Nanotechnology Platform for Advancing Vaccine Development against the COVID-19 Virus
Abstract
1. Introduction
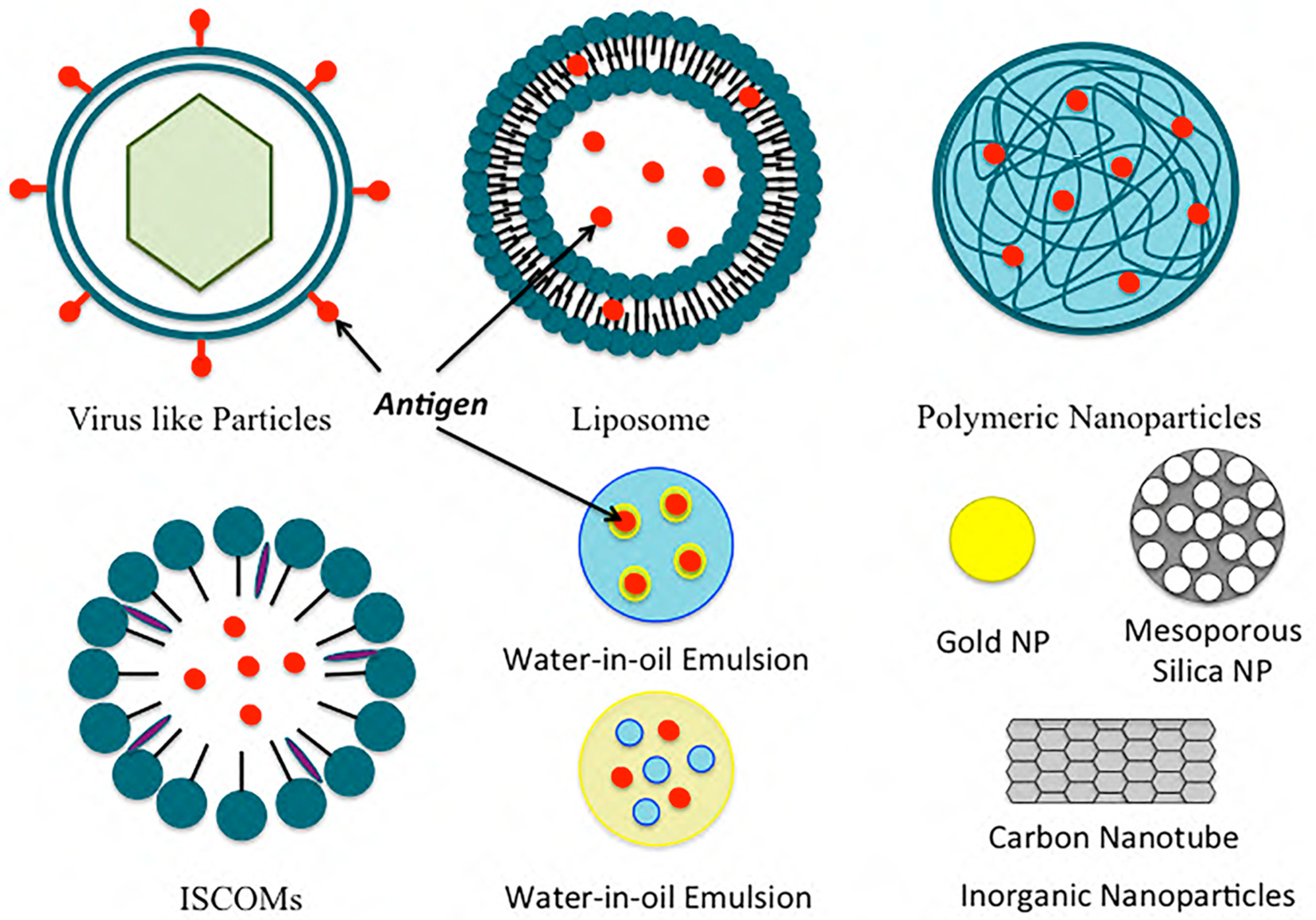
2. Nanoparticle Vaccine Adjuvants and Delivery Systems
2.1. Polymeric Nanoparticles
2.2. Inorganic Nanoparticles
2.3. Liposomes
2.4. Immunostimulatory Complexes (ISCOMs)
2.5. Emulsions
2.6. Virus-like Particles and Virosomes
3. The Vaccine Development Approach for Coronavirus
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19. 2020. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed on 5 August 2023).
- Chattopadhyay, S.; Chen, J.Y.; Chen, H.W.; Hu, C.J. Nanoparticle Vaccines Adopting Virus-like Features for Enhanced Immune Potentiation. Nanotheranostics 2017, 1, 244–260. [Google Scholar] [CrossRef] [PubMed]
- Vartak, A.; Sucheck, S.J. Recent Advances in Subunit Vaccine Carriers. Vaccines 2016, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Boni, M.F. Vaccination and antigenic drift in influenza. Vaccine 2008, 26 (Suppl. S3), C8–C14. [Google Scholar] [CrossRef] [PubMed]
- Demento, S.L.; Cui, W.; Criscione, J.M.; Stern, E.; Tulipan, J.; Kaech, S.M.; Fahmy, T.M. Role of sustained antigen release from nanoparticle vaccines in shaping the T cell memory phenotype. Biomaterials 2012, 33, 4957–4964. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Rawat, A.; Hope-Weeks, L.; Ahsan, F. Aerosolized PLA and PLGA nanoparticles enhance humoral, mucosal and cytokine responses to hepatitis B vaccine. Mol. Pharm. 2011, 8, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Bershteyn, A.; Hanson, M.C.; Crespo, M.P.; Moon, J.J.; Li, A.V.; Suh, H.; Irvine, D.J. Robust IgG responses to nanograms of antigen using a biomimetic lipid-coated particle vaccine. J. Control. Release 2012, 157, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.J.; Suh, H.; Polhemus, M.E.; Ockenhouse, C.F.; Yadava, A.; Irvine, D.J. Antigen-displaying lipid-enveloped PLGA nanoparticles as delivery agents for a Plasmodium vivax malaria vaccine. PLoS ONE 2012, 7, e31472. [Google Scholar] [CrossRef]
- Mann, J.F.; McKay, P.F.; Arokiasamy, S.; Patel, R.K.; Klein, K.; Shattock, R.J. Pulmonary delivery of DNA vaccine constructs using deacylated PEI elicits immune responses and protects against viral challenge infection. J. Control. Release 2013, 170, 452–459. [Google Scholar] [CrossRef]
- Saini, V.; Jain, V.; Sudheesh, M.S.; Dixit, S.; Gaur, R.L.; Sahoo, M.K.; Joseph, S.K.; Verma, S.K.; Jaganathan, K.S.; Murthy, P.K.; et al. Humoral and cell-mediated immune-responses after administration of a single-shot recombinant hepatitis B surface antigen vaccine formulated with cationic poly(l-lactide) microspheres. J. Drug Target. 2010, 18, 212–222. [Google Scholar] [CrossRef]
- Gupta, P.N.; Vyas, S.P. Investigation of lectinized liposomes as M-cell targeted carrier-adjuvant for mucosal immunization. Colloids Surf. B Biointerfaces 2011, 82, 118–125. [Google Scholar] [CrossRef]
- Ulery, B.D.; Kumar, D.; Ramer-Tait, A.E.; Metzger, D.W.; Wannemuehler, M.J.; Narasimhan, B. Design of a protective single-dose intranasal nanoparticle-based vaccine platform for respiratory infectious diseases. PLoS ONE 2011, 6, e17642. [Google Scholar] [CrossRef] [PubMed]
- Lugade, A.A.; Bharali, D.J.; Pradhan, V.; Elkin, G.; Mousa, S.A.; Thanavala, Y. Single low-dose un-adjuvanted HBsAg nanoparticle vaccine elicits robust, durable immunity. Nanomedicine 2013, 9, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Mehrabi, M.; Dounighi, N.M.; Rezayat, S.M.; Doroud, D.; Amani, A.; Khoobi, M.; Ajdary, S. Novel approach to improve vaccine immunogenicity: Mannosylated chitosan nanoparticles loaded with recombinant hepatitis B antigen as a targeted vaccine delivery system. J. Drug Deliv. Sci. Technol. 2018, 44, 19–26. [Google Scholar] [CrossRef]
- Nochi, T.; Yuki, Y.; Takahashi, H.; Sawada, S.; Mejima, M.; Kohda, T.; Harada, N.; Kong, I.G.; Sato, A.; Kataoka, N.; et al. Nanogel antigenic protein-delivery system for adjuvant-free intranasal vaccines. Nat. Mater. 2010, 9, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Slütter, B.; Bal, S.; Keijzer, C.; Mallants, R.; Hagenaars, N.; Que, I.; Kaijzel, E.; van Eden, W.; Augustijns, P.; Löwik, C.; et al. Nasal vaccination with N-trimethyl chitosan and PLGA based nanoparticles: Nanoparticle characteristics determine quality and strength of the antibody response in mice against the encapsulated antigen. Vaccine 2010, 28, 6282–6291. [Google Scholar] [CrossRef] [PubMed]
- Sarei, F.; Dounighi, N.M.; Zolfagharian, H.; Khaki, P.; Bidhendi, S.M. Alginate nanoparticles as a promising adjuvant and vaccine delivery system. Indian J. Pharm. Sci. 2013, 75, 442–449. [Google Scholar] [CrossRef]
- Moon, S.H.; Shin, E.C.; Noh, Y.W.; Lim, Y.T. Evaluation of hyaluronic acid-based combination adjuvant containing monophosphoryl lipid A and aluminum salt for hepatitis B vaccine. Vaccine 2015, 33, 4762–4769. [Google Scholar] [CrossRef]
- Sanchez-Villamil, J.I.; Tapia, D.; Torres, A.G. Development of a Gold Nanoparticle Vaccine against Enterohemorrhagic Escherichia coli O157:H7. mBio 2019, 10, e01869-19. [Google Scholar] [CrossRef]
- Niikura, K.; Matsunaga, T.; Suzuki, T.; Kobayashi, S.; Yamaguchi, H.; Orba, Y.; Kawaguchi, A.; Hasegawa, H.; Kajino, K.; Ninomiya, T.; et al. Gold nanoparticles as a vaccine platform: Influence of size and shape on immunological responses in vitro and in vivo. ACS Nano 2013, 7, 3926–3938. [Google Scholar] [CrossRef]
- Wang, T.; Zou, M.; Jiang, H.; Ji, Z.; Gao, P.; Cheng, G. Synthesis of a novel kind of carbon nanoparticle with large mesopores and macropores and its application as an oral vaccine adjuvant. Eur. J. Pharm. Sci. 2011, 44, 653–659. [Google Scholar] [CrossRef]
- Schreiber, H.A.; Prechl, J.; Jiang, H.; Zozulya, A.; Fabry, Z.; Denes, F.; Sandor, M. Using carbon magnetic nanoparticles to target, track, and manipulate dendritic cells. J. Immunol. Methods 2010, 356, 47–59. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Montalvo-Quirós, S.; Vallet-Regí, M.; Palacios, A.; Anguita, J.; Prados-Rosales, R.C.; González, B.; Luque-Garcia, J.L. Mesoporous Silica Nanoparticles as a Potential Platform for Vaccine Development against Tuberculosis. Pharmaceutics 2020, 12, 1218. [Google Scholar] [CrossRef] [PubMed]
- Neuhaus, V.; Chichester, J.A.; Ebensen, T.; Schwarz, K.; Hartman, C.E.; Shoji, Y.; Guzmán, C.A.; Yusibov, V.; Sewald, K.; Braun, A. A new adjuvanted nanoparticle-based H1N1 influenza vaccine induced antigen-specific local mucosal and systemic immune responses after administration into the lung. Vaccine 2014, 32, 3216–3222. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, N.P.; Olsen, A.; Buonsanti, C.; Follmann, F.; Zhang, Y.; Coler, R.N.; Fox, C.B.; Meinke, A.; D’Oro, U.; Casini, D.; et al. Different human vaccine adjuvants promote distinct antigen-independent immunological signatures tailored to different pathogens. Sci. Rep. 2016, 6, 19570. [Google Scholar] [CrossRef] [PubMed]
- Morçöl, T.; Hurst, B.L.; Tarbet, E.B. Calcium phosphate nanoparticle (CaPNP) for dose-sparing of inactivated whole virus pandemic influenza A (H1N1) 2009 vaccine in mice. Vaccine 2017, 35, 4569–4577. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.J.; Suh, H.; Bershteyn, A.; Stephan, M.T.; Liu, H.; Huang, B.; Sohail, M.; Luo, S.; Um, S.H.; Khant, H.; et al. Interbilayer-crosslinked multilamellar vesicles as synthetic vaccines for potent humoral and cellular immune responses. Nat. Mater. 2011, 10, 243–251. [Google Scholar] [CrossRef]
- Mašek, J.; Bartheldyová, E.; Turánek-Knotigová, P.; Skrabalová, M.; Korvasová, Z.; Plocková, J.; Koudelka, S.; Skodová, P.; Kulich, P.; Křupka, M.; et al. Metallochelating liposomes with associated lipophilised norAbuMDP as biocompatible platform for construction of vaccines with recombinant His-tagged antigens: Preparation, structural study and immune response towards rHsp90. J. Control. Release 2011, 151, 193–201. [Google Scholar] [CrossRef]
- Kaur, R.; Bramwell, V.W.; Kirby, D.J.; Perrie, Y. Pegylation of DDA:TDB liposomal adjuvants reduces the vaccine depot effect and alters the Th1/Th2 immune responses. J. Control. Release 2012, 158, 72–77. [Google Scholar] [CrossRef]
- Milicic, A.; Kaur, R.; Reyes-Sandoval, A.; Tang, C.K.; Honeycutt, J.; Perrie, Y.; Hill, A.V. Small cationic DDA:TDB liposomes as protein vaccine adjuvants obviate the need for TLR agonists in inducing cellular and humoral responses. PLoS ONE 2012, 7, e34255. [Google Scholar] [CrossRef]
- McNeil, S.E.; Rosenkrands, I.; Agger, E.M.; Andersen, P.; Perrie, Y. Subunit vaccines: Distearoylphosphatidylcholine-based liposomes entrapping antigen offer a neutral alternative to dimethyldioctadecylammonium-based cationic liposomes as an adjuvant delivery system. J. Pharm. Sci. 2011, 100, 1856–1865. [Google Scholar] [CrossRef]
- Rosenkrands, I.; Vingsbo-Lundberg, C.; Bundgaard, T.J.; Lindenstrøm, T.; Enouf, V.; van der Werf, S.; Andersen, P.; Agger, E.M. Enhanced humoral and cell-mediated immune responses after immunization with trivalent influenza vaccine adjuvanted with cationic liposomes. Vaccine 2011, 29, 6283–6291. [Google Scholar] [CrossRef] [PubMed]
- Henriksen-Lacey, M.; Devitt, A.; Perrie, Y. The vesicle size of DDA:TDB liposomal adjuvants plays a role in the cell-mediated immune response but has no significant effect on antibody production. J. Control. Release 2011, 154, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Christensen, D.; Henriksen-Lacey, M.; Kamath, A.T.; Lindenstrøm, T.; Korsholm, K.S.; Christensen, J.P.; Rochat, A.F.; Lambert, P.H.; Andersen, P.; Siegrist, C.A.; et al. A cationic vaccine adjuvant based on a saturated quaternary ammonium lipid have different in vivo distribution kinetics and display a distinct CD4 T cell-inducing capacity compared to its unsaturated analog. J. Control. Release 2012, 160, 468–476. [Google Scholar] [CrossRef] [PubMed]
- de Veer, M.; Neeland, M.; Burke, M.; Pleasance, J.; Nathanielsz, J.; Elhay, M.; Meeusen, E. Cell recruitment and antigen trafficking in afferent lymph after injection of antigen and poly(I:C) containing liposomes, in aqueous or oil-based formulations. Vaccine 2013, 31, 1012–1018. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.; Coulter, A.; Macfarlan, R.; Beezum, L.; Bates, J.; Wong, T.-Y.; Drane, D. Development of an Influenza-Iscom™ Vaccine. In Vaccine Design: The Role of Cytokine Networks; Gregoriadis, G., McCormack, B., Allison, A.C., Eds.; Springer: Boston, MA, USA, 1997; pp. 33–49. [Google Scholar]
- Frazer, I.H.; Quinn, M.; Nicklin, J.L.; Tan, J.; Perrin, L.C.; Ng, P.; O’Connor, V.M.; White, O.; Wendt, N.; Martin, J.; et al. Phase 1 study of HPV16-specific immunotherapy with E6E7 fusion protein and ISCOMATRIX adjuvant in women with cervical intraepithelial neoplasia. Vaccine 2004, 23, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Brito, L.A.; Chan, M.; Baudner, B.; Gallorini, S.; Santos, G.; O’Hagan, D.T.; Singh, M. An alternative renewable source of squalene for use in emulsion adjuvants. Vaccine 2011, 29, 6262–6268. [Google Scholar] [CrossRef] [PubMed]
- Calabro, S.; Tritto, E.; Pezzotti, A.; Taccone, M.; Muzzi, A.; Bertholet, S.; De Gregorio, E.; O’Hagan, D.T.; Baudner, B.; Seubert, A. The adjuvant effect of MF59 is due to the oil-in-water emulsion formulation, none of the individual components induce a comparable adjuvant effect. Vaccine 2013, 31, 3363–3369. [Google Scholar] [CrossRef]
- Singh, M.; Kazzaz, J.; Ugozzoli, M.; Baudner, B.; Pizza, M.; Giuliani, M.; Hawkins, L.D.; Otten, G.; O’Hagan, D.T. MF59 oil-in-water emulsion in combination with a synthetic TLR4 agonist (E6020) is a potent adjuvant for a combination Meningococcus vaccine. Hum. Vaccines Immunother. 2012, 8, 486–490. [Google Scholar] [CrossRef]
- Myc, A.; Kukowska-Latallo, J.F.; Smith, D.M.; Passmore, C.; Pham, T.; Wong, P.; Bielinska, A.U.; Baker, J.R., Jr. Nanoemulsion nasal adjuvant W₈₀5EC induces dendritic cell engulfment of antigen-primed epithelial cells. Vaccine 2013, 31, 1072–1079. [Google Scholar] [CrossRef]
- Makidon, P.E.; Nigavekar, S.S.; Bielinska, A.U.; Mank, N.; Shetty, A.M.; Suman, J.; Knowlton, J.; Myc, A.; Rook, T.; Baker, J.R., Jr. Characterization of stability and nasal delivery systems for immunization with nanoemulsion-based vaccines. J. Aerosol Med. Pulm. Drug Deliv. 2010, 23, 77–89. [Google Scholar] [CrossRef]
- Lousada-Dietrich, S.; Jogdand, P.S.; Jepsen, S.; Pinto, V.V.; Ditlev, S.B.; Christiansen, M.; Larsen, S.O.; Fox, C.B.; Raman, V.S.; Howard, R.F.; et al. A synthetic TLR4 agonist formulated in an emulsion enhances humoral and Type 1 cellular immune responses against GMZ2—A GLURP-MSP3 fusion protein malaria vaccine candidate. Vaccine 2011, 29, 3284–3292. [Google Scholar] [CrossRef] [PubMed]
- Treanor, J.J.; Essink, B.; Hull, S.; Reed, S.; Izikson, R.; Patriarca, P.; Goldenthal, K.L.; Kohberger, R.; Dunkle, L.M. Evaluation of safety and immunogenicity of recombinant influenza hemagglutinin (H5/Indonesia/05/2005) formulated with and without a stable oil-in-water emulsion containing glucopyranosyl-lipid A (SE+GLA) adjuvant. Vaccine 2013, 31, 5760–5765. [Google Scholar] [CrossRef] [PubMed]
- Lumsden, J.M.; Nurmukhambetova, S.; Klein, J.H.; Sattabongkot, J.; Bennett, J.W.; Bertholet, S.; Fox, C.B.; Reed, S.G.; Ockenhouse, C.F.; Howard, R.F.; et al. Evaluation of immune responses to a Plasmodium vivax CSP-based recombinant protein vaccine candidate in combination with second-generation adjuvants in mice. Vaccine 2012, 30, 3311–3319. [Google Scholar] [CrossRef] [PubMed]
- Bovier, P.A. Epaxal: A virosomal vaccine to prevent hepatitis A infection. Expert Rev. Vaccines 2008, 7, 1141–1150. [Google Scholar] [CrossRef] [PubMed]
- Herzog, C.; Hartmann, K.; Künzi, V.; Kürsteiner, O.; Mischler, R.; Lazar, H.; Glück, R. Eleven years of Inflexal V-a virosomal adjuvanted influenza vaccine. Vaccine 2009, 27, 4381–4387. [Google Scholar] [CrossRef] [PubMed]
- Glück, R. Intranasal immunization against influenza. J. Aerosol Med. 2002, 15, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Mutsch, M.; Zhou, W.; Rhodes, P.; Bopp, M.; Chen, R.T.; Linder, T.; Spyr, C.; Steffen, R. Use of the inactivated intranasal influenza vaccine and the risk of Bell’s palsy in Switzerland. N. Engl. J. Med. 2004, 350, 896–903. [Google Scholar] [CrossRef]
- de Bruijn, I.A.; Nauta, J.; Gerez, L.; Palache, A.M. The virosomal influenza vaccine Invivac®: Immunogenicity and tolerability compared to an adjuvanted influenza vaccine (Fluad®) in elderly subjects. Vaccine 2006, 24, 6629–6631. [Google Scholar] [CrossRef]
- de Bruijn, I.; Meyer, I.; Gerez, L.; Nauta, J.; Giezeman, K.; Palache, B. Antibody induction by virosomal, MF59-adjuvanted, or conventional influenza vaccines in the elderly. Vaccine 2007, 26, 119–127. [Google Scholar] [CrossRef]
- Jain, H.; Kumavat, V.; Singh, T.; Versteilen, A.; Sarnecki, M. Immunogenicity and safety of a pediatric dose of a virosomal hepatitis A vaccine in healthy children in India. Hum. Vaccines Immunother. 2014, 10, 2089–2097. [Google Scholar] [CrossRef]
- Draget, K.I.; Taylor, C. Chemical, physical and biological properties of alginates and their biomedical implications. Food Hydrocoll. 2011, 25, 251–256. [Google Scholar] [CrossRef]
- Downs, E.C.; Robertson, N.E.; Riss, T.L.; Plunkett, M.L. Calcium alginate beads as a slow-release system for delivering angiogenic molecules In Vivo and In Vitro. J. Cell. Physiol. 1992, 152, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Liang, J.; Noble, P.W. Hyaluronan as an immune regulator in human diseases. Physiol. Rev. 2011, 91, 221–264. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.R.; Trowbridge, J.M.; Rudisill, J.A.; Termeer, C.C.; Simon, J.C.; Gallo, R.L. Hyaluronan fragments stimulate endothelial recognition of injury through TLR4. J. Biol. Chem. 2004, 279, 17079–17084. [Google Scholar] [CrossRef] [PubMed]
- Gu, P.; Liu, Z.; Sun, Y.; Ou, N.; Hu, Y.; Liu, J.; Wu, Y.; Wang, D. Angelica sinensis polysaccharide encapsulated into PLGA nanoparticles as a vaccine delivery and adjuvant system for ovalbumin to promote immune responses. Int. J. Pharm. 2019, 554, 72–80. [Google Scholar] [CrossRef] [PubMed]
- An, M.; Li, M.; Xi, J.; Liu, H. Silica Nanoparticle as a Lymph Node Targeting Platform for Vaccine Delivery. ACS Appl. Mater. Interfaces 2017, 9, 23466–23475. [Google Scholar] [CrossRef] [PubMed]
- Popat, A.; Hartono, S.; Stahr, F.; Liu, J.; Qiao, S.; Lu, M. Mesoporous Silica Nanoparticles for Bioadsorption, Enzyme Immobilisation, and Delivery Carriers. Nanoscale 2011, 3, 2801–2818. [Google Scholar] [CrossRef]
- Huang, X.; Cavalcante, D.P.; Townley, H.E. Macrophage-like THP-1 cells show effective uptake of silica nanoparticles carrying inactivated diphtheria toxoid for vaccination. J. Nanoparticle Res. 2020, 22, 23. [Google Scholar] [CrossRef]
- Thurmond, K.B.; Remsen, E.E.; Kowalewski, T.; Wooley, K.L. Packaging of DNA by shell crosslinked nanoparticles. Nucleic Acids Res. 1999, 27, 2966–2971. [Google Scholar] [CrossRef][Green Version]
- Wang, T.; Jiang, H.; Zhao, Q.; Wang, S.; Zou, M.; Cheng, G. Enhanced mucosal and systemic immune responses obtained by porous silica nanoparticles used as an oral vaccine adjuvant: Effect of silica architecture on immunological properties. Int. J. Pharm. 2012, 436, 351–358. [Google Scholar] [CrossRef]
- Jiménez-Periáñez, A.; Abos Gracia, B.; López Relaño, J.; Diez-Rivero, C.M.; Reche, P.A.; Martínez-Naves, E.; Matveyeva, E.; Gómez del Moral, M. Mesoporous silicon microparticles enhance MHC class I cross-antigen presentation by human dendritic cells. Clin. Dev. Immunol. 2013, 2013, 362163. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Ji, Z.; Liao, Y.P.; Wang, M.; Wang, X.; Dong, J.; Chang, C.H.; Li, R.; Zhang, H.; Nel, A.E.; et al. Engineering an effective immune adjuvant by designed control of shape and crystallinity of aluminum oxyhydroxide nanoparticles. ACS Nano 2013, 7, 10834–10849. [Google Scholar] [CrossRef] [PubMed]
- Allison, A.C.; Gregoriadis, G. Liposomes as immunological adjuvants. Nature 1974, 252, 252. [Google Scholar] [CrossRef]
- Oussoren, C.; Zuidema, J.; Crommelin, D.J.A.; Storm, G. Lymphatic uptake and biodistribution of liposomes after subcutaneous injection.: II. Influence of liposomal size, lipid composition and lipid dose. Biochim. Et Biophys. Acta (BBA)—Biomembr. 1997, 1328, 261–272. [Google Scholar] [CrossRef]
- Henriksen-Lacey, M.; Bramwell, V.W.; Christensen, D.; Agger, E.M.; Andersen, P.; Perrie, Y. Liposomes based on dimethyldioctadecylammonium promote a depot effect and enhance immunogenicity of soluble antigen. J. Control. Release 2010, 142, 180–186. [Google Scholar] [CrossRef]
- Henriksen-Lacey, M.; Christensen, D.; Bramwell, V.W.; Lindenstrøm, T.; Agger, E.M.; Andersen, P.; Perrie, Y. Comparison of the depot effect and immunogenicity of liposomes based on dimethyldioctadecylammonium (DDA), 3β-[N-(N’,N’-Dimethylaminoethane)carbomyl] cholesterol (DC-Chol), and 1,2-Dioleoyl-3-trimethylammonium propane (DOTAP): Prolonged liposome retention mediates stronger Th1 responses. Mol. Pharm. 2011, 8, 153–161. [Google Scholar] [CrossRef]
- Badiee, A.; Khamesipour, A.; Samiei, A.; Soroush, D.; Shargh, V.H.; Kheiri, M.T.; Barkhordari, F.; Robert Mc Master, W.; Mahboudi, F.; Jaafari, M.R. The role of liposome size on the type of immune response induced in BALB/c mice against leishmaniasis: Rgp63 as a model antigen. Exp. Parasitol. 2012, 132, 403–409. [Google Scholar] [CrossRef]
- Spang, A.; Martijn, J.; Saw, J.H.; Lind, A.E.; Guy, L.; Ettema, T.J. Close encounters of the third domain: The emerging genomic view of archaeal diversity and evolution. Archaea 2013, 2013, 202358. [Google Scholar] [CrossRef]
- Patel, G.B.; Zhou, H.; KuoLee, R.; Chen, W. Archaeosomes as adjuvants for combination vaccines. J. Liposome Res. 2004, 14, 191–202. [Google Scholar] [CrossRef]
- Butts, C.; Murray, N.; Maksymiuk, A.; Goss, G.; Marshall, E.; Soulières, D.; Cormier, Y.; Ellis, P.; Price, A.; Sawhney, R.; et al. Randomized phase IIB trial of BLP25 liposome vaccine in stage IIIB and IV non-small-cell lung cancer. J. Clin. Oncol. 2005, 23, 6674–6681. [Google Scholar] [CrossRef]
- Morein, B.; Sundquist, B.; Höglund, S.; Dalsgaard, K.; Osterhaus, A. Iscom, a novel structure for antigenic presentation of membrane proteins from enveloped viruses. Nature 1984, 308, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Morein, B.; Lövgren, K.; Rönnberg, B.; Sjölander, A.; Villacrés-Eriksson, M. Immunostimulating Complexes. Clin. Immunother. 1995, 3, 461–475. [Google Scholar] [CrossRef]
- Trudel, M.; Nadon, F.; Séguin, C.; Simard, C.; Lussier, G. Experimental polyvalent ISCOMs subunit vaccine induces antibodies that neutralize human and bovine respiratory syncytial virus. Vaccine 1989, 7, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Pearse, M.J.; Drane, D. ISCOMATRIX adjuvant for antigen delivery. Adv. Drug Deliv. Rev. 2005, 57, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.T.; Brown, L.E.; Deliyannis, G.; Pearse, M.J. ISCOM-based vaccines: The second decade. Immunol. Cell. Biol. 2005, 83, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Ennis, F.A.; Cruz, J.; Jameson, J.; Klein, M.; Burt, D.; Thipphawong, J. Augmentation of human influenza A virus-specific cytotoxic T lymphocyte memory by influenza vaccine and adjuvanted carriers (ISCOMS). Virology 1999, 259, 256–261. [Google Scholar] [CrossRef]
- Cox, J.C.; Sjölander, A.; Barr, I.G. ISCOMs and other saponin based adjuvants. Adv. Drug Deliv. Rev. 1998, 32, 247–271. [Google Scholar] [CrossRef]
- Sjölander, A.; Drane, D.; Maraskovsky, E.; Scheerlinck, J.P.; Suhrbier, A.; Tennent, J.; Pearse, M. Immune responses to ISCOM formulations in animal and primate models. Vaccine 2001, 19, 2661–2665. [Google Scholar] [CrossRef]
- Drane, D.; Gittleson, C.; Boyle, J.; Maraskovsky, E. ISCOMATRIX adjuvant for prophylactic and therapeutic vaccines. Expert Rev. Vaccines 2007, 6, 761–772. [Google Scholar] [CrossRef]
- Skene, C.D.; Sutton, P. Saponin-adjuvanted particulate vaccines for clinical use. Methods 2006, 40, 53–59. [Google Scholar] [CrossRef]
- Morein, B.; Hu, K.F.; Abusugra, I. Current status and potential application of ISCOMs in veterinary medicine. Adv. Drug Deliv. Rev. 2004, 56, 1367–1382. [Google Scholar] [CrossRef] [PubMed]
- Vesikari, T.; Knuf, M.; Wutzler, P.; Karvonen, A.; Kieninger-Baum, D.; Schmitt, H.J.; Baehner, F.; Borkowski, A.; Tsai, T.F.; Clemens, R. Oil-in-water emulsion adjuvant with influenza vaccine in young children. N. Engl. J. Med. 2011, 365, 1406–1416. [Google Scholar] [CrossRef] [PubMed]
- Grgacic, E.V.; Anderson, D.A. Virus-like particles: Passport to immune recognition. Methods 2006, 40, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Huckriede, A.; Bungener, L.; Stegmann, T.; Daemen, T.; Medema, J.; Palache, A.M.; Wilschut, J. The virosome concept for influenza vaccines. Vaccine 2005, 23 (Suppl. S1), S26–S38. [Google Scholar] [CrossRef] [PubMed]
- Glück, R.; Moser, C.; Metcalfe, I.C. Influenza virosomes as an efficient system for adjuvanted vaccine delivery. Expert Opin. Biol. Ther. 2004, 4, 1139–1145. [Google Scholar] [CrossRef]
- de Bruijn, I.A.; Nauta, J.; Cramer, W.C.; Gerez, L.; Palache, A.M. Clinical experience with inactivated, virosomal influenza vaccine. Vaccine 2005, 23 (Suppl. S1), S39–S49. [Google Scholar] [CrossRef]
- Choi, Y.; Chang, J. Viral vectors for vaccine applications. Clin. Exp. Vaccine Res. 2013, 2, 97–105. [Google Scholar] [CrossRef]
- Schuldt, N.J.; Amalfitano, A. Malaria vaccines: Focus on adenovirus based vectors. Vaccine 2012, 30, 5191–5198. [Google Scholar] [CrossRef]
- Simon, J.; Edelman, R. Clinical Evaluation of Adjuvants; Academic Press: Cambridge, MA, USA, 2006; pp. 319–342. [Google Scholar]
- Schijns, V.E.J.C.; O’Hagan, D.T. (Eds.) Immunopotentiators in Modern Vaccines; Academic Press: Cambridge, MA, USA, 2006. [Google Scholar]
- Villa, L.L.; Costa, R.L.; Petta, C.A.; Andrade, R.P.; Ault, K.A.; Giuliano, A.R.; Wheeler, C.M.; Koutsky, L.A.; Malm, C.; Lehtinen, M.; et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: A randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol. 2005, 6, 271–278. [Google Scholar] [CrossRef]
- Guerrini, G.; Magrì, D.; Gioria, S.; Medaglini, D.; Calzolai, L. Characterization of nanoparticles-based vaccines for COVID-19. Nat. Nanotechnol. 2022, 17, 570–576. [Google Scholar] [CrossRef]
- Huynh, A.; Kelton, J.G.; Arnold, D.M.; Daka, M.; Nazy, I. Antibody epitopes in vaccine-induced immune thrombotic thrombocytopaenia. Nature 2021, 596, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Nanomedicine and the COVID-19 vaccines. Nat. Nanotechnol. 2020, 15, 963. [CrossRef] [PubMed]
- Gopalakrishnan, A.M.; Kundu, A.K.; Mandal, T.K.; Kumar, N. Novel nanosomes for gene delivery to Plasmodium falciparum-infected red blood cells. Sci. Rep. 2013, 3, 1534. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y. Nucleic Acid Drugs-Current Status, Issues, and Expectations for Exosomes. Cancers 2021, 13, 5002. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, J.A.; Witzigmann, D.; Thomson, S.B.; Chen, S.; Leavitt, B.R.; Cullis, P.R.; van der Meel, R. The current landscape of nucleic acid therapeutics. Nat. Nanotechnol. 2021, 16, 630–643. [Google Scholar] [CrossRef] [PubMed]
- Chandra, P.K.; Kundu, A.K.; Hazari, S.; Chandra, S.; Bao, L.; Ooms, T.; Morris, G.F.; Wu, T.; Mandal, T.K.; Dash, S. Inhibition of hepatitis C virus replication by intracellular delivery of multiple siRNAs by nanosomes. Mol. Ther. 2012, 20, 1724–1736. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef]
- Akinc, A.; Maier, M.A.; Manoharan, M.; Fitzgerald, K.; Jayaraman, M.; Barros, S.; Ansell, S.; Du, X.; Hope, M.J.; Madden, T.D.; et al. The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat. Nanotechnol. 2019, 14, 1084–1087. [Google Scholar] [CrossRef]
- Mendes, B.B.; Conniot, J.; Avital, A.; Yao, D.; Jiang, X.; Zhou, X.; Sharf-Pauker, N.; Xiao, Y.; Adir, O.; Liang, H.; et al. Nanodelivery of nucleic acids. Nat. Rev. Methods Primers 2022, 2, 24. [Google Scholar] [CrossRef]
- Yang, K.; Wang, C.; White, K.I.; Pfuetzner, R.A.; Esquivies, L.; Brunger, A.T. Structural conservation among variants of the SARS-CoV-2 spike postfusion bundle. Proc. Natl. Acad. Sci. USA 2022, 119, e2119467119. [Google Scholar] [CrossRef]
- Kandwal, S.; Fayne, D. Genetic conservation across SARS-CoV-2 non-structural proteins—Insights into possible targets for treatment of future viral outbreaks. Virology 2023, 581, 97–115. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, N.; Weissman, D.; Whitehead, K.A. mRNA vaccines for infectious diseases: Principles, delivery and clinical translation. Nat. Rev. Drug Discov. 2021, 20, 817–838. [Google Scholar] [CrossRef] [PubMed]
- Roundtree, I.A.; Evans, M.E.; Pan, T.; He, C. Dynamic RNA Modifications in Gene Expression Regulation. Cell 2017, 169, 1187–1200. [Google Scholar] [CrossRef] [PubMed]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Evert, B.; Adeniyi, A.; Salla-Martret, M.; Lua, L.H.; Ozberk, V.; Pandey, M.; Good, M.F.; Suhrbier, A.; Halfmann, P.; et al. Ambient Temperature Stable, Scalable COVID-19 Polymer Particle Vaccines Induce Protective Immunity. Adv. Healthc. Mater. 2022, 11, e2102089. [Google Scholar] [CrossRef]
- Chavda, V.P.; Bezbaruah, R.; Valu, D.; Patel, B.; Kumar, A.; Prasad, S.; Kakoti, B.B.; Kaushik, A.; Jesawadawala, M. Adenoviral Vector-Based Vaccine Platform for COVID-19: Current Status. Vaccines 2023, 11, 432. [Google Scholar] [CrossRef] [PubMed]
- Fausther-Bovendo, H.; Kobinger, G.P. Pre-existing immunity against Ad vectors: Humoral, cellular, and innate response, what’s important? Hum. Vaccines Immunother. 2014, 10, 2875–2884. [Google Scholar] [CrossRef]
- Yin, Q.; Luo, W.; Mallajosyula, V.; Bo, Y.; Guo, J.; Xie, J.; Sun, M.; Verma, R.; Li, C.; Constantz, C.M.; et al. A TLR7-nanoparticle adjuvant promotes a broad immune response against heterologous strains of influenza and SARS-CoV-2. Nat. Mater. 2023, 22, 380–390. [Google Scholar] [CrossRef]
- Yang, M.C.; Wang, C.C.; Tang, W.C.; Chen, K.M.; Chen, C.Y.; Lin, H.H.; Hsieh, Y.C.; Wang, N.H.; Kuo, Y.C.; Chu, P.T.; et al. Immunogenicity of a spike protein subunit-based COVID-19 vaccine with broad protection against various SARS-CoV-2 variants in animal studies. PLoS ONE 2023, 18, e0283473. [Google Scholar] [CrossRef]
- Peek, L.J.; Middaugh, C.R.; Berkland, C. Nanotechnology in vaccine delivery. Adv. Drug Deliv. Rev. 2008, 60, 915–928. [Google Scholar] [CrossRef]
- Shin, M.D.; Shukla, S.; Chung, Y.H.; Beiss, V.; Chan, S.K.; Ortega-Rivera, O.A.; Wirth, D.M.; Chen, A.; Sack, M.; Pokorski, J.K.; et al. COVID-19 vaccine development and a potential nanomaterial path forward. Nat. Nanotechnol. 2020, 15, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Dong, Y.; Shao, R.; Zhang, S.; Wu, X.; Huang, X.; Sun, B.; Zeng, B.; Zhao, J. Application of nanoparticles in drug delivery for the treatment of osteosarcoma: Focussing on the liposomes. J. Drug Target 2022, 30, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Shimon, M.B.; Shapira, S.; Seni, J.; Arber, N. The Big Potential of Small Particles: Lipid-Based Nanoparticles and Exosomes in Vaccination. Vaccines 2022, 10, 1119. [Google Scholar] [CrossRef]
- García-Pinel, B.; Porras-Alcalá, C.; Ortega-Rodríguez, A.; Sarabia, F.; Prados, J.; Melguizo, C.; López-Romero, J.M. Lipid-Based Nanoparticles: Application and Recent Advances in Cancer Treatment. Nanomaterials 2019, 9, 638. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef]
- Panahi, Y.; Einollahi, B.; Beiraghdar, F.; Darvishi, M.; Fathi, S.; Javanbakht, M.; Shafiee, S.; Akhavan-Sigari, R. Fully understanding the efficacy profile of the COVID-19 vaccination and its associated factors in multiple real-world settings. Front. Immunol. 2022, 13, 947602. [Google Scholar] [CrossRef]
| Delivery System | Composition | Antigen | References |
|---|---|---|---|
| Polymeric-Based System | PLGA | OVA | Demento et al. [5] |
| PLGA, polylactic acid | Hepatitis B surface antigen | Thomas et al. [6] | |
| Lipid-coated PLGA | OVA | Bershteyn et al. [7] | |
| Lipid-coated PLGA | Malaria antigen | Moon et al. [8] | |
| Deacylated cationic polyethyleneimine | HIV CN54gp140 antigen | Mann et al. [9] | |
| Polylactic acid | Hepatitis B surface antigen | Saini et al. [10] | |
| Chitosan-coated polycaprolactone | H1N1 hemagglutinin | Gupta et al. [11] | |
| Polyanhydrides | Yersinia pestis antigen | Ulery et al. [12] | |
| Chitosan nanoparticles | HBsAg | Lugade et al. [13] | |
| Mannosylated chitosan nanoparticles | Recombinant hepatitis B virus surface antigen | Mehrabi et al. [14] | |
| Cholesteryl-conjugated pullulan | Clostridium botulinum type-A neurotoxin subunit antigen | Nochi et al. [15] | |
| N-trimethyl chitosan | OVA | Slutter et al. [16] | |
| Alginate nanoparticles | Diphtheria toxoid | Sarei et al. [17] | |
| Hyaluronic acid (HA), monophosphoryl lipid A (MPLA), aluminum salt (Alum) | Hepatitis B antigen | Moon et al. [18] | |
| Inorganic Nanoparticles | Gold nanoparticles | Escherichia coli-specific immunogenic antigens | Sanchez-Villamil et al. [19] |
| Gold nanoparticles | West Nile virus envelops protein | Niikura et al. [20] | |
| Carbon nanoparticles | Bovine serum albumin | Wang et al. [21] | |
| Carbon magnetic nanoparticles | Hen egg lysozyme | Schreiber et al. [22] | |
| Mesoporous silica nanoparticles | Schitosoma mansoni | Montalvo-Quiros et al. [23] | |
| Silica nanoparticle-based drug delivery system | H1N1 influenza hemagglutinin antigen | Neuhaus et al. [24] | |
| Alum | Combination of an influenza antigen | Knudsen et al. [25] | |
| Calcium phosphate nanoparticle | H1N1 hemagglutinin antigen | Morcol et al. [26] | |
| Liposomes | DOPC, DOPG, MPB | OVA | Moon et al. [27] |
| EPC, DOGS-NTA-Ni | His-tagged heat shock protein | Mašek et al. [28] | |
| Pegylated DDA, TDB | Ag85B-ESAT-6 | Kaur et al. [29] | |
| DDA, TDB | OVA | Milicic et al. [30] | |
| DDA, DSPC, cholesterol, TDB | Ag85B-ESAT-6 | McNeil et al. [31] | |
| DDA, TDB | Trivalent influenza vaccine | Rosenkrands et al. [32] | |
| DDA, TDB | Ag85B-ESAT-6 | Henriksen-Lacey et al. [33] | |
| DDA, DODA, TDB | Ag85B-ESAT-6 | Christensen et al. [34] | |
| Lecithin, cholesterol | Diphtheria toxoid | de Veer et al. [35] | |
| Immunostimulatory Complexes (ISCOMS) | Cholesterol, phospholipids, saponins | hemagglutinin antigen | Cox et al. [36] |
| ISCOMATRIX | HPV16 E6 and E7 recombinant bacterial fusion protein | Frazer et al. [37] | |
| Emulsion | MF59 | Recombinant meningococcal B protein | Brito et al. [38] |
| MF59 | Hemagglutinin | Calabro et al. [39] | |
| MF59 | Recombinant meningococcal B protein | Singh et al. [40] | |
| W805EC | OVA | Myc et al. [41] | |
| W805EC | OVA | Makidon et al. [42] | |
| GLA | Falciparum subunit | Lousada-Dietrich et al. [43] | |
| GLA-SE | Recombinant hemagglutinin | Treanor et al. [44] | |
| GLA-SE | Plasmodium vivax subunit | Lumsden et al. [45] | |
| Virus-Like Particles And Virosomes | Epaxal® (Crucell, Leiden, The Netherlands) A (H1N1) virosomes + inactivated hepatitis A virus | Bovier et al. [46] | |
| Inflexal® V (Crucell) Virosomes from three influenza strains: A (H1N1), A (H3N2), and B | Herzog et al. [47] | ||
| Nasalflu® (Berna Biotech, Bern, Switzerland) Virosomes from three influenza strains: A (H1N1), A (H3N2), and B + heat labile toxin adjuvant | Gluck et al. [48]; Mutsch et al. [49] | ||
| Invivac® (Solvay, Brussels, Belgium) Virosomes from three influenza strains: A (H1N1), A (H3N2), and B | de Bruijn et al. [50]; de Bruijn et al. [51] | ||
| Epaxal® Junior (Crucell) A (H1N1) virosomes + inactivated hepatitis A virus | Bovier et al. [46]; Van der Wielen et al. [52] |
| Study Title | Clinical Trails Gov ID | Clinical Trial | Interventions |
|---|---|---|---|
| Training the Innate Immune System Against SARS-CoV-2 (COVID-19) Using the Shingrix Vaccine in Nursing Home Residents (NH-Shingrix) | NCT04523246 | Early Phase 1 | Biological: SHINGRIX (zoster vaccine Recombinant, adjuvanted) Drug: normal saline |
| A Study Assessing the Safety, Tolerability, Immunogenicity of COVID-19 Vaccine Candidate PRIME-2-CoV_Beta, Orf Virus Expressing SARS-CoV_2 Spike and Nucleocapsid Proteins | NCT05367843 | Phase 1 | Drug: PRIME-2-CoV_Beta |
| Phase 1 Study of Intranasal PIV5 COVID-19 Vaccine Expressing SARS-CoV-2 Spike Protein in Healthy Adults and Adolescents (CVXGA1-001) | NCT04954287 | Phase 1 | Biological: CVXGA1 low dose Biological: CVXGA1 high dose |
| Safety And Immunogenicity Of HDT-301 Targeting A SARS-CoV-2 Variant Spike Protein | NCT05132907 | Phase 1 | Biological: HDT-301 |
| Delayed Heterologous SARS-CoV-2 Vaccine Dosing (Boost) After Receipt of EUA Vaccines | NCT04889209 | Phase 1 Phase 2 | Biological: Ad26.COV2.S Biological: BNT162b2 Biological: mRNA-1273 Biological: mRNA-1273.211 Biological: mRNA-1273.222 Biological: SARS-CoV-2 rS/M1 |
| GLS-5310 Vaccine in Healthy Volunteers as a Booster for SARS-CoV-2 (COVID-19) | NCT05182567 | Phase 1 | Drug: GLS-5310 (Group 1) Drug: GLS-5310 (Group 2) Drug: GLS-5310 (Group 3) Drug: GLS-5310 (Group 4) |
| COVID-19 Variant Immunologic Landscape Trial (COVAIL Trial) | NCT05289037 | Phase 1 Phase 2 | Drug: AS03 Biological: BNT162b2 Biological: BNT162b2 (B.1.1.529) Biological: BNT162b2 (B.1.351) Biological: BNT162b2 bivalent (wild type and Omicron BA.1) Biological: BNT162b2 bivalent (wild type and Omicron BA.4/BA.5) Biological: CoV2 preS dTM [B.1.351] Biological: CoV2 preS dTM/D614 Biological: CoV2 preS dTM/D614 + B.1.351 Biological: mRNA-1273 Biological: mRNA-1273.351 Biological: mRNA-1273.529 Biological: mRNA-1273.617.2 Other: sodium chloride, 0.9% |
| A Safety, Reactogenicity, and Immunogenicity Study of mRNA-1045 (Influenza and Respiratory Syncytial Virus [RSV]) or mRNA-1230 (Influenza, RSV, and Severe Acute Respiratory Syndrome Coronavirus 2 [SARS-CoV-2]) Vaccine in Adults 50 to 75 Years Old | NCT05585632 | Phase 1 | Biological: mRNA-1010 Biological: mRNA-1345 Biological: mRNA-1273.214 Biological: mRNA-1045 Biological: mRNA-1230 |
| Chimpanzee Adenovirus and Self-Amplifying mRNA Prime-Boost Prophylactic Vaccines Against SARS-CoV-2 in Healthy Adults | NCT04776317 | Phase 1 | Biological: ChAdV68-S Biological: ChAdV68-S-TCE Biological: SAM-LNP-S Biological: SAM-LNP-S-TCE Other: sodium chloride, 0.9% |
| A Live Recombinant Newcastle Disease Virus-vectored COVID-19 Vaccine Phase 1 Study | NCT05181709 | Phase 1 | Drug: sodium chloride Biological: NDV-HXP-S IN low dose Biological: NDV-HXP-S IM low dose Biological: NDV-HXP-S IN high dose Biological: NDV-HXP-S IM high dose |
| Safety and Immunogenicity Study of a Booster Dose of the Investigational CV0501 mRNA COVID-19 Vaccine in Adults at Least 18 Years Old | NCT05477186 | Phase 1 | Biological: CV0501 (3 μg) Biological: CV0501 (6 μg) Biological: CV0501 (12 μg) Biological: CV0501 (25 μg) Biological: CV0501 (50 μg) Biological: CV0501 (75 μg) Biological: CV0501 (100 μg) Biological: CV0501 (150 μg) Biological: CV0501 (200 μg) |
| SARS-CoV-2-Spike-Ferritin-Nanoparticle (SpFN) Vaccine With ALFQ Adjuvant for Prevention of COVID-19 in Healthy Adults | NCT04784767 | Phase 1 | Biological: 25 µg SpFN_1B-06-PL + ALFQ (QS21 adjuvant) Drug: sodium chloride, USP, for injection (0.9% NaCl) Biological: 50 µg SpFN_1B-06-PL + ALFQ (QS21 adjuvant) |
| A Study of Modified mRNA Vaccines in Healthy Adults | NCT05397223 | Phase 1 | Biological: mRNA-1273 Biological: mRNA-1010 Biological: mRNA-1345 Biological: FLUAD® Biological: mRNA-1647 |
| Study of Recombinant Protein Vaccines With Adjuvant as a Primary Series and as a Booster Dose Against COVID-19 in Adults 18 Years of Age and Older (VAT00002) | NCT04762680 | Phase 2 Phase 3 | Biological: SARS-CoV-2 recombinant protein vaccine Phase 2 Formulation 1 Biological: SARS-CoV-2 recombinant protein vaccine Phase 2 Formulation 2 Biological: SARS-CoV-2 recombinant protein vaccine Phase 2 Formulation 3 Biological: SARS-CoV-2 adjuvanted recombinant protein vaccine, monovalent (D614)-AS03, Dosage A Biological: SARS-CoV-2 adjuvanted recombinant protein vaccine, monovalent (B.1.351)-AS03 Biological: SARS-CoV-2 adjuvanted recombinant protein vaccine, monovalent (D614)-AS03, Dosage B Biological: SARS-CoV-2 adjuvanted recombinant protein vaccine, monovalent (B.1.351)-AS03 Alternative Exploratory Formulation 1 Biological: SARS-CoV-2 adjuvanted recombinant protein vaccine, monovalent (B.1.351)-AS03 Alternative Exploratory Formulation 2 Biological: SARS-CoV-2 adjuvanted recombinant protein vaccine, monovalent (B.1.351)-AS03 Alternative Exploratory Formulation 3 Biological: SARS-CoV-2 adjuvanted recombinant protein vaccine, monovalent (B.1.351)-AS03 Alternative Exploratory Formulation 4 Biological: SARS-CoV-2 adjuvanted recombinant protein vaccine, bivalent (D614 + B.1.351)-AS03 |
| Study of a Recombinant Coronavirus-Like Particle COVID-19 Vaccine in Adults | NCT04636697 | Phase 2 Phase 3 | Drug: intramuscular injection Biological: intramuscular vaccine |
| A Ph 2 Trial With an Oral Tableted COVID-19 Vaccine | NCT05067933 | Phase 2 | Drug: VXA-CoV2-1.1-S Other: placebo tablets |
| Safety and Immunogenicity of RNA-based Vaccines Against SARS-CoV-2 Variants in Healthy Participants | NCT05004181 | Phase 2 | Biological: BNT162b2 Biological: BNT162b2 (B.1.1.7 + B.1.617.2) Biological: BNT162b2 (B.1.1.7) Biological: BNT162b2 (B.1.617.2) Biological: BNT162b2 (B.1.1.529) Other: observational |
| COVID-19 VAX Booster Dosing in Patients With Hematologic Malignancies | NCT05028374 | Phase 2 | Drug: A single “booster” dose of the Moderna mRNA COVID-19 vaccine |
| A Study to Evaluate Safety and Effectiveness of mRNA-1273 COVID-19 Vaccine in Healthy Children Between 6 Months of Age and Less Than 12 Years of Age | NCT04796896 | Phase 2 Phase 3 | Biological: mRNA-1273 Biological: placebo Biological: mRNA-1273.214 |
| A Phase 1/2/3 Study to Evaluate the Safety, Tolerability, and Immunogenicity of an RNA Vaccine Candidate Against COVID-19 in Healthy Children | NCT04816643 | Phase 2 Phase 3 | Biological: biological/vaccine: BNT162b2 10mcg Biological: BNT162b2 20mcg Biological: BNT162b2 30mcg Other: placebo Biological: biological/vaccine: BNT162b2 3mcg |
| A Study to Evaluate the Immunogenicity and Safety of mRNA Vaccine Boosters for SARS-CoV-2 (COVID-19) Variants | NCT04927065 | Phase 2 Phase 3 | Biological: mRNA-1273.211 Biological: mRNA-1273 Biological: mRNA-1273.617.2 Biological: mRNA-1273.213 Biological: mRNA-1273.529 Biological: mRNA-1273.214 Biological: mRNA-1273.222 Biological: mRNA-1273.815 Biological: mRNA-1273.231 |
| Study to Evaluate Safety, Tolerability & Immunogenicity of BNT162b2 in Immunocompromised Participants ≥2 Years | NCT04895982 | Phase 2 | Biological: BNT162b2 |
| COVID-19 Booster Vaccine in Autoimmune Disease Non-Responders | NCT05000216 | Phase 2 | Biological: Moderna mRNA-1273 Biological: BNT162b2 Biological: Ad26.COV2.S Drug: continue IS (MMF or MPA) Drug: continue IS (MTX) Biological: continue IS (B cell depletion therapy) Biological: monovalent (B.1.351) CoV2 preS dTM-AS03 Drug: withhold IS (MMF or MPA) Drug: withhold IS (MTX) Drug: withhold IS (B cell depletion therapy) Biological: Moderna mRNA-1273, bivalent Biological: BNT162b2, bivalent |
| A Study to Learn About Two or More Vaccines That Are Put Together as One Shot Against Infectious Lung Illnesses, Including COVID-19 and Respiratory Syncytial Virus (RSV) | NCT05886777 | Phase 2 | Biological: combination (RSVpreF + BNTb162b2) Biological: bivalent BNT162b2 (original/Omi BA.4/BA.5) Biological: RSVpreF Biological: QIV Biological: normal saline placebo |
| Study of Monovalent and Bivalent Recombinant Protein Vaccines Against COVID-19 in Adults 18 Years of Age and Older (VAT00008) | NCT04904549 | Phase 3 | Biological: SARS-CoV-2 adjuvanted recombinant protein vaccine (monovalent D614) (primary series) Biological: SARS-CoV-2 adjuvanted recombinant protein vaccine (bivalent D614 + B.1.351) (primary series) Biological: placebo Biological: SARS-CoV-2 adjuvanted recombinant protein vaccine (monovalent B.1.351) (booster dose) ≥4 months after last vaccination Biological: SARS-CoV-2 adjuvanted recombinant protein vaccine (monovalent D614) (primary series) and SARS-CoV-2 adjuvanted recombinant protein vaccine (monovalent B.1.351) (booster dose) ≥4 months after last vaccination |
| Phase 3 Study of Novavax Vaccine(s) as Booster Dose After mRNA Vaccines | NCT05875701 | Phase 3 | Biological: NVX-CoV2373 Biological: SARS-CoV-2 rS antigen/Matrix-M adjuvant |
| A Study to Evaluate Safety and Immunogenicity of mRNA-1273 Vaccine to Prevent COVID-19 in Adult Organ Transplant Recipients and in Healthy Adult Participants | NCT04860297 | Phase 3 | Biological: mRNA-1273 |
| A Study to Evaluate the Safety and Immunogenicity of the mRNA-1273.214 COVID-19 Vaccine in Healthy Children Between 6 Months to Less Than 6 Years of Age | NCT05436834 | Phase 3 | Biological: mRNA-1273.214 |
| ABNCoV2 Vaccine in Adult Subjects Previously Vaccinated for SARS-CoV-2 | NCT05329220 | Phase 3 | Biological: ABNCoV2 Biological: Comirnaty |
| A Study to Evaluate the Efficacy, Immune Response, and Safety of a COVID-19 Vaccine in Adults ≥18 Years With a Pediatric Expansion in Adolescents (12 to <18 Years) at Risk for SARS-CoV-2 | NCT04611802 | Phase 3 | Biological: SARS-CoV-2 rS/Matrix-M1 adjuvant (initial vaccination period) Other: placebo (initial vaccination period) Biological: SARS-CoV-2 rS/Matrix-M1 adjuvant (crossover vaccination period) Other: placebo (crossover vaccination period) Biological: SARS-CoV-2 rS/Matrix-M1 adjuvant (booster vaccination) Biological: SARS-CoV-2 rS/Matrix-M1 adjuvant (second booster vaccination) |
| Safety and Immunogenicity of 9-valent Human Papillomavirus (9vHPV) Vaccine Coadministered With Messenger Ribonucleic Acid (mRNA)-1273 Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) (COVID-19) Vaccine (V503-076) | NCT05119855 | Phase 3 | Biological: 9vHPV vaccine Biological: mRNA-1273 vaccine |
| Platform Trial to Compare Homologous Boost of Authorized COVID-19 Vaccines and Heterologous Boost With UB-612 Vaccine | NCT05293665 | Phase 3 | Biological: UB-612 Biological: BNT162b2 vaccine Biological: ChAdOx1-S vaccine Biological: Sinopharm BIBP |
| BCG Vaccine for Health Care Workers as Defense Against COVID 19 (BADAS) | NCT04348370 | Phase 4 | Biological: BCG vaccine Biological: placebo vaccine |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chowdhury, N.; Kundu, A. Nanotechnology Platform for Advancing Vaccine Development against the COVID-19 Virus. Diseases 2023, 11, 177. https://doi.org/10.3390/diseases11040177
Chowdhury N, Kundu A. Nanotechnology Platform for Advancing Vaccine Development against the COVID-19 Virus. Diseases. 2023; 11(4):177. https://doi.org/10.3390/diseases11040177
Chicago/Turabian StyleChowdhury, Nusrat, and Anup Kundu. 2023. "Nanotechnology Platform for Advancing Vaccine Development against the COVID-19 Virus" Diseases 11, no. 4: 177. https://doi.org/10.3390/diseases11040177
APA StyleChowdhury, N., & Kundu, A. (2023). Nanotechnology Platform for Advancing Vaccine Development against the COVID-19 Virus. Diseases, 11(4), 177. https://doi.org/10.3390/diseases11040177






