Potential Diets to Improve Mitochondrial Activity in Amyotrophic Lateral Sclerosis
Abstract
1. Introduction
2. Mitochondrial Dysfunction Involved in the Pathogenesis of ALS
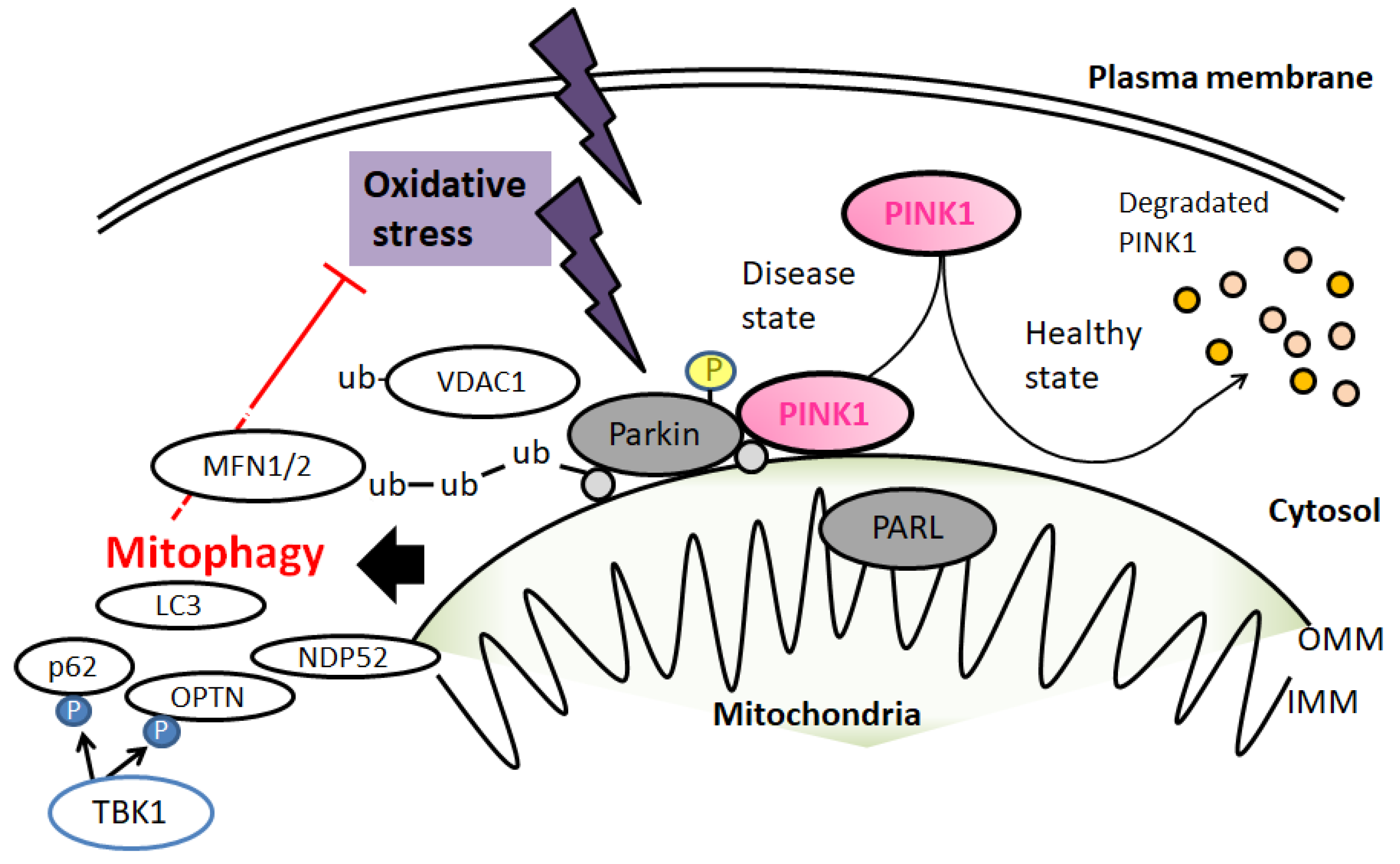
3. Mitochondrial Quality Control by Mitophagy in ALS
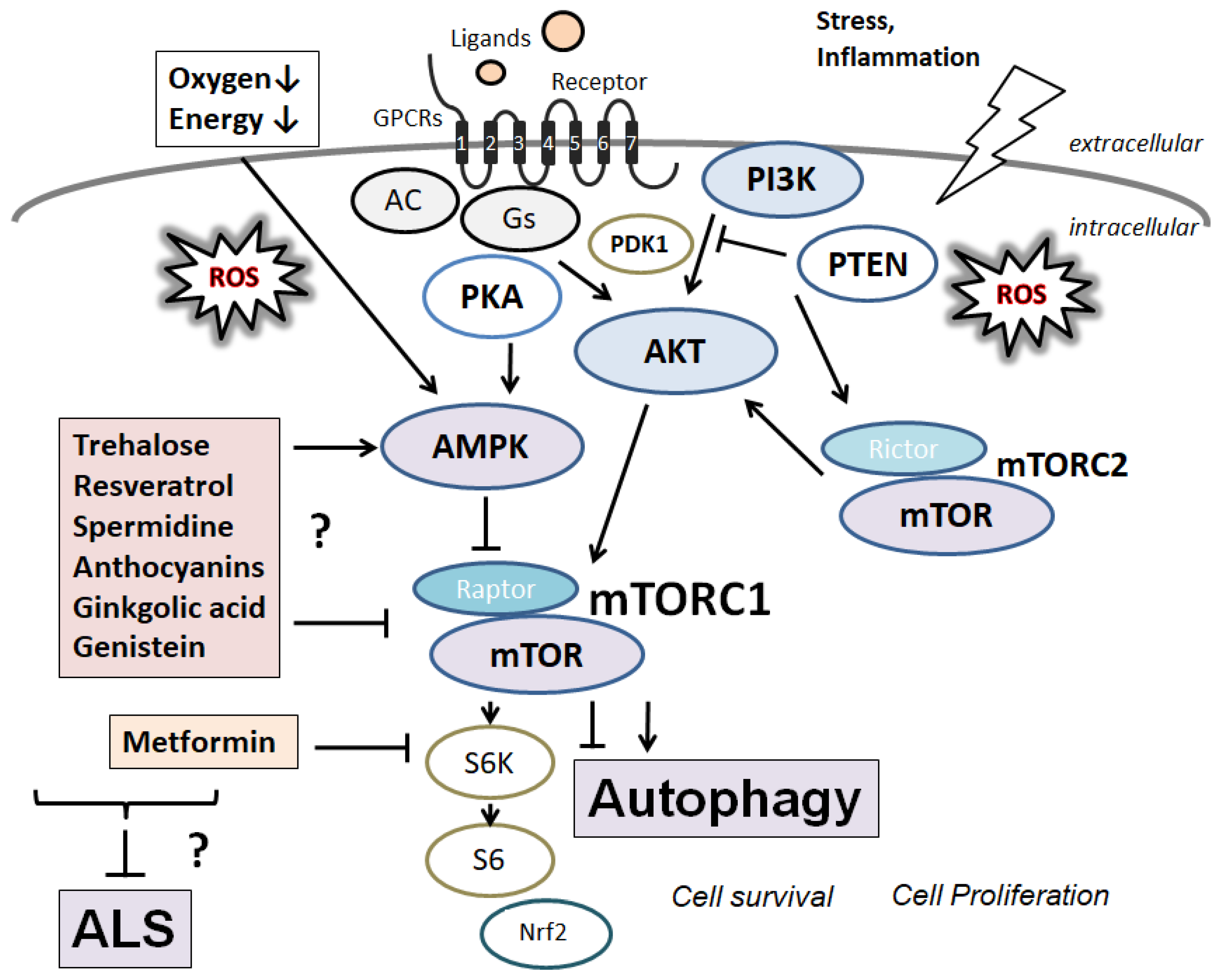
4. Beneficial Components from Natural Products for the Treatment of ALS
5. Current Therapeutic Possibilities and Limitations for ALS
6. Next Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALS: amyotrophic lateral sclerosis | |
| ATP: adenosine triphosphate | |
| DNA: deoxyribonucleic acid | |
| HDAC6; histone deacetylase 6 | |
| mTOR: mechanistic/mammalian target of rapamycin | |
| MFN1: mitofusin 1 | |
| mTORC: mechanistic/mammalian target of rapamycin complex | |
| NDP52: nuclear dot protein 52 | |
| PARL: presenilin | associated rhomboid |
| like | |
| OPTN: optineurin | |
| PDK1: 3 | phosphoinositide-dependent protein kinase |
| 1 | |
| PINK1: PTEN | induced kinase 1 |
| PTEN: phosphatase and tensin homolog | |
| QOL: quality of life | |
| ROS: reactive oxygen species | |
| SIRT1: Sirtuin 1 | |
| TBK1: TANK | binding kinase 1 |
| TSC2: tuberous sclerosis protein 2 | |
| VDAC1: voltage | dependent anion channel 1 |
| Ub: ubiquitin |
References
- Harms, M.J.; Li, Q.; Lee, S.; Zhang, C.; Kull, B.; Hallen, S.; Thorell, A.; Alexandersson, I.; Hagberg, C.E.; Peng, X.R.; et al. Mature Human White Adipocytes Cultured under Membranes Maintain Identity, Function, and Can Transdifferentiate into Brown-like Adipocytes. Cell Rep. 2019, 27, 213–225.e5. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, V.; Menzies, K.J.; Auwerx, J. Repairing Mitochondrial Dysfunction in Disease. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 353–389. [Google Scholar] [CrossRef] [PubMed]
- Osbakken, M.; Ito, K.; Zhang, D.; Ponomarenko, I.; Ivanics, T.; Jahngen, E.G.; Cohn, M. Creatine and cyclocreatine effects on ischemic myocardium: 31P nuclear magnetic resonance evaluation of intact heart. Cardiology 1992, 80, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Lambert-Smith, I.A.; Saunders, D.N.; Yerbury, J.J. Proteostasis impairment and ALS. Prog. Biophys. Mol. Biol. 2022, 174, 3–27. [Google Scholar] [CrossRef]
- Kirtonia, A.; Gala, K.; Fernandes, S.G.; Pandya, G.; Pandey, A.K.; Sethi, G.; Khattar, E.; Garg, M. Repurposing of drugs: An attractive pharmacological strategy for cancer therapeutics. Semin. Cancer Biol. 2021, 68, 258–278. [Google Scholar] [CrossRef]
- Russo, E.; Nguyen, H.; Lippert, T.; Tuazon, J.; Borlongan, C.V.; Napoli, E. Mitochondrial targeting as a novel therapy for stroke. Brain Circ. 2018, 4, 84–94. [Google Scholar]
- Chua, J.P.; De Calbiac, H.; Kabashi, E.; Barmada, S.J. Autophagy and ALS: Mechanistic insights and therapeutic implications. Autophagy 2022, 18, 254–282. [Google Scholar] [CrossRef]
- Prakash, Y.S.; Pabelick, C.M.; Sieck, G.C. Mitochondrial Dysfunction in Airway Disease. Chest 2017, 152, 618–626. [Google Scholar] [CrossRef]
- Kirtonia, A.; Sethi, G.; Garg, M. The multifaceted role of reactive oxygen species in tumorigenesis. Cell. Mol. Life Sci. 2020, 77, 4459–4483. [Google Scholar] [CrossRef]
- Perrelli, A.; Retta, S.F. Polymorphisms in genes related to oxidative stress and inflammation: Emerging links with the pathogenesis and severity of Cerebral Cavernous Malformation disease. Free Radic. Biol. Med. 2021, 172, 403–417. [Google Scholar] [CrossRef]
- Chen, X.; Wang, X.; Yang, L.; Xu, H.; Wu, Y.; Wu, J.; Chen, L.; Xu, C. Magnesium isoglycyrrhizinate prevents cadmium-induced activation of JNK and apoptotic hepatocyte death by reversing ROS-inactivated PP2A. J. Pharm. Pharmacol. 2021, 73, 1663–1674. [Google Scholar] [CrossRef]
- Rabinowitz, A.R.; Li, X.; Levin, H.S. Sport and nonsport etiologies of mild traumatic brain injury: Similarities and differences. Annu. Rev. Psychol. 2014, 65, 301–331. [Google Scholar] [CrossRef]
- Pan, Q.; Ban, Y.; Xu, L. Silibinin-Albumin Nanoparticles: Characterization and Biological Evaluation Against Oxidative Stress-Stimulated Neurotoxicity Associated with Alzheimer’s Disease. J. Biomed. Nanotechnol. 2021, 17, 1123–1130. [Google Scholar] [CrossRef]
- Rae, C.D.; Bröer, S. Creatine as a booster for human brain function. How might it work? Neurochem. Int. 2015, 89, 249–259. [Google Scholar] [CrossRef]
- Shah, S.I.; Paine, J.G.; Perez, C.; Ullah, G. Mitochondrial fragmentation and network architecture in degenerative diseases. PLoS ONE 2019, 14, e0223014. [Google Scholar] [CrossRef]
- Liu, Z.; Butow, R.A. Mitochondrial retrograde signaling. Annu. Rev. Genet. 2006, 40, 159–185. [Google Scholar] [CrossRef]
- Kalyanaraman, B.; Cheng, G.; Zielonka, J.; Bennett, B. Low-Temperature EPR Spectroscopy as a Probe-Free Technique for Monitoring Oxidants Formed in Tumor Cells and Tissues: Implications in Drug Resistance and OXPHOS-Targeted Therapies. Cell Biochem. Biophys. 2019, 77, 89–98. [Google Scholar] [CrossRef]
- Tan, Y.Q.; Zhang, X.; Zhang, S.; Zhu, T.; Garg, M.; Lobie, P.E.; Pandey, V. Mitochondria: The metabolic switch of cellular oncogenic transformation. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188534. [Google Scholar] [CrossRef]
- Bloch-Damti, A.; Bashan, N. Proposed mechanisms for the induction of insulin resistance by oxidative stress. Antioxid. Redox Signal. 2005, 7, 1553–1567. [Google Scholar] [CrossRef]
- Mrakic-Sposta, S.; Vezzoli, A.; Rizzato, A.; Della Noce, C.; Malacrida, S.; Montorsi, M.; Paganini, M.; Cancellara, P.; Bosco, G. Oxidative stress assessment in breath-hold diving. Eur. J. Appl. Physiol. 2019, 119, 2449–2456. [Google Scholar] [CrossRef]
- Kittimongkolsuk, P.; Pattarachotanant, N.; Chuchawankul, S.; Wink, M.; Tencomnao, T. Neuroprotective Effects of Extracts from Tiger Milk Mushroom Lignosus rhinocerus Against Glutamate-Induced Toxicity in HT22 Hippocampal Neuronal Cells and Neurodegenerative Diseases in Caenorhabditis elegans. Biology 2021, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.C. Mitochondrial Dynamics and Its Involvement in Disease. Annu. Rev. Pathol. 2020, 15, 235–259. [Google Scholar] [CrossRef] [PubMed]
- Boillée, S.; Vande, C.V.; Cleveland, D.W. ALS: A disease of motor neurons and their nonneuronal neighbors. Neuron 2006, 52, 39–59. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.F.; Shaw, P.J.; De Vos, K.J. The role of mitochondria in amyotrophic lateral sclerosis. Neurosci. Lett. 2019, 710, 132933. [Google Scholar] [CrossRef] [PubMed]
- Jena, K.K.; Mehto, S.; Kolapalli, S.P.; Nath, P.; Sahu, R.; Chauhan, N.R.; Sahoo, P.K.; Dhar, K.; Das, S.K.; Chauhan, S.; et al. TRIM16 governs the biogenesis and disposal of stress-induced protein aggregates to evade cytotoxicity: Implication for neurodegeneration and cancer. Autophagy 2019, 15, 924–926. [Google Scholar] [CrossRef]
- Su, Z.; Nie, Y.; Huang, X.; Zhu, Y.; Feng, B.; Tang, L.; Zheng, G. Mitophagy in Hepatic Insulin Resistance: Therapeutic Potential and Concerns. Front. Pharmacol. 2019, 10, 1193. [Google Scholar] [CrossRef]
- Kim, J.; Kim, T.Y.; Cho, K.S.; Kim, H.N.; Koh, J.Y. Autophagy activation and neuroprotection by progesterone in the G93A-SOD1 transgenic mouse model of amyotrophic lateral sclerosis. Neurobiol. Dis. 2013, 59, 80–85. [Google Scholar] [CrossRef]
- Perera, N.D.; Tomas, D.; Wanniarachchillage, N.; Cuic, B.; Luikinga, S.J.; Rytova, V.; Turner, B.J. Stimulation of mTOR-independent autophagy and mitophagy by rilmenidine exacerbates the phenotype of transgenic TDP-43 mice. Neurobiol. Dis. 2021, 154, 105359. [Google Scholar] [CrossRef]
- Matsuda, S.; Kitagishi, Y.; Kobayashi, M. Function and characteristics of PINK1 in mitochondria. Oxid. Med. Cell. Longev. 2013, 2013, 601587. [Google Scholar] [CrossRef]
- Matsuda, S.; Nakanishi, A.; Minami, A.; Wada, Y.; Kitagishi, Y. Functions and characteristics of PINK1 and Parkin in cancer. Front. Biosci. 2015, 20, 491–501. [Google Scholar] [CrossRef]
- Rüb, C.; Wilkening, A.; Voos, W. Mitochondrial quality control by the Pink1/Parkin system. Cell Tissue Res. 2017, 367, 111–123. [Google Scholar] [CrossRef]
- Narendra, D.; Tanaka, A.; Suen, D.F.; Youle, R.J. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J. Cell Biol. 2008, 183, 795–803. [Google Scholar] [CrossRef]
- Nardin, A.; Schrepfer, E.; Ziviani, E. Counteracting PINK/Parkin Deficiency in the Activation of Mitophagy: A Potential Therapeutic Intervention for Parkinson’s Disease. Curr. Neuropharmacol. 2016, 14, 250–259. [Google Scholar] [CrossRef]
- Moreira, O.C.; Estébanez, B.; Martínez-Florez, S.; de Paz, J.A.; Cuevas, M.J.; González-Gallego, J. Mitochondrial Function and Mitophagy in the Elderly: Effects of Exercise. Oxid. Med. Cell Longev. 2017, 2017, 2012798. [Google Scholar] [CrossRef]
- Wang, H.; Ni, H.M.; Chao, X.; Ma, X.; Rodriguez, Y.A.; Chavan, H.; Wang, S.; Krishnamurthy, P.; Dobrowsky, R.; Xu, D.X.; et al. Double deletion of PINK1 and Parkin impairs hepatic mitophagy and exacerbates acetaminophen-induced liver injury in mice. Redox Biol. 2019, 22, 101148. [Google Scholar] [CrossRef]
- Eldeeb, M.A.; Ragheb, M.A. N-degron-mediated degradation and regulation of mitochondrial PINK1 kinase. Curr. Genet. 2020, 66, 693–701. [Google Scholar] [CrossRef]
- Li, X.; Huang, L.; Lan, J.; Feng, X.; Li, P.; Wu, L.; Peng, Y. Molecular mechanisms of mitophagy and its roles in neurodegenerative diseases. Pharmacol. Res. 2021, 163, 105240. [Google Scholar] [CrossRef]
- Oakes, J.A.; Davies, M.C.; Collins, M.O. TBK1: A new player in ALS linking autophagy and neuroinflammation. Mol. Brain 2017, 10, 5. [Google Scholar] [CrossRef]
- Xie, Y.; Zhou, B.; Lin, M.Y.; Wang, S.; Foust, K.D.; Sheng, Z.H. Endolysosomal Deficits Augment Mitochondria Pathology in Spinal Motor Neurons of Asymptomatic fALS Mice. Neuron 2015, 87, 355–370. [Google Scholar] [CrossRef]
- Chennampally, P.; Sayed-Zahid, A.; Soundararajan, P.; Sharp, J.; Cox, G.A.; Collins, S.D.; Smith, R.L. A microfluidic approach to rescue ALS motor neuron degeneration using rapamycin. Sci. Rep. 2021, 11, 18168. [Google Scholar] [CrossRef]
- Mugume, Y.; Kazibwe, Z.; Bassham, D.C. Target of Rapamycin in Control of Autophagy: Puppet Master and Signal Integrator. Int. J. Mol. Sci. 2020, 21, 8259. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, A.; Mattesich, M.; Zwerschke, W. Silencing of the small GTPase DIRAS3 induces cellular senescence in human white adipose stromal/progenitor cells. Aging 2017, 9, 860–879. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barzilai, N.; Crandall, J.P.; Kritchevsky, S.B.; Espeland, M.A. Metformin as a Tool to Target Aging. Cell Metab. 2016, 23, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Ning, X.; He, J.; Shi, X.; Yang, G. Regulation of Adipogenesis by Quinine through the ERK/S6 Pathway. Int. J. Mol. Sci. 2016, 17, 504. [Google Scholar] [CrossRef] [PubMed]
- Guertin, D.A.; Sabatini, D.M. The pharmacology of mTOR inhibition. Sci. Signal. 2009, 2, pe24. [Google Scholar] [CrossRef]
- Stokoe, D.; Stephens, L.R.; Copeland, T.; Gaffney, P.R.; Reese, C.B.; Painter, G.F.; Holmes, A.B.; McCormick, F.; Hawkins, P.T. Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science 1997, 277, 567–570. [Google Scholar] [CrossRef]
- Long, X.; Lin, Y.; Ortiz-Vega, S.; Yonezawa, K.; Avruch, J. Rheb binds and regulates the mTOR kinase. Curr. Biol. 2005, 15, 702–713. [Google Scholar] [CrossRef]
- Egan, D.; Kim, J.; Shaw, R.J.; Guan, K.L. The autophagy initiating kinase ULK1 is regulated via opposing phosphorylation by AMPK and mTOR. Autophagy 2011, 7, 643–644. [Google Scholar] [CrossRef]
- Høyer-Hansen, M.; Bastholm, L.; Szyniarowski, P.; Campanella, M.; Szabadkai, G.; Farkas, T.; Bianchi, K.; Fehrenbacher, N.; Ellingm, F.; Rizzuto, R.; et al. Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-beta, and Bcl-2. Mol. Cell 2007, 25, 193–205. [Google Scholar] [CrossRef]
- Fang, J.; Yang, J.; Wu, X.; Zhang, G.; Li, T.; Wang, X.; Zhang, H.; Wang, C.C.; Liu, G.H.; Wang, L. Metformin alleviates human cellular aging by upregulating the endoplasmic reticulum glutathione peroxidase 7. Aging Cell 2018, 17, e12765. [Google Scholar] [CrossRef]
- Zhang, K.; Zhou, X.; Wang, J.; Zhou, Y.; Qi, W.; Chen, H.; Nie, S.; Xie, M. Dendrobium officinale polysaccharide triggers mitochondrial disorder to induce colon cancer cell death via ROS-AMPK-autophagy pathway. Carbohydr. Polym. 2021, 264, 118018. [Google Scholar] [CrossRef]
- Williams, A.; Sarkar, S.; Cuddon, P.; Ttofi, E.K.; Saiki, S.; Siddiqi, F.H.; Jahreiss, L.; Fleming, A.; Pask, D.; Goldsmith, P.; et al. Novel targets for Huntington’s disease in an mTOR-independent autophagy pathway. Nat. Chem. Biol. 2008, 4, 295–305. [Google Scholar] [CrossRef]
- Hsueh, K.W.; Chiou, T.W.; Chiang, S.F.; Yamashita, T.; Abe, K.; Borlongan, C.V.; Sanberg, P.R.; Huang, A.Y.; Lin, S.Z.; Harn, H.J. Autophagic down-regulation in motor neurons remarkably prolongs the survival of ALS mice. Neuropharmacology. 2016, 108, 152–160. [Google Scholar] [CrossRef]
- Suntar, I.; Sureda, A.; Belwal, T.; Sanches Silva, A.; Vacca, R.A.; Tewari, D.; Sobarzo-Sánchez, E.; Nabavi, S.F.; Shirooie, S.; Dehpour, A.R.; et al. Natural products, PGC-1 α, and Duchenne muscular dystrophy. Acta Pharm. Sin. B 2020, 10, 734–745. [Google Scholar] [CrossRef]
- Lilamand, M.; Mouton-Liger, F.; Di Valentin, E.; Sànchez, O.M.; Paquet, C. Efficacy and Safety of Ketone Supplementation or Ketogenic Diets for Alzheimer’s Disease: A Mini Review. Front. Nutr. 2022, 8, 807970. [Google Scholar] [CrossRef]
- Chen, N.; Qi, Y.; Ma, X.; Xiao, X.; Liu, Q.; Xia, T.; Xiang, J.; Zeng, J.; Tang, J. Rediscovery of Traditional Plant Medicine: An Underestimated Anticancer Drug of Chelerythrine. Front. Pharmacol. 2022, 13, 906301. [Google Scholar] [CrossRef]
- Hsu, C.M.; Yen, C.H.; Wang, S.C.; Liu, Y.C.; Huang, C.T.; Wang, M.H.; Chuang, T.M.; Ke, Y.L.; Yeh, T.J.; Gau, Y.C.; et al. Emodin Ameliorates the Efficacy of Carfilzomib in Multiple Myeloma Cells via Apoptosis and Autophagy. Biomedicines 2022, 10, 1638. [Google Scholar] [CrossRef]
- Hosseinpour-Moghaddam, K.; Caraglia, M.; Sahebkar, A. Autophagy induction by trehalose: Molecular mechanisms and therapeutic impacts. J. Cell. Physiol. 2018, 233, 6524–6543. [Google Scholar] [CrossRef]
- Rusmini, P.; Cortese, K.; Crippa, V.; Cristofani, R.; Cicardi, M.E.; Ferrari, V.; Vezzoli, G.; Tedesco, B.; Meroni, M.; Messi, E.; et al. Trehalose induces autophagy via lysosomal-mediated TFEB activation in models of motoneuron degeneration. Autophagy 2019, 15, 631–651. [Google Scholar] [CrossRef]
- Bordoni, M.; Pansarasa, O.; Scarian, E.; Cristofani, R.; Leone, R.; Fantini, V.; Garofalo, M.; Diamanti, L.; Bernuzzi, S.; Gagliardi, S.; et al. Lysosomes Dysfunction Causes Mitophagy Impairment in PBMCs of Sporadic ALS Patients. Cells 2022, 11, 1272. [Google Scholar] [CrossRef]
- DeBosch, B.J.; Heitmeier, M.R.; Mayer, A.L.; Higgins, C.B.; Crowley, J.R.; Kraft, T.E.; Chi, M.; Newberry, E.P.; Chen, Z.; Finck, B.N.; et al. Trehalose inhibits solute carrier 2A (SLC2A) proteins to induce autophagy and prevent hepatic steatosis. Sci. Signal. 2016, 9, ra21. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.J.; Stitham, J.; Evans, T.D.; Zhang, X.; Rodriguez-Velez, A.; Yeh, Y.S.; Tao, J.; Takabatake, K.; Epelman, S.; Lodhi, I.J.; et al. Trehalose causes low-grade lysosomal stress to activate TFEB and the autophagy-lysosome biogenesis response. Autophagy 2021, 17, 3740–3752. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Chen, S.; Gao, K.; Zhou, Z.; Wang, C.; Shen, Z.; Guo, Y.; Li, Z.; Wan, Z.; Liu, C.; et al. Resveratrol protects against spinal cord injury by activating autophagy and inhibiting apoptosis mediated by the SIRT1/AMPK signaling pathway. Neuroscience 2017, 348, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Maher, P.; Dargusch, R.; Bodai, L.; Gerard, P.E.; Purcell, J.M.; Marsh, J.L. ERK activation by the polyphenols fisetin and resveratrol provides neuroprotection in multiple models of Huntington’s disease. Hum. Mol. Genet. 2011, 20, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Hecht, J.T.; Coustry, F.; Veerisetty, A.C.; Hossain, M.G.; Posey, K.L. Resveratrol Reduces COMPopathy in Mice Through Activation of Autophagy. JBMR Plus 2021, 5, e10456. [Google Scholar] [CrossRef]
- Suvorova, I.I.; Knyazeva, A.R.; Petukhov, A.V.; Aksenov, N.D.; Pospelov, V.A. Resveratrol enhances pluripotency of mouse embryonic stem cells by activating AMPK/Ulk1 pathway. Cell Death Discov. 2019, 5, 61. [Google Scholar] [CrossRef]
- Atiya, A.M.; Poortvliet, E.; Strömberg, R.; Yngve, A. Polyamines in foods: Development of a food database. Food Nutr. Res. 2011, 55, 5572. [Google Scholar] [CrossRef]
- Wirth, M.; Schwarz, C.; Benson, G.; Horn, N.; Buchert, R.; Lange, C.; Köbe, T.; Hetzer, S.; Maglione, M.; Michael, E.; et al. Effects of spermidine supplementation on cognition and biomarkers in older adults with subjective cognitive decline (SmartAge)-study protocol for a randomized controlled trial. Alzheimers Res. Ther. 2019, 11, 36. [Google Scholar] [CrossRef]
- Yan, J.; Yan, J.Y.; Wang, Y.X.; Ling, Y.N.; Song, X.D.; Wang, S.Y.; Liu, H.Q.; Liu, Q.C.; Zhang, Y.; Yang, P.Z.; et al. Spermidine-enhanced autophagic flux improves cardiac dysfunction following myocardial infarction by targeting the AMPK/mTOR signalling pathway. Br. J. Pharmacol. 2019, 176, 3126–3142. [Google Scholar] [CrossRef]
- Eisenberg, T.; Abdellatif, M.; Schroeder, S.; Primessnig, U.; Stekovic, S.; Pendl, T.; Harger, A.; Schipke, J.; Zimmermann, A.; Schmidt, A.; et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat. Med. 2016, 22, 1428–1438. [Google Scholar] [CrossRef]
- Pietrocola, F.; Lachkar, S.; Enot, D.P.; Niso-Santano, M.; Bravo-San Pedro, J.M.; Sica, V.; Izzo, V.; Maiuri, M.C.; Madeo, F.; Mariño, G.; et al. Spermidine induces autophagy by inhibiting the acetyltransferase EP300. Cell Death Differ. 2015, 22, 509–516. [Google Scholar] [CrossRef]
- Cerdá, B.; Periago, P.; Espín, J.C.; Tomás-Barberán, F.A. Identification of urolithin a as a metabolite produced by human colon microflora from ellagic acid and related compounds. J. Agric. Food Chem. 2005, 53, 5571–5576. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, H.; Jin, Y.; Shen, K.; Chen, X.; Xu, Z.; Jin, B.; Pan, H. Role of TFEB in autophagic modulation of ischemia reperfusion injury in mice kidney and protection by urolithin A. Food Chem. Toxicol. 2019, 131, 110591. [Google Scholar] [CrossRef]
- Kujawska, M.; Jourdes, M.; Kurpik, M.; Szulc, M.; Szaefer, H.; Chmielarz, P.; Kreiner, G.; Krajka-Kuźniak, V.; Mikołajczak, P.Ł.; Teissedre, P.L.; et al. Neuroprotective Effects of Pomegranate Juice against Parkinson’s Disease and Presence of Ellagitannins-Derived Metabolite-Urolithin A-In the Brain. Int. J. Mol. Sci. 2019, 21, 202. [Google Scholar] [CrossRef]
- Ryu, D.; Mouchiroud, L.; Andreux, P.A.; Katsyuba, E.; Moullan, N.; Nicolet-Dit-Félix, A.A.; Williams, E.G.; Jha, P.; Lo Sasso, G.; Huzard, D.; et al. Urolithin A induces mitophagy and prolongs lifespan in C. elegans and increases muscle function in rodents. Nat. Med. 2016, 22, 879–888. [Google Scholar] [CrossRef]
- Tuohetaerbaike, B.; Zhang, Y.; Tian, Y.; Zhang, N.N.; Kang, J.; Mao, X.; Zhang, Y.; Li, X. Pancreas protective effects of Urolithin A on type 2 diabetic mice induced by high fat and streptozotocin via regulating autophagy and AKT/mTOR signaling pathway. J. Ethnopharmacol. 2020, 250, 112479. [Google Scholar] [CrossRef]
- Kim, Y.K.; Yoon, H.H.; Lee, Y.D.; Youn, D.Y.; Ha, T.J.; Kim, H.S.; Lee, J.H. Anthocyanin Extracts from Black Soybean (Glycine max L.) Protect Human Glial Cells Against Oxygen-Glucose Deprivation by Promoting Autophagy. Biomol. Ther. 2012, 20, 68–74. [Google Scholar] [CrossRef]
- Youdim, K.A.; Shukitt-Hale, B.; Joseph, J.A. Flavonoids and the brain: Interactions at the blood-brain barrier and their physiological effects on the central nervous system. Free Radic. Biol. Med. 2004, 37, 1683–1693. [Google Scholar] [CrossRef]
- Li, J.; Zhao, R.; Zhao, H.; Chen, G.; Jiang, Y.; Lyu, X.; Wu, T. Reduction of Aging-Induced Oxidative Stress and Activation of Autophagy by Bilberry Anthocyanin Supplementation via the AMPK-mTOR Signaling Pathway in Aged Female Rats. J. Agric. Food Chem. 2019, 67, 7832–7843. [Google Scholar] [CrossRef]
- Wu, Y.; Hu, Y.; Zhou, H.; Zhu, J.; Tong, Z.; Qin, S.; Liu, D. Organosulfur compounds induce cytoprotective autophagy against apoptosis by inhibiting mTOR phosphorylation activity in macrophages. Acta Biochim. Biophys. Sin. 2018, 50, 1085–1093. [Google Scholar] [CrossRef]
- Chu, Y.L.; Ho, C.T.; Chung, J.G.; Raghu, R.; Lo, Y.C.; Sheen, L.Y. Allicin induces anti-human liver cancer cells through the p53 gene modulating apoptosis and autophagy. J. Agric. Food Chem. 2013, 61, 9839–9848. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Leng, B.; Li, Y.; Jiang, H.; Duan, W.; Guo, Y.; Li, C.; Hong, K. Diallyl Trisulfide Protects Motor Neurons from the Neurotoxic Protein TDP-43 via Activating Lysosomal Degradation and the Antioxidant Response. Neurochem. Res. 2018, 43, 2304–2312. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, B.; Zhang, L.; Cong, X.; Liu, Z.; Hu, Y.; Zhang, J.; Hu, H. Ginkgolic acid induces interplay between apoptosis and autophagy regulated by ROS generation in colon cancer. Biochem. Biophys. Res. Commun. 2018, 498, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumaran, S.; Nakamura, Y.; Henley, J.M.; Pountney, D.L. Ginkgolic acid promotes autophagy-dependent clearance of intracellular alpha-synuclein aggregates. Mol. Cell. Neurosci. 2019, 101, 103416. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, X.; Pang, X.; Zhao, Z.; Yu, H.; Zhou, H. Genistein protects against ox-LDL-induced senescence through enhancing SIRT1/LKB1/AMPK-mediated autophagy flux in HUVECs. Mol. Cell. Biochem. 2019, 455, 127–134. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Zhang, T.; Chi, Y.; Liu, M.; Liu, Y. Genistein and Myd88 Activate Autophagy in High Glucose-Induced Renal Podocytes In Vitro. Med. Sci. Monit. 2018, 24, 4823–4831. [Google Scholar] [CrossRef]
- Jopowicz, A.; Wiśniowska, J.; Tarnacka, B. Cognitive and Physical Intervention in Metals’ Dysfunction and Neurodegeneration. Brain Sci. 2022, 12, 345. [Google Scholar] [CrossRef]
- Aridegbe, T.; Kandler, R.; Walters, S.J.; Walsh, T.; Shaw, P.J.; McDermott, C.J. The natural history of motor neuron disease: Assessing the impact of specialist care. Amyotroph. Lateral Scler. Front. Degener. 2013, 14, 13–19. [Google Scholar] [CrossRef]
- Van Es, M.A.; Hardiman, O.; Chio, A.; Al-Chalabi, A.; Pasterkamp, R.J.; Veldink, J.H.; van den Berg, L.H. Amyotrophic lateral sclerosis. Lancet 2017, 390, 2084–2098. [Google Scholar] [CrossRef]
- Scott, A. Drug therapy: On the treatment trail for ALS. Nature 2017, 550, S120–S121. [Google Scholar] [CrossRef]
- Wobst, H.J.; Mack, K.L.; Brown, D.G.; Brandon, N.J.; Shorter, J. The clinical trial landscape in amyotrophic lateral sclerosis-Past, present, and future. Med. Res. Rev. 2020, 40, 1352–1384. [Google Scholar] [CrossRef]
- Bellingham, M.C. A review of the neural mechanisms of action and clinical efficiency of riluzole in treating amyotrophic lateral sclerosis: What have we learned in the last decade? CNS Neurosci. Ther. 2011, 17, 4–31. [Google Scholar] [CrossRef]
- Andrews, J.A.; Jackson, C.E.; Heiman-Patterson, T.D.; Bettica, P.; Brooks, B.R.; Pioro, E.P. Real-world evidence of riluzole effectiveness in treating amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Front. Degener. 2020, 21, 509–518. [Google Scholar] [CrossRef]
- West, R.J.H.; Ugbode, C.; Fort-Aznar, L.; Sweeney, S.T. Neuroprotective activity of ursodeoxycholic acid in CHMP2BIntron5 models of frontotemporal dementia. Neurobiol. Dis. 2020, 144, 105047. [Google Scholar] [CrossRef]
- Sala, G.; Arosio, A.; Conti, E.; Beretta, S.; Lunetta, C.; Riva, N.; Ferrarese, C.; Tremolizzo, L. Riluzole Selective Antioxidant Effects in Cell Models Expressing Amyotrophic Lateral Sclerosis Endophenotypes. Clin. Psychopharmacol. Neurosci. 2019, 17, 438–442. [Google Scholar] [CrossRef]
- Rothstein, J.D. Edaravone: A new drug approved for ALS. Cell 2017, 171, 725. [Google Scholar] [CrossRef]
- Sawada, H. Clinical efficacy of edaravone for the treatment of amyotrophic lateral sclerosis. Expert Opin. Pharmacother. 2017, 18, 735–738. [Google Scholar] [CrossRef]
- Guo, Z.; Wu, H.T.; Li, X.X.; Yu, Y.; Gu, R.Z.; Lan, R.; Qin, X.Y. Edaravone protects rat astrocytes from oxidative or neurotoxic inflammatory insults by restoring Akt/Bcl-2/Caspase-3 signaling axis. IBRO Rep. 2020, 8, 122–128. [Google Scholar] [CrossRef]
- Matsumoto, S.; Murozono, M.; Kanazawa, M.; Nara, T.; Ozawa, T.; Watanabe, Y. Edaravone and cyclosporine A as neuroprotective agents for acute ischemic stroke. Acute Med. Surg. 2018, 5, 213–221. [Google Scholar] [CrossRef]
- Xu, X.; Shen, D.; Gao, Y.; Zhou, Q.; Ni, Y.; Meng, H.; Shi, H.; Le, W.; Chen, S.; Chen, S. A perspective on therapies for amyotrophic lateral sclerosis: Can disease progression be curbed? Transl. Neurodegener. 2021, 10, 29. [Google Scholar] [CrossRef]
- Arthur, K.C.; Calvo, A.; Price, T.R.; Geiger, J.T.; Chiò, A.; Traynor, B.J. Projected increase in amyotrophic lateral sclerosis from 2015 to 2040. Nat. Commun. 2016, 7, 12408. [Google Scholar] [CrossRef] [PubMed]
- Roberts, B.; Theunissen, F.; Mastaglia, F.L.; Akkari, P.A.; Flynn, L.L. Synucleinopathy in Amyotrophic Lateral Sclerosis: A Potential Avenue for Antisense Therapeutics? Int. J. Mol. Sci. 2022, 23, 9364. [Google Scholar] [CrossRef] [PubMed]
- Gordon, P.H. Amyotrophic Lateral Sclerosis: An update for 2013 Clinical Features, Pathophysiology, Management and Therapeutic Trials. Aging Dis. 2013, 4, 295–310. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.; Perera, N.D.; Beart, P.M.; Turner, B.J.; Shabanpoor, F. Amyotrophic Lateral Sclerosis and Autophagy: Dysfunction and Therapeutic Targeting. Cells 2020, 9, 2413. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshikawa, S.; Taniguchi, K.; Sawamura, H.; Ikeda, Y.; Tsuji, A.; Matsuda, S. Potential Diets to Improve Mitochondrial Activity in Amyotrophic Lateral Sclerosis. Diseases 2022, 10, 117. https://doi.org/10.3390/diseases10040117
Yoshikawa S, Taniguchi K, Sawamura H, Ikeda Y, Tsuji A, Matsuda S. Potential Diets to Improve Mitochondrial Activity in Amyotrophic Lateral Sclerosis. Diseases. 2022; 10(4):117. https://doi.org/10.3390/diseases10040117
Chicago/Turabian StyleYoshikawa, Sayuri, Kurumi Taniguchi, Haruka Sawamura, Yuka Ikeda, Ai Tsuji, and Satoru Matsuda. 2022. "Potential Diets to Improve Mitochondrial Activity in Amyotrophic Lateral Sclerosis" Diseases 10, no. 4: 117. https://doi.org/10.3390/diseases10040117
APA StyleYoshikawa, S., Taniguchi, K., Sawamura, H., Ikeda, Y., Tsuji, A., & Matsuda, S. (2022). Potential Diets to Improve Mitochondrial Activity in Amyotrophic Lateral Sclerosis. Diseases, 10(4), 117. https://doi.org/10.3390/diseases10040117





