CO2 Laser for Esthetic Healing of Injuries and Surgical Wounds with Small Parenchymal Defects in Oral Soft Tissues
Abstract
:1. Introduction
2. Wound Healing and Scarring
2.1. Primary Healing and Scar Formation
2.2. Secondary Healing and Scar Formation
3. Characteristics of CO2 Laser
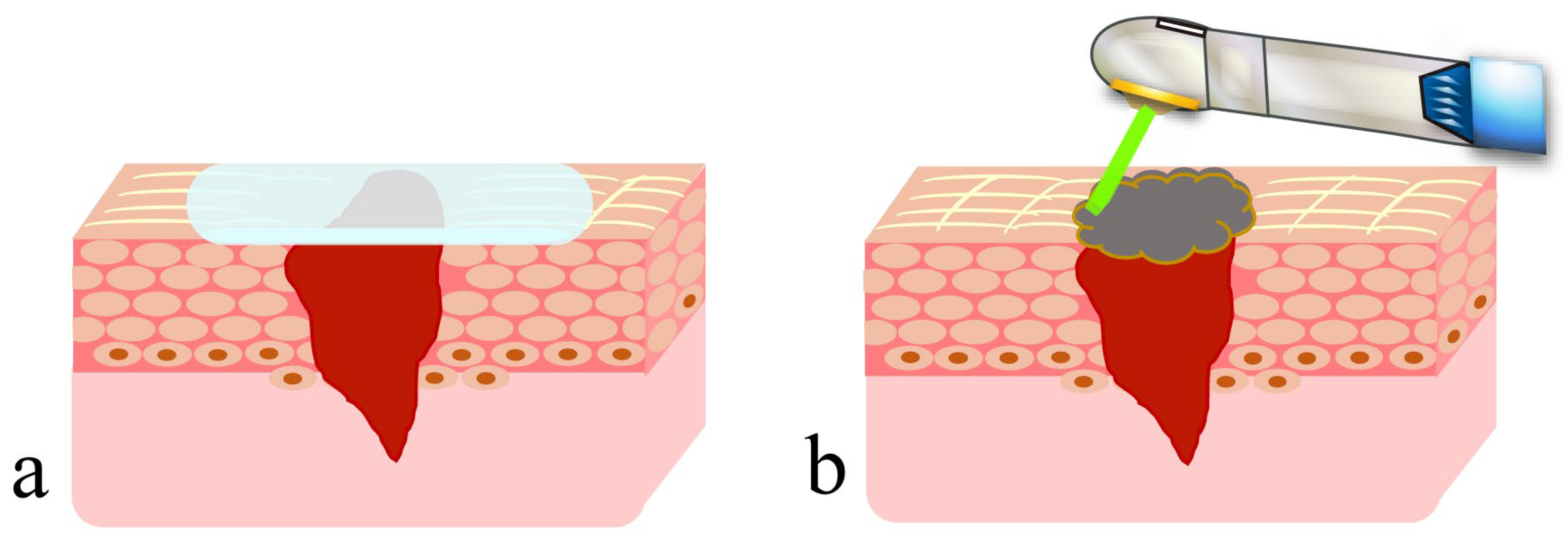
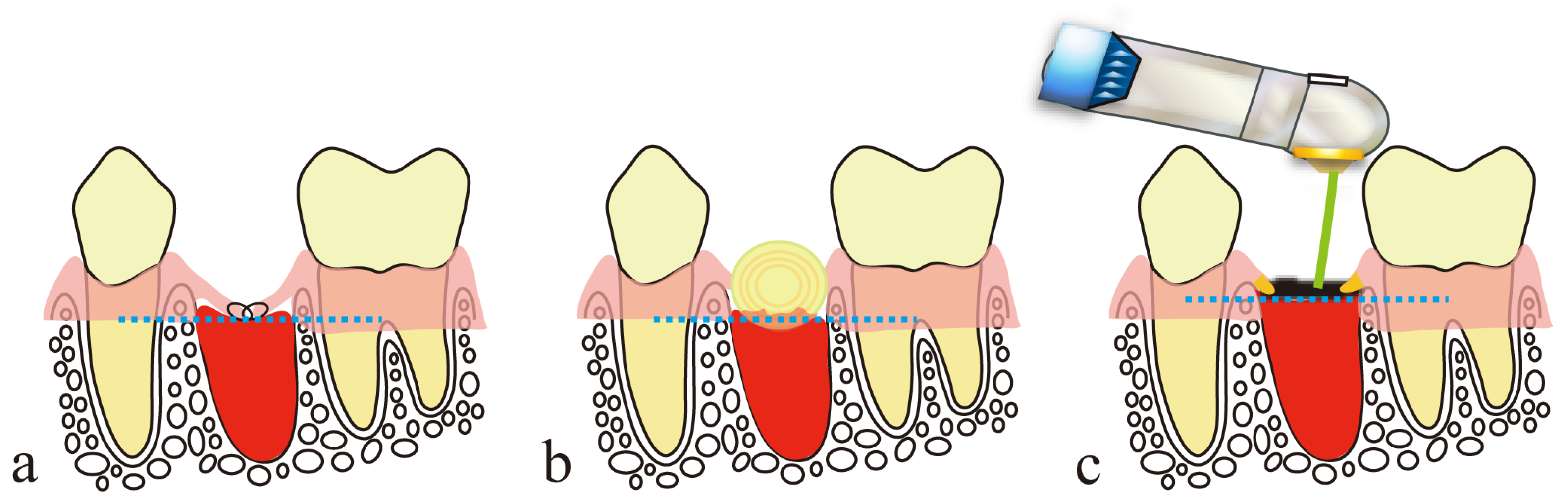
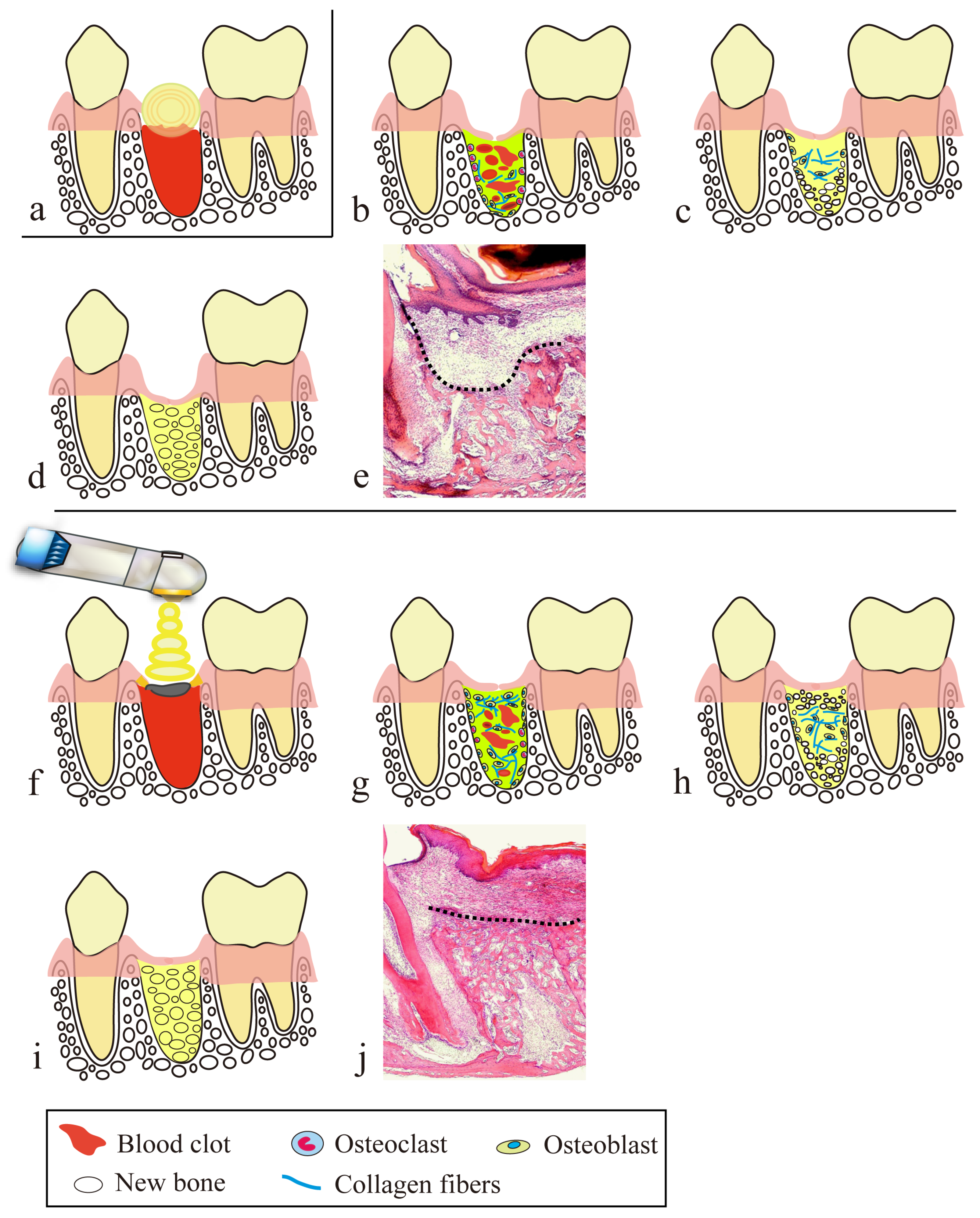
4. Secondary Healing-like Effect Using a CO2 Laser
4.1. Presence of Artificial Scabs
4.2. Preventive Effect of PBMT on Scarring
5. Treatment of the Lips Using a CO2 Laser
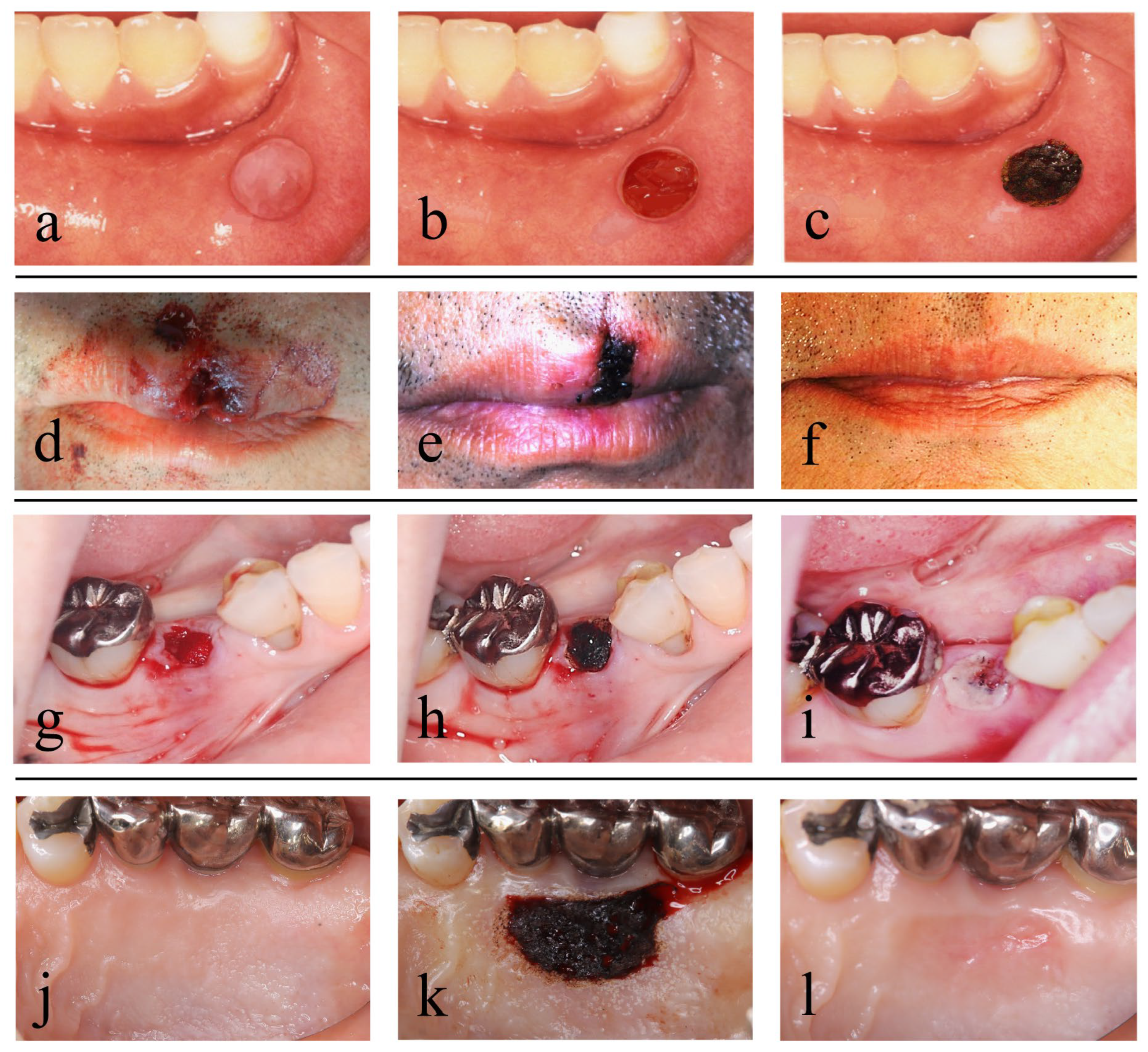
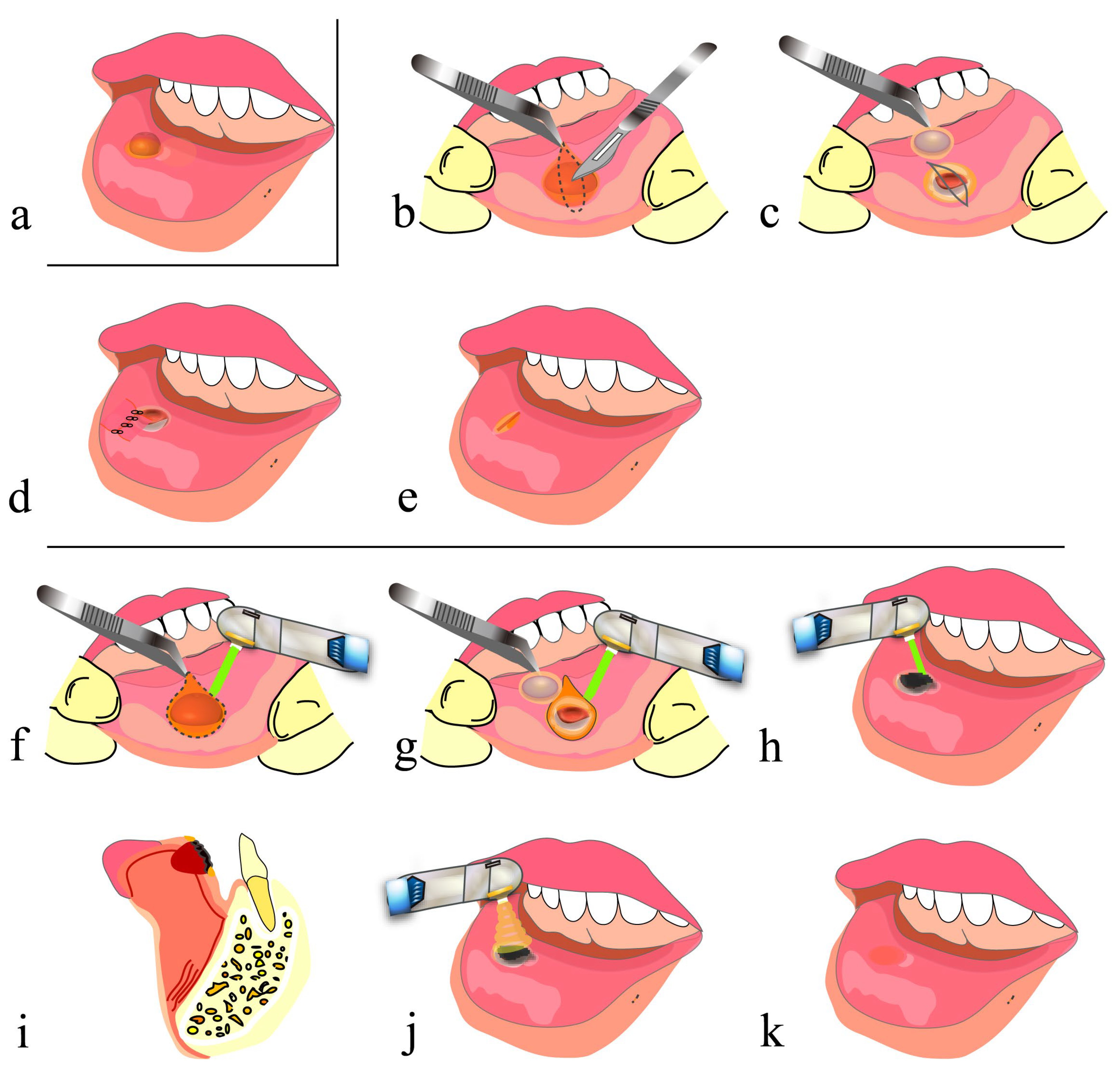
5.1. Mucocele of the Lip
5.2. Open Lip Vermillion Wounds
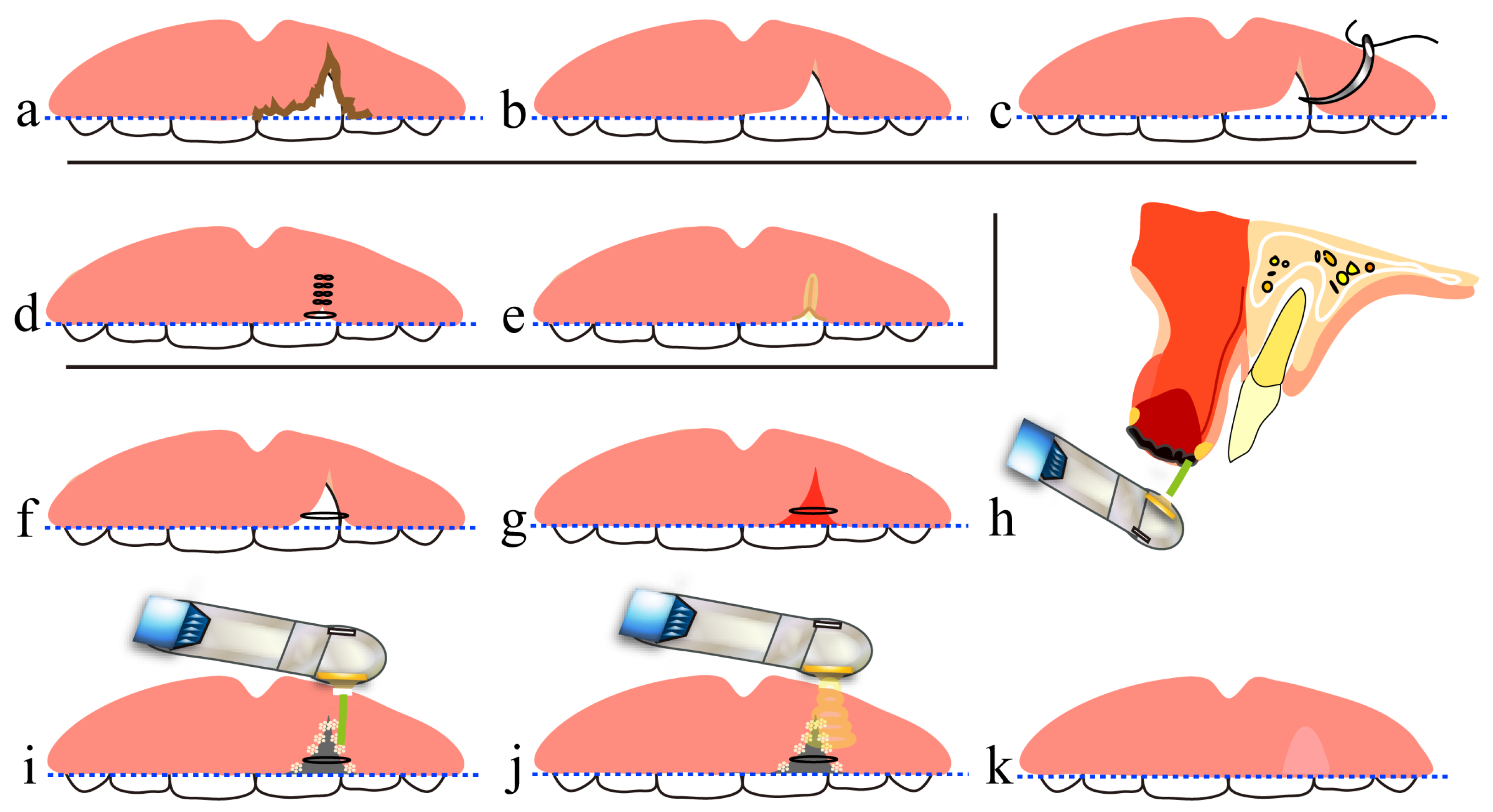
6. Treatment Using a CO2 Laser after Tooth Extraction
7. Free Gingival Grafting Using a CO2 Laser
8. Conclusions
- Blood is allowed to accumulate at the exact site of a small parenchymal defect, and the blood surface is coagulated and carbonized by HILT to form an artificial scab.
- The artificial scab is soldered to the surrounding mucosa so that it will not separate from the surrounding mucosa.
- Artificial scabs have a space-making effect for the accumulation of blood and effusion necessary for tissue regeneration.
- Artificial scabs facilitate the accumulation of blood and effusion, thereby preserving a moist environment (a moist wound-healing-like effect).
- Artificial scabs protect wounds from contamination and infection.
- PBMT contributes to suppressing scar formation at the wound site and to promoting wound healing.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Bayramicli, M.; Numanoğlu, A.; Tezel, E. The mental V-Y island advancement flap in functional lower lip reconstruction. Plast. Reconstr. Surg. 1997, 100, 1682–1690. [Google Scholar] [CrossRef]
- Kim, J.H.; Ahn, C.H.; Kim, S.; Lee, W.S.; Oh, S.H. Effective method for reconstruction of remaining lower lip vermilion defect after a mental V-Y advancement flap. Arch. Craniofac. Surg. 2019, 20, 76–83. [Google Scholar] [CrossRef]
- Dalisson, B.; Barralet, J. Bioinorganics and wound healing. Adv. Healthc. Mater. 2019, 8, e1900764. [Google Scholar] [CrossRef]
- Nuutila, K.; Eriksson, E. Moist wound healing with commonly available dressings. Adv. Wound Care 2021, 10, 685–698. [Google Scholar] [CrossRef]
- Makboul, M.; Makboul, R.; Abdelhafez, A.H.K.; Hassan, S.S.; Youssif, S.M. Evaluation of the effect of fractional CO2 laser on histopathological picture and TGF-β1 expression in hypertrophic scar. J. Cosmet. Dermatol. 2014, 13, 169–179. [Google Scholar] [CrossRef]
- Qu, L.; Liu, A.; Zhou, L.; He, C.; Grossman, P.H.; Moy, R.L.; Mi, Q.S.; Ozog, D. Clinical and molecular effects on mature burn scars after treatment with a fractional CO2 laser. Lasers Surg. Med. 2012, 44, 517–524. [Google Scholar] [CrossRef]
- Sabry, H.H.; Rahman, S.H.A.; Hussein, M.S.; Sanad, R.R.; Abd El Azez, T.A. The efficacy of combining fractional carbon dioxide laser with verapamil hydrochloride or 5-fluorouracil in the treatment of hypertrophic scars and keloids: A clinical and immunohistochemical study. Dermatol. Surg. 2019, 45, 536–546. [Google Scholar] [CrossRef]
- Karmisholt, K.E.; Taudorf, E.H.; Wulff, C.B.; Wenande, E.; Philipsen, P.A.; Haedersdal, M. Fractional CO2 laser treatment of caesarean section scars-A randomized controlled split-scar trial with long term follow-up assessment. Lasers Surg. Med. 2017, 49, 189–197. [Google Scholar] [CrossRef]
- Tawfic, S.O.; El-Tawdy, A.; Shalaby, S.; Foad, A.; Shaker, O.; Sayed, S.S.; Metwally, D. Evaluation of fractional CO2 versus long pulsed Nd:YAG lasers in treatment of hypertrophic scars and keloids: A randomized clinical trial. Lasers Surg. Med. 2020, 52, 959–965. [Google Scholar] [CrossRef]
- Peng, W.; Zhang, X.; Kong, X.; Shi, K. The efficacy and safety of fractional CO2 laser therapy in the treatment of burn scars: A meta-analysis. Burns 2021, 47, 1469–1477. [Google Scholar] [CrossRef]
- Issler-Fisher, A.C.; Fisher, O.M.; Haertsch, P.A.; Li, Z.; Maitz, P.K.M. Effectiveness and safety of ablative fractional CO2 laser for the treatment of burn scars: A case-control study. Burns 2021, 47, 785–795. [Google Scholar] [CrossRef]
- Jahanbin, A.; Eslami, N.; Layegh, P.; Saeidi, M.; Kazemi, M.; Shahabi, M.; Raisolsadat, S.M.A. Fractional CO2 laser treatment for post-surgical lip scars in cleft lip and palate patients. Lasers Med. Sci. 2019, 34, 1699–1703. [Google Scholar] [CrossRef]
- Zhang, N.; Yu, X.; Zhao, J.; Yu, J.; Shi, K.; Liu, T. Fractional CO2 laser therapy for cesarean scar under the guidance of multiple evaluation methods: A retrospective study. J. Cosmet. Dermatol. 2021, 20, 2119–2124. [Google Scholar] [CrossRef]
- Hedelund, L.; Haak, C.S.; Togsverd-Bo, K.; Bogh, M.K.; Bjerring, P.; Hædersdal, M. Fractional CO2 laser resurfacing for atrophic acne scars: A randomized controlled trial with blinded response evaluation. Lasers Surg. Med. 2012, 44, 447–452. [Google Scholar] [CrossRef]
- Cox, C.; Bettiol, P.; Le, A.; MacKay, B.J.; Griswold, J.; McKee, D. CO2 laser resurfacing for burn and traumatic scars of the hand and upper extremity. Scars Burn. Heal. 2022, 8, 20595131211047694. [Google Scholar] [CrossRef]
- Bohan, P.M.K.; Cooper, L.E.; Lu, K.N.; Raper, D.M.; Batchinsky, M.; Carlsson, A.H.; Cancio, L.C.; Chan, R.K. Fractionated ablative carbon dioxide laser therapy decreases ultrasound thickness of hypertrophic burn scar: A prospective process improvement initiative. Ann. Plast. Surg. 2021, 86, 273–278. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, S.; Xia, Z.; Peng, Z.; Cheng, X.; Yang, X.; Luo, W.; Yang, R. 595-nm pulsed dye laser combined with fractional CO2 laser reduces hypertrophic scar through down-regulating TGFβ1 and PCNA. Lasers Med. Sci. 2021, 36, 1625–1632. [Google Scholar] [CrossRef]
- Chen, H.Y.; Lei, Y.; OuYang, H.W.; Gold, M.H.; Tan, J. Experimental comparative study of the effect of fractional CO2 laser combined with pulsed dye laser versus each laser alone on the treatment of hypertrophic scar of rabbit ears. J. Cosmet. Dermatol. 2022, 21, 979–990. [Google Scholar] [CrossRef]
- Zhang, J.; Xia, Z.; Zhou, S.; Luo, W.; Peng, Z.; Yang, R. Effect of artesunate combined with fractional CO2 laser on the hypertrophic scar in a rabbit model. Lasers Surg. Med. 2021; online ahead of print. [Google Scholar] [CrossRef]
- Huang, J.; Chen, J.; Wo, Y.; Wang, X.; Zhang, Y.; Chen, X.; Zhang, Z.; Biskup, E. CO2 fractional laser combined with 5-fluorouracil ethosomal gel treatment of hypertrophic scar macro-, microscopic, and molecular mechanism of action in a rabbit animal model. Rejuvenation Res. 2021, 24, 131–138. [Google Scholar] [CrossRef]
- Xiong, J.; Li, X.; Xu, G.; Wang, Y.; Wen, H. Effectiveness of fractional carbon dioxide laser combined with botulinum toxin type A in a rabbit ear model with the underlying mechanism. J. Cosmet. Dermatol. 2023; online ahead of print. [Google Scholar] [CrossRef]
- de Freitas, A.C.; Pinheiro, A.L.B.; Gerardt de Oliveira, M.; Pedreira Ramalho, L.M. Assessment of the behavior of myofibroblasts on scalpel and CO2 laser wounds: An immunohistochemical study in rats. J. Clin. Laser Med. Surg. 2002, 20, 221–225. [Google Scholar] [CrossRef]
- Nowak, K.C.; McCormack, M.; Koch, R.J. The effect of superpulsed carbon dioxide laser energy on keloid and normal dermal fibroblast secretion of growth factors: A serum-free study. Plast. Reconstr. Surg. 2000, 105, 2039–2048. [Google Scholar] [CrossRef]
- Cheng, E.T.; Nowak, K.C.; Koch, R.J. Effect of blended carbon dioxide and erbium: YAG laser energy on preauricular and ear lobule keloid fibroblast secretion of growth factors: A serum-free study. Arch. Facial Plast. Surg. 2001, 3, 252–257. [Google Scholar] [CrossRef]
- Fukuoka, H.; Daigo, Y.; Enoki, N.; Taniguchi, K.; Sato, H. Influence of carbon dioxide laser irradiation on the healing process of extraction sockets. Acta Odontol. Scand. 2011, 69, 33–40. [Google Scholar] [CrossRef]
- Daigo, Y.; Daigo, E.; Hasegawa, A.; Fukuoka, H.; Ishikawa, M.; Takahashi, K. Utility of high-intensity laser therapy combined with photobiomodulation therapy for socket preservation after tooth extraction. Photobiomodul. Photomed. Laser Surg. 2020, 38, 75–83. [Google Scholar] [CrossRef]
- Daigo, Y.; Daigo, E.; Fukuoka, H.; Fukuoka, N.; Ishikawa, M.; Takahashi, K. Wound healing and cell dynamics including mesenchymal and dental pulp stem cells induced by photobiomodulation therapy: An example of socket-preserving effects after tooth extraction in rats and a literature review. Int. J. Mol. Sci. 2020, 21, 6850. [Google Scholar] [CrossRef]
- Taniguchi, Y.; Matsuzaki, E.; Daigo, Y.; Tsutsumi, T.; Fukuoka, H.; Kakura, K.; Egashira, K.; Takahashi, K.; Kido, H. Space-making effect for new bone formation by suppressing scar contraction of mucosal epithelium of rat tooth extraction wound using diode laser and CO2 laser treatment. J. Dent. Sci. 2022, 17, 1001–1008. [Google Scholar] [CrossRef]
- Fukuoka, H.; Fukuoka, N.; Daigo, Y.; Daigo, E.; Ishikawa, M.; Kibe, T. Healing of open upper lip vermillion wounds irradiated with CO2 laser immediately after injury. Photobiomodul. Photomed. Laser Surg. 2021, 39, 612–616. [Google Scholar] [CrossRef]
- Powell, D.W.; Mifflin, R.C.; Valentich, J.D.; Crowe, S.E.; Saada, J.I.; West, A.B. Myofibroblasts. I. Paracrine cells important in health and disease. Am. J. Physiol. 1999, 277, C1–C9. [Google Scholar] [CrossRef]
- Phan, S.H. Biology of fibroblasts and myofibroblasts. Proc. Am. Thorac. Soc. 2008, 5, 334–337. [Google Scholar] [CrossRef]
- Hinz, B. Myofibroblasts. Exp. Eye Res. 2016, 142, 56–70. [Google Scholar] [CrossRef]
- Hinz, B. The role of myofibroblasts in wound healing. Curr. Res. Transl. Med. 2016, 64, 171–177. [Google Scholar] [CrossRef]
- Zitelli, J. Wound healing for the clinician. Adv. Dermatol. 1987, 2, 243–267. [Google Scholar]
- Wang, Y.; Beekman, J.; Hew, J.; Jackson, S.; Issler-Fisher, A.C.; Parungao, R.; Lajevardi, S.S.; Li, Z.; Maitz, P.K.M. Burn injury: Challenges and advances in burn wound healing, infection, pain and scarring. Adv. Drug Deliv. Rev. 2018, 123, 3–17. [Google Scholar] [CrossRef]
- Alster, T.S.; Tanzi, E.L. Hypertrophic scars and keloids: Etiology and management. Am. J. Clin. Dermatol. 2003, 4, 235–243. [Google Scholar] [CrossRef]
- Tuncer, I.; Özçakır-Tomruk, C.; Şencift, K.; Çöloğlu, S. Comparison of conventional surgery and CO2 laser on intraoral soft tissue pathologies and evaluation of the collateral thermal damage. Photomed. Laser Surg. 2010, 28, 75–79. [Google Scholar] [CrossRef]
- Bornstein, M.M.; Winzap-Kälin, C.; Cochran, D.L.; Buser, D. The CO2 laser for excisional biopsies of oral lesions: A case series study. Int. J. Periodontics Restor. Dent. 2005, 25, 221–229. [Google Scholar]
- Suter, V.G.A.; Altermatt, H.J.; Bornstein, M.M. A randomized controlled clinical and histopathological trial comparing excisional biopsies of oral fibrous hyperplasias using CO2 and Er:YAG laser. Lasers Med. Sci. 2017, 32, 573–581. [Google Scholar] [CrossRef]
- Levanon, D.; Katzir, A.; Ravid, A. A scanning electron microscopy study of CO2 laser-albumin soldering in the rabbit model. Photomed. Laser Surg. 2004, 22, 461–469. [Google Scholar] [CrossRef]
- McNally, K.M.; Sorg, B.S.; Welch, A.J.; Dawes, J.M.; Owen, E.R. Photothermal effects of laser tissue soldering. Phys. Med. Biol. 1999, 44, 983–1002. [Google Scholar] [CrossRef]
- Han, B.; Fan, J.; Liu, L.; Tian, J.; Gan, C.; Yang, Z.; Jiao, H.; Zhang, T.; Liu, Z.; Zhang, H. Adipose-derived mesenchymal stem cells treatments for fibroblasts of fibrotic scar via downregulating TGF-β1 and Notch-1 expression enhanced by photobiomodulation therapy. Lasers Med. Sci. 2019, 1, 1–10. [Google Scholar] [CrossRef]
- Santiago, R.; Gomes, S.; Ozsarfati, J.; Zitney, M. Photobiomodulation for modulation of neuropathic pain and improvement of scar tissue. Scars Burn Heal. 2022, 8, 20595131221134052. [Google Scholar] [CrossRef]
- Rocha Júnior, A.M.; Vieira, B.J.; de Andrade, L.C.; Aarestrup, F.M. Low-level laser therapy increases transforming growth factor-beta2 expression and induces apoptosis of epithelial cells during the tissue repair process. Photomed. Laser Surg. 2009, 27, 303–307. [Google Scholar] [CrossRef]
- Keshri, G.K.; Gupta, A.; Yadav, A.; Sharma, S.K.; Singh, S.B. Photobiomodulation with pulsed and continuous wave near-infrared laser (810 nm, Al-Ga-As) augments dermal wound healing in immunosuppressed rats. PLoS ONE 2016, 11, e0166705. [Google Scholar] [CrossRef]
- Ying, B. Adjacent flaps for lower lip reconstruction after mucocele resection. J. Craniofac. Surg. 2012, 23, 556–557. [Google Scholar] [CrossRef]
- Nammour, S.; El Mobadder, M.; Namour, M.; Namour, A.; Arnabat-Dominguez, J.; Grzech-Leśniak, K.; Vanheusden, A.; Vescovi, P. Aesthetic treatment outcomes of capillary hemangioma, venous lake, and venous malformation of the lip using different surgical procedures and laser wavelengths (Nd:YAG, Er,Cr:YSGG, CO2, and diode 980 nm). Int. J. Environ. Res. Public Health 2020, 17, 8665. [Google Scholar] [CrossRef]
- Huang, I.Y.; Chen, C.M.; Kao, Y.H.; Worthington, P. Treatment of mucocele of the lower lip with carbon dioxide laser. J. Oral Maxillofac. Surg. 2007, 65, 855–858. [Google Scholar] [CrossRef]
- Yagüe-García, J.; España-Tost, A.J.; Berini-Aytés, L.; Gay-Escoda, C. Treatment of oral mucocele-scalpel versus CO2 laser. Med. Oral Patol. Oral Cir. Bucal. 2009, 14, e469–e474. [Google Scholar]
- Ramkumar, S.; Ramkumar, L.; Malathi, N.; Suganya, R. Excision of mucocele using diode laser in lower lip. Case Rep. Dent. 2016, 2016, 1746316. [Google Scholar] [CrossRef]
- Boj, J.R.; Poirier, C.; Espasa, E.; Hernandez, M.; Espanya, A. Lower lip mucocele treated with an erbium laser. Pediatr. Dent. 2009, 31, 249–252. [Google Scholar]
- Monteiro, K.; Delgado, M.L.; Garcês, F.; Machado, M.; Ferreira, F.; Martins, M.; Salazar, F.; Pacheco, J.J. A histological evaluation of the surgical margins from human oral fibrous-epithelial lesions excised with CO2 laser, diode laser, Er:YAG laser, Nd:YAG laser, electrosurgical scalpel and cold scalpel. Med. Oral Patol. Oral Cir. Bucal. 2019, 24, e271–e280. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Li, X.; Du, J.; Guo, L.; Liu, Y. A histological evaluation of the mice oral mucosal tissue wounds excised with diode laser, Er:YAG laser, and cold scalpel. Lasers Med. Sci. 2022, 37, 2707–2715. [Google Scholar] [CrossRef]
- Suter, V.G.A.; Altermatt, H.J.; Bornstein, M.M. A randomized controlled trial comparing surgical excisional biopsies using CO2 laser, Er:YAG laser and scalpel. Int. J. Oral Maxillofac. Surg. 2020, 49, 99–106. [Google Scholar] [CrossRef]
- Bartkowska, P.; Komisarek, O. Scar management in patients after cleft lip repair-Systematic review Cleft lip scar management. J. Cosmet. Dermatol. 2020, 19, 1866–1876. [Google Scholar] [CrossRef]
- Frans, F.A.; van Zuijlen, P.P.M.; Griot, J.P.W.D.; van Der Horst, C.M.A.M. Assessment of scar quality after cleft lip closure. Cleft Palate Craniofac. J. 2012, 49, 171–176. [Google Scholar] [CrossRef]
- Yoshino, H.; Hasuike, A.; Sanjo, N.; Sato, D.; Kubota, T.; Nagashima, H.; Sato, S. CO2 laser de-epithelization technique for subepithelial connective tissue graft: A study of 21 recessions. In Vivo 2020, 34, 869–875. [Google Scholar] [CrossRef]
- Visser, H.; Mausberg, R. Free gingival grafts using a CO2 laser: Results of a clinical study. J. Clin. Laser Med. Surg. 1996, 14, 85–88. [Google Scholar] [CrossRef]
- Zhao, H.; Hu, J.; Zhao, L. The effect of low-level laser therapy as an adjunct to periodontal surgery in the management of postoperative pain and wound healing: A systematic review and meta-analysis. Lasers Med. Sci. 2021, 36, 175–187. [Google Scholar] [CrossRef]
- Keskiner, I.; Lutfioğlu, M.; Aydogdu, A.; Saygun, N.I.; Serdar, M.A. Effect of photobiomodulation on transforming growth factor-β1, platelet-derived growth factor-BB, and interleukin-8 release in palatal wounds after free gingival graft harvesting: A randomized clinical study. Photomed. Laser Surg. 2016, 34, 263–271. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daigo, Y.; Daigo, E.; Fukuoka, H.; Fukuoka, N.; Idogaki, J.; Taniguchi, Y.; Tsutsumi, T.; Ishikawa, M.; Takahashi, K. CO2 Laser for Esthetic Healing of Injuries and Surgical Wounds with Small Parenchymal Defects in Oral Soft Tissues. Diseases 2023, 11, 172. https://doi.org/10.3390/diseases11040172
Daigo Y, Daigo E, Fukuoka H, Fukuoka N, Idogaki J, Taniguchi Y, Tsutsumi T, Ishikawa M, Takahashi K. CO2 Laser for Esthetic Healing of Injuries and Surgical Wounds with Small Parenchymal Defects in Oral Soft Tissues. Diseases. 2023; 11(4):172. https://doi.org/10.3390/diseases11040172
Chicago/Turabian StyleDaigo, Yuki, Erina Daigo, Hiroshi Fukuoka, Nobuko Fukuoka, Jun Idogaki, Yusuke Taniguchi, Takashi Tsutsumi, Masatsugu Ishikawa, and Kazuya Takahashi. 2023. "CO2 Laser for Esthetic Healing of Injuries and Surgical Wounds with Small Parenchymal Defects in Oral Soft Tissues" Diseases 11, no. 4: 172. https://doi.org/10.3390/diseases11040172
APA StyleDaigo, Y., Daigo, E., Fukuoka, H., Fukuoka, N., Idogaki, J., Taniguchi, Y., Tsutsumi, T., Ishikawa, M., & Takahashi, K. (2023). CO2 Laser for Esthetic Healing of Injuries and Surgical Wounds with Small Parenchymal Defects in Oral Soft Tissues. Diseases, 11(4), 172. https://doi.org/10.3390/diseases11040172



