 21.6 (19.0–24.5) kg/m2. The patient characteristics based on different BMIs and lean body masses are shown in Table 2.
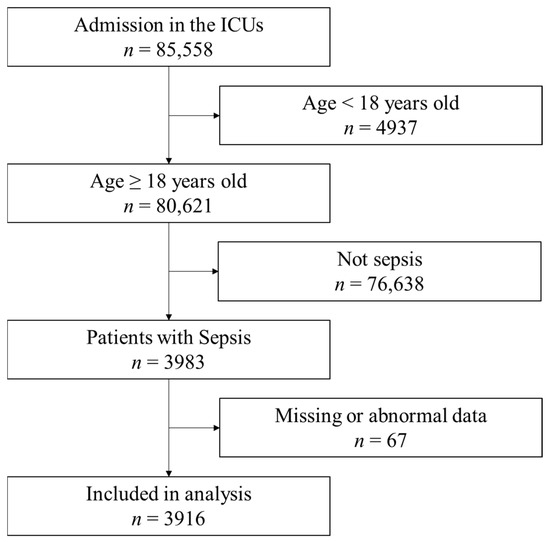
Figure 1.
Flow of data selection from the Japanese Intensive Care Patient Database. Out of the 85,558 registry entries, the analysis included 3,916 patients. ICU: intensive care unit.

Table 1.
Characteristics of Patients.

Table 2.
Characteristics of patients with different BMIs and lean body masses.
3.2. Outcomes
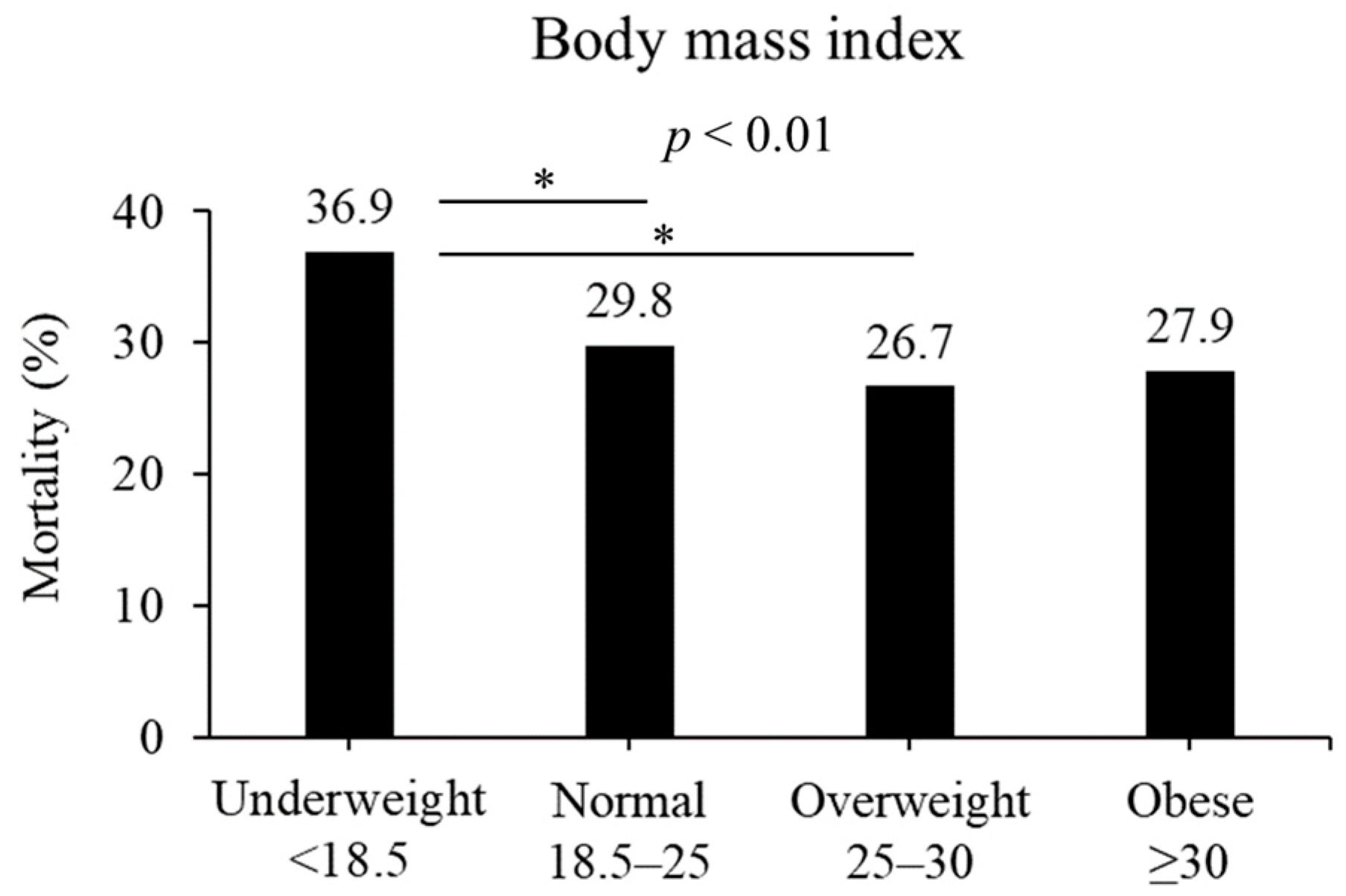
In terms of BMI, the in-hospital mortality rates differed among various weight categories: 36.9%, 29.8%, 26.7%, and 27.9% for underweight, normal weight, overweight, and obesity, respectively (p < 0.01, Figure 2). Among them, patients classified as being overweight and normal weight exhibited a significant difference compared with those classified as being underweight (overweight, p < 0.05; normal, p < 0.05).
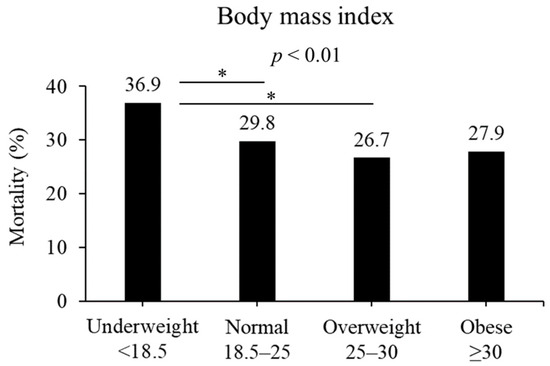
Figure 2.
Mortality based on BMI (kg/m2). Mortality was different in the four BMI groups (p < 0.01). Underweight patients had significantly higher mortality than normal or overweight in post hoc analysis. * p < 0.05 in post hoc Bonferroni tests.
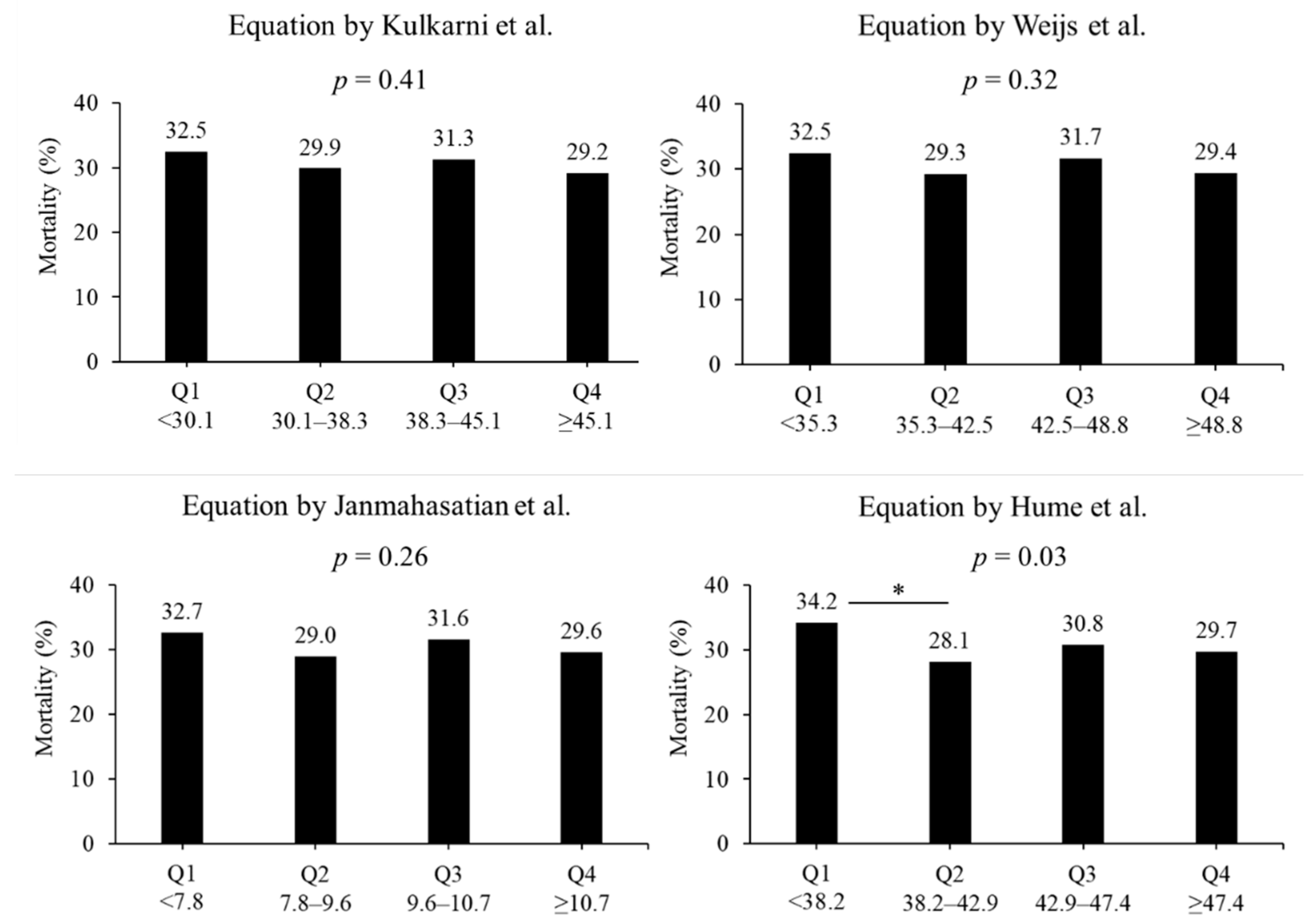
In terms of the lean body mass equation, no difference in the in-hospital mortality rate was observed. In the equation by Kulkarni et al., the mortality rates were 32.5%, 29.9%, 31.3%, and 29.2% in quartiles 1, 2, 3, and 4, respectively (p = 0.41, Figure 3). In the equation by Weijs et al., the mortality rates were 32.5%, 29.3%, 31.7%, and 29.4% in quartiles 1, 2, 3, and 4, respectively (p = 0.32). In the equation by Janmahasatian et al., the mortality rates were 32.7%, 29.0%, 31.6%, and 29.6% in quartiles 1, 2, 3, and 4, respectively (p = 0.41). In the equation by Hume et al., the mortality rates were 34.2%, 28.1%, 30.8%, and 29.7% in quartiles 1, 2, 3, and 4, respectively (p = 0.03). The morality rates between quartiles 1 and 2 had a significant difference in the equation by Hume et al. (p < 0.05).
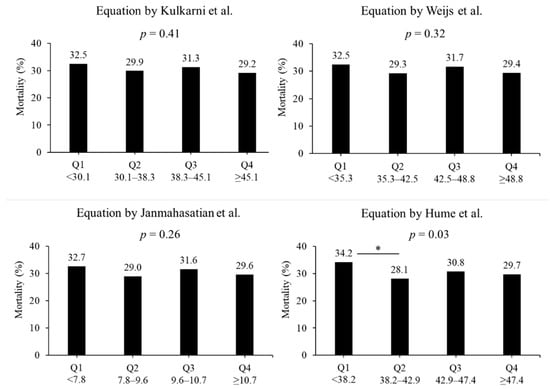
Figure 3.
Mortality based on four lean body mass equations. Four equations include the formula reported by Kulkarni et al. [22], Weijs et al. [23], Janmahasatian et al. [24], and Hume et al. [25] Groups were divided into lowest quartile, Q1, to highest quartile, Q4. * p < 0.05 in post hoc Bonferroni tests. Q: quartile.
4. Discussion
In this study, we observed that BMI at ICU admission was associated with in-hospital mortality in patients with sepsis. However, contrary to our hypothesis, most calculated lean body mass values were not associated with in-hospital mortality, and all equations did not show lower mortality with increased lean body mass. As lean body mass is reportedly associated with mortality in critically ill patients, the utility of lean body mass calculation was unreliable in this population. BMI was a reliable indicator of in-hospital mortality in critically ill adults with sepsis.
Consistent with a previous meta-analysis [26], BMI was a strong indicator of in-hospital mortality in patients with sepsis. In our study, the obese category had slightly higher mortality than the overweight category, and the obese category did not have a statistical difference in in-hospital mortality. This can be explained by the obesity paradox. Owing to the limited sample size, the obese category included very obese patients (≥40 kg/m2). This very obese category reportedly has increased mortality, known as the obesity paradox [29]. As the BMI results were consistent with those of previous studies [30,31,32], our data are reliable.
In a previous study, the four equations used for estimating lean body mass were reportedly correlated with lean body mass quantified using computed tomography (0.680–0.756, p < 0.001) [26]. However, they reported that the equation overestimated the lean body mass. This overestimation may have contributed to the nonsignificant outcomes in patients with sepsis. Although a significant difference was partially observed in the equation by Hume et al., the significant difference was inconsistent in the higher quartile population, suggesting unreliable results. Therefore, this equation is considered insufficient for predicting mortality.
To reliably estimate lean body mass, ICU-specific equations are needed. The equation is important not only for predicting mortality but also for assessing nutritional status [13]. Malnutrition is common in critically ill patients, reported to be 38–78% [33] and is associated with poor clinical outcomes, including postoperative complications and mortality [34,35]. As these equations include only age, sex, body weight, and height, reliable equations may require blood tests or anthropometric measurements. These equations will contribute to the prediction of outcomes and the assessment of nutrition status in critically ill patients with sepsis.
Because there are no reliable ICU-specific equations to estimate lean body mass, the establishment of muscle mass assessment may be another strategy for muscle mass assessment at the ICU admission. Bioelectrical impedance analysis, dual-energy X-ray absorptiometry, computed tomography, and ultrasonography are available methods for muscle mass assessment [36]. These methods have pros and cons in terms of their utility [37]. Ultrasonography is recommended because it is noninvasive and not affected by dynamic fluid changes [18]. Although accurate assessment using ultrasonography is influenced by its technical skills, the skill can be acquired through ultrasonography training [38]. Ultrasonography-based muscle mass assessment can be conducted prospectively in the upper [39] or lower limbs [40], which reflect whole-body muscle mass at the ICU admission. Therefore, ultrasonography may become an alternative method for reliable ICU-specific equations.
This study has some limitations. First, the data may include incorrect information. Although data quality was controlled by JIPAD quality control measures, the data may include some incorrect data because we excluded abnormal data. Second, the applicability to different populations requires further studies. The proportion of obesity is different among the populations. Using the Japanese population database, we observed that the BMI is generally lower than that in other countries. Therefore, validity in different populations is required. Third, other potential confounders, including disease severity and comorbidities, may have affected mortality. In this study, we focused on the difference between BMI and calculated lean body mass. Therefore, we did not perform a multivariate analysis. Fourth, this database distinguished between sepsis, sepsis along with a urinary tract infection, septic shock, and septic shock along with a urinary tract infection. Therefore, we could not identify the cause of sepsis except for a urinary tract infection. Because sepsis is caused by various sources, further studies are required on the different infection sources.
5. Conclusions
In critically ill patients with sepsis, BMI was a better predictor than calculated lean body mass at ICU admission. To reliably estimate lean body mass and mortality, ICU-specific equations are needed.
Author Contributions
Conceptualization, N.N.; methodology, N.N.; data curation, R.S.; writing—original draft preparation, R.S.; supervision, M.I., J.O. and J.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was partly supported by a crowdfunding project entitled the Muscle Atrophy Zero Project using the platform “Otsucle” https://otsucle.jp/cf/project/2553.html (accessed on 27 March 2020).
Institutional Review Board Statement
This study was approved by the clinical research ethics committees of Tokushima University Hospital on 25 May 2020 (approval number 3721).
Informed Consent Statement
Patient consent was waived owing to the deidentified nature of the data analyzed.
Data Availability Statement
The datasets used in this study are not available since the dataset was obtained from the Japanese Intensive Care Patient Database through a formal request/approval process.
Acknowledgments
The authors thank all those who supported the Muscle Atrophy Zero Project, which aims to prevent muscle atrophy in patients.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Pedersen, B.K. Muscle as a secretory organ. Compr. Physiol. 2013, 3, 1337–1362. [Google Scholar] [CrossRef]
- Wojtara, T.; Alnajjar, F.; Shimoda, S.; Kimura, H. Muscle synergy stability and human balance maintenance. J. NeuroEngineering Rehabil. 2014, 11, 129. [Google Scholar] [CrossRef]
- Rai, M.; Demontis, F. Muscle-to-brain signaling via myokines and myometabolites. Brain Plast. 2022, 8, 43–63. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef]
- Yuan, S.; Larsson, S.C. Epidemiology of sarcopenia: Prevalence, risk factors, and consequences. Metabolism 2023, 144, 155533. [Google Scholar] [CrossRef]
- Au, P.C.; Li, H.L.; Lee, G.K.; Li, G.H.; Chan, M.; Cheung, B.M.; Wong, I.C.; Lee, V.H.; Mok, J.; Yip, B.H.; et al. Sarcopenia and mortality in cancer: A meta-analysis. Osteoporos. Sarcopenia 2021, 7, S28–S33. [Google Scholar] [CrossRef]
- Jogiat, U.M.; Sasewich, H.; Turner, S.R.; Baracos, V.; Eurich, D.T.; Filafilo, H.; Bédard, E.L.R. Sarcopenia determined by skeletal muscle index predicts overall survival, disease-free survival, and postoperative complications in resectable esophageal cancer: A systematic review and meta-analysis. Ann. Surg. 2022, 276, e311–e318. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Huang, Y.; Chen, Y.; Shen, X.; Pan, H.; Yu, W. Impact of muscle mass on survival in patients with sepsis: A systematic review and meta-analysis. Ann. Nutr. Metab. 2021, 77, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Looijaard, W.; Molinger, J.; Weijs, P.J.M. Measuring and monitoring lean body mass in critical illness. Curr. Opin. Crit. Care 2018, 24, 241–247. [Google Scholar] [CrossRef]
- Jaitovich, A.; Dumas, C.L.; Itty, R.; Chieng, H.C.; Khan, M.M.H.S.; Naqvi, A.; Fantauzzi, J.; Hall, J.B.; Feustel, P.J.; Judson, M.A. ICU admission body composition: Skeletal muscle, bone, and fat effects on mortality and disability at hospital discharge—A prospective, cohort study. Crit. Care 2020, 24, 566. [Google Scholar] [CrossRef]
- Thackeray, M.; Mohebbi, M.; Orford, N.; Kotowicz, M.A.; Pasco, J.A. Lean mass as a risk factor for intensive care unit admission: An observational study. Crit. Care 2021, 25, 364. [Google Scholar] [CrossRef]
- Deutz, N.E.P.; Ashurst, I.; Ballesteros, M.D.; Bear, D.E.; Cruz-Jentoft, A.J.; Genton, L.; Landi, F.; Laviano, A.; Norman, K.; Prado, C.M. The underappreciated role of low muscle mass in the management of malnutrition. J. Am. Med. Dir. Assoc. 2019, 20, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Jensen, G.L.; Correia, M.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 38, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Compher, C.; Cederholm, T.; Correia, M.; Gonzalez, M.C.; Higashiguch, T.; Shi, H.P.; Bischoff, S.C.; Boirie, Y.; Carrasco, F.; Cruz-Jentoft, A.; et al. Guidance for assessment of the muscle mass phenotypic criterion for the Global Leadership Initiative on Malnutrition diagnosis of malnutrition. JPEN J. Parenter. Enteral Nutr. 2022, 46, 1232–1242. [Google Scholar] [CrossRef]
- Narayan, S.K.; Gudivada, K.K.; Krishna, B. Assessment of nutritional status in the critically ill. Indian. J. Crit. Care Med. 2020, 24, S152–S156. [Google Scholar] [CrossRef]
- Mueller, C.; Compher, C.; Ellen, D.M.; American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) Board of Directors. A.S.P.E.N. Clinical guidelines: Nutrition screening, assessment, and intervention in adults. JPEN J. Parenter. Enteral Nutr. 2011, 35, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2018, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, N.; Tsutsumi, R.; Okayama, Y.; Takashima, T.; Ueno, Y.; Itagaki, T.; Tsutsumi, Y.; Sakaue, H.; Oto, J. Monitoring of muscle mass in critically ill patients: Comparison of ultrasound and two bioelectrical impedance analysis devices. J. Intensive Care 2019, 7, 61. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, J.; Gu, Q.; Gu, Y.; Zhao, Y.; Ge, X.; Sun, X.; Lian, J.; Zeng, Q. Changes in muscle ultrasound for the diagnosis of intensive care unit acquired weakness in critically ill patients. Sci. Rep. 2021, 11, 18280. [Google Scholar] [CrossRef]
- Sánchez Romero, E.A.; Alonso Pérez, J.L.; Muñoz Fernández, A.C.; Battaglino, A.; Castaldo, M.; Cleland, J.A.; Villafañe, J.H. Reliability of sonography measures of the lumbar multifidus and transversus abdominis during static and dynamic activities in subjects with non-specific chronic low back pain. Diagnostics 2021, 11, 632. [Google Scholar] [CrossRef]
- Nawata, K.; Nakanishi, N.; Inoue, S.; Liu, K.; Nozoe, M.; Ono, Y.; Yamada, I.; Katsukawa, H.; Kotani, J. Current practice and barriers in the implementation of ultrasound-based assessment of muscle mass in Japan: A nationwide, web-based cross-sectional study. PLoS ONE 2022, 17, e0276855. [Google Scholar] [CrossRef]
- Kulkarni, B.; Kuper, H.; Taylor, A.; Wells, J.C.; Radhakrishna, K.V.; Kinra, S.; Ben-Shlomo, Y.; Smith, G.D.; Ebrahim, S.; Byrne, N.M.; et al. Development and validation of anthropometric prediction equations for estimation of lean body mass and appendicular lean soft tissue in Indian men and women. J. Appl. Physiol. 2013, 115, 1156–1162. [Google Scholar] [CrossRef]
- Weijs, P.J.; Sauerwein, H.P.; Kondrup, J. Protein recommendations in the ICU: G protein/kg body weight—Which body weight for underweight and obese patients? Clin. Nutr. 2012, 31, 774–775. [Google Scholar] [CrossRef]
- Janmahasatian, S.; Duffull, S.B.; Ash, S.; Ward, L.C.; Byrne, N.M.; Green, B. Quantification of lean bodyweight. Clin. Pharmacokinet. 2005, 44, 1051–1065. [Google Scholar] [CrossRef] [PubMed]
- Hume, R. Prediction of lean body mass from height and weight. J. Clin. Pathol. 1966, 19, 389–391. [Google Scholar] [CrossRef]
- Moisey, L.L.; Mourtzakis, M.; Kozar, R.A.; Compher, C.; Heyland, D.K. Existing equations to estimate lean body mass are not accurate in the critically ill: Results of a multicenter observational study. Clin. Nutr. 2017, 36, 1701–1706. [Google Scholar] [CrossRef] [PubMed]
- Irie, H.; Okamoto, H.; Uchino, S.; Endo, H.; Uchida, M.; Kawasaki, T.; Kumasawa, J.; Tagami, T.; Shigemitsu, H.; Hashiba, E.; et al. The Japanese Intensive care PAtient Database (JIPAD): A national intensive care unit registry in Japan. J. Crit. Care 2020, 55, 86–94. [Google Scholar] [CrossRef]
- Executive summary of the clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. Arch. Intern. Med. 1998, 158, 1855–1867. [CrossRef]
- Hutagalung, R.; Marques, J.; Kobylka, K.; Zeidan, M.; Kabisch, B.; Brunkhorst, F.; Reinhart, K.; Sakr, Y. The obesity paradox in surgical intensive care unit patients. Intensive Care Med. 2011, 37, 1793–1799. [Google Scholar] [CrossRef] [PubMed]
- Yeo, H.J.; Kim, T.H.; Jang, J.H.; Jeon, K.; Oh, D.K.; Park, M.H.; Lim, C.-M.; Kim, K.; Cho, W.H.; Korean Sepsis Alliance (KSA) Investigators. Obesity paradox and functional outcomes in sepsis: A multicenter prospective study. Crit. Care Med. 2023, 51, 742–752. [Google Scholar] [CrossRef]
- Wang, S.; Liu, X.; Chen, Q.; Liu, C.; Huang, C.; Fang, X. The role of increased body mass index in outcomes of sepsis: A systematic review and meta-analysis. BMC Anesthesiol. 2017, 17, 118. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Kudo, D.; Kushimoto, S.; Hasegawa, M.; Ito, F.; Yamanouchi, S.; Honda, H.; Andoh, K.; Furukawa, H.; Yamada, Y.; et al. Associations between low body mass index and mortality in patients with sepsis: A retrospective analysis of a cohort study in Japan. PLoS ONE 2021, 16, e0252955. [Google Scholar] [CrossRef] [PubMed]
- Lew, C.C.H.; Yandell, R.; Fraser, R.J.L.; Chua, A.P.; Chong, M.F.F.; Miller, M. Association between malnutrition and clinical outcomes in the intensive care unit: A systematic review. JPEN J. Parenter. Enteral Nutr. 2017, 41, 744–758. [Google Scholar] [CrossRef] [PubMed]
- Soloff, M.A.; Vargas, M.V.; Wei, C.; Ohnona, A.; Tyan, P.; Gu, A.; Georgakopoulos, B.; Thomas, C.A.; Quan, T.; Barishansky, S.; et al. Malnutrition is associated with poor postoperative outcomes following laparoscopic hysterectomy. Jsls 2021, 25, e2020.00084. [Google Scholar] [CrossRef]
- Lew, C.C.H.; Wong, G.J.Y.; Cheung, K.P.; Chua, A.P.; Chong, M.F.F.; Miller, M. Association between malnutrition and 28-Day mortality and intensive care length-of-stay in the critically ill: A prospective cohort study. Nutrients 2017, 10, 10. [Google Scholar] [CrossRef]
- Nakanishi, N.; Okura, K.; Okamura, M.; Nawata, K.; Shinohara, A.; Tanaka, K.; Katayama, S. Measuring and monitoring skeletal muscle mass after stroke: A review of current methods and clinical applications. J. Stroke Cerebrovasc. Dis. 2021, 30, 105736. [Google Scholar] [CrossRef]
- Holmes, C.J.; Racette, S.B. The utility of body composition assessment in nutrition and clinical practice: An overview of current methodology. Nutrients 2021, 13, 2493. [Google Scholar] [CrossRef]
- Nakanishi, N.; Inoue, S.; Tsutsumi, R.; Akimoto, Y.; Ono, Y.; Kotani, J.; Sakaue, H.; Oto, J. Rectus femoris mimicking ultrasound phantom for muscle mass assessment: Design, research, and training application. J. Clin. Med. 2021, 10, 2721. [Google Scholar] [CrossRef]
- Nakanishi, N.; Inoue, S.; Ono, Y.; Sugiyama, J.; Takayama, K.; Arai, Y.; Nakamura, K.; Oto, J.; Kotani, J. Ultrasound-based upper limb muscle thickness is useful for screening low muscularity during intensive care unit admission: A retrospective study. Clin. Nutr. ESPEN 2023, 57, 569–574. [Google Scholar] [CrossRef]
- Arai, Y.; Nakanishi, N.; Ono, Y.; Inoue, S.; Kotani, J.; Harada, M.; Oto, J. Ultrasound assessment of muscle mass has potential to identify patients with low muscularity at intensive care unit admission: A retrospective study. Clin. Nutr. ESPEN 2021, 45, 177–183. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).