The Association of Combined Per- and Polyfluoroalkyl Substances and Metals with Allostatic Load Using Bayesian Kernel Machine Regression
Abstract
1. Introduction
1.1. Background
1.2. Human Exposure Pathways to PFAS and Metals
1.3. Bayesian Kernel Machine Regression (BKMR): A Mechanism for Monitoring Multiple Environmental Exposures
2. Materials and Methods
2.1. Study Cohort and Design
2.2. PFAS and Metals Measurements
2.2.1. PFAS Quantification
2.2.2. Metals Quantification
2.3. Determining Allostatic Load Levels
2.4. Data Analysis
BKMR Modeling for Binary Outcomes
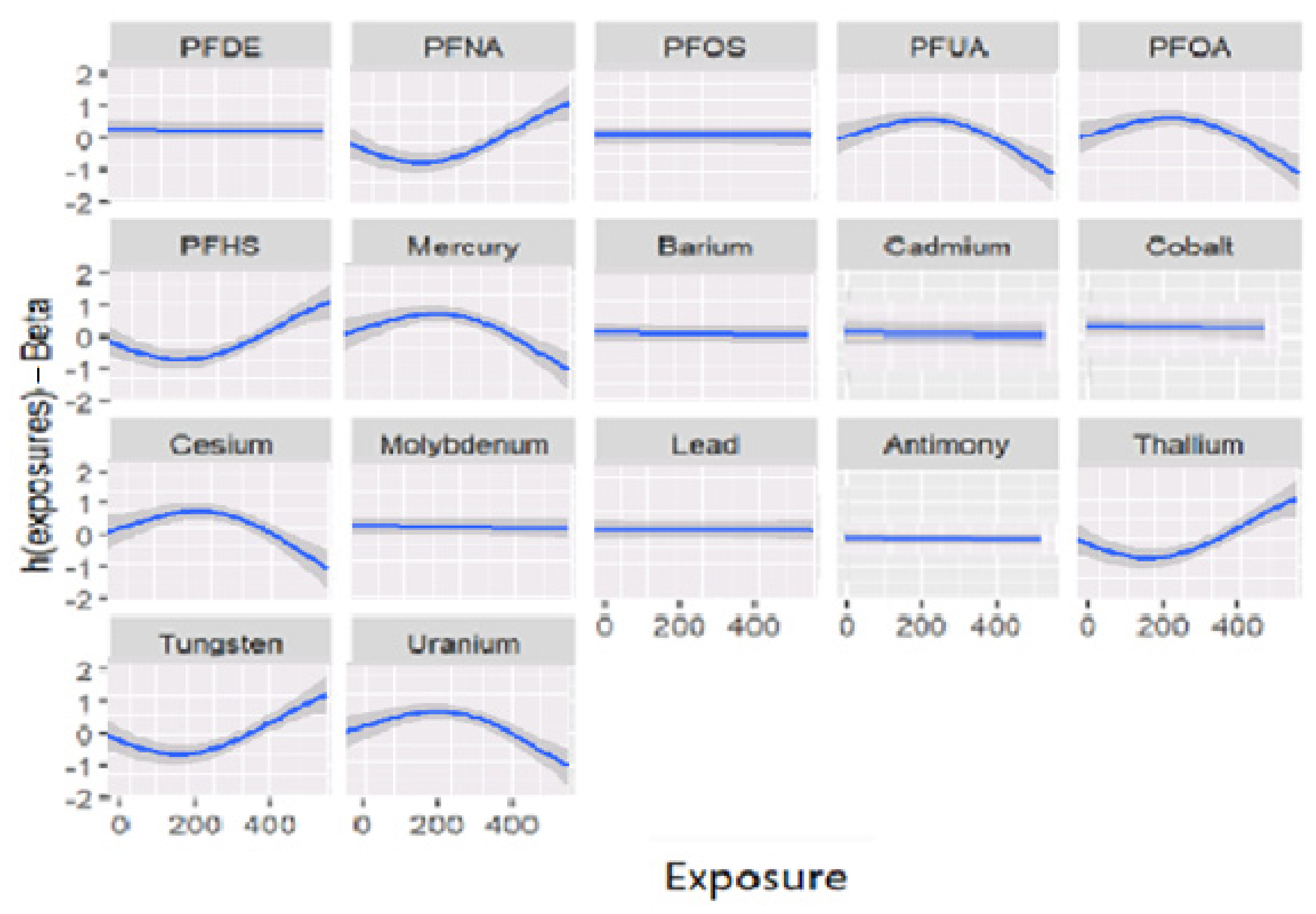
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wild, C.P. The exposome: From concept to utility. Int. J. Epidemiol. 2012, 41, 24–32. [Google Scholar] [CrossRef]
- Bashir, T.; Obeng-Gyasi, E. The Association between Multiple Per- and Polyfluoroalkyl Substances’ Serum Levels and Allostatic Load. Int. J. Environ. Res. Public Health 2022, 19, 5455. [Google Scholar] [CrossRef] [PubMed]
- Szyf, M.; McGowan, P.; Meaney, M.J. The social environment and the epigenome. Environ. Mol. Mutagen. 2008, 49, 46–60. [Google Scholar] [CrossRef] [PubMed]
- Caplin, A.; Ghandehari, M.; Lim, C.; Glimcher, P.; Thurston, G. Advancing environmental exposure assessment science to benefit society. Nat. Commun. 2019, 10, 1236. [Google Scholar] [CrossRef]
- Juster, R.-P.; McEwen, B.S.; Lupien, S.J. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci. Biobehav. Rev. 2010, 35, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Utumatwishima, J.N.; Baker, R.L.; Bingham, B.A.; Chung, S.T.; Berrigan, D.; Sumner, A.E. Stress Measured by Allostatic Load Score Varies by Reason for Immigration: The Africans in America Study. J. Racial Ethn. Health Disparities 2018, 5, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Cacioppo, J.T.; Berntson, G.G.; Adolphs, R.; Carter, C.S.; Davidson, R.J.; McClintock, M.; McEwen, B.S.; Meaney, M.; Schacter, D.L.; Sternberg, E.M.; et al. Protective and Damaging Effects of Stress Mediators. N. Engl. J. Med. 1998, 338, 171–179. [Google Scholar] [CrossRef]
- Duong, M.T.; Bingham, B.A.; Aldana, P.C.; Chung, S.T.; Sumner, A.E. Variation in the Calculation of Allostatic Load Score: 21 Examples from NHANES. J. Racial Ethn. Health Disparities 2016, 4, 455–461. [Google Scholar] [CrossRef]
- de Hollander, A.E.M.; Melse, J.M.; Lebret, E.; Kramers, P.G.N. An Aggregate Public Health Indicator to Represent the Impact of Multiple Environmental Exposures. Epidemiology 1999, 10, 606–617. [Google Scholar] [CrossRef]
- Su, F.; Zeeshan, M.; Xiong, L.-H.; Lv, J.-Y.; Wu, Y.; Tang, X.-J.; Zhou, Y.; Ou, Y.-Q.; Huang, W.-Z.; Feng, W.-R.; et al. Co-exposure to perfluoroalkyl acids and heavy metals mixtures associated with impaired kidney function in adults: A community-based population study in China. Sci. Total Environ. 2022, 839, 156299. [Google Scholar] [CrossRef] [PubMed]
- Bobb, J.F.; Henn, B.C.; Valeri, L.; Coull, B.A. Statistical software for analyzing the health effects of multiple concurrent exposures via Bayesian kernel machine regression. Environ. Health 2018, 17, 67. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. Mol. Clin. Environ. Toxicol. 2012, 3, 133–164. [Google Scholar]
- Stone, J.; Sutrave, P.; Gascoigne, E.; Givens, M.B.; Fry, R.C.; Manuck, T.A. Exposure to toxic metals and per- and polyfluoroalkyl substances and the risk of preeclampsia and preterm birth in the United States: A review. Am. J. Obstet. Gynecol. MFM 2021, 3, 100308. [Google Scholar] [CrossRef] [PubMed]
- Obeng-Gyasi, E.; Roostaei, J.; Gibson, J.M. Lead Distribution in Urban Soil in a Medium-Sized City: Household-Scale Analysis. Environ. Sci. Technol. 2021, 55, 3696–3705. [Google Scholar] [CrossRef] [PubMed]
- Genualdi, S.; Beekman, J.; Carlos, K.; Fisher, C.M.; Young, W.; DeJager, L.; Begley, T. Analysis of per- and poly-fluoroalkyl substances (PFAS) in processed foods from FDA’s Total Diet Study. Anal. Bioanal. Chem. 2021, 414, 1189–1199. [Google Scholar] [CrossRef] [PubMed]
- Tansel, B. PFAS use in electronic products and exposure risks during handling and processing of e-waste: A review. J. Environ. Manag. 2022, 316, 115291. [Google Scholar] [CrossRef]
- Garg, S.; Kumar, P.; Mishra, V.; Guijt, R.; Singh, P.; Dumée, L.F.; Sharma, R.S. A review on the sources, occurrence and health risks of per-/poly-fluoroalkyl substances (PFAS) arising from the manufacture and disposal of electric and electronic products. J. Water Process Eng. 2020, 38, 101683. [Google Scholar] [CrossRef]
- Bobb, J.F.; Valeri, L.; Henn, B.C.; Christiani, D.C.; Wright, R.O.; Mazumdar, M.; Godleski, J.J.; Coull, B.A. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics 2015, 16, 493–508. [Google Scholar] [CrossRef]
- George, E.I.; McCulloch, R.E. Approaches for Bayesian variable selection. Stat. Sin. 1997, 7, 339–373. [Google Scholar]
- Stafoggia, M.; Breitner, S.; Hampel, R.; Basagaña, X. Statistical Approaches to Address Multi-Pollutant Mixtures and Multiple Exposures: The State of the Science. Curr. Environ. Health Rep. 2017, 4, 481–490. [Google Scholar] [CrossRef]
- Sun, Z.; Tao, Y.; Li, S.; Ferguson, K.K.; Meeker, J.D.; Park, S.K.; Batterman, S.A.; Mukherjee, B. Statistical strategies for constructing health risk models with multiple pollutants and their interactions: Possible choices and comparisons. Environ. Health 2013, 12, 85. [Google Scholar] [CrossRef]
- Bobb, J. Introduction to Bayesian Kernel Machine Regression and the bkmr R Package. 2017. Available online: https://jenfb.github.io/bkmr/overview.html (accessed on 16 February 2023).
- Centers for Disease Control and Prevention. Laboratory Procedure Manual for Perfluoroalkyl and Polyfluoroalkyl Substances (NHANES 2013−2014); (Method No. 6304.06); CDC: Atlanta, GA, USA, 2014. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2013-2014/labmethods/PFAS_H_MET.pdf (accessed on 16 February 2023).
- Mottaleb, M.A.; Petriello, M.C.; Morris, A.J. High-Throughput UHPLC-MS/MS Measurement of Per- and Poly-Fluorinated Alkyl Substances in Human Serum. J. Anal. Toxicol. 2020, 44, 339–347. [Google Scholar] [CrossRef]
- Obeng-Gyasi, E.; Obeng-Gyasi, B. Chronic Stress and Cardiovascular Disease among Individuals Exposed to Lead: A Pilot Study. Diseases 2020, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Obeng-Gyasi, E. Cumulative Effects of Low-Level Lead Exposure and Chronic Physiological Stress on Hepatic Dysfunction—A Preliminary Study. Med. Sci. 2020, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Bashir, T.; Asiseh, F.; Jefferson-Moore, K.; Obeng-Gyasi, E. The Association of Per- and Polyfluoroalkyl Substances Public Health Serum Levels and Allostatic Load by Country of Birth and the Length of Time in the United States. Int. J. Environ. Res. Public Health 2022, 19, 9438. [Google Scholar] [CrossRef]
- Sakshaug, J.W.; Wiśniowski, A.; Ruiz, D.A.P.; Blom, A.G. Supplementing Small Probability Samples with Nonprobability Samples: A Bayesian Approach. J. Off. Stat. 2019, 35, 653–681. [Google Scholar] [CrossRef]
- Scott, J.G.; Berger, J.O. Bayes and empirical-Bayes multiplicity adjustment in the variable-selection problem. Ann. Stat. 2010, 38, 2587–2619. [Google Scholar] [CrossRef]
- Rodriquez, E.J.; Livaudais-Toman, J.; Gregorich, S.E.; Jackson, J.S.; Nápoles, A.M.; Pérez-Stable, E.J. Relationships between allostatic load, unhealthy behaviors, and depressive disorder in U.S. adults, 2005–2012 NHANES. Prev. Med. 2018, 110, 9–15. [Google Scholar] [CrossRef]
- Wadhwa, P.D.; Culhane, J.F.; Rauh, V.; Barve, S.S.; Hogan, V.; Sandman, C.A.; Hobel, C.J.; Chicz-DeMet, A.; Dunkel-Schetter, C.; Garite, T.J.; et al. Stress, infection and preterm birth: A biobehavioural perspective. Paediatr. Périnat. Epidemiol. 2001, 15, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Logan, J.G.; Barksdale, D.J. Allostasis and allostatic load: Expanding the discourse on stress and cardiovascular disease. J. Clin. Nurs. 2008, 17, 201–208. [Google Scholar] [CrossRef]
- Mattei, J.; Demissie, S.; Falcon, L.M.; Ordovas, J.M.; Tucker, K. Allostatic load is associated with chronic conditions in the Boston Puerto Rican Health Study. Soc. Sci. Med. 2010, 70, 1988–1996. [Google Scholar] [CrossRef]
- Nelson, K.M.; Reiber, G.; Kohler, T.; Boyko, E.J. Peripheral arterial disease in a multiethnic national sample: The role of conventional risk factors and allostatic load. Ethn. Dis. 2007, 17, 669. [Google Scholar] [PubMed]
- Slade, G.D.; Sanders, A.E.; By, K. Role of Allostatic Load in Sociodemographic Patterns of Pain Prevalence in the U.S. Population. J. Pain 2012, 13, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Obeng-Gyasi, E. Factors associated with elevated Per- and Polyfluoroalkyl substances serum levels in older adults. Aging Health Res. 2022, 2, 100086. [Google Scholar] [CrossRef]
- Crimmins, E.M.; Johnston, M.; Hayward, M.; Seeman, T. Age differences in allostatic load: An index of physiological dysregulation. Exp. Gerontol. 2003, 38, 731–734. [Google Scholar] [CrossRef]
- Salazar, C.R.; Strizich, G.; Seeman, T.E.; Isasi, C.R.; Gallo, L.C.; Avilés-Santa, L.M.; Cai, J.; Penedo, F.J.; Arguelles, W.; Sanders, A.E.; et al. Nativity differences in allostatic load by age, sex, and Hispanic background from the Hispanic Community Health Study/Study of Latinos. SSM-Popul. Health 2016, 2, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Rogers, C.R.; Moore, J.X.; Gilmore, D.R.; Petersen, E.; Brooks, E.; Kennedy, C.; Thorpe, R.J. Investigation of Differences in Allostatic Load among Black Men by Level of Educational Attainment: High School Graduates Experience the Highest Levels of Stress. Int. J. Environ. Res. Public Health 2022, 19, 3580. [Google Scholar] [CrossRef]
- Petrovic, D.; Pivin, E.; Ponte, B.; Dhayat, N.; Pruijm, M.; Ehret, G.; Ackermann, D.; Guessous, I.; Younes, S.E.; Pechère-Bertschi, A.; et al. Sociodemographic, behavioral and genetic determinants of allostatic load in a Swiss population-based study. Psychoneuroendocrinology 2016, 67, 76–85. [Google Scholar] [CrossRef]
- Pampel, F.C.; Krueger, P.M.; Denney, J.T. Socioeconomic Disparities in Health Behaviors. Annu. Rev. Sociol. 2010, 36, 349–370. [Google Scholar] [CrossRef]

| Overall Effects | ||||||
|---|---|---|---|---|---|---|
| Models | ||||||
| BKMR 1 | Oracle 2 | Linear 3 | TRUE 4 | |||
| 0.680 | −0.539 | −0.572 | −0.843 | |||
| Individual effect | ||||||
| Models | ||||||
| variable # | variable | PIP 5 | BKMR | Oracle | Linear | TRUE |
| 1 | PFDE | 0.700 | 0.068 | 0.153 | −0.693 | −1.784 |
| 2 | PFNA | 0.824 | 0.023 | 0.239 | 0.124 | 0.264 |
| 3 | PFOS | 0.651 | 0.087 | −0.131 | 0.032 | 0.059 |
| 4 | PFUA | 0.795 | 0.019 | 0.098 | 0.235 | −0.324 |
| 5 | PFOA | 0.754 | 0.033 | 0.955 | 0.015 | −0.021 |
| 6 | PFHS | 0.854 | 0.115 | −0.169 | −0.037 | −0.068 |
| 7 | Mercury | 0.807 | 0.022 | −0.278 | −0.084 | −0.144 |
| 8 | Barium | 0.719 | 0.014 | 0.427 | 0.033 | 0.054 |
| 9 | Cadmium | 0.727 | 0.390 | 0.356 | 0.114 | 0.199 |
| 10 | Cobalt | 0.706 | 0.022 | −0.038 | −0.169 | −0.273 |
| 11 | Cesium | 1.000 | 0.350 | 0.101 | 0.003 | 0.008 |
| 12 | Molybdenum | 1.000 | 0.238 | −0.386 | 0.004 | 0.006 |
| 13 | Lead | 0.674 | 0.035 | 0.736 | −0.008 | −0.036 |
| 14 | Antimony | 0.701 | 0.203 | −0.258 | 0.343 | 0.543 |
| 15 | Thallium | 0.749 | 0.044 | −0.163 | 0.419 | 0.663 |
| 16 | Tungsten | 0.762 | 0.012 | −0.195 | −0.349 | −0.689 |
| 17 | Uranium | 0.723 | 0.016 | −0.413 | 4.204 | 7.213 |
| Metals and PFAS | ||||||
|---|---|---|---|---|---|---|
| Variable | Molybdenum | Cesium | Mercury | PFNA | PFOA | PFHS |
| Activities | Mean | |||||
| 1 day | 57.80 | 5.08 | 0.59 | 0.14 | 0.98 | 0.80 |
| 2 days | 76.70 | 4.72 | 0.62 | 0.13 | 0.89 | 0.77 |
| 3 days | 55.70 | 5.05 | 0.57 | 0.13 | 0.85 | 0.70 |
| 4 days | 45.30 | 5.20 | 1.33 | 0.12 | 0.84 | 0.72 |
| 5 days | 60.40 | 4.26 | 0.57 | 0.13 | 0.90 | 0.79 |
| 6 days | 40.10 | 3.56 | 0.44 | 0.13 | 1.00 | 0.82 |
| 7 days | 57.40 | 3.60 | 0.63 | 0.14 | 0.86 | 0.67 |
| Smoke | ||||||
| yes | 51.70 | 4.95 | 0.56 | 0.13 | 0.93 | 0.78 |
| no | 61.00 | 5.03 | 0.64 | 0.14 | 0.81 | 0.67 |
| AL | ||||||
| high | 67.54 | 5.35 | 0.60 | 0.13 | 0.83 | 0.73 |
| low | 49.45 | 4.73 | 0.61 | 0.13 | 0.88 | 0.71 |
| Ethnicity | ||||||
| Mexican | 59.10 | 5.07 | 0.58 | 0.09 | 0.78 | 0.59 |
| Black | 57.70 | 4.78 | 0.66 | 0.14 | 0.84 | 0.72 |
| White | 50.20 | 4.89 | 0.54 | 0.12 | 0.97 | 0.82 |
| Hispanic | 61.80 | 5.19 | 0.43 | 0.11 | 0.80 | 0.59 |
| Other and Asian | 65.90 | 5.37 | 0.60 | 0.26 | 0.65 | 0.60 |
| Sex | ||||||
| Female | 54.30 | 4.76 | 0.63 | 0.12 | 0.76 | 0.56 |
| Male | 59.90 | 5.23 | 0.58 | 0.14 | 0.97 | 0.88 |
| Age Groups | |||
|---|---|---|---|
| 20 to 39 | 40 to 59 | 60 and older | |
| Ethnicity | AL mean | ||
| Mexican | 2.9 | 3.49 | 3.58 |
| Black | 3.32 | 3.92 | 3.83 |
| White | 2.66 | 3.26 | 3.37 |
| Hispanic | 2.69 | 3.47 | 3.64 |
| Other and Asian | 2.63 | 3.08 | 3.13 |
| Metals and PFAS | ||||||
|---|---|---|---|---|---|---|
| PFNA | PFOA | PFHS | Cesium | Molybdenum | Mercury | |
| PFNA | 1.000 | 0.124 | 0.028 | 0.042 | −0.006 | 0.064 |
| PFOA | 0.124 | 1.000 | 0.327 | 0.003 | 0.032 | −0.032 |
| PFHS | 0.028 | 0.327 | 1.000 | 0.029 | −0.013 | −0.069 |
| Cesium | 0.042 | 0.003 | 0.029 | 1.000 | 0.320 | 0.438 |
| Molybdenum | −0.006 | 0.032 | −0.013 | 0.320 | 1.000 | 0.221 |
| Mercury | 0.064 | −0.032 | −0.069 | 0.438 | 0.221 | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bashir, T.; Obeng-Gyasi, E. The Association of Combined Per- and Polyfluoroalkyl Substances and Metals with Allostatic Load Using Bayesian Kernel Machine Regression. Diseases 2023, 11, 52. https://doi.org/10.3390/diseases11010052
Bashir T, Obeng-Gyasi E. The Association of Combined Per- and Polyfluoroalkyl Substances and Metals with Allostatic Load Using Bayesian Kernel Machine Regression. Diseases. 2023; 11(1):52. https://doi.org/10.3390/diseases11010052
Chicago/Turabian StyleBashir, Tahir, and Emmanuel Obeng-Gyasi. 2023. "The Association of Combined Per- and Polyfluoroalkyl Substances and Metals with Allostatic Load Using Bayesian Kernel Machine Regression" Diseases 11, no. 1: 52. https://doi.org/10.3390/diseases11010052
APA StyleBashir, T., & Obeng-Gyasi, E. (2023). The Association of Combined Per- and Polyfluoroalkyl Substances and Metals with Allostatic Load Using Bayesian Kernel Machine Regression. Diseases, 11(1), 52. https://doi.org/10.3390/diseases11010052







