1. Introduction
Acute cholangitis has been increasingly associated with multidrug-resistant (MDR) organisms, complicating treatment strategies [
1]. The prevalence of acute cholangitis has seen an upward trend, with studies indicating an increase in hospitalizations for cholangitis in the United States between 2005 and 2014, reflecting a growing burden of gallstone disease and associated complications [
2,
3,
4]. This rise is paralleled by a growing concern over the emergence of MDR pathogens, which pose a significant threat to patient outcomes [
5,
6].
The microbial spectrum in acute cholangitis has evolved over the years. Initially dominated by
Escherichia coli and
Klebsiella pneumoniae, recent studies have reported a diversification in the microbial species involved, including the rise of anaerobic bacteria [
7,
8,
9]. This shift in the microbial landscape has important implications for antibiotic therapy, as the efficacy of traditional regimens is increasingly being questioned [
10]. The role of cholecystectomy, a common treatment for gallstone-related diseases, in altering microbial patterns and resistance profiles in acute cholangitis patients remains underexplored [
11].
Multidrug resistance in acute cholangitis is a growing concern, with studies reporting resistance rates as high as 30–40% in certain regions [
12]. The mechanisms of resistance are varied, including extended-spectrum beta-lactamases (ESBLs) and carbapenemases, which significantly limit treatment options [
13,
14]. The clinical impact of these MDR organisms is profound, often leading to prolonged hospital stays, increased healthcare costs, and higher mortality rates [
15,
16]. The relationship between cholecystectomy and the development of MDR in cholangitis patients is a critical area of investigation, with potential implications for both surgical and pharmacological management.
Evidence suggests that patient outcomes in acute cholangitis are heavily influenced by the timely administration of appropriate antimicrobial therapy [
17]. However, the rise of MDR organisms has made the selection of effective antibiotics increasingly complex [
18]. The need for empirical therapy that covers a broad range of potential pathogens, while also considering local resistance patterns, is essential [
19]. This challenge is further compounded in post-cholecystectomy patients, where alterations in bile flow and gut microbiota may affect the susceptibility and presence of various microbial species [
20].
Recent advances in microbial sequencing and resistance pattern analysis offer new opportunities to understand the complex interplay between microbial species and drug resistance in acute cholangitis [
21]. These technologies hold promise for the development of more targeted and effective treatment strategies, particularly in the context of post-cholecystectomy patients [
22,
23,
24]. However, there remains a significant gap in comprehensive studies that integrate microbial species analysis with multidrug resistance patterns in this patient group.
Given the lack of published data on acute cholangitis in the healthcare setting of Romania, our study aims to fill this significant gap by analyzing the specific microbial spectrum and multidrug resistance patterns prevalent in the Romanian population. This approach is critical, as local bacterial profiles and resistance trends can markedly differ from global patterns, and are in constant change, necessitating an ongoing data report, and different strategies for effective management and treatment of acute cholangitis, particularly in the context of post-cholecystectomy care.
In this study, we hypothesize that acute cholangitis in post-cholecystectomy patients is characterized by a unique microbial spectrum and distinct multidrug resistance patterns compared to non-cholecystectomy patients. Our aim is to conduct a comparative analysis of microbial species and multidrug resistance patterns in these two patient groups, providing insights that could inform more effective management strategies for acute cholangitis in the context of cholecystectomy.
2. Materials and Methods
2.1. Research Framework and Ethical Considerations
This retrospective study was conducted at a major regional hospital in western Romania, specializing in gastroenterology and hepatobiliary diseases. The study period spanned from 2020 to 2023, focusing on patients diagnosed with acute cholangitis who had a history of cholecystectomy. Ethical approval was obtained from the hospital’s Institutional Review Board, in alignment with the principles of the Declaration of Helsinki. Confidentiality and privacy of patient data were strictly maintained. Informed consent was obtained from all participants before data collection.
Patients in both the case and control groups had a confirmed diagnosis of acute cholangitis as per the Tokyo guidelines and were treated with antibiotics, according to standard of care. The control group included patients with a history of cholecystectomy, chosen to compare the impact of gallbladder removal and biliary tree manipulation on microbial spectrum and resistance patterns. Bile samples were collected from all patients as a necessary procedure in the diagnosis and management of acute cholangitis, providing data on the bacterial profile and antibiotic sensitivity testing.
2.2. Participant Selection and Sample Collection
For this study, acute cholangitis (AC) was diagnosed by adhering to the criteria outlined in the latest clinical guidelines (Tokyo Guidelines TG18) [
25]. Enrolled participants were categorized into two groups: Group A (patients with a history of cholecystectomy) and Group B (patients without a history of cholecystectomy), identified at their initial hospital admission. Those with a history of AC post-endoscopic retrograde cholangiopancreatography (ERCP) or currently on antibiotics for other conditions were excluded. Patients were also excluded from the study if they refused to share their data, if they lacked the ability to consent, and had missing medical information.
Following the diagnosis of acute cholangitis, all participants received antibiotic therapy tailored to their specific classification of AC severity, in accordance with the latest guidelines. The treatment regimens varied from ampicillin/sulbactam, ciprofloxacin, or levofloxacin for milder cases, to more potent antibiotics such as ceftriaxone or meropenem for severe instances. Bile and blood samples were collected for microbial analysis.
Upon hospitalization for moderate to severe acute cholangitis, in accordance with the TG18 recommendations, blood cultures were promptly initiated. Bile samples were collected after cannulation using a sphincterotome, prior to initiating any therapeutic intervention. To minimize contamination, the first 5 mL of bile was discarded, and the subsequent 5 mL was collected into a sterile container designed for both anaerobic and aerobic bacterial culturing. These specimens were then incubated at 37 °C for at least seven days or until microbial growth was detected. The VITEK
® 2 system (bioMérieux, Marcy-l′Étoile, France) was employed to determine the antibiotic susceptibility, specifically the minimum inhibitory concentration (MIC), of the isolates. The results were interpreted in line with current clinical guidelines [
26]. Antibiotic resistance and susceptibility were established based on the standards and criteria set forth by the Clinical and Laboratory Standards Institute (CLSI) for all bacteria cultured [
27].
Additional diagnostic measures, such as B-mode sonography and endoscopic ultrasound (EUS), were employed to determine the nature of biliary obstruction. ERCP, conducted with high-precision instruments, was used both for diagnostic and therapeutic purposes. Antibiotic sensitivity was determined using the disk diffusion method.
2.3. Data Acquisition, and Study Variables
Data was collected from the hospital database and patients’ medical records and reviewed by two independent researchers. All inconsistencies between findings were evaluated by a third physician, who reviewed the data. Demographic data (age, sex, age range), clinical history (including cholecystectomy history for Group A), presenting symptoms, ERCP timing, the Tokyo severity score, etiology of obstruction, and duration of illness were recorded. Laboratory data included white blood count, inflammatory tests (CRP), and liver function tests (total bilirubin, number of platelets, and INR). The primary variables of interest were the identification of microbial species in bile samples and their antibiotic resistance patterns as follows: ESBL—extended-spectrum beta-lactamases; MRSA—methicillin-resistant Staphylococcus Aureus; VRE—vancomycin-resistant Enterococci; CRE—carbapenem-resistant Enterobacteriaceae; MDR—multidrug-resistant.
2.4. Statistical Analysis
Data analysis was performed using SPSS version 27. Descriptive statistics provided a summary of demographic and clinical characteristics. The proportions of microbial species and resistance patterns between the two groups were compared using the Chi-square test or Fisher’s exact test, based on the frequency assumptions. For continuous data, we calculated the independent samples t-test when comparing two means, and the Mann–Whitney U test to compare two medians. A p-value < 0.05 was considered statistically significant.
For hypothesis testing, it was assumed that post-cholecystectomy patients have a higher likelihood of presenting with more complex microbial profiles in their bile cultures, as well as having a higher risk of developing antibiotic resistance, compared to non-cholecystectomy patients. The dependent variable was the complexity of the microbial profile in bile cultures, categorized based on the number of different bacterial species identified (sterile, 1 bacterium, 2 bacteria, ≥3 bacteria). The independent variable of primary interest was the cholecystectomy status. The chosen covariates were age, gender, total bilirubin, CRP levels, and the Tokyo severity score. These variables were included to adjust for potential confounders. Logistic regression models were used to identify factors independently associated with microbial complexity and resistance patterns.
3. Results
Table 1 presents the background characteristics of patients, divided into those with and without cholecystectomy. The analysis included a total of 488 patients, with 102 who had a history of cholecystectomy and 386 who did not go through the procedure. Age distribution showed a slightly higher mean age in the cholecystectomy group (68.7 years) compared to the non-cholecystectomy group (66.3 years), but this difference was not statistically significant (
p-value = 0.062). Gender distribution was fairly balanced in both groups, with males constituting 47.1% of the cholecystectomy group and 53.1% of the non-cholecystectomy group.
The prevalence of symptoms like abdominal pain, jaundice, and fever and chills were similar in both groups, with no significant differences (p-values: 0.704, 0.722, and 0.948, respectively). In terms of ERCP (endoscopic retrograde cholangiopancreatography) timing, a comparable distribution was observed across emergent, urgent, and late categories in both groups (p-value = 0.755).
The duration of hospital stay was slightly higher in the cholecystectomy group (7.3 days, IQR 4.9) compared to the non-cholecystectomy group (7.1 days, IQR 5.1), but this difference was not statistically significant (p-value = 0.543). Tokyo severity scores, categorized into grades I, II, and III, showed a balanced distribution across both groups, with no significant differences observed (p-value = 0.379). A higher percentage of malignant causes was observed in the non-cholecystectomy group (52.6%) compared to the cholecystectomy group (42.2%), while benign causes were more common in the cholecystectomy group (57.8%) compared to the non-cholecystectomy group (47.4%), with no statistical significance (p-value = 0.060).
C-reactive protein (CRP) levels were significantly higher in the cholecystectomy group (129.5 mg/L) compared to the non-cholecystectomy group (109.1 mg/L), with a p-value of 0.032, suggesting a significantly stronger inflammatory response in patients who had a history of cholecystectomy. Total bilirubin levels were also significantly higher among those who had cholecystectomy (7.9 g/dL vs. 6.7 g/dL, p-value = 0.041), indicating a potential difference in liver function or biliary obstruction between the two groups.
In terms of bile culture results, a significant difference was observed between the study groups (p-value < 0.001). In the cholecystectomy group, 18.6% had sterile cultures, while 33.4% of the non-cholecystectomy group had sterile cultures. The presence of one bacterium was slightly more common in the cholecystectomy group (41.2% vs. 38.3%), whereas 27.5% of cholecystectomy patients had two bacteria compared to 25.1% in the non-cholecystectomy group. Notably, a higher proportion of patients in the cholecystectomy group had cultures with ≥ three bacteria (12.7%) compared to the non-cholecystectomy group (3.1%).
Blood culture results also showed a significant difference between acute cholangitis patients with a previous history of cholecystectomy and those without (
p-value = 0.005). Sterile cultures were more common in the non-cholecystectomy group (60.0%) compared to the cholecystectomy group (56.0%). In the cholecystectomy group, 36.0% had cultures with one bacterium, compared to 28.8% in the non-cholecystectomy group. Both groups had a low prevalence of cultures with two bacteria and no cases with ≥ three bacteria, as presented in
Table 2.
Table 3 presents a comparison in the etiology of obstruction in patients with and without cholecystectomy. The majority of cases in both groups were due to choledocholithiasis, with a notably high prevalence in the cholecystectomy group (96.6% or 57 cases) compared to the non-cholecystectomy group (91.3% or 167 cases). However, this difference was not statistically significant (
p-value = 0.172). Other benign causes, such as Vaterian ampulloma, benign choledochal stenosis, Mirizzi syndrome, and liver abscess were relatively rare, and their proportions did not significantly differ between the two groups, with
p-values of 0.199, 0.426, 0.976, and 0.569, respectively.
In terms of malignant obstructions, pancreatic cancer was the most common cause in both groups, with 53.5% (23 cases) in the cholecystectomy group and 55.7% (113 cases) in the non-cholecystectomy group. This similarity was statistically non-significant (p-value = 0.794). Other malignant causes, including cholangiocarcinoma, malignant Vaterian ampulloma, malignant extrinsic compression, and gallbladder cancer, were also compared. None of these causes showed a significant difference in prevalence between the cholecystectomy and non-cholecystectomy groups.
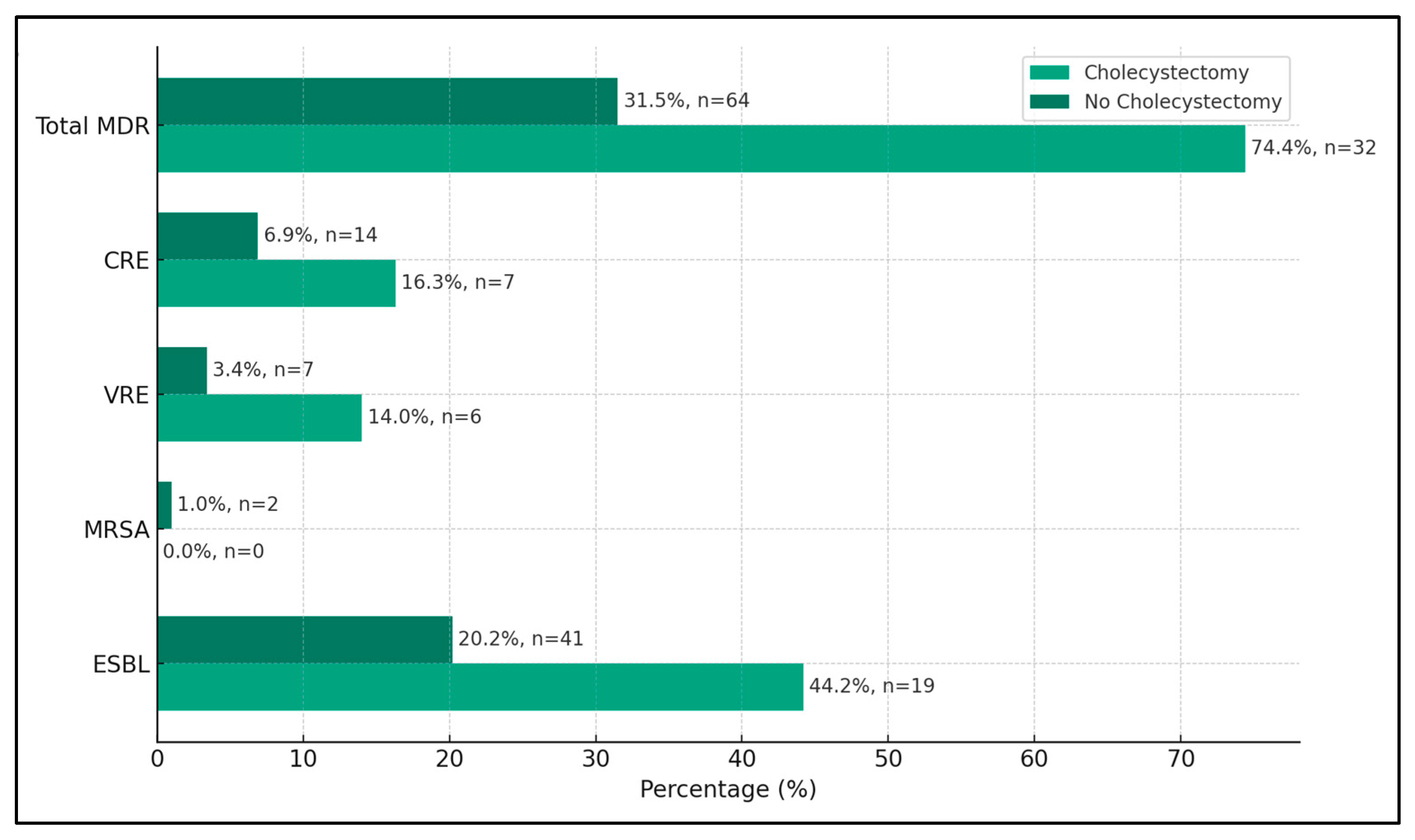
A significant finding was the high prevalence of extended-spectrum beta-lactamases (ESBLs)-producing organisms in the cholecystectomy group, accounting for 44.2% (19 cases) of the isolates, compared to 20.2% (41 cases) in the non-cholecystectomy group. This difference was statistically significant (p-value < 0.001), indicating a stronger association of ESBLs with patients who underwent cholecystectomy. The presence of methicillin-resistant Staphylococcus Aureus (MRSA) was relatively low in both groups, with no cases in the cholecystectomy group and 1.0% (two cases) in the non-cholecystectomy group. This difference was not statistically significant (p-value = 0.513), suggesting that the cholecystectomy status did not significantly impact the occurrence of MRSA in bile cultures.
Vancomycin-resistant Enterococci (VRE) were more prevalent in the cholecystectomy group, with 14.0% (six cases) compared to 3.4% (7 cases) in the non-cholecystectomy group. This difference was statistically significant (
p-value = 0.005), suggesting a possible link between cholecystectomy and the presence of VRE. Carbapenem-resistant Enterobacteriaceae (CRE) were also found more frequently in the cholecystectomy group, at 16.3% (seven cases) compared to 6.9% (14 cases) in the non-cholecystectomy group, with a statistically significant difference (
p-value = 0.046). Overall, the total incidence of multidrug-resistant organisms was markedly higher in the cholecystectomy group at 74.4% (32 cases) compared to 31.5% (64 cases) in the non-cholecystectomy group (
p-value < 0.001), as seen in
Table 4 and
Figure 1.
Among the Gram-negative bacteria, E. coli was identified significantly more frequently in patients with cholecystectomy (40.2%) compared to those without (28.2%), with a p-value of 0.019. Klebsiella spp. were found in 15.7% (16 cases) of the cholecystectomy group and 19.2% (74 cases) of the non-cholecystectomy group, but this difference was not statistically significant (p-value = 0.419). Pseudomonas spp. and Enterobacter spp. showed a higher prevalence in the cholecystectomy group, with significant differences (21.6% vs. 2.8% and 13.7% vs. 4.4%, respectively; p-values < 0.001). Acinetobacter spp. and Citrobacter spp. were present in similar proportions in both groups, with no significant differences (p-values 0.133 and 0.379, respectively).
For Gram-positive bacteria,
Enterococcus spp. were significantly more prevalent in the cholecystectomy group (32.4% or 33 cases) compared to the non-cholecystectomy group (21.2% or 82 cases), with a
p-value of 0.018.
Streptococcus spp. and
Staphylococcus spp. were also more commonly found in the cholecystectomy group, with significant differences observed (12.7% vs. 1.6% and 8.8% vs. 2.8%, respectively;
p-values < 0.001 and 0.007), as seen in
Table 5.
Piperacillin/Tazobactam resistance was significantly higher in the cholecystectomy group (23.5%) compared to the non-cholecystectomy group (11.7%), with a p-value of 0.002. Resistance to Fluoroquinolones (Ciprofloxacin/Levofloxacin) was observed in 6.9% of the cholecystectomy group, significantly lower than the 16.8% in the non-cholecystectomy group (p-value = 0.011). Penems (Meropenem/Imipenem) resistance was significantly higher in the cholecystectomy group (22.5%) than in the non-cholecystectomy group (9.6%), with a p-value of less than 0.001.
There was no significant difference in resistance to second generation cephalosporins (p-value = 0.473) and third generation cephalosporins (p-value = 0.203) between the two groups. However, resistance to fourth generation cephalosporins was significantly different, with 7.8% in the cholecystectomy group compared to 17.9% in the non-cholecystectomy group (p-value = 0.013).
Aminoglycoside (Gentamicin/Amikacin) resistance showed no significant difference between the groups (
p-value = 0.483). Resistance to Ticarcillin/Clavulanic acid was also not significant, with 20.6% in the cholecystectomy group and 11.4% in the non-cholecystectomy group (
p-value = 0.087). Piperacillin resistance was significantly higher in the cholecystectomy group (56.9%) compared to the non-cholecystectomy group (27.5%), with a
p-value of less than 0.001, as presented in
Table 6.
Table 7 focuses on the development of antibiotic resistance. The odds ratio (OR) for cholecystectomy status was 3.17 (CI = 1.30 to 4.46), indicating that patients with cholecystectomy were more than three times as likely to develop antibiotic resistance compared to those without (
p-value < 0.001). The total bilirubin levels had an OR of 1.08 (1.02–1.64) and were statistically significant (
p-value = 0.008), suggesting a modest association with the development of antibiotic resistance.
Resistance to Piperacillin/Tazobactam had an OR of 3.25 (1.75–6.05) with a
p-value of less than 0.001, indicating a strong association with the development of resistance. Similarly, resistance to Penems (OR = 2.80, 1.50–5.21,
p-value = 0.001) and Piperacillin (OR = 2.45, 1.35–4.40,
p-value = 0.003) were significantly associated with increased odds of resistance. Conversely, resistance to fourth generation cephalosporins (OR = 0.50, 0.28–0.96,
p-value = 0.020) and Fluoroquinolones (OR = 0.45, 0.25–0.83,
p-value = 0.007) were associated with a reduced likelihood of developing resistance, as presented in
Figure 2.
The regression analysis found that cholecystectomy status had a significant association with the development of complex bacterial infections. Patients with cholecystectomy had an odds ratio of 2.45 (CI = 1.56–3.84), indicating they were more than twice as likely to develop complex infections compared to those without cholecystectomy, with a p-value of 0.002. Age and gender, with ORs of 1.02 (0.99–1.05) and 1.18 (0.74–1.89) respectively, did not show a significant association with the development of complex bacterial infections.
Total bilirubin levels were found to be significantly associated with the development of complex infections, with an OR of 1.10 (CI = 1.03–1.18,
p-value = 0.007), suggesting that higher bilirubin levels might be a risk factor for more complex infections. CRP levels also showed a significant association, with an OR of 1.04 (1.01–1.07) and a
p-value of 0.015, indicating that elevated CRP levels might be predictive of more complex bacterial profiles. However, the Tokyo severity score, a clinical measure used in the management of cholangitis, showed an OR of 1.29 (0.82–2.03) but was not statistically significant in predicting the development of complex infections (
p-value = 0.273), as seen in
Table 8.