Abstract
The strong benefits of exercise, in addition to the development of both the therapeutic applications of physical activity and molecular biology tools, means that it has become very important to explore the underlying molecular patterns linking exercise and its induced phenotypic changes. Within this context, secreted protein acidic and rich in cysteine (SPARC) has been characterized as an exercise-induced protein that would mediate and induce some important effects of exercise. Herein, we suggest some underlying pathways to explain such SPARC-induced exercise-like effects. Such mechanistic mapping would not only allow us to understand the molecular processes of exercise and SPARC effects but would also highlight the potential to develop novel molecular therapies. These therapies would be based on mimicking the exercise benefits via either introducing SPARC or pharmacologically targeting the SPARC-related pathways to produce exercise-like effects. This is of a particular importance for those who do not have the ability to perform the required physical activity due to disabilities or diseases. The main objective of this work is to highlight selected potential therapeutic applications deriving from SPARC properties that have been reported in various publications.
1. Secreted Protein Acidic and Rich in Cysteine (SPARC): An Exercise-Induced Biomolecule
Beyond being a social activity or a hobby, strong evidence has linked exercise to a variety of health benefits and has made physical activity a part of different therapies including for the treatment of obesity [1,2,3], diabetes [4], depression [5,6] anxiety [5,7], Parkinson disease [8], Alzheimer’s disease [9], Coronary heart disease [10], ageing and sarcopenia [11,12,13]. To reveal the mechanisms beyond the exercise benefits there was a need to explore the molecular and cellular changes underlying the exercise-induced changes. As genes are important factors of biomolecular and biochemical pathways, the changes in gene expression in response to exercise have been explored. Within this context, functional genomics has identified genes that are overexpressed with exercise [14,15]. Exploring these genes represents a significant starting point towards the mechanistic understanding of exercise.
The most important of these gene expressions would be the secreted protein acidic and rich in cysteine (SPARC) [16]. Following the identification of Sparc as an exercise-induced gene (induced during endurance training), and as SPARC expression is also known to decline with age [17], SPARC implications have been explored in the contexts of both exercise and ageing. Briefly, studies using genetically-modified mice suggested that exercise-induced muscle phenotype changes are SPARC-dependent [18] and showed that SPARC overexpression mimics exercise effects in mice, whereas Sparc KO leads to an accelerated ageing phenotype which is improved by exercise [19]. Together, these data suggest that at least a part of the exercise benefits are mediated by SPARC, which would be anti-aging, and with effects against various metabolic disorders and age-related diseases [20,21]. SPARC is expressed in various situations and has even been suggested as a molecular physiological and pathological biomarker [22] for which its measure could optimize personalized medicine [23]. Herein, we suggest literature-based mechanisms to explain the exercise effects, the SPARC effects, as well as the molecular pathways beyond the exercise-induced effects that are mediated by SPARC, which is considered as an exercise-induced protein.
2. Related Pathological Concepts
Exercise is known for its benefits in enhancing metabolic functions and body fitness and for improving many risk factors including obesity, body fat, metabolic syndrome, lipidic profiles and insulin resistance [15,16]. These benefits correlate with exercise-induced gene expression regulation that include, for instance, increase in peroxisome proliferator-activated receptor gamma (PPARγ), coactivator 1-alpha (PGC1α), metabolic-related (TCA cycle, β-oxidation, electron transport and oxidative phosphorylation), antioxidant enzymes and contractile apparatus-encoded genes [16,24] among other genes [15]. Moreover, some exercise benefits such as those related to the improvement of muscular oxidative phosphorylation, calcium signalling, and tissue development have been found to remain even following training cessation [25]. The global trend towards physical inactivity has driven a dramatic increase in the incidence of many chronic diseases such as obesity, type 2 diabetes (T2D), hypertension, cardiovascular diseases (CVD), immune dysfunction, certain types of cancer, pulmonary diseases, musculoskeletal diseases and several types of neurodegenerative disorders [26].
Aging, defined as a chronic biological process of progressive functional decline in intrinsic physiological functions [14,27,28], also leads to chronic diseases, thus increasing the age-specific mortality rate [29]. The skeletal muscles are among the most age-sensitive tissues. Sarcopenia, a gradual loss of muscle mass, strength and function due to aging [30], is associated with reduced muscle regenerative capacity, mitochondrial dysfunction [31] and muscle fiber atrophy [31,32,33]. The most evident metabolic explanation is an imbalance between protein synthesis and breakdown rates. However, other causes include neurodegenerative processes, the reduction in anabolic hormone productions or sensitivity such as insulin, growth hormones and sex hormones, the dysregulation of cytokine secretions, modification in the response to inflammatory events, inadequate nutritional intakes and sedentary lifestyle [34,35,36].
Sarcopenia represents not only a risk factor for falling, a decrease of independence, and disability caused by immobility, but also for metabolic disorders, such as T2D and obesity [37,38]. Due to the skeletal muscle constituting the largest insulin-sensitive tissue in the body [39], and being the primary site for insulin-stimulated glucose utilization [39], T2D can be a consequence of muscle atrophy. Moreover, as skeletal muscle accounts for 40–50% of body weight and 20–30% of total resting energy expenditure [40], obesity can result from accumulated minor imbalances of energy intake over expenditure [41]. Under the obese status, the adipose tissue macrophages are a prominent source of the proinflammatory cytokines such as tumour necrosis factor α (TNF-α) and interleukin 6 (IL-6), both which can block the tissue insulin action and cause systemic insulin resistance, thus providing a potential link between inflammation and insulin resistance [42]. In the case of the etiology of atherosclerosis, the inflammation also plays a central role in developing CVD [43].
In addition, mitochondrial dysfunction resulting from oxidative damage to the mitochondrial DNA (mtDNA) caused by the reactive oxygen species (ROS) is one of the factors driving the aging process [44,45]. In an autoimmune disease, mitochondrial dysfunctions increase the ROS production that drive type I interferon-inducible gene expression and muscle inflammation [46]. The excessive ROS production can trigger mitochondrial dysfunction, apoptosis, autophagy, inflammation and muscle atrophy [47,48,49,50]. Moreover, mice lacking the cytoplasmic antioxidant enzyme, superoxide dismutase (SOD1), showed increased oxidative damage to proteins, lipids and DNAs, resulting in progressive muscle denervation, weakness and loss [51]. Thus, accumulation of the mtDNA mutations have also been linked to the pathogenesis of sarcopenia [52].
3. SPARC-Mediated Effects among the Exercise Benefits
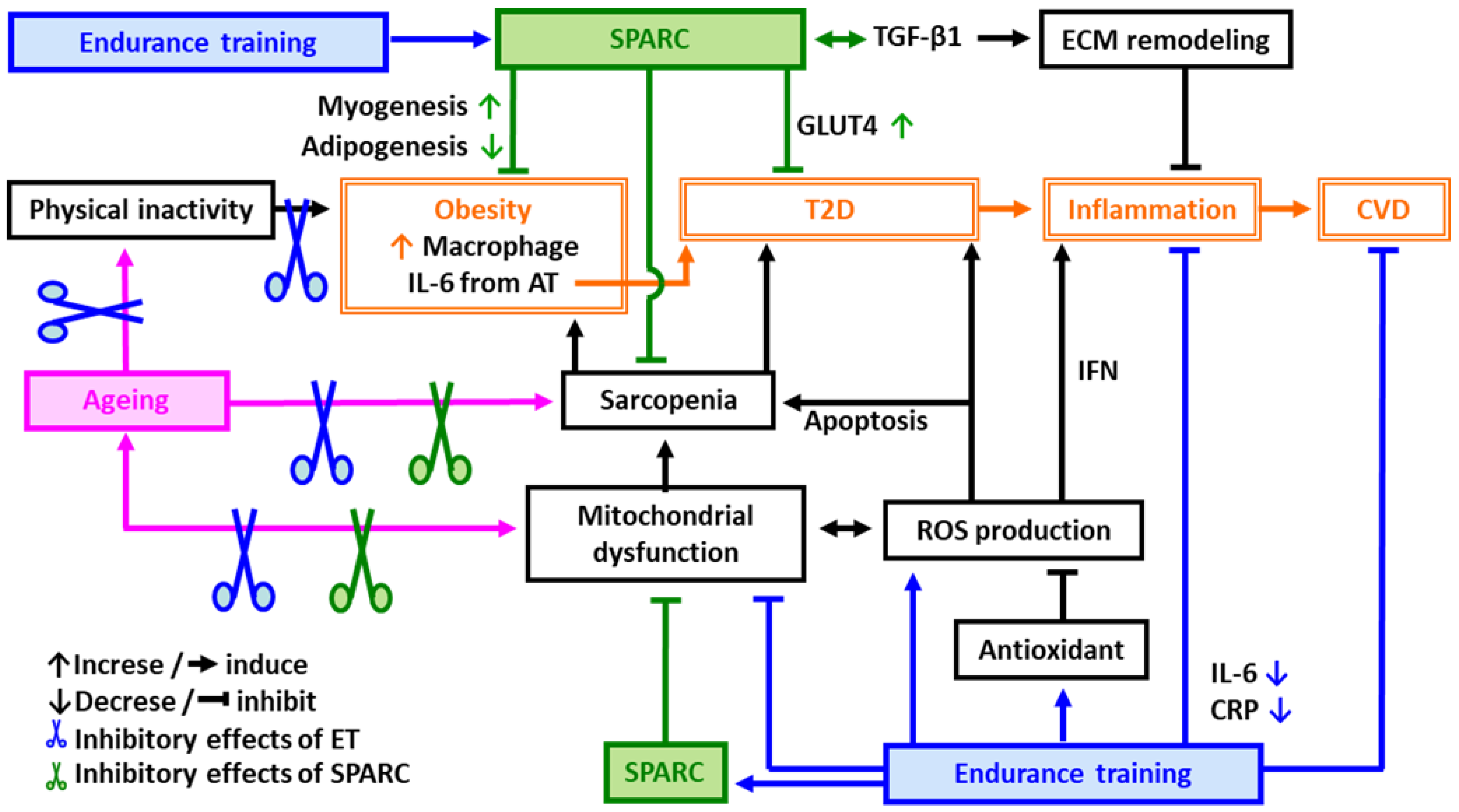
Exercise is significantly superior to all known pharmacological, nutritional and hormonal interventions for stabilizing and reversing sarcopenia [53]. Endurance training (ET) is well known to activate the mitochondrial biogenesis/function and to reduce serum inflammatory mediators such as C-reactive protein (CRP) and IL-6 [54] that are both impaired with ageing [26,55]. The improved mitochondrial function/systemic inflammation ameliorates insulin sensitivity and the lipid profile [16,56,57], and contributes to a decrease in mortality rates [58]. Thus, ET improves both the anti-oxidative and the anti-inflammatory response in addition to ameliorating obesity, CVD risks and sarcopenia (Figure 1).
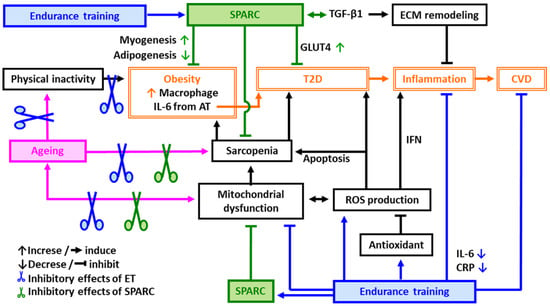
Figure 1.
Possible mechanisms of protective effects of endurance training (ET) and SPARC against age-related metabolic disorders. Abbreviations: AT: adipose tissue, CVD: cardiovascular diseases, CRP: C-reactive protein, ECM: extracellular matrix, ET: endurance training, GLUT4: glucose transporter type 4, IL-6: interleukin 6, IFN: interferon, ROS: reactive oxygen species, SPARC: secreted protein acidic and rich in cysteine, T2D: type 2 diabetes, TGF-β1: transforming growth factor beta 1.
Physical inactivity leads to obesity and T2D resulting in an acceleration of inflammation. Inflammation plays a central role in developing CVD. ET is a key factor to prevent age-related metabolic disorders such as obesity, T2D and CVD through the improvement of mitochondrial function and sarcopenia as well as through the induction of antioxidants that eliminate the ROS and decrease apoptosis. SPARC also improves mitochondrial function, sarcopenia, obesity, T2D, CVD and inflammation.
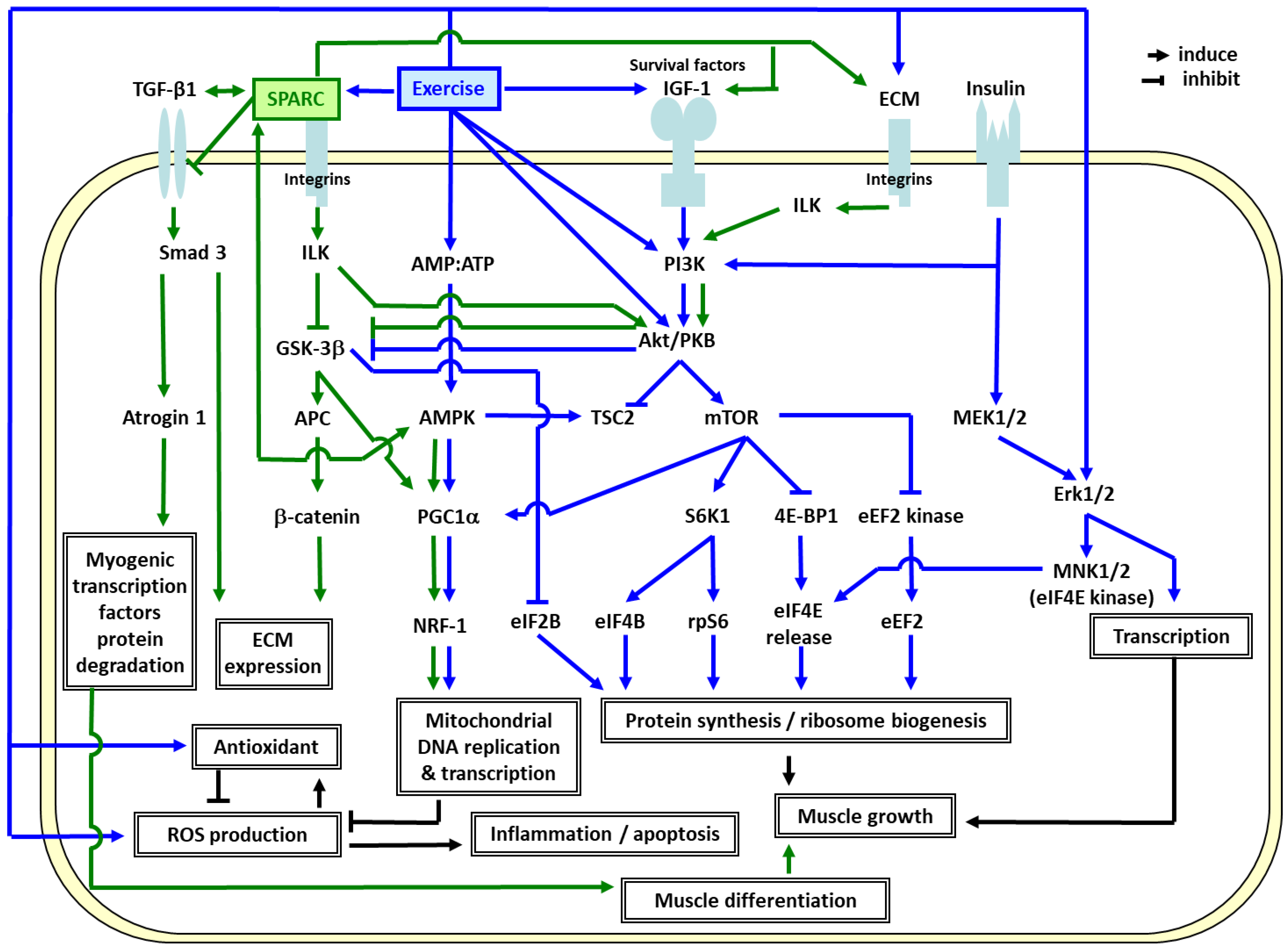
In order to elucidate the molecular mechanisms responsible for the ET effects [14,28], we identified the genes specifically modulated by ET in elderly muscle compared to young adults, and highlighted the importance of mitochondrial oxidative phosphorylation (OXPHOS) and extracellular matrix (ECM) remodeling in the skeletal muscle [16,24,57,59]. As shown in Figure 2, the ET-induced genes in elderly men [16,24,57,59], SPARC, specifically binds several of the ECM molecules including the collagens [60]. Thus, they influence lamina organization by binding to the growth factors such as insulin-like growth factor 1 (IGF-1) and transforming growth factor beta 1 (TGF-β1) [61,62,63]. TGF-β1 (profibrotic and anti-inflammatory protein) induces SPARC expression and vice versa [64]. While the extracellular SPARC functions as a matricellular protein, the intracellular and membrane-associated SPARC regulates cellular apoptotic pathways [64].
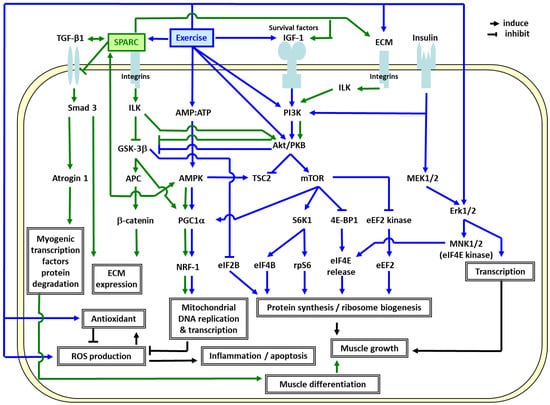
Figure 2.
Possible mechanisms linking extracellular matrix (ECM), mitochondrial biogenesis, the effects of SPARC and exercise training. Abbreviations: 4E-BP1: eIF4E-binding protein 1, Akt/PKB: RAC-alpha serine/threonine-protein kinase/protein kinase B, AMP: adenosine monophosphate, AMPK: AMP-activated protein kinase, APC: adenomatous polyposis coli, ATP: adenosine triphosphate, CL-1: collagenase 1/matrix metalloproteinase 1, Dvl: dishevelled, dsh homolog 1 (Drosophila), ECM: extracellular matrix, eEF2B: eukaryotic translation elongation factor 2B, eIF2B/4B/4E: eukaryotic translation initiation factor 2B/4B/4E, Erk1/2: extracellular-signal-regulated kinases 1/2, FAK: focal adhesion kinase, GSK-3β: glycogen synthase kinase 3 beta, IGF-1: insulin-like growth factor 1, ILK: integrin-linked kinase, MEK1/2: mitogen-activated protein (MAP) kinase kinase 1/2, MNK1/2: MAP kinase-interacting kinase 1/2, mTOR: mammalian target of rapamycin, NRF-1: regulating nuclear respiratory factor 1, PGC1α: peroxisome proliferator-activated receptor gamma coactivator-1α, PI3K: phosphoinositide 3-kinase, ROS: reactive oxygen species, rpS6: ribosomal protein S6, S6K1: ribosomal protein S6 kinase beta-1, Smad3: SMAD family member 3, SPARC: secreted protein acidic and rich in cysteine, TGF-β1: transforming growth factor beta 1, TSC2: tuberous sclerosis protein 2.
After myocardial infarction, SPARC is expressed by inflammatory cells [65], suggesting that SPARC produced by infiltrating leukocytes has a role in the inflammatory response and fibrosis in the heart. Thus, the absence of SPARC results in increased cardiac rupture and dysfunction [65]. By facilitating monocyte recruitment and/or macrophage differentiation and tissue retention [66], SPARC and TGF-β1 may be involved in minimizing inflammation [67]. SPARC also functions in the production and remodeling of the adipose tissue as well as in the regulation of preadipocytes differentiation [68]. In the absence of SPARC, mice show enhanced diet-induced obesity [68]. Furthermore, we have shown that SPARC increases type I collagen and OXPHOS expressions in proliferating and differentiating myoblasts in addition to accelerating differentiation, whereas inhibition leads to the opposite effects [69]. Moreover, we have confirmed an induction of myokine, Sparc, and PGC1α expressions in myoblasts after 48 h of electrical pulse stimulation, which is a suitable exercise model in vitro [70].
The results suggest that the exercise-induced SPARC plays a crucial role in the muscle integrity through ECM remodeling and mitochondrial biogenesis. Figure 2 also illustrates how ET promotes muscle growth and mitochondrial biogenesis via IGF1-phosphoinositide 3 kinase (PI3K)-Akt-mammalian target of rapamycin (mTOR) [71,72,73,74,75,76,77,78,79] and AMP-activated protein kinase (AMPK)-PGC1α [80,81,82] pathways. Exercise-induced SPARC regulates ECM remodeling via integrin-linked kinase (ILK)-glycogen synthase kinase 3 beta (GSK 3β)-β catenin [83,84], and transforming growth factor beta 1 (TGF β1)-SMAD family member 3 (Smad3) pathways [85]. SPARC binds to TGF-β1 co-receptor and inhibits the binding of TGF-β1 to its receptor. Thus, the TGF β1-Smad3-atrogin 1 pathway, in turn suppresses myogenic transcription factors (Myo D and myogenin) degradation and promotes muscle differentiation [86]. SPARC also interacts with AMPK [70,87] which induces PGC1α [80] and stimulates the ILK-GSK3β-PGC1 pathway [88], which may lead to mitochondrial biogenesis through a powerful induction of regulating nuclear respiratory factor 1 (NRF1) [82]. ET also induces antioxidants which eliminate the ROS, and consequently decrease apoptosis and inflammation. Other metabolic effects of SPARC on the cells have been shown in murine in cultured 3T3-L1 white and HIB1B brown adipocytes and the results suggest that recombinant SPARC both activates brown adipocytes and upregulates white adipocytes browning [89].
In order to further clarify the in vivo roles of SPARC and their similarities with ET, the impacts of Sparc knock-out (KO) in relation to sarcopenia and age-related metabolic disorders in young and old mice have also been investigated [18,19,90]. As expected, aging and/or Sparc KO led to sarcopenia (decreased muscle mass and strength), decreased glucose tolerance, and decreased expressions of muscle glucose transporter type 4 (GLUT4), collagen and OXPHOS, whereas ET had the opposite effects. Such Sparc KO-induced phenotype is important to understand the roles of SPARC in sarcopenia and glucose metabolism and to understand the link between ECM remodeling and mitochondrial function. Overall, SPARC can mimic the effects of ET which include the modulation of the ECM, mitochondrial function, inflammation, the tissue integrity and the immune response, as well as myogenesis, adiposity and glucose homeostasis [18,69,70,90,91,92,93,94,95,96]. Therefore, SPARC would be a key molecular link between physical exercise, obesity, T2D, CVD and inflammation (Figure 1 and Figure 2).
Nevertheless, a true confirmation regarding the potential of SPARC to be an exercise surrogate would be the addition/introduction of SPARC into a biological system. Thus, transgenic (Tg) mice over-expressing Sparc gene were created, and compared to both Sparc KO mice and an ET-induced phenotype [19]. The young Sparc Tg mice had increased muscle strength, muscle mass, and muscle glucose transporter and OXPHOS expressions, but lower glycemia and adiposity, an effect especially found in males [19]. Collectively, these findings showed that Sparc KO mice manifested an aging-like phenotype, whereas SPARC overexpression and exercise generated similar benefits [19]. The benefits were in regard to counteracting both SPARC deficiency-induced aging-like phenotype in addition to reversing age-related changes [19]. Indeed, Sparc overexpression would counteract some of the aging effects, most likely by activating the ILK-ECM pathway [91] via SPARC induction, as well as the mTOR-protein synthesis pathway (Figure 2). The Sparc KO lowered the muscle mitochondrial OXPHOS proteins, whereas the aging effects were only seen in the WT mice, since their levels in young Sparc KO mice were already as low as the old WT mice [97]. Theoretically, Sparc overexpression and ET would counteract the aging effects, most likely by inducing the antioxidant enzymes and the mitochondrial biogenesis inducer, PGC1α (Figure 2).
Both ET and Sparc overexpression would counteract aging-related dyslipidemia and glucose intolerance (Figure 1). We expected that SPARC would be, in part, involved in the mechanisms mediating ET-induced skeletal muscle adaptation, which in turn, would improve age-related diseases. Muscle atrophy occurs when a balance between anabolism and catabolism shifts toward excessive catabolism. The ubiquitin-proteasome system is the main regulatory mechanism of protein degradation in the skeletal muscle. The muscle specific ubiquitin-ligase enzymes (E3s), the muscle RING finger-1 (MuRF1) and the muscle atrophy F-box (MAFbx, also known as atrogin 1), regulate skeletal muscle atrophy in various pathological and physiological conditions by inducing the degradations of the structural proteins such as myosin light chain 2 and troponin I as well as myogenic differentiation proteins such as MyoD and myogenin, respectively [98,99,100]. The loss of SPARC in the mouse skeletal muscle causes myofiber atrophy by enhancing TGF-β1 signaling via phosphorylation of Smad3. Thus, it upregulates the atrogin 1 expression which may, in turn, cause muscle atrophy [86]. In mice, hindlimb immobilization leads to a 36% reduction in myofiber size and to an early inflammatory process during atrophy [101]. Moreover, exercise training, prior to the immobilization, alleviates muscle atrophy [102]. Therefore, we also hypothesize that SPARC may optimize or act similarly to exercise in order to attenuate muscle atrophy, which, in turn, prevents sarcopenia and age-related diseases (Figure 1).
4. Perspectives and Significance
Understanding SPARC implications during various biological processes and its potential roles in preventing or treating different diseases and health conditions such as obesity, sarcopenia, ageing and metabolic disorders could lead to important therapeutic tools to deal with such challenging health problems. The SPARC properties can be of use for pharmaceutical companies to develop molecules that mimic SPARC or that target SPARC-related pathways to counteract those specific health problems. Among such health problems, obesity is a condition involving different factors (biochemistry [103], genetics [104], hormones [105], DNA damage [106], etc.) and it has developmental patterns that have even been compared to cancer [107].
Indeed, exploring the pathways described above would allow for a better understanding of how SPARC mediates exercise-induced benefits and would reveal the molecular pathways linking physical activity to its induced phenotype. Such mechanistic understanding would help to develop a new generation of molecular therapies that mimic exercise. The principle underlying these therapies would be to either administer SPARC or pharmacologically target SPARC-related pathways to generate exercise-like benefits. Within this context, the importance of other considerations such as the predictive analysis of the SPARC molecular structure (for instance via AlphaFold [108]) could suggest potential mechanistic hypotheses for SPARC mechanisms of action.
Therapeutic applications can be speculated on based on the various functions and properties that SPARC has been associated with such as anti-inflammatory [94], regeneration [92], anticancer [109] and metabolism [110,111]. Further studies are required to investigate whether systemic injection or expression is the best option, or whether it is the targeting of specific tissues that would improve the outcomes depending on the targeted health problems.
Author Contributions
A.G. designed the manuscript structure and wrote it. A.G., M.Y. and J.S.-A. discussed the content, edited and critically revised the paper. J.S.-A. gave the final approval for the version to be published. All authors have read and agreed to the published version of the manuscript.
Funding
This work received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Abdelaziz Ghanemi received a scholarship under the Merit Scholarship Program for foreign students from the Ministry of Education and Higher Education of Quebec, Canada. The Fonds de recherche du Québec–Nature et technologies (FRQNT) is responsible for managing the program (Bourses d’excellence pour étudiants étrangers du Ministère de l’Éducation et de l’Enseignement supérieur du Québec, Le Fonds de recherche du Québec–Nature et technologies (FRQNT) est responsable de la gestion du programme). Abdelaziz Ghanemi received the scholarship « Bourse Tremplin -Stage en milieu de pratique» (Internship scholarship) from the Fonds de recherche du Québec–Sante (FRQS), Quebec, Canada. Abdelaziz Ghanemi received the scholarship “Inspirational journey” («Un parcours inspirant») from la Caisse Desjardins de l’Université Laval, Quebec, Canada.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Johnson, N.A.; Sultana, R.N.; Brown, W.J.; Bauman, A.E.; Gill, T. Physical activity in the management of obesity in adults: A position statement from Exercise and Sport Science Australia. J. Sci. Med. Sport 2021, 24, 1245–1254. [Google Scholar] [CrossRef] [PubMed]
- Brandt, C.; Pedersen, B.K. Physical Activity, Obesity and Weight Loss Maintenance. Handb. Exp. Pharmacol. 2022, 274, 349–369. [Google Scholar] [CrossRef] [PubMed]
- Swift, D.L.; McGee, J.E.; Earnest, C.P.; Carlisle, E.; Nygard, M.; Johannsen, N.M. The Effects of Exercise and Physical Activity on Weight Loss and Maintenance. Prog. Cardiovasc. Dis. 2018, 61, 206–213. [Google Scholar] [CrossRef]
- Kanaley, J.A.; Colberg, S.R.; Corcoran, M.H.; Malin, S.K.; Rodriguez, N.R.; Crespo, C.J.; Kirwan, J.P.; Zierath, J.R. Exercise/Physical Activity in Individuals with Type 2 Diabetes: A Consensus Statement from the American College of Sports Medicine. Med. Sci. Sports Exerc. 2022, 54, 353–368. [Google Scholar] [CrossRef] [PubMed]
- Carek, P.J.; Laibstain, S.E.; Carek, S.M. Exercise for the treatment of depression and anxiety. Int. J. Psychiatry Med. 2011, 41, 15–28. [Google Scholar] [CrossRef]
- Knapen, J.; Vancampfort, D.; Moriën, Y.; Marchal, Y. Exercise therapy improves both mental and physical health in patients with major depression. Disabil. Rehabil. 2015, 37, 1490–1495. [Google Scholar] [CrossRef]
- Stonerock, G.L.; Hoffman, B.M.; Smith, P.J.; Blumenthal, J.A. Exercise as Treatment for Anxiety: Systematic Review and Analysis. Ann. Behav. Med. 2015, 49, 542–556. [Google Scholar] [CrossRef]
- Tsukita, K.; Sakamaki-Tsukita, H.; Takahashi, R. Long-term Effect of Regular Physical Activity and Exercise Habits in Patients With Early Parkinson Disease. Neurology 2022, 98, e859–e871. [Google Scholar] [CrossRef]
- Valenzuela, P.L.; Castillo-García, A.; Morales, J.S.; de la Villa, P.; Hampel, H.; Emanuele, E.; Lista, S.; Lucia, A. Exercise benefits on Alzheimer’s disease: State-of-the-science. Ageing Res. Rev. 2020, 62, 101108. [Google Scholar] [CrossRef]
- Wang, L.; Ai, D.; Zhang, N. Exercise Benefits Coronary Heart Disease. Adv. Exp. Med. Biol. 2017, 1000, 3–7. [Google Scholar] [CrossRef]
- Rogeri, P.S.; Zanella, R., Jr.; Martins, G.L.; Garcia, M.D.A.; Leite, G.; Lugaresi, R.; Gasparini, S.O.; Sperandio, G.A.; Ferreira, L.H.B.; Souza-Junior, T.P.; et al. Strategies to Prevent Sarcopenia in the Aging Process: Role of Protein Intake and Exercise. Nutrients 2021, 14, 52. [Google Scholar] [CrossRef] [PubMed]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Ageing and Obesity Shared Patterns: From Molecular Pathogenesis to Epigenetics. Diseases 2021, 9, 87. [Google Scholar] [CrossRef]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Exercise, Diet and Sleeping as Regenerative Medicine Adjuvants: Obesity and Ageing as Illustrations. Medicines 2022, 9, 7. [Google Scholar] [CrossRef]
- Melouane, A.; Ghanemi, A.; Aubé, S.; Yoshioka, M.; St-Amand, J. Differential gene expression analysis in ageing muscle and drug discovery perspectives. Ageing Res. Rev. 2018, 41, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Ghanemi, A.; Melouane, A.; Yoshioka, M.; St-Amand, J. Exercise and High-Fat Diet in Obesity: Functional Genomics Perspectives of Two Energy Homeostasis Pillars. Genes 2020, 11, 875. [Google Scholar] [CrossRef] [PubMed]
- Riedl, I.; Yoshioka, M.; Nishida, Y.; Tobina, T.; Paradis, R.; Shono, N.; Tanaka, H.; St-Amand, J. Regulation of skeletal muscle transcriptome in elderly men after 6 weeks of endurance training at lactate threshold intensity. Exp. Gerontol. 2010, 45, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Scime, A.; Desrosiers, J.; Trensz, F.; Palidwor, G.A.; Caron, A.Z.; Andrade-Navarro, M.A.; Grenier, G. Transcriptional profiling of skeletal muscle reveals factors that are necessary to maintain satellite cell integrity during ageing. Mech. Ageing Dev. 2010, 131, 9–20. [Google Scholar] [CrossRef]
- Ghanemi, A.; Melouane, A.; Yoshioka, M.; St-Amand, J. Exercise Training of Secreted Protein Acidic and Rich in Cysteine (Sparc) KO Mice Suggests That Exercise-Induced Muscle Phenotype Changes Are SPARC-Dependent. Appl. Sci. 2020, 10, 9108. [Google Scholar] [CrossRef]
- Ghanemi, A.; Melouane, A.; Yoshioka, M.; St-Amand, J. Secreted Protein Acidic and Rich in Cysteine (Sparc) KO Leads to an Accelerated Ageing Phenotype Which Is Improved by Exercise Whereas SPARC Overexpression Mimics Exercise Effects in Mice. Metabolites 2022, 12, 125. [Google Scholar] [CrossRef]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Genetic Expression between Ageing and Exercise: Secreted Protein Acidic and Rich in Cysteine as a Potential “Exercise Substitute” Antiageing Therapy. Genes 2022, 13, 950. [Google Scholar] [CrossRef]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Secreted Protein Acidic and Rich in Cysteine as an Exercise-Induced Gene: Towards Novel Molecular Therapies for Immobilization-Related Muscle Atrophy in Elderly Patients. Genes 2022, 13, 1014. [Google Scholar] [CrossRef]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Secreted Protein Acidic and Rich in Cysteine as a Molecular Physiological and Pathological Biomarker. Biomolecules 2021, 11, 1689. [Google Scholar] [CrossRef] [PubMed]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Measuring Exercise-Induced Secreted Protein Acidic and Rich in Cysteine Expression as a Molecular Tool to Optimize Personalized Medicine. Genes 2021, 12, 1832. [Google Scholar] [CrossRef] [PubMed]
- Nishida, Y.; Tanaka, H.; Tobina, T.; Murakami, K.; Shono, N.; Shindo, M.; Ogawa, W.; Yoshioka, M.; St-Amand, J. Regulation of muscle genes by moderate exercise. Int. J. Sport Med. 2010, 31, 656–670. [Google Scholar] [CrossRef]
- St-Amand, J.; Yoshioka, M.; Nishida, Y.; Tobina, T.; Shono, N.; Tanaka, H. Effects of mild-exercise training cessation in human skeletal muscle. Eur. J. Appl. Physiol. 2012, 112, 853–869. [Google Scholar] [CrossRef] [PubMed]
- Handschin, C.; Spiegelman, B.M. The role of exercise and PGC1alpha in inflammation and chronic disease. Nature 2008, 454, 463–469. [Google Scholar] [CrossRef]
- Finch, C.E. The regulation of physiological changes during mammalian aging. Q. Rev. Biol. 1976, 51, 49–83. [Google Scholar] [CrossRef]
- Melouane, A.; St-Amand, J. Rôle du Gène Induit par l’Exercice, SPARC, Contre la Sarcopénie: Lien Possible Entre la Matrice Extracellulaire et la Fonction Mitochondriale. Ph.D. Thesis, Faculté de m, Université Laval, Québec, QC, Canada, 2020. Available online: http://hdl.handle.net/20.500.11794/38212 (accessed on 16 November 2022).
- Flatt, T. A New Definition of Aging? Front. Genet. 2012, 3, 148. [Google Scholar] [CrossRef]
- Bijlsma, A.Y.; Meskers, C.G.; Ling, C.H.; Narici, M.; Kurrle, S.E.; Cameron, I.D.; Westendorp, R.G.; Maier, A.B. Defining sarcopenia: The impact of different diagnostic criteria on the prevalence of sarcopenia in a large middle aged cohort. Age 2013, 35, 871–881. [Google Scholar] [CrossRef]
- Gueugneau, M.; Coudy-Gandilhon, C.; Theron, L.; Meunier, B.; Barboiron, C.; Combaret, L.; Taillandier, D.; Polge, C.; Attaix, D.; Picard, B.; et al. Skeletal muscle lipid content and oxidative activity in relation to muscle fiber type in aging and metabolic syndrome. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Carosio, S.; Berardinelli, M.G.; Aucello, M.; Musaro, A. Impact of ageing on muscle cell regeneration. Ageing Res. Rev. 2011, 10, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Listrat, A.; Meunier, B.; Gueugneau, M.; Coudy-Gandilhon, C.; Combaret, L.; Taillandier, D.; Polge, C.; Attaix, D.; Lethias, C.; et al. Apoptosis in capillary endothelial cells in ageing skeletal muscle. Aging Cell 2014, 13, 254–262. [Google Scholar] [CrossRef]
- Evans, W.J.; Paolisso, G.; Abbatecola, A.M.; Corsonello, A.; Bustacchini, S.; Strollo, F.; Lattanzio, F. Frailty and muscle metabolism dysregulation in the elderly. Biogerontology 2010, 11, 527–536. [Google Scholar] [CrossRef]
- Boirie, Y. Physiopathological mechanism of sarcopenia. J. Nutr. Health Aging 2009, 13, 717–723. [Google Scholar] [CrossRef]
- Meng, S.J.; Yu, L.J. Oxidative stress, molecular inflammation and sarcopenia. Int. J. Mol. Sci. 2010, 11, 1509–1526. [Google Scholar] [CrossRef]
- Dutta, C. Significance of sarcopenia in the elderly. J. Nutr. 1997, 127, 992S–993S. [Google Scholar] [PubMed]
- Taekema, D.G.; Gussekloo, J.; Maier, A.B.; Westendorp, R.G.; de Craen, A.J. Handgrip strength as a predictor of functional, psychological and social health. A prospective population-based study among the oldest old. Age Ageing 2010, 39, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Stump, C.S.; Henriksen, E.J.; Wei, Y.; Sowers, J.R. The metabolic syndrome: Role of skeletal muscle metabolism. Ann. Med. 2006, 38, 389–402. [Google Scholar] [CrossRef]
- Gallagher, D.; Belmonte, D.; Deurenberg, P.; Wang, Z.; Krasnow, N.; Pi-Sunyer, F.X.; Heymsfield, S.B. Organ-tissue mass measurement allows modeling of REE and metabolically active tissue mass. Am. J. Physiol. 1998, 275, E249–E258. [Google Scholar] [CrossRef]
- Jequier, E. Pathways to obesity. Int. J. Obes. Relat. Metab. Disord. 2002, 26 (Suppl. 2), S12–S17. [Google Scholar] [CrossRef]
- Ota, T. Obesity-induced inflammation and insulin resistance. Front. Endocrinol. 2014, 5, 204. [Google Scholar] [CrossRef] [PubMed]
- Haffner, S.M. The metabolic syndrome: Inflammation, diabetes mellitus, and cardiovascular disease. Am. J. Cardiol. 2006, 97, 3A–11A. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. The biologic clock: The mitochondria? J. Am. Geriatr. Soc. 1972, 20, 145–147. [Google Scholar] [CrossRef]
- Miquel, J.; Economos, A.C.; Fleming, J.; Johnson, J.E., Jr. Mitochondrial role in cell aging. Exp. Gerontol. 1980, 15, 575–591. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.; Laverny, G.; Allenbach, Y.; Grelet, E.; Ueberschlag, V.; Echaniz-Laguna, A.; Lannes, B.; Alsaleh, G.; Charles, A.L.; Singh, F.; et al. IFN-beta-induced reactive oxygen species and mitochondrial damage contribute to muscle impairment and inflammation maintenance in dermatomyositis. Acta Neuropathol. 2017, 134, 655–666. [Google Scholar] [CrossRef]
- Pingitore, A.; Lima, G.P.; Mastorci, F.; Quinones, A.; Iervasi, G.; Vassalle, C. Exercise and oxidative stress: Potential effects of antioxidant dietary strategies in sports. Nutrition 2015, 31, 916–922. [Google Scholar] [CrossRef] [PubMed]
- Mason, S.; Wadley, G.D. Skeletal muscle reactive oxygen species: A target of good cop/bad cop for exercise and disease. Redox Rep. 2014, 19, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, E.; Sestili, P. Reactive oxygen species in skeletal muscle signaling. J. Signal Transduct. 2012, 2012, 982794. [Google Scholar] [CrossRef]
- Sallam, N.; Laher, I. Exercise Modulates Oxidative Stress and Inflammation in Aging and Cardiovascular Diseases. Oxid. Med. Cell. Longev. 2016, 2016, 7239639. [Google Scholar] [CrossRef] [PubMed]
- Muller, F.L.; Song, W.; Liu, Y.; Chaudhuri, A.; Pieke-Dahl, S.; Strong, R.; Huang, T.T.; Epstein, C.J.; Roberts, L.J., 2nd; Csete, M.; et al. Absence of CuZn superoxide dismutase leads to elevated oxidative stress and acceleration of age-dependent skeletal muscle atrophy. Free. Radic. Biol. Med. 2006, 40, 1993–2004. [Google Scholar] [CrossRef] [PubMed]
- Kujoth, G.C.; Hiona, A.; Pugh, T.D.; Someya, S.; Panzer, K.; Wohlgemuth, S.E.; Hofer, T.; Seo, A.Y.; Sullivan, R.; Jobling, W.A.; et al. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science 2005, 309, 481–484. [Google Scholar] [CrossRef]
- Arnold, A.S.; Egger, A.; Handschin, C. PGC-1alpha and Myokines in the Aging Muscle—A Mini-Review. Gerontology 2011, 57, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Kohut, M.L.; McCann, D.A.; Russell, D.W.; Konopka, D.N.; Cunnick, J.E.; Franke, W.D.; Castillo, M.C.; Reighard, A.E.; Vanderah, E. Aerobic exercise, but not flexibility/resistance exercise, reduces serum IL-18, CRP, and IL-6 independent of beta-blockers, BMI, and psychosocial factors in older adults. Brain. Behav. Immun. 2006, 20, 201–209. [Google Scholar] [CrossRef]
- Vina, J.; Gomez-Cabrera, M.C.; Borras, C.; Froio, T.; Sanchis-Gomar, F.; Martinez-Bello, V.E.; Pallardo, F.V. Mitochondrial biogenesis in exercise and in ageing. Adv. Drug Deliv. Rev. 2009, 61, 1369–1374. [Google Scholar] [CrossRef]
- Hagberg, J.M. Physical activity, fitness, health and aging. In Physical Activity, Fitness, and Health: International Proceedings and Consensus Statement; Bouchard, C., Shephard, R.J., Stephens, T., Eds.; Human Kinetics Publishers Inc: Champaign, IL, USA, 1994; pp. 993–1005. [Google Scholar]
- Kwon, J.H.; Moon, K.M.; Min, K.W. Exercise-Induced Myokines can Explain the Importance of Physical Activity in the Elderly: An Overview. Healthcare 2020, 8, 378. [Google Scholar] [CrossRef]
- Paffenbarger, R.S.J.; Lee, I.-M. Age-specific physical activities and life style patterns as related to all-causes mortality and to longevity. In Exercise for Preventing Common Diseases; Tanaka, H., Shindo, M., Eds.; Springer: Tokyo, Japan, 1999; pp. 121–130. [Google Scholar]
- Verbrugge SAJ, Alhusen JA, Kempin S, Pillon NJ, Rozman J, Wackerhage H, Kleinert M: Genes controlling skeletal muscle glucose uptake and their regulation by endurance and resistance exercise. J. Cell Biochem. 2022, 123, 202–214. [CrossRef]
- Sage, H.; Vernon, R.B.; Funk, S.E.; Everitt, E.A.; Angello, J. SPARC, a secreted protein associated with cellular proliferation, inhibits cell spreading in vitro and exhibits Ca+2-dependent binding to the extracellular matrix. J. Cell Biol. 1989, 109, 341–356. [Google Scholar] [CrossRef]
- Francki, A.; Motamed, K.; McClure, T.D.; Kaya, M.; Murri, C.; Blake, D.J.; Carbon, J.G.; Sage, E.H. SPARC regulates cell cycle progression in mesangial cells via its inhibition of IGF-dependent signaling. J. Cell Biochem. 2003, 88, 802–811. [Google Scholar] [CrossRef]
- Mason, I.J.; Taylor, A.; Williams, J.G.; Sage, H.; Hogan, B.L. Evidence from molecular cloning that SPARC, a major product of mouse embryo parietal endoderm, is related to an endothelial cell ‘culture shock’ glycoprotein of Mr 43,000. Embo J. 1986, 5, 1465–1472. [Google Scholar] [CrossRef] [PubMed]
- McCurdy, S.; Baicu, C.F.; Heymans, S.; Bradshaw, A.D. Cardiac extracellular matrix remodeling: Fibrillar collagens and Secreted Protein Acidic and Rich in Cysteine (SPARC). J. Mol. Cell. Cardiol. 2010, 48, 544–549. [Google Scholar] [CrossRef]
- Wong, S.L.; Sukkar, M.B. The SPARC protein: An overview of its role in lung cancer and pulmonary fibrosis and its potential role in chronic airways disease. Br. J. Pharmacol. 2017, 174, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Schellings, M.W.; Vanhoutte, D.; Swinnen, M.; Cleutjens, J.P.; Debets, J.; van Leeuwen, R.E.; d’Hooge, J.; Van de Werf, F.; Carmeliet, P.; Pinto, Y.M.; et al. Absence of SPARC results in increased cardiac rupture and dysfunction after acute myocardial infarction. J. Exp. Med. 2009, 206, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Riley, H.J.; Bradshaw, A.D. The Influence of the Extracellular Matrix in Inflammation: Findings from the SPARC-Null Mouse. Anat. Rec. 2020, 303, 1624–1629. [Google Scholar] [CrossRef] [PubMed]
- Aseer, K.R.; Kim, S.W.; Choi, M.S.; Yun, J.W. Opposite Expression of SPARC between the Liver and Pancreas in Streptozotocin-Induced Diabetic Rats. PLoS ONE 2015, 10, e0131189. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Bradshaw, A.D.; Delany, A.M.; Sage, E.H. Inactivation of SPARC enhances high-fat diet-induced obesity in mice. Connect. Tissue Res. 2011, 52, 99–108. [Google Scholar] [CrossRef]
- Melouane, A.; Carbonell, A.; Yoshioka, M.; Puymirat, J.; St-Amand, J. Implication of SPARC in the modulation of the extracellular matrix and mitochondrial function in muscle cells. PLoS ONE 2018, 13, e0192714. [Google Scholar] [CrossRef]
- Melouane, A.; Yoshioka, M.; Kanzaki, M.; St-Amand, J. Sparc, an EPS-induced gene, modulates the extracellular matrix and mitochondrial function via ILK/AMPK pathways in C2C12 cells. Life Sci. 2019, 229, 277–287. [Google Scholar] [CrossRef]
- Shah, O.J.; Anthony, J.C.; Kimball, S.R.; Jefferson, L.S. 4E-BP1 and S6K1: Translational integration sites for nutritional and hormonal information in muscle. Am. J. Physiol. Endocrinol. Metab. 2000, 279, E715–E729. [Google Scholar] [CrossRef]
- Greiwe, J.S.; Kwon, G.; McDaniel, M.L.; Semenkovich, C.F. Leucine and insulin activate p70 S6 kinase through different pathways in human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2001, 281, E466–E471. [Google Scholar] [CrossRef]
- Barclay, R.D.; Burd, N.A.; Tyler, C.; Tillin, N.A.; Mackenzie, R.W. The Role of the IGF-1 Signaling Cascade in Muscle Protein Synthesis and Anabolic Resistance in Aging Skeletal Muscle. Front. Nutr. 2019, 6, 146. [Google Scholar] [CrossRef]
- Anthony, J.C.; Anthony, T.G.; Kimball, S.R.; Jefferson, L.S. Signaling pathways involved in translational control of protein synthesis in skeletal muscle by leucine. J. Nutr. 2001, 131, 856S–860S. [Google Scholar] [CrossRef]
- Kimball, S.R.; Jefferson, L.S.; Fadden, P.; Haystead, T.A.; Lawrence, J.C., Jr. Insulin and diabetes cause reciprocal changes in the association of eIF-4E and PHAS-I in rat skeletal muscle. Am. J. Physiol. 1996, 270, C705–C709. [Google Scholar] [CrossRef] [PubMed]
- Brunn, G.J.; Hudson, C.C.; Sekulic, A.; Williams, J.M.; Hosoi, H.; Houghton, P.J.; Lawrence, J.C., Jr.; Abraham, R.T. Phosphorylation of the translational repressor PHAS-I by the mammalian target of rapamycin. Science 1997, 277, 99–101. [Google Scholar] [CrossRef]
- Xu, G.; Marshall, C.A.; Lin, T.A.; Kwon, G.; Munivenkatappa, R.B.; Hill, J.R.; Lawrence, J.C., Jr.; McDaniel, M.L. Insulin mediates glucose-stimulated phosphorylation of PHAS-I by pancreatic beta cells. An insulin-receptor mechanism for autoregulation of protein synthesis by translation. J. Biol. Chem. 1998, 273, 4485–4491. [Google Scholar] [CrossRef] [PubMed]
- Kimball, S.R.; Shantz, L.M.; Horetsky, R.L.; Jefferson, L.S. Leucine regulates translation of specific mRNAs in L6 myoblasts through mTOR-mediated changes in availability of eIF4E and phosphorylation of ribosomal protein S6. J. Biol. Chem. 1999, 274, 11647–11652. [Google Scholar] [CrossRef] [PubMed]
- Long, W.; Saffer, L.; Wei, L.; Barrett, E.J. Amino acids regulate skeletal muscle PHAS-I and p70 S6-kinase phosphorylation independently of insulin. Am. J. Physiol. Endocrinol. Metab. 2000, 279, E301–E306. [Google Scholar] [CrossRef] [PubMed]
- Lira, V.A.; Benton, C.R.; Yan, Z.; Bonen, A. PGC-1alpha regulation by exercise training and its influences on muscle function and insulin sensitivity. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E145–E161. [Google Scholar] [CrossRef]
- Winder, W.W.; Hardie, D.G. Inactivation of acetyl-CoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am. J. Physiol. 1996, 270, E299–E304. [Google Scholar] [CrossRef]
- Wu, Z.; Puigserver, P.; Andersson, U.; Zhang, C.; Adelmant, G.; Mootha, V.; Troy, A.; Cinti, S.; Lowell, B.; Scarpulla, R.C.; et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 1999, 98, 115–124. [Google Scholar] [CrossRef]
- Nie, J.; Sage, E.H. SPARC inhibits adipogenesis by its enhancement of beta-catenin signaling. J. Biol. Chem. 2009, 284, 1279–1290. [Google Scholar] [CrossRef]
- Konigshoff, M.; Balsara, N.; Pfaff, E.M.; Kramer, M.; Chrobak, I.; Seeger, W.; Eickelberg, O. Functional Wnt signaling is increased in idiopathic pulmonary fibrosis. PLoS ONE 2008, 3, e2142. [Google Scholar] [CrossRef]
- Verrecchia, F.; Mauviel, A. Transforming growth factor-beta signaling through the Smad pathway: Role in extracellular matrix gene expression and regulation. J. Investig. Dermatol. 2002, 118, 211–215. [Google Scholar] [CrossRef]
- Nakamura, K.; Nakano, S.; Miyoshi, T.; Yamanouchi, K.; Nishihara, M. Loss of SPARC in mouse skeletal muscle causes myofiber atrophy. Muscle Nerve 2013, 48, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Guan, Y.; Zhang, L.; Li, K.; Dong, C. SPARC interacts with AMPK and regulates GLUT4 expression. Biochem. Biophys. Res. Commun. 2010, 396, 961–966. [Google Scholar] [CrossRef] [PubMed]
- Theeuwes, W.F.; Gosker, H.R.; Langen, R.C.J.; Pansters, N.A.M.; Schols, A.; Remels, A.H.V. Inactivation of glycogen synthase kinase 3beta (GSK-3beta) enhances mitochondrial biogenesis during myogenesis. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 2913–2926. [Google Scholar] [CrossRef]
- Mukherjee, S.; Choi, M.J.; Kim, S.W.; Yun, J.W. Secreted protein acidic and rich in cysteine (SPARC) regulates thermogenesis in white and brown adipocytes. Mol. Cell. Endocrinol. 2020, 506, 110757. [Google Scholar] [CrossRef] [PubMed]
- Ghanemi, A.; St-Amand, J. Obesity and Functional Genomics-Identified Genes: A Focus on the High-Fat Diet-Induced Gene Trefoil Factor 2 (Tff2) and the Exercise-Induced Gene Secreted Protein Acidic and Rich in Cysteine (Sparc) within the Context of Energy Metabolism. Ph.D. Thesis, Faculté de md, Université Laval, Québec, QC, Canada. Available online: http://hdl.handle.net/20.500.11794/107345 (accessed on 16 November 2022).
- Hayashi, Y.; Furue, M.K. Biological Effects of Culture Substrates on Human Pluripotent Stem Cells. Stem Cells Int. 2016, 2016, 5380560. [Google Scholar] [CrossRef] [PubMed]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Secreted Protein Acidic and Rich in Cysteine as A Regeneration Factor: Beyond the Tissue Repair. Life 2021, 11, 38. [Google Scholar] [CrossRef]
- Barker, T.H.; Baneyx, G.; Cardo-Vila, M.; Workman, G.A.; Weaver, M.; Menon, P.M.; Dedhar, S.; Rempel, S.A.; Arap, W.; Pasqualini, R.; et al. SPARC regulates extracellular matrix organization through its modulation of integrin-linked kinase activity. J. Biol. Chem. 2005, 280, 36483–36493. [Google Scholar] [CrossRef]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Secreted protein acidic and rich in cysteine and inflammation: Another homeostatic property? Cytokine 2020, 133, 155179. [Google Scholar] [CrossRef]
- Bradshaw, A.D.; Graves, D.C.; Motamed, K.; Sage, E.H. SPARC-null mice exhibit increased adiposity without significant differences in overall body weight. Proc. Natl. Acad. Sci. USA 2003, 100, 6045–6050. [Google Scholar] [CrossRef] [PubMed]
- Atorrasagasti, C.; Onorato, A.; Gimeno, M.L.; Andreone, L.; Garcia, M.; Malvicini, M.; Fiore, E.; Bayo, J.; Perone, M.J.; Mazzolini, G.D. SPARC is required for the maintenance of glucose homeostasis and insulin secretion in mice. Clin. Sci. 2019, 133, 351–365. [Google Scholar] [CrossRef]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Effect of the secreted protein acidic and rich in cysteine (Sparc) knock-out and physical exercise on adiposity and metabolism patterns in young and old mice. In Proceedings of the International Congress on Obesity and Metabolic Syndrome, Seoul, Korea, 30 August 2019. [Google Scholar]
- Kedar, V.; McDonough, H.; Arya, R.; Li, H.H.; Rockman, H.A.; Patterson, C. Muscle-specific RING finger 1 is a bona fide ubiquitin ligase that degrades cardiac troponin I. Proc. Natl. Acad. Sci. USA 2004, 101, 18135–18140. [Google Scholar] [CrossRef]
- Tintignac, L.A.; Lagirand, J.; Batonnet, S.; Sirri, V.; Leibovitch, M.P.; Leibovitch, S.A. Degradation of MyoD mediated by the SCF (MAFbx) ubiquitin ligase. J. Biol. Chem. 2005, 280, 2847–2856. [Google Scholar] [CrossRef]
- Jogo, M.; Shiraishi, S.; Tamura, T.A. Identification of MAFbx as a myogenin-engaged F-box protein in SCF ubiquitin ligase. FEBS Lett. 2009, 583, 2715–2719. [Google Scholar] [CrossRef] [PubMed]
- Caron, A.Z.; Drouin, G.; Desrosiers, J.; Trensz, F.; Grenier, G. A novel hindlimb immobilization procedure for studying skeletal muscle atrophy and recovery in mouse. J. Appl. Physiol. (1985) 2009, 106, 2049–2059. [Google Scholar] [CrossRef]
- Son, J.S.; Kim, J.H.; Kim, H.J.; Yoon, D.H.; Kim, J.S.; Song, H.S.; Song, W. Effect of resistance ladder training on sparc expression in skeletal muscle of hindlimb immobilized rats. Muscle Nerve 2016, 53, 951–957. [Google Scholar] [CrossRef]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. In Vitro Mimicking of Obesity-Induced Biochemical Environment to Study Obesity Impacts on Cells and Tissues. Diseases 2022, 10, 76. [Google Scholar] [CrossRef]
- Singh, R.K.; Kumar, P.; Mahalingam, K. Molecular genetics of human obesity: A comprehensive review. Comptes Rendus Biol. 2017, 340, 87–108. [Google Scholar] [CrossRef]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Tricking the Brain with Leptin to Limit Post Liposuction and Post Bariatric Surgery Weight Regain? Diseases 2022, 10, 80. [Google Scholar] [CrossRef]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. DNA Damage as a Mechanistic Link between Air Pollution and Obesity? Medicines 2022, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Boubertakh, B.; Silvestri, C.; Di Marzo, V. Obesity: The Fat Tissue Disease Version of Cancer. Cells 2022, 11, 1872. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Secreted protein acidic and rich in cysteine and cancer: A homeostatic hormone? Cytokine 2020, 127, 154996. [Google Scholar] [CrossRef] [PubMed]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Secreted Protein Acidic and Rich in Cysteine: Metabolic and Homeostatic Properties beyond the Extracellular Matrix Structure. Appl. Sci. 2020, 10, 2388. [Google Scholar] [CrossRef]
- Ghanemi, A.; Melouane, A.; Yoshioka, M.; St-Amand, J. Secreted protein acidic and rich in cysteine and bioenergetics: Extracellular matrix, adipocytes remodeling and skeletal muscle metabolism. Int. J. Biochem. Cell Biol. 2019, 117, 105627. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).