Spatial Co-Clustering of Tuberculosis and HIV in Ethiopia
Abstract
1. Background
2. Material and Methodology
2.1. Study Area
2.2. Data Sources
3. Statistical Analyses
Ethical Consideration
4. Results
4.1. Descriptive Statistics
4.1.1. Global Pattern Analyses
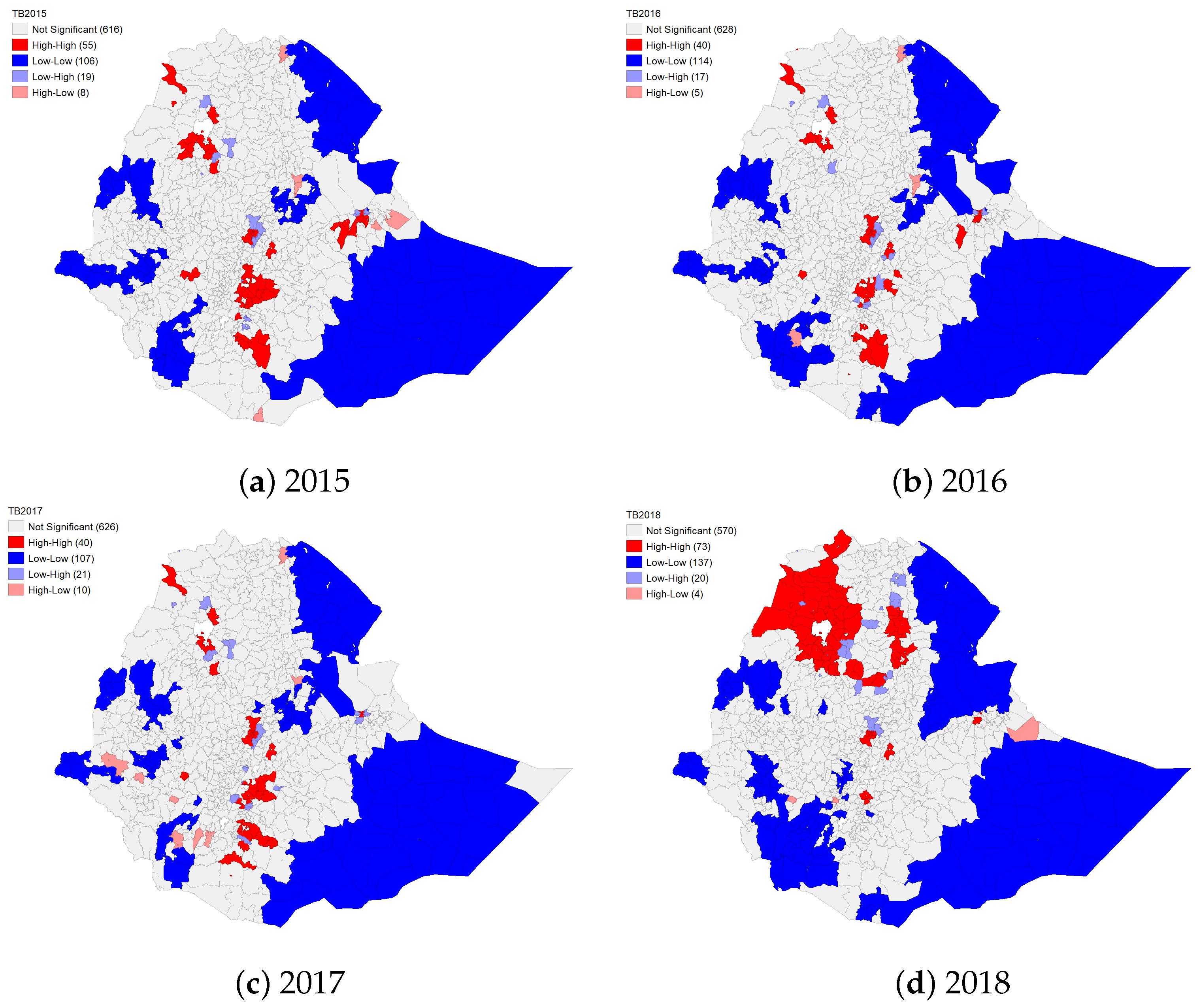
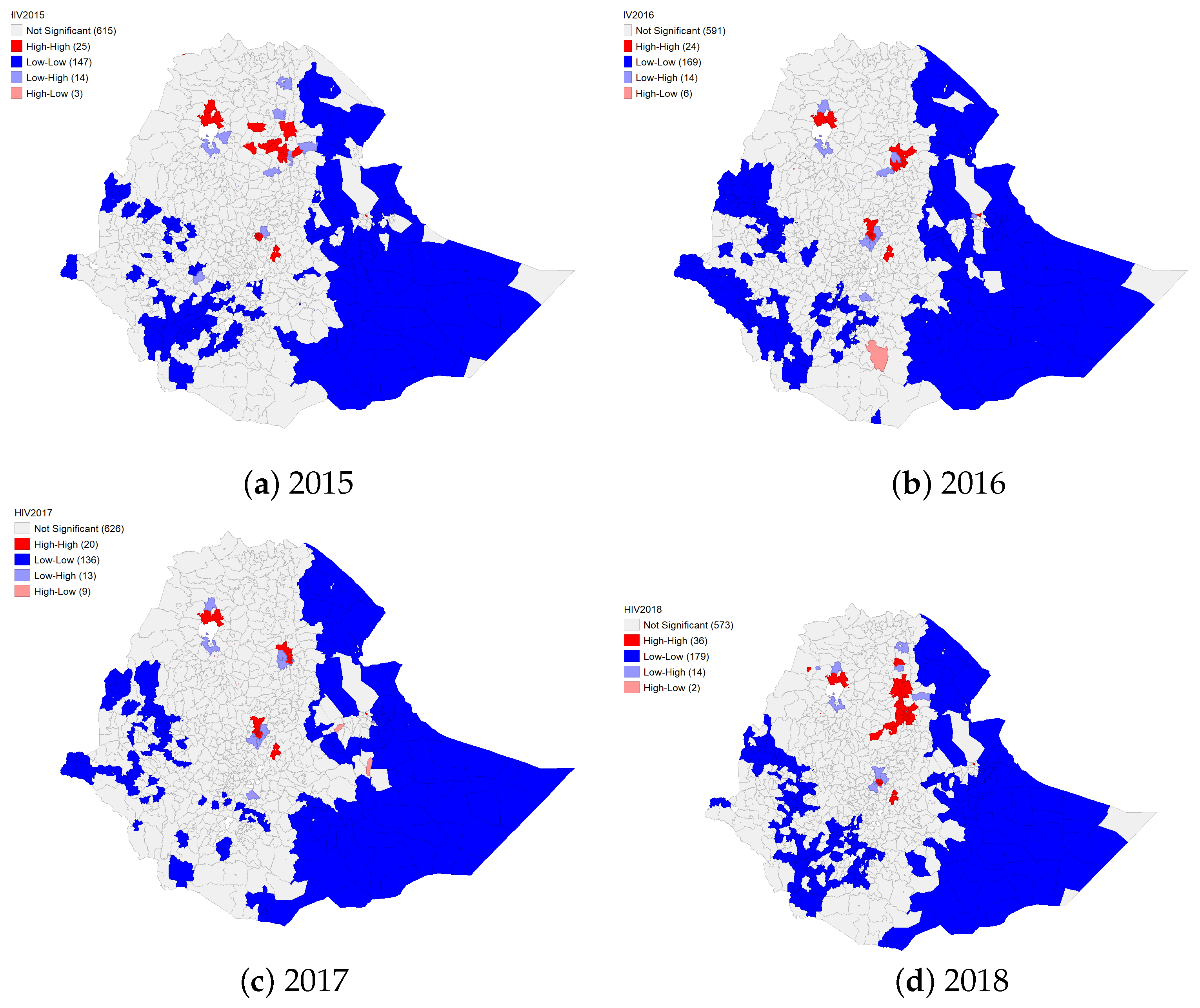
4.1.2. Spatial Clustering of TB and HIV
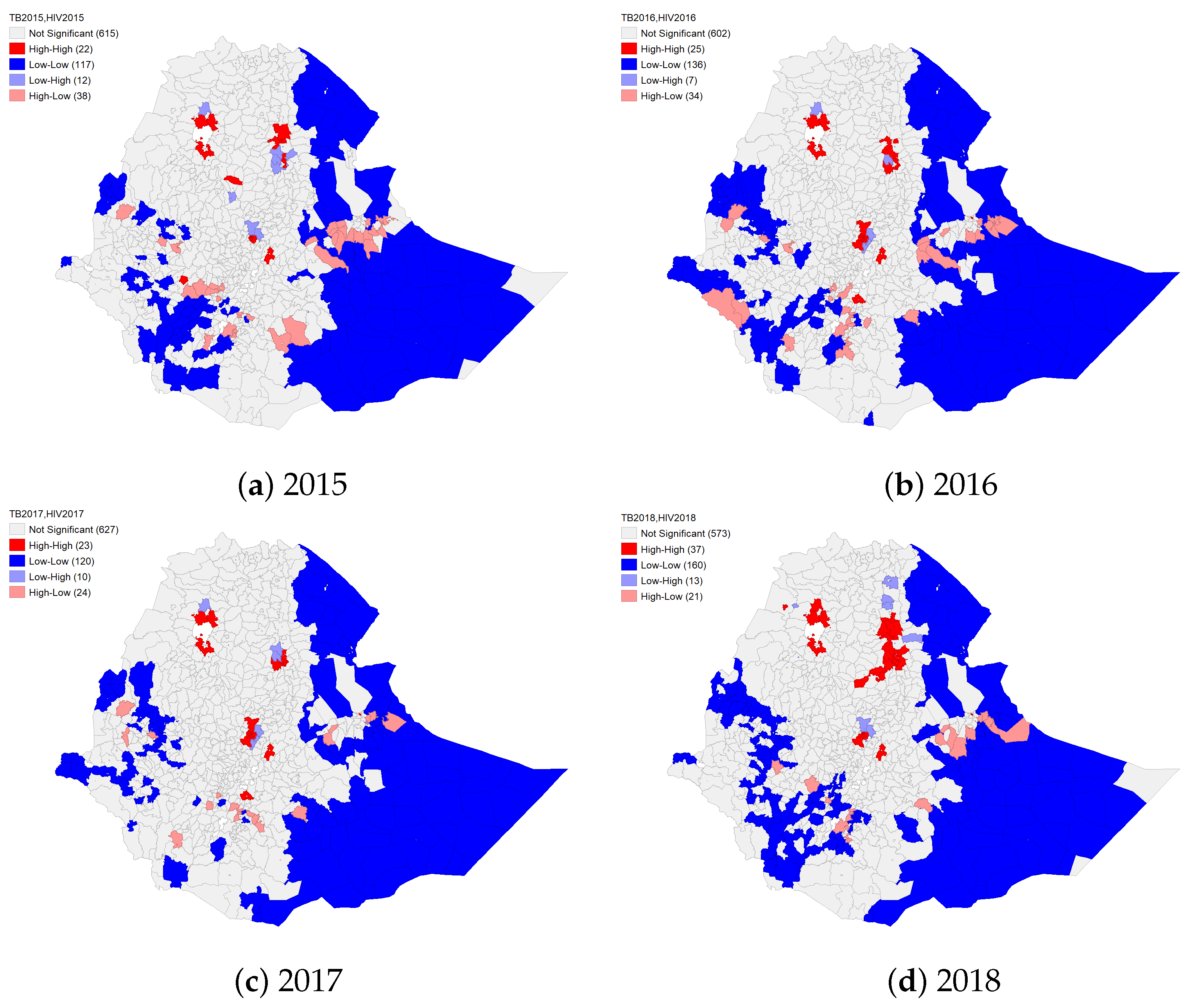
4.2. Spatial Co-Clustering of HIV and TB
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIDS | Acquired immunodeficiency syndrome |
| DOTS | Directly Observed Therapy, Short Course |
| HMI | Health Management Information System |
| HIV | Human Immunodeficiency Virus |
| LISA | local indicators of spatial analysis |
References
- World Health Organization (WHO). Global Tuberculosis Report 2021. 2021. Available online: file:///C:/Users/debuslk/Downloads/9789240037021-eng%20(1).pdf (accessed on 24 March 2022).
- United Nations Programme on HIV/AIDS (UNAIDS): Fact Sheet—World AIDS Day 2021. 2021. Available online: https://www.unaids.org/sites/default/files/mediaasset/UNAIDSFactSheeten.pdf (accessed on 24 March 2022).
- United Nations Programme on HIV/AIDS (UNAIDS): Feature Story. 2021. Available online: https://www.unaids.org/en/resources/presscentre/featurestories/2021/march/20210324tuberculosis-deathspeople-living-with-hiv (accessed on 5 August 2022).
- World Health Organization (WHO): HIV Key Facts 27 July 2022. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/hiv-aids (accessed on 5 August 2022).
- United Nations Office for the Coordination of Humanitarian Affairs (OCHA): TB Disease and Deaths Declining among People Living with HIV, but There Have Been some Reversals due to the COVID-19 Pandemic. 2022. Available online: https://reliefweb.int/report/world/tb-disease-and-deaths-declining-among-people-living-hiv-there-have-beensome-reversals (accessed on 5 August 2022).
- The World Bank: Incidence of Tuberculosis. 2022. Available online: https://data.worldbank.org/indicator/SH.TBS.INCD?locations=ET (accessed on 5 August 2022).
- United Nations Programme on HIV/AIDS (UNAIDS): Country Fact Sheets Ethiopia 2019. 2020. Available online: https://www.usaid.gov/sites/default/files/documents/Ethiopia-Fact-SheetHIV-AIDSOct-2020.pdf (accessed on 13 April 2022).
- Federal Democratic Republic of Ethipia Ministry of Health (FDREMoH). Guidelines for Clinical and Programmatic Management of TB, Leprosy and TB/HIV in Ethiopia; Ministry of Health of Ethiopia: Addis Ababa, Ethiopia, 2012.
- World Health Organization. Guide to Monitoring and Evaluation for Collaborative TB/HIV Activities—2015 Update; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Dangisso, M.H.; Datiko, D.G.; Lindtjørn, B. Trends of tuberculosis case notification and treatment outcomes in the Sidama Zone, southern Ethiopia: Ten-year retrospective trend analysis in urban-rural settings. PLoS ONE 2014, 9, e114225. [Google Scholar] [CrossRef] [PubMed]
- Tadesse, T.; Demissie, M.; Berhane, Y.; Kebede, Y.; Abebe, M. The clustering of smear-positive tuberculosis in Dabat, Ethiopia: A population based cross sectional study. PLoS ONE 2013, 8, e65022. [Google Scholar] [CrossRef] [PubMed]
- Dangisso, M.H.; Datiko, D.G.; Lindtjørn, B. Spatio-temporal analysis of smear-positive tuberculosis in the Sidama Zone, southern Ethiopia. PLoS ONE 2015, 10, e0126369. [Google Scholar] [CrossRef] [PubMed]
- Alene, K.A.; Viney, K.; McBryde, E.S.; Clements, A.C. Spatial patterns of multidrug resistant tuberculosis and relationships to socio-economic, demographic and household factors in northwest Ethiopia. PLoS ONE 2017, 12, e0171800. [Google Scholar] [CrossRef] [PubMed]
- Alene, K.A.; Viney, K.; McBryde, E.S.; Clements, A.C. Spatiotemporal transmission and socio-climatic factors related to paediatric tuberculosis in north-western Ethiopia. Geospat. Health 2017, 12, 575. [Google Scholar] [CrossRef]
- Shaweno, D.; Shaweno, T.; Trauer, J.M.; Denholm, J.T.; McBryde, E.S. Heterogeneity of distribution of tuberculosis in Sheka Zone, Ethiopia: Drivers and temporal trends. Int. J. Tuberc. Lung Dis. 2017, 21, 79–85. [Google Scholar] [CrossRef]
- Gelaw, Y.A.; Williams, G.; Assefa, Y.; Asressie, M.; Soares Magalhães, R.J. Sociodemographic profiling of tuberculosis hotspots in Ethiopia, 2014–2017. Trans. R. Soc. Trop. Med. Hyg. 2019, 113, 379–391. [Google Scholar] [CrossRef]
- Gelaw, Y.A.; Magalhães, R.J.S.; Assefa, Y.; Williams, G. Spatial clustering and socio-demographic determinants of HIV infection in Ethiopia, 2015–2017. Int. J. Infect. Dis. 2019, 82, 33–39. [Google Scholar] [CrossRef]
- Alene, K.A.; Clements, A.C. Spatial clustering of notified tuberculosis in Ethiopia: A nationwide study. PLoS ONE 2019, 14, e0221027. [Google Scholar] [CrossRef]
- Gelibo, T.; Lulseged, S.; Eshetu, F.; Abdella, S.; Melaku, Z.; Ajiboye, S.; Demissie, M.; Solmo, C.; Ahmed, J.; Getaneh, Y.; et al. Spatial distribution and determinants of HIV prevalence among adults in urban Ethiopia: Findings from the Ethiopia Population-based HIV Impact Assessment Survey (2017–2018). PLoS ONE 2022, 17, e0271221. [Google Scholar] [CrossRef]
- Arcêncio, R.A.; Berra, T.Z.; Terena, N.D.F.M.; Rocha, M.; Ferraz de Araújo Alecrim, T.; de Souza Kihara, F.M.; Mascarello, K.C.; Martins Sales, C.M.; Maciel, E.L.N. Spatial clustering and temporal trend analysis of international migrants diagnosed with tuberculosis in Brazil. PLoS ONE 2021, 16, e0252712. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, M.; Wu, S.S.; Jiang, H.; Zhang, J.; Wang, S.; Ma, W.; Li, Q.; Ma, Y.; Liu, Y.; et al. Spatial distribution of tuberculosis and its association with meteorological factors in mainland China. BMC Infect. Dis. 2019, 19, 379. [Google Scholar] [CrossRef]
- Ibrahim, S.; Hamisu, I.U.L. Spatial pattern of tuberculosis prevalence in nigeria: A comparative analysis of spatial autocorrelation indices. Am. J. Geogr. Inf. Syst. 2015, 4, 87–94. [Google Scholar]
- Daniel, O.J.; Adejumo, O.A.; Alabi, A.D.; Bamidele, J.O.; Oritogun, K.S. Spatial analysis of tuberculosis and risk factors at the lowest administrative level in Nigeria. Afr. J. Health Sci. 2022, 35, 70–82. [Google Scholar]
- Nelson, K.N.; Shah, N.S.; Mathema, B.; Ismail, N.; Brust, J.C.; Brown, T.S.; Auld, S.C.; Omar, S.V.; Morris, N.; Campbell, A.; et al. Spatial patterns of extensively drug-resistant tuberculosis transmission in KwaZulu-Natal, South Africa. J. Infect. Dis. 2018, 218, 1964–1973. [Google Scholar] [CrossRef]
- Barankanira, E.; Molinari, N.; Niyongabo, T.; Laurent, C. Spatial analysis of HIV infection and associated individual characteristics in Burundi: Indications for effective prevention. BMC Public Health 2015, 16, 118. [Google Scholar] [CrossRef]
- Chen, W.; Yang, J.; Jiang, J.; He, L.; Xu, Y.; Zheng, J.; Jiang, J.; Pan, X. A spatial analysis of the epidemiology of HIV-infected students in Zhejiang province, China. BMC Infect. Dis. 2021, 21, 430. [Google Scholar] [CrossRef]
- Zulu, L.C.; Kalipeni, E.; Johannes, E. Analyzing spatial clustering and the spatiotemporal nature and trends of HIV/AIDS prevalence using GIS: The case of Malawi, 1994–2010. BMC Infect. Dis. 2014, 14, 285. [Google Scholar] [CrossRef]
- González, R.; Augusto, O.J.; Munguambe, K.; Pierrat, C.; Pedro, E.N.; Sacoor, C.; De Lazzari, E.; Aponte, J.J.; Macete, E.; Alonso, L.; et al. HIV incidence and spatial clustering in a rural area of southern Mozambique. PLoS ONE 2015, 10, e0132053. [Google Scholar] [CrossRef]
- Tanser, F.; Bärnighausen, T.; Cooke, G.S.; Newell, M.L. Localized spatial clustering of HIV infections in a widely disseminated rural South African epidemic. Int. J. Epidemiol. 2009, 38, 1008–1016. [Google Scholar] [CrossRef]
- Chimoyi, L.A.; Musenge, E. Spatial analysis of factors associated with HIV infection among young people in Uganda, 2011. BMC Public Health 2014, 14, 555. [Google Scholar] [CrossRef] [PubMed]
- Feske, M.L.; Teeter, L.D.; Musser, J.M.; Graviss, E.A. Including the third dimension: A spatial analysis of TB cases in Houston Harris County. Tuberculosis 2011, 91, S24–S33. [Google Scholar] [CrossRef] [PubMed]
- Dangisso, M.H.; Datiko, D.G.; Lindtjørn, B. Identifying geographical heterogeneity of pulmonary tuberculosis in southern Ethiopia: A method to identify clustering for targeted interventions. Glob. Health Action 2020, 13, 1785737. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, T.; Fuji, Y.; Nzou, S.M.; Tanigawa, C.; Kiche, I.; Mwau, M.; Mwangi, A.W.; Karama, M.; Hirayama, K.; Goto, K.; et al. Spatial distributions of HIV infection in an endemic area of Western Kenya: Guiding information for localized HIV control and prevention. PLoS ONE 2016, 11, e0148636. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, R.; Gregson, S.; Takaruza, A.; Rhead, R.; Masoka, T.; Schur, N.; Anderson, S.J.; Nyamukapa, C. Spatial patterns of HIV prevalence and service use in East Zimbabwe: Implications for future targeting of interventions. J. Int. Aids Soc. 2017, 20, 21409. [Google Scholar] [CrossRef]
- O’Brien-Carelli, C.; Steuben, K.; Stafford, K.A.; Aliogo, R.; Alagi, M.; Johanns, C.K.; Ibrahim, J.; Shiraishi, R.; Ehoche, A.; Greby, S.; et al. Mapping HIV prevalence in Nigeria using small area estimates to develop a targeted HIV intervention strategy. PLoS ONE 2022, 17, e0268892. [Google Scholar] [CrossRef]
- Pedroso, A.O.; Gomes, D.; Sousa, S.M.L.; Ferreira, G.R.O.N.; Ramos, A.M.C.; Polaro, S.H.I.; Nogueira, L.M.V.; Botelho, E. Temporal and Spatial Analysis Techniques as Potential Tools for Combating the HIV Epidemic among Young Brazilian Amazonian People: An Ecological Study. Trop. Med. Infect. Dis. 2022, 7, 137. [Google Scholar] [CrossRef]
- De Cock, K.M.; Chaisson, R.E. Will DOTS do it? A reappraisal of tuberculosis control in countries with high rates of HIV infection [Counterpoint]. Int. J. Tuberc. Lung Dis. 1999, 3, 457–465. [Google Scholar]
- Lawn, S.D.; Wilkinson, R. Extensively drug resistant tuberculosis. BMJ 2006, 333, 559–560. [Google Scholar] [CrossRef]
- Ramkissoon, S.; Mwambi, H.G.; Matthews, A. Modelling HIV and MTB co-infection including combined treatment strategies. PLoS ONE 2012, 7, e49492. [Google Scholar] [CrossRef]
- Belay, M.; Bjune, G.; Abebe, F. Prevalence of tuberculosis, HIV, and TB-HIV co-infection among pulmonary tuberculosis suspects in a predominantly pastoralist area, northeast Ethiopia. Glob. Health Action 2015, 8, 27949. [Google Scholar] [CrossRef]
- Fite, R.O.; Chichiabellu, T.Y.; Demissie, B.W.; Hanfore, L.K. Tuberculosis and HIV Co-infection and associated factors among HIV reactive patients in Ethiopia. J. Nurs. Midwifery Sci. 2019, 6, 15. [Google Scholar] [CrossRef]
- Alene, K.A.; Viney, K.; Moore, H.C.; Wagaw, M.; Clements, A.C. Spatial patterns of tuberculosis and HIV co-infection in Ethiopia. PLoS ONE 2019, 14, e0226127. [Google Scholar] [CrossRef]
- Wei, W.; Zhang, W.-S.; Alayi, A.; Yan, C.; Zhang, W.-W.; Cao, M.-Q. The characteristics of TB epidemic and TB/HIV co-infection epidemic: A 2007–2013 retrospective study in Urumqi, Xinjiang Province, China. PLoS ONE 2016, 11, e0164947. [Google Scholar] [CrossRef] [PubMed]
- Aturinde, A.; Farnaghi, M.; Pilesjö, P.; Mansourian, A. Spatial analysis of HIV-TB co-clustering in Uganda. BMC Infect. Dis. 2019, 19, 612. [Google Scholar] [CrossRef]
- Cavalin, R.F.; Pellini, A.C.G.; Lemos, R.R.G.D.; Sato, A.S. TB-HIV co-infection: Spatial and temporal distribution in the largest Brazilian metropolis. Rev. Saúde Pública 2020, 54. [Google Scholar] [CrossRef]
- Addis Alene, K.; Nega, A.; Wasie Taye, B. Incidence and predictors of tuberculosis among adult people living with human immunodeficiency virus at the University of Gondar Referral Hospital, Northwest Ethiopia. BMC Infect. Dis. 2013, 13, 292. [Google Scholar] [CrossRef]
- Ayalaw, S.G.; Alene, K.A.; Adane, A.A. Incidence and predictors of tuberculosis among HIV positive children at University of Gondar Referral Hospital, northwest Ethiopia: A retrospective follow-up study. Int. Sch. Res. Not. 2015, 2015, 307810. [Google Scholar] [CrossRef]
- Gatrell, A.C.; Bailey, T.C. Interactive spatial data analysis in medical geography. Soc. Sci. Med. 1996, 42, 843–855. [Google Scholar] [CrossRef]
- Cuadros, D.F.; Li, J.; Branscum, A.J.; Akullian, A.; Jia, P.; Mziray, E.N.; Tanser, F. Mapping the spatial variability of HIV infection in Sub-Saharan Africa: Effective information for localized HIV prevention and control. Sci. Rep. 2017, 7, 9093. [Google Scholar] [CrossRef]
- Belay, H.; Azim, T.; Kassahun, H. Assessment of Health Management Information System (HMIS) Performance in SNNPR, Ethiopia; USAID Measure Evaluation, SNNP Regional Health Bureau: Washington, DC, USA, 2013. [Google Scholar]
- Tadesse, K.; Gebeyoh, E.; Tadesse, G. Assessment of health management information system implementation in Ayder referral hospital, Mekelle, Ethiopia. Int. Intell. Inf. Syst. 2014, 3, 34. [Google Scholar] [CrossRef]
- Anselin, L.; Syabri, I.; Kho, Y. GeoDa: An introduction to spatial data analysis. Geogr. Anal. 2006, 38, 5–22. [Google Scholar] [CrossRef]
- Moran, A. Notes on continuous stochastic phenomena. Biometrika 1950, 37, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Anselin, L. Local indicators of spatial association—LISA. Geogr. Anal. 1995, 27, 93–115. [Google Scholar] [CrossRef]
- Haining, R.; Li, G. Regression Modelling Wih Spatial and Spatial-Temporal Data: A Bayesian Approach; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Anselin, L. Global Spatial Autocorrelation (2): Bivariate, Differential and EB Moran Scatter Plot. GeoDa Workbook. 2019. Available online: https://geodacenter.github.io/workbook/5b_global_adv/lab5b.html (accessed on 7 August 2022).
- Bivand, R.S.; Wong, D.W. Comparing implementations of global and local indicators of spatial association. Test 2018, 27, 716–748. [Google Scholar] [CrossRef]
- Herrero, M.B.; Arrossi, S.; Ramos, S.; Braga, J.U. Spatial analysis of the tuberculosis treatment dropout, Buenos Aires, Argentina. Rev. Saúde Pública 2015, 49, 49. [Google Scholar] [CrossRef]
- Harling, G.; Castro, M.C. A spatial analysis of social and economic determinants of tuberculosis in Brazil. Health Place 2014, 25, 56–67. [Google Scholar] [CrossRef]
- Wang, X.; Yin, S.; Li, Y.; Wang, W.; Du, M.; Guo, W.; Xue, M.; Wu, J.; Liang, D.; Wang, R.; et al. Spatiotemporal epidemiology of, and factors associated with, the tuberculosis prevalence in northern China, 2010–2014. BMC Infect. Dis. 2019, 19, 365. [Google Scholar] [CrossRef]
- Cain, K.; Marano, N.; Kamene, M.; Sitienei, J.; Mukherjee, S.; Galev, A.; Burton, J.; Nasibov, O.; Kioko, J.; De Cock, K.M. The movement of multidrug-resistant tuberculosis across borders in East Africa needs a regional and global solution. PLoS Med. 2015, 12, e1001791. [Google Scholar] [CrossRef]
- da Roza, D.L.; Caccia-Bava, M.D.C.G.; Martinez, E.Z. Spatio-temporal patterns of tuberculosis incidence in Ribeirao Preto, state of Sao Paulo, southeast Brazil, and their relationship with social vulnerability: A Bayesian analysis. Rev. Soc. Bras. Med. Trop. 2012, 45, 607–615. [Google Scholar] [CrossRef]
- Posey, D.L.; Marano, N.; Cetron, M.S. Cross-border solutions needed to address tuberculosis in migrating populations. Int. J. Tuberc. Lung Dis. 2017, 21, 485–486. [Google Scholar] [CrossRef]
- Gezie, L.D.; Taye, B.W.; Ayele, T.A. Time to unsafe sexual practice among cross-border female sex workers in Metemma Yohannes, North West Ethiopia. BMC Public Health 2015, 15, 710. [Google Scholar] [CrossRef]
- Central Statistical Agency (CSA) [Ethiopia] and ICF. Ethiopia Demographic and Health Survey 2016: Key Indicators Report; CSA and ICF: Addis Ababa, Ethiopia, 2016. [Google Scholar]
- Hailu, B.A.; Tadese, F.; Bogale, G.G.; Molla, A.; Miheretu, B.A.; Beyene, J. Spatial patterns and associated factors of HIV Seropositivity among adults in Ethiopia from EDHS 2016: A spatial and multilevel analysis. BMC Infect. Dis. 2020, 20, 751. [Google Scholar] [CrossRef]
- Idris, S.H.; Sambo, M.N.; Obi, P. Comportment of heavy goods vehicle drivers in HIV spread along settlements around Kaduna: Kano road transport corridor in Nigeria. Int. J. Med. Public Health 2013, 3, 26–32. [Google Scholar]
- Alemayehu, M.; Wubshet, M.; Mesfin, N.; Gebayehu, A. Prevalence of Human Immunodeficiency Virus and associated factors among Visceral Leishmaniasis infected patients in Northwest Ethiopia: A facility based cross-sectional study. BMC Infect. Dis. 2017, 17, 152. [Google Scholar] [CrossRef]
- Sing, R.K.; Patra, S. What factors are responsible for higher prevalence of HIV infection among urban women than rural women in Tanzania? Ethiop. J. Health Sci. 2015, 25, 321–328. [Google Scholar] [CrossRef]
- Deribew, A. Distribution of Most-at-Risk Population Groups and Their Perceptions towards HIV/AIDS: A Baseline Survey in Amhara Region for the Implementation of Mobile HIV Counselling and Testing; Private Sector Program-Ethiopia, Abt Associates Inc.: Bethesda, MD, USA, 2009. [Google Scholar]
- Tesfaye, B.; Alebel, A.; Gebrie, A.; Zegeye, A.; Tesema, C.; Kassie, B. The twin epidemics: Prevalence of TB/HIV co-infection and its associated factors in Ethiopia; A systematic review and meta-analysis. PLoS ONE 2018, 13, e0203986. [Google Scholar] [CrossRef]
- Federal Democratic Republic of Ethiopia Ministry of Health. National Strategic Plan Tuberculosis and Leprosy Control 2006–2013 EC (2013/14–2020)); Federal democratic Republic of Ethiopia, Ministry of Health: Addis Ababa, Ethiopia, 2017.
- Migliori, G.B.; Thong, M.; Alffenaar, J.W.; Denholm, J.; Tadolini, M.; Alyaquobi, F.; Blanc, F.X.; Buonsenso, D.; Cho, J.G.; Codecasa, L.R.; et al. Gauging the impact of the COVID-19 pandemic on tuberculosis services: A global study. Eur. Respir. J. 2021, 58, 2101786. [Google Scholar] [CrossRef]
- Glaziou, P. Predicted impact of the COVID-19 pandemic on global tuberculosis deaths in 2020. MedRxiv 2021. [Google Scholar] [CrossRef]
- Kadota, J.L.; Reza, T.F.; Nalugwa, T.; Kityamuwesi, A.; Nanyunja, G.; Kiwanuka, N.; Shete, P.; Davis, J.L.; Dowdy, D.; Turyahabwe, S.; et al. Impact of shelter-in-place on TB case notifications and mortality during the COVID-19 pandemic. Int. J. Tuberc. Lung Dis. 2020, 24, 1212–1214. [Google Scholar] [CrossRef]
| Year | ||||
|---|---|---|---|---|
| 2015 | 2016 | 2017 | 2018 | |
| Global Moran’s I | ||||
| Tuberculosis | 0.407 (<) | 0.423 (<) | 0.411 (<) | 0.432 (<) |
| HIV | 0.102 (0.0036) | 0.247 (<) | 0.238 (<) | 0.202 (0.0006) |
| Bivariate Global Moran’s I | ||||
| TB/HIV | 0.152 (<) | 0.251 (<) | 0.230 (<) | 0.224 (<) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gemechu, L.L.; Debusho, L.K. Spatial Co-Clustering of Tuberculosis and HIV in Ethiopia. Diseases 2022, 10, 106. https://doi.org/10.3390/diseases10040106
Gemechu LL, Debusho LK. Spatial Co-Clustering of Tuberculosis and HIV in Ethiopia. Diseases. 2022; 10(4):106. https://doi.org/10.3390/diseases10040106
Chicago/Turabian StyleGemechu, Leta Lencha, and Legesse Kassa Debusho. 2022. "Spatial Co-Clustering of Tuberculosis and HIV in Ethiopia" Diseases 10, no. 4: 106. https://doi.org/10.3390/diseases10040106
APA StyleGemechu, L. L., & Debusho, L. K. (2022). Spatial Co-Clustering of Tuberculosis and HIV in Ethiopia. Diseases, 10(4), 106. https://doi.org/10.3390/diseases10040106





