Abstract
Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2), the causative agent for the Coronavirus Disease 2019 (COVID-19) pandemic, has sparked a medical emergency worldwide. With the rise in COVID-19 infections and an eventual increase in hospitalized critically ill patients, a trend of bacterial, fungal, and viral superinfection has been noted. One important agent of co-infection identified is Candida auris. Due to its multidrug-resistant nature and easy transmissibility, C. auris is difficult to manage in COVID-positive patients. Patients with comorbidities, immunosuppressive states, intubated and on ventilators are more likely to contract the fungal infection. Therefore, it is essential to the first screen, diagnose, and isolate patients with C. auris infection and manage and treat them while preventing the spread of the disease. Failure to recognize and prevent its spread may lead to an eventual epidemic or even a pandemic during the current COVID-pandemic, which the exhausted healthcare system can most definitely not handle. This systematic review investigates the prevalence of C. auris, its pathophysiology, diagnosis, prevention, and treatment during the COVID-19 pandemic.
1. Introduction
Since its first report in China’s Wuhan Province in 2019 [1], severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection, the cause of the Coronavirus Disease 2019 (COVID-19), has enveloped the world in a massive pandemic. It has proudly challenged even the most advanced healthcare systems globally, leaving a lot of them in shackles. However, it has not acted alone. It has exacerbated several other healthcare burdens that were gaining increasing attention in the pre-COVID-19 world, one of which is the secondary transmission of multidrug-resistant organisms such as the fungus Candida auris (C. auris) in hospital settings in COVID-19 ICUs [2]. Secondary infections, such as those by C. auris, combined with a lack of natural immunity, lead to severe lung injury, acute respiratory distress syndrome (ARDS), and high mortality rates in COVID-19 patients [3].
C. auris has continued to make its mark across the world. This fungus has been reported on all continents of the world except Antarctica [4] since it was first discovered in a patient’s ear canal in Japan in 2009. In India, a study spanning four months showed a 60% case-fatality rate of COVID-19 patients, among which a shocking two-thirds had been diagnosed with C. auris infection [5]. An alarming finding from Mexico revealed a high mortality rate of over 83% in patients with COVID-19-associated C. auris bloodstream infection, despite antifungal therapy [6]. In four healthcare institutions in the C. auris endemic region of Northern Colombia [7], six (3.33%) out of 20 cases of fungemia were reported in hospitalized patients with SARS-CoV-2 from June to September 2020, who had C. auris infection. Various major outbreaks of C. auris bloodstream infections have also been reported in India, the U.K., Colombia, South Africa, and the USA These cases are nothing but the tip of the iceberg pointing toward a much larger issue than anticipated. It is, thus, imperative to identify patients quickly and correctly with C. auris if we hope to contain its spread [5].
Fungal infections can complicate the diagnosis, treatment, and progression of COVID-19. Established data from past coronavirus outbreaks (SARS-CoV and MERS) have demonstrated that systemic fungal infections, such as invasive aspergillosis and candidemia, have contributed to severe outcomes for I.C.U. patients [8]. Hence, it is no surprise that C. auris-associated superinfections have been associated with alarmingly high 30-day mortality rates in critically ill COVID-19 patients, usually above 50% [9]. It has exponentially exacerbated the burden on already saturated healthcare systems due to the ongoing COVID-19 pandemic.
C. auris patients continuously shed viable yeast cells from their skin and contaminate hospital environments, especially I.C.U.s that house critically ill patients. Unfortunately, I.C.U. teams in developing countries have particularly faced the challenge of over-occupancy of beds and relatively increased compromised infection prevention practices, encouraging C. auris to thrive and spread quickly. It is also noteworthy that several risk factors are shared between COVID-19 and C. auris patients in I.C.U.s, such as diabetes mellitus and chronic renal disease, enabling C. auris to harbor itself alongside SARS-CoV-2, forming a potentially lethal force to be reckoned with.
Despite its widespread emergence, there is still an alarming lack of identification strategies available globally, which mandates an immediate need for effective therapeutic strategies against this pathogen [10]. The lack of identification strategy also accounts for high misidentification rates due to difficulties in correctly identifying C. auris strains. However, that is not all. This, unbearably, is just one of the four troublesome aspects associated with C. auris, the other three being its high rate of antifungal drug resistance [11], its ability to colonize the skin and other bodily sites, and its aptitude to live on abiotic surfaces and equipment for weeks [12], thus explaining the mode and speed of its transmission among healthcare workers and immunosuppressed hospitalized patients [13].
The Centre for Disease Control and Prevention (C.D.C.) in the USA has recommended strict isolation of patients colonized with or treated for C. auris. However, the pandemic has made it severely challenging to implement these guidelines as hospital settings are already excessively overwhelmed and overburdened.
Therefore, in this systematic review, we aim to discuss the prevalence of C. auris, and how its pathophysiology, diagnosis, prevention, and treatment differ or are to be modified with a SARS-CoV-2 coinfection.
2. Methods
This systematic review was carried out along the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (P.R.I.S.M.A.) guidelines statement [14]. An extensive literature search was conducted using electronic databases such as PUBMED/MEDLINE, Google Scholar, and Scopus, from inception till February 2022. The following keywords were used as a search string: (“COVID-19” or “SARS-CoV-2”) AND (“Candida auris” or “Candidemia”). Additional search strings included: (“COVID-19” or “SARS-CoV-2”) AND (“Candida auris” or “Candidemia”) AND (“Pathophysiology” OR “Clinical Manifestations” OR “Geographical Distribution” OR “Diagnosis” OR “Management” OR “Treatment”). Data extracted include title, study type, study duration, region, the prevalence of cases, clinical presentation, diagnostic techniques, and mortality, as presented in Table 1.

Table 1.
Regions, clinical manifestations, diagnostic tools, and risk factors of C. auris in coronavirus patients.
3. Results
The search yielded 633 results, of which 42 studies were included, as shown in Figure 1 The search results applied no filters or limitations to date, time, location, or study type. Studies that did not report candida infection and co-infection of COVID-19 and candida were excluded. Case reports, case series, observational studies, retrospective studies, and systematic reviews which reported co-infection of COVID-19 with Candida were included in this study. Hand-searching of review articles was performed to extract relevant studies. Relevant studies were imported to Endnote X9 (Clarivate Analytics, Philadelphia, PA, USA) to remove duplicates further. The included studies comprise 5 letters-to-the-editors (LTEs), 12 original articles, 9 observational studies, 11 case reports, 1 case-control study, 3 retrospective cohort studies, and 1 systematic review.
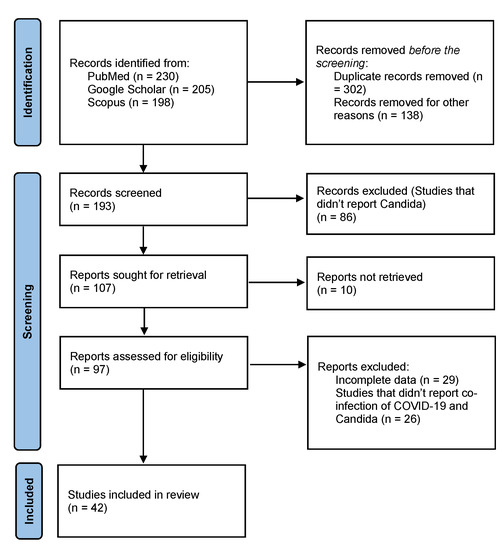
Figure 1.
PRISMA Flowchart.
4. COVID and Co-Infections
Previous studies have established that viral illnesses can lead to microbial co-infection, as observed in MERS, SARS, and influenza epidemics. The emergence of recent data in the present SARS-CoV-2 pandemic indicates an increased incidence of microbial co-infection in hospitalized COVID patients [15]. The coinfection rate ranges from 7% in hospitalized patients to 14% in critically ill patients [16]. The rate of hospital-acquired infection in COVID-19-positive patients admitted to the I.C.U. is higher because they receive invasive procedures such as intubation, mechanical ventilation, and extracorporeal membrane oxygenation that can allow microbiota to grow in instruments used in invasive procedures, allowing the hospital flora to invade the systems of critically ill patients [17].
The co-infection can primarily result from the immune dysregulation found in individuals diagnosed with COVID-19. Recent studies regarding COVID 19-associated immune dysfunction identify lymphopenia and immunosuppression due to leukocyte apoptosis and T-cell exhaustion, paving the pathway for secondary or concurrent bacterial, fungal, and viral infections [15,18]. Co-infections complicate disease progression, resulting in poor prognosis, diagnosis difficulty, and treatment with increased severity, and mortality [19]. We will further discuss in detail different fungal organisms other than Candida auris along with some light on bacterial and viral organisms causing infection in COVID-positive individuals to navigate how they exacerbate COVID -19 infection, the role of hospital flora in co-infection, and antibiotic/antifungal resistance.
Bacterial co-infection among respiratory viruses is common because viral pneumonia weakens the immune system and makes individuals susceptible to concurrent superinfection. Several studies have observed an increased rate of bacterial coinfection in SARS-CoV-2-positive patients suspected of sepsis, community or hospital/ventilator-associated pneumonia, bacteremia, urinary tract infection, and infective endocarditis [17,20,21,22,23]. A single-center study conducted in New York observed around 152 COVID-19 patients that received central venous catheterization also showed signs of bacteremia [24]. Another study with a similar aim stated that about 392 (1.2%) COVID-19 positive individuals had laboratory-confirmed microbial co-infection showing single and multiple pathogens in the culture. Out of which 171 COVID-19 patients suspected bacterial infection when more than one pathogen had been identified from different body sites. The most predominant bacteria found were gram-negative; Haemophilus influenzae, followed by Staphylococcus aureus [25].
Another factor contributing to bacterial growth is the widespread use of antibiotics resulting in antibiotic resistance in COVID-19 patients, considering that patients who received antibiotics 30 days before their positive microbiological cultures suffered bacterial co-infection from multi-drug resistant strains [9].
The Candida auris outbreak during the SARS-CoV-2 pandemic has raised a concern about other fungal species also causing co-infection in this population. The previous data concerning fungal infections shows that the immunocompromised population is more susceptible to fungemia. The SARS-CoV-2 positive patients in critical condition, requiring invasive mechanical ventilation and a prolonged hospital stay, are optimum hosts to opportunistic fungal infection. A study showed 10% fungemia in SARS-CoV-2 positive individuals with a mortality rate of 70.4%, which was higher than the mortality rate observed in bacterial co-infection [26]. In Iran, about 5% of COVID-19 patients with immunocompromised status showed oropharyngeal thrush suggesting opportunistic yeast infection in the oral mucosa. However, a similar yeast infection is also observed in individuals with H.I.V. infection, marking the role of immunosuppression in fungal co-infection [27]. During the second wave of the SARS-CoV-2 pandemic, the antifungal consumption rate increased to 15% in the entire hospital and 75% in the Intensive Care Unit. The highly consumed antifungals were echinocandins (caspofungin), azoles (fluconazole, voriconazole), and Amphotericin B (a second-line drug) in the Intensive Care Unit where most patients suffered from C. auris associated candidemia. Even though there was an increment in fungal co-infection and antifungal consumption, a few antifungal resistant strains were isolated. Such as Fluconazole-resistant C. auris, echinocandin-resistant C. auris, and caspofungin-resistant C. dubliniensis [27,28].
As SARS-CoV-2 damages the respiratory epithelium by decreasing mucociliary clearance that favors other opportunistic respiratory viral infections [19]. Mussuza et al. also reported a viral co-infection rate of 10% and viral superinfection of 4% when additional RT-PCR assay tests showed different viruses other than SARS-CoV-2 [29]. Seasonality may play its role in viral illness outbreaks because respiratory viruses other than COVID 19 such as influenza are common in the cold season and may result in variations in viral co-infection rates. About 22.3% of COVID-19 patients had influenza A virus, and 19.3% were alive individuals. The higher mortality was associated with the seasonal outbreak, bacterial superinfection, comorbidities, and a lack of vaccination against influenza in that area [15,26,30]. These viruses usually cause flu-like symptoms such as cough, sore throat, fever, rhinorrhea, dyspnea, lung infection, and splenomegaly that can exacerbate the COVID-19 symptoms [19,30].
Table 2 highlights all the microbial species causing co-infection with their specific symptoms in COVID-19 positive patients [16,17,19,29,30,31,32,33,34].

Table 2.
Microbial co-infection in patients.
5. Types of Invasive Mycoses
SARS-CoV-2 is aggressive towards lung parenchyma, making respiratory epithelium vulnerable to opportunistic invasive fungal infections (I.F.I.) such as invasive candidiasis, invasive aspergillosis, cryptococcosis, and mucormycosis [36,37]. Usually, these fungi are associated with immunocompromised individuals with an underlying cause such as T-cell exhaustion in severe COVID-19 infection, tumors, H.I.V., organ transplantation, and other comorbidities. However, I.F.I. also affects immunocompetent individuals [38]. These fungal infections usually enter the bloodstream and cause systemic inflammation [31]. Kula et al. reported Invasive Mold Disease in patients with fatal COVID-19 indicating 2% of individuals with evident invasive pulmonary aspergillosis, mycosis, and disseminated mucormycosis in eyes, and sinuses, lungs, hilar lymph nodes, brain, and kidneys. These individuals received IL-1 inhibitors, IL-6 inhibitors, and corticosteroid therapy which predisposed them to invasive mycoses [28,39].
The yeast infection and COVID-associated pulmonary aspergillosis (CAPA) were prevalent in 26.7% of individuals positive for SARS-CoV-2. The combined mortality rate for both Invasive Fungal Diseases was 52.8%, which increased without proper antifungal treatment [40]. In a single-centered prospective study, 3.3% of 239 non-immunocompromised individuals presented with COVID-associated pulmonary aspergillosis (CAPA). Those who developed acute respiratory distress syndrome (a complication of COVID-19 infection) received tocilizumab and mechanical ventilation which may have predisposed them to invasive pulmonary aspergillosis [41]. In Chile, 11% (16) I.C.U. admitted SARS-CoV-2 positive patient COVID-associated invasive mold infection (C.A.I.M.I.). The patients were diagnosed with mold infections from non-aspergillus species such as Rhizopus and scedosporium species [42]. Certain invasive mycoses such as coccidiomycosis, blastomycosis, and histoplasmosis have similar COVID symptoms. Hence, they can mimic COVID 19 infection [38]. Table 3 summarizes the opportunistic invasive mycoses causing invasive fungal diseases in COVID-19 patients [36,40,41,42,43,44].

Table 3.
Opportunistic mycoses in COVID-19 infection.
6. Epidemiology
With the increasing incidence of fungal and yeast infections globally, C. auris was first detected in 2009 in a patient from Japan after isolating an ear sample [45]. Since then, 30 Candida species have been identified [46]. Of all the Candida species, the multi-drug resistant C. auris, with its easy transmissibility, emerges as a rising global challenge [47]. According to a study [48], phylogenetic analysis from 54 isolates of C. auris from four regions around the world showed four major clades of the fungus separated by tens of thousands of single nucleotide polymorphisms (SNPs), each of which is localized to distinct geographical locations, hence supporting the hypothesis that these clades emerged simultaneously and independently in separate geographical human populations. It remains the second most prevalent species of Candidemia [49] and requires immediate action to control its spread.
At present, the spread of SARS-CoV-2 has curated breeding grounds for C. auris in immunocompromised patients. The deadly combo of COVID-19 and C. auris spreads in hospital settings through humans and inanimate surfaces [50]. While personal protective equipment has reduced human-to-human transmission, cross-contamination through equipment remains an unavoidable phenomenon [5]. The use of equipment without proper disinfection exacerbated the coronavirus pandemic when hospital facilities were already overburdened. Oxygen tubing, C.T. scans, X-ray machines, and reusable axillary thermometers are suspected of being used for multiple patients without disinfection [50]. Cultures collected from hospital bed railings, floor, call buttons, and bins tested positive for C. auris [5].
Until January 2022, COVID-19 associated with C. auris has affected over ten countries, including but not limited to Mexico, Italy, Lebanon, USA, China, and Brazil. This fungal infection has affected over 1010 individuals worldwide, with at least 75 deaths. The USA Spain, Colombia, and Italy rank on the top charts with 179, 166, 148, and 111 cases respectively. The case series in Lebanon [51] reported five deaths, one resulting from respiratory failure. Table 3 summarizes the regions, clinical manifestations, diagnostic tools, and risk factors of C. auris in coronavirus patients.
7. Pathophysiology
7.1. Pathophysiology of SARS-CoV-2
The pathogenesis of SARS-CoV-2, the COVID-19-causing pathogen, is complicated and not fully understood. However, with the current literature, effective therapeutic strategies are constantly being worked upon and introduced, which is a positive indicator of progress and a reminder that more research is needed to combat this pandemic actively.
SARS-CoV-2, upon entering the body via the respiratory pathway, acts on ACE-2 receptors and releases its genetic material (R.N.A.) inside epithelial cells of the airway. There, it multiplies and is then released back out, further infecting neighboring cells and entering the alveolar area (respiratory zone) of the lung [52]. After binding with the ACE-2 receptor inside the airway epithelial cells, the SARS-CoV-2 triggers localized inflammation, endothelial activation and damage, tissue damage, and cytokine release, thereby participating in the pathophysiology of acute respiratory distress syndrome (ARDS). It is noteworthy that many COVID-19 patients died due to ARDS [53]. Furthermore, the endothelial damage may also trigger Disseminated Intravascular Coagulation (D.I.C.). As a result of the D.I.C. and congestion of the small capillaries by inflammatory cells, there is a risk of possible thrombosis in larger vessels, causing lung ischemia, which may stimulate angiogenesis and epithelial cell hyperplasia [54]. To make matters worse, the virus invades the bloodstream from the respiratory tract (by infecting and damaging epithelial cells). It disseminates throughout the body to organs including the heart, brain, kidney, liver, and gastrointestinal tract, causing multiple extrapulmonary manifestations such as cerebral hemorrhage, ischemic stroke, paralysis, coma, and possibly, death [55].
The enhanced vascular permeability and leakage in patients with severe COVID-19 may happen due to multiple proposed mechanisms [56]. As the virus binds the ACE-2 receptor to enter the host cells, it reduces the receptor’s activity and indirectly triggers the kallikrein-bradykinin pathway, leading to increased vascular permeability. Also, the damaged epithelial cells recruit neutrophils, which, when activated, produce cytotoxic mediators including reactive oxygen species (R.O.S.) [56]. The ‘cytokine storm’, propagates the damage progression and leads to other epithelial cell dysfunction, inflammation, D.I.C., and vasodilation of the pulmonary capillaries. Altogether, this combination of events ultimately leads to multi-organ failure and death (due to alveolar dysfunction and ARDS with hypoxic respiratory failure) seen in patients with severe COVID-19.
7.2. Pathophysiology of Candida auris
Candida auris, usually a hospital-acquired fungus, has been found in several infection sites throughout the body, including urine, bile, blood, wounds, nares, axilla, skin, and rectum-infected individuals (reviewed by [57]). C. auris, unlike Candida albicans, does not primarily colonize the gastrointestinal or genitourinary tracts of healthy individuals. Instead, it is thought to colonize the skin primarily and predominantly. It has rarely been isolated from the oral, esophageal, and gut mucosa of infected individuals [48]. A study showed how in-vivo experiments and clinical manifestations together suggest that C. auris is incapable of colonizing anaerobic body environments, such as the gut [58]. Even in the oral mucosa, histatin-5, a salivary antimicrobial peptide, has been shown to have a potent antifungal effect on C. auris, hence limiting its colonization [59].
In clinical settings, C. auris is most associated with bloodstream infections [60]. C. auris has been observed to cause infections ranging from mild cutaneous otitis to CSF meningitis. The very first case of C. auris reported in Iran was a female with otomycosis. The candida strain was identified from external ear canal discharge [61]. A pediatric patient in Iran was reported to have candida-associated meningitis. Thereafter, the number of CSF meningitis caused by C. auris increased with reports coming in from the UK, India, and Iran [62] Candida auris has been shown to cause infections in patients of all ages and genders but it has been reported to predominantly affect male patients and critically ill patients in ICUs [63]. In terms of pathogenic potential, C. auris has shown to be less virulent than C. albicans, but significantly more virulent than C. glabrata and C. haemulonii in animal models [64].
The delayed occurrence of candidemia in patients is suggestive of nosocomial transmission [63].
In terms of virulence attributes, there are many similarities between C. auris and C. albicans, including their tissue invasion, enzyme secretion, nutrient acquisition, histidine kinase-2 component system, multi-drug efflux, genes, and pathways involved in nutrient acquisition and cell wall modeling [65]. A study performed on a C. auris isolate from a case of vulvovaginitis showed proteinase, phospholipase, and haemolysin activity [66]. Another study showed that the production of proteinases and phospholipases is strain-dependent [67]. Even though most strains of C. auris form biofilms, there are reports of a lack of biofilm formation in some strains [65,67,68]. In-vitro studies on isolates of C. auris have shown that isolates can be phenotypically divided into aggregating and non-aggregating strains [68], with the aggregative strain having the capability not to be physically disrupted, even by detergent treatment or by vortexing [69]. This property may contribute to the survival of C. auris isolates in hospital environments. The non-aggregating isolates, on the other hand, have shown to exhibit more pathogenicity than aggregating isolates and similar pathogenicity to C. albicans [69]. Some reports have also suggested that these non-aggregating isolates can be more pathogenic than C. albicans [68]. However, the exact pathogenesis of C. auris is still poorly understood and understandably requires research.
8. Risk Factors
The coronavirus’s most striking characteristic is its spike protein’s ability to attach to the ACE-2 receptors in the lungs, which downregulates its expression and overproduces angiotensin-2, leading to lung injury. Additionally, activating macrophages by the antigen presentation cells produces a ’cytokine storm’ of CCL2, IFN-α, IFN-γ, and IL-6 [69]. Diarrhea and vomiting follow the infection as pro-inflammatory cytokines disrupt the mucosa. This provides a route of entry for fungal infections such as candidemia which spread from the gut to the lumen [70]. COVID-19 patients reportedly have lower expression of CD4+ and CD8+ T-cells. COVID-19 patients have a lymphocyte count below normal, accounting for lymphocytopenia [71]. Overproduction of pro-inflammatory mediators, specifically IL-6, associates them with a worse prognosis of COVID-19 and puts them at risk for opportunistic infections [31].
While there is no specific treatment for COVID-19, antifungals, broad-spectrum antibiotics, and corticosteroids are being used to treat mild to severe cases [72]. Tocilizumab, an IL-6 receptor monoclonal antibody, has clinically reduced damage to the immune system. While the promising results show decreased oxygen requirements in severe COVID-19 patients [73], it increases the susceptibility of candidemia in such immunocompromised patients as their innate immune response to fungi and viruses is suppressed [74] Similarly, corticosteroid therapy such as hydrocortisone and dexamethasone suppress the immune system, posing a threat for superimposed fungal infections such as candidemia. Meropenem or moxifloxacin, broad-spectrum antibiotics, are frequently used in severe coronavirus patients. This allows oropharyngeal candidemia to cross barriers through the disrupted mucosal wall [75] A study in Iran showed that using wide-spectrum antibiotics in COVID-19 patients was the leading risk factor of oropharyngeal candidemia in 92.5% of patients [76].
An impaired immune response to COVID-19 is further endangered in the presence of underlying comorbidities, the most threatening being respiratory illnesses, diabetes, chronic kidney disease, and cancer. Hyperglycemia, lymphocytopenia, and inflammatory cytokines create a perfect storm for opportunistic candidemia. In non-COVID circumstances, chemotherapy and cancer accounted for one-third of the candidemia cases [31,77]; while non-clinical risk factors of C. auris include the patient’s age and sex. A retrospective study from India reported that COVID-19 patients above the age of 60 and the male sex were associated with a candidemia infection [78].
A worse prognosis of COVID-19 manifests as moderate to severe pneumonia, which requires I.C.U. the admission followed by mechanical ventilation in critical cases. The median stay of COVID-19 patients at an intensive care unit ranged from one to three weeks [79]. Transfer of patients from hospitals or areas endemic with candidemia, previous I.C.U. admission, prolonged hospital stays, and invasive procedures such as intubation and indwelling central lines and urinary catheters may bring the patient in contact with the hospital environment, which poses a risk of developing candidemia infection through cross-contamination [78,80]. As the rising coronavirus cases burdened the healthcare system, many facilities were scrambling for resources. This became evident as improperly disinfected axillary thermometers became increasingly common. Similarly, a study in Brazil reported positive cultures for reusable probes and bed railings in a COVID-19 setting [5]. Table 4 gives a summary of clinical and non-clinical risk factors of C. auris in COVID-19 patients.

Table 4.
Risk factors of C. auris in COVID-19 patients.
9. Clinical Presentation and Complications
COVID-19 caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV 2) is predominantly a respiratory illness, with clinical signs and symptoms that may vary from person to person. Some patients remained asymptomatic with a self-limiting illness throughout the disease, while some showed visible symptoms [86]. The most frequently seen symptoms included fever, cough, fatigue, expectoration, anorexia, sputum production, and shortness of breath. Headache, sore throat, confusion, and hemoptysis were some of the less commonly seen symptoms [86]. The clinical presentation of the patient depends on the severity of the illness. In severe cases, patients experience complications including acute respiratory distress syndrome, sudden cardiac death, liver dysfunction, and multi-organ failure [87].
Superimposed fungal infection in a COVID-19 patient can exacerbate the severity of the illness. A worrisome complication of COVID-19 was seen with the emergence of a fungal co-infection that caused COVID patients to suffer from candidemia. Laboratory reports of about 85% of the COVID patients showed lymphopenia making them more vulnerable to opportunistic nosocomial infections [87]. C. auris outbreaks tend to occur in critically ill hospitalized patients with mortality rates as high as 72% [4]. The mortality rate of COVID-19-associated candidemia (C.A.C.) remained high even after treatment with antifungal drugs [9,88]. There is limited data for the symptomatic presentation of a COVID-19 patient co-infected with C. auris. However, based on a few case reports, it was observed that the patients who eventually succumbed to fungal infection by C. auris presented to the hospital with complaints of progressive dyspnea, fever, and cough, all indicative of COVID-19 and had to be given critical care [89]. They were assisted with mechanical ventilation to maintain their oxygen saturation, and were given steroids, antibiotics, and an extended hospital stay [87,89,90]. All these factors made them vulnerable to colonization and infection by C. auris. It was observed that even after the resolution of coronavirus, the patients remained critically ill and could not maintain their oxygen saturation. One of the patients diagnosed with fungal co-infection by C. auris on day 20 of admission died due to cardiorespiratory arrest [89]. Since fungal growth was detected in their blood cultures, it can be thought that candidiasis was the cause of debilitation in these patients [89,90].
A variety of invasive fungal infections have been associated with C. auris [57]. Invasive candidiasis is a worrisome infection that can affect the brain, heart, blood, ears, eyes, bones, and other body parts [91]. The most feared and common complication of invasive candidiasis is bloodstream infection; candidemia [91]. A potentially fatal condition presents with a fever that does not subside with antibiotics, chills, pains, redness, swelling, and a general feeling of malaise and fatigue [92]. Candidiasis is also associated with respiratory and urinary tract infections, ear canal, and pericarditis [57]. A case report by Breazzano et al. suggested a link between endogenous panopthalmitis and C. auris. The histopathological analysis of the ocular tissue layers of this patient showed massive polymorphonuclear infiltration. The patient’s ocular specimens showed that he was infected with C. auris and Pseudomonas aeruginosa. Due to the lack of literature on the association of panopthalmitis with fungal and bacterial infections the cause of panopthalmitis in this patient remains uncertain [93]. However, C. auris could be highly suspected of causing panopthalmitis in immunocompromised patients [94]. Spondylodiscitis can also be a rare complication of C. auris. The first report published on the association of spondylodiscitis with C. auris suggested that about 1% of all reported spondylodiscitis cases are associated with Candida species [95]. With the increasing spread of C. auris and simultaneous rise in otologic infections, it was reported that this fungal isolate can also be responsible for causing fungal otomastoiditis, a rare disease of immunocompromised patients [96,97]. C. auris is an opportunistic pathogen that causes opportunistic infections. Some of the infections observed in a patient in Belgium include vulvovaginitis, pleuritis, intra-abdominal infections, pericarditis, ventriculitis, surgical wound infections, and osteomyelitis [97].
Admitted COVID patients presenting any of the signs mentioned earlier/infections or consistently deteriorating in health regardless of the provided treatment should be suspected of fungal co-infection by Candida auris and immediately screened for early diagnosis and effective treatment.
10. Diagnosis
Proper diagnosis of any disease is necessary for adequate treatment and prevention interventions. The diagnosis of candidiasis can be achieved by using the culture method, which includes fungal culturing of blood or other body fluids (urine, CSF, Perineal fluid) or pus from the affected site, as well as swab surveillance samples were taken to detect skin colonization from the axilla, groin, rectum, vagina, nares, and oropharynx under sterile conditions [98,99]. Although the culture method is the gold standard for diagnosis, sometimes invasive candidiasis cannot be diagnosed due to the low concentration of yeast cells in the blood or tissue organs. Therefore, non-culture diagnostic tests have also been introduced as adjuncts to cultures, including mannan and anti-mannan IgG tests, B.D.G., and PCR-based assays [100].
C. auris can be identified by various techniques, namely, phenotypic/culture, commercial biochemical, and molecular methods [101]. The phenotypic/ culture method identifies this isolate based on the appearance and color of colonies formed in various culture broths [102]. On culturing C. auris in a Sabouraud dextrose/glucose agar, smooth and white/cream-colored colonies are seen. Whereas isolates of this species, when cultured on commercial chromogenic candida agar medium, produce pink, beige, red, or pale rose-colored colonies [103]. The sabouraud dextrose agar has a sensitivity of 100% and a specificity of less than or equal to 100% [101]. Another culture broth formed by combining two culture media, CHROMagar candida media supplemented with Pal’s medium, gave a sensitivity and specificity of 100% and was best to differentiate between C. auris and Candida Haemulonii. C. auris isolates produced white-cream colonies at 420 C, whereas C. haemulonii isolates produced light pink colonies and stopped growing at 420 C [101]. However, these methods cannot solely be used to identify C. auris since it is difficult to distinguish it from other candida species without other techniques [102].
Traditional biochemical methods of identification, including API 20C, Vitek 2 (bioMérieux, Marcy-l’Étoile, France), Phoenix (B.D. Diagnostics, Ukraine), and MicroScan (Beckman Coulter, Pasadena, CA, USA), are known to misdiagnose C. auris as other yeasts [57]. This isolate confirms identification is made using accepted methods such as MALDI-TOF or molecular identification methods such as PCR (polymerase chain reaction), sequencing, or A.F.L.P. (amplified fragment length polymorphism) [98].
MALDI-TOF MS technique is an accurate and reliable method for identifying C. auris [60]. The matrix-associated laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) method is available in two platforms, namely, Bruker biotyperTM, which is more commonly used than the VITEK MS platform [101]. The C. auris isolate can be identified with 100% specificity within a few minutes using this technology [101]. The MALDI-TOF method compares the obtained spectra with a reference database that contains the C. auris strain spectrum [57,103]. Identification will only be possible if the ‘research use only (R.U.O.) database contains C. auris spectrum [57,103]. Laboratories should therefore confirm beforehand whether the used reference database or research library contains the C. auris strain spectrum or not [103].
The molecular method used for species identification, i.e., Conventional PCR, A.F.L.P., W.G.S., Real time-PCR, has proved to be an authentic technique for detecting C. auris. Genomic sequencing of the D1−D2 region of the 28 S ribosomal D.N.A. (rDNA) or the internal transcribed region (ITS) by conventional PCR and sequencing of the amplicons is currently the gold standard for identifying C. auris providing 100% sensitivity and specificity with a short turnaround time [101]. The sequenced amplicons of the isolate are compared with the available sequences at the GEN bank to identify the isolate as C. auris [101]. Therefore, the isolate is usually identified by the MALDI-TOF technique, and its presence is further confirmed by the molecular amplification and sequencing method [5].
Once C. auris infection is suspected, it can be diagnosed using the aforementioned techniques. However, there are certain hurdles and barriers faced by the healthcare community regarding diagnostics. Since the technology needed to identify this isolate is very advanced, resource-limited countries face a significant challenge in identifying and controlling and prevention [4]. Developing or low-income countries with limited diagnostic resources lead to the under-recognition of fungal co-infections in COVID-19 patients [4]. Furthermore, even though facilitated with all the required diagnostic technology, some healthcare setups fail to correctly identify the isolate as C. auris is frequently misdiagnosed as Candida haemulonii/Candida famata/Candida sake or Rhodotorula glutinis due to errors in the automated identification systems [104]. Another major problem in the diagnosis of fungal co-infection is the overlapping of clinical symptoms. Similar or overlapping physical findings, clinical symptoms, routine laboratory, and radiographic results have made differentiating between fungal and bacterial co-infections a matter of great difficulty [104]. With the severity of disease increasing in a COVID-19 patient, more frequently thought is given to bacterium or even mycoplasma as the causative co-infection agent with little or no regard given to fungal co-infections [76]. This leads to a delay in diagnosing fungal co-infection and increases the risk of mortality [76].
In a study conducted to determine the various co-infections in COVID-19 patients, it was observed that co-infection could cause a false negative result of real-time reverse-transcriptase polymerase chain reaction for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [105]. According to the reports, the co-infectious agent that resulted in a false negative report was the most common viral pathogen, namely, the influenza A virus [105]. Due to the lack of data concerning fungal co-infections in COVID-19 patients, nothing can be said about a false-negative COVID-19 test result during candidiasis caused by C. auris [106]. It is, therefore, necessary to carry out a detailed and proper investigation of the disease in case there is a doubt of co-infection. The high mortality rates in COVID-19 patients due to fungal co-infections, which are either delayed or neglected, make early diagnosis and appropriate management necessary for patient survival [87]. The French High Council has recommended systematic screening for fungal co-infection in COVID-19 patients for Public Health for early diagnosis [51]. To curb the spread of this infection, rapid detection and efficient reporting of new cases are essential [51]. COVID-19 patients who are deteriorating should be suspected of fungal and bacterial co-infection and screened without delay [79].
11. Treatment and Prevention
The treatment of C. auris infection in a COVID-19 diagnosed patient is the same as in a non-COVID-19 patient. The options of treatment comprise the following categories of antifungal agents: polyenes (amphotericin B, deoxycholate, and liposomal amphotericin B), triazoles (fluconazole, voriconazole, itraconazole), echinocandins (caspofungin, anidulafungin, and micafungin), and flucytosine [37,107].
Echinocandin is labeled as the first-line therapy for C. auris infection [108]. If the patient is clinically unresponsive to this treatment, combination therapy of echinocandin and liposomal amphotericin B should be prescribed as synergistic interactions have better efficacy [109]. Due to the emerging yet few cases of resistance to echinocandins, patients should undergo close follow-up and microbiological culture-based reassessment to detect the therapeutic failure and eventual development of resistance.
Treating C. auris infection has become difficult due to its multiresistant nature. As per a report published in C.D.C., more than 99% of the C. auris isolates in New York were shown to be resistant to fluconazole, and around 61% were resistant to amphotericin B, and up to 4% were resistant to echinocandins [6]. It is to be noted that a high resistance rate exists with fluconazole and amphotericin B amongst C. auris worldwide. In a study conducted by Magnasco et al. in Italy in 2020, 6 of 14 critically ill COVID-19 patients had a C. auris colonization and infection. All strains of C. auris identified proved to be resistant to amphotericin B and azoles but susceptible to echinocandins [110]. A low number (about 4%) of isolates show resistance to all classes of antifungals; it is the only species of Candida that has isolates shown to be resistant to all classes of human antifungal drugs [111,112].
Considering multi- and pan-resistant cases of C. auris, the development of other antifungals has been highlighted in medicine. Fosmanogepix (APX001), an N-phosphonooxymethyl prodrug, is an antifungal agent currently in phase 2 of clinical development to treat life-threatening invasive fungal infections. It is active against a broad range of pathogenic yeast and molds, including Candida, by inhibiting the highly conserved fungal enzyme Gwt1, which is essential for the biosynthesis of glycosylphosphatidylinositol anchors [113]. Another drug currently in phase 3 of clinical trials is ibrexafungerp (SCY-078). It is the first representative of a novel class of structurally distinct glucan synthase inhibitors with broad and potent activity against Candida species, including echinocandin-resistant strains of Candida spp [114]. In addition, Rezafungin, which is currently in phase 3 of clinical trials, is a novel once-weekly echinocandin and has also proven effective against Candida spp. and Aspergillus spp. including subsets of echinocandin-resistant C. auris [115].
The possibility of transmission and outbreak of C. auris in admitted COVID-19 patients is likely due to all risk factors, such as broad-spectrum antibacterial treatment, central venous or bladder catheters, comorbidities, and mechanical ventilation [36]. Strict prevention and control methods must be put in play by hospitals and health care facilities to control nosocomial spread. As per C.D.C.’s guidelines, patients with C. auris should be isolated in a single patient room with appropriate contact precautions. Regular hand hygiene should be emphasized by healthcare personnel and patients. Daily disinfection of the patient care environment should occur, and contacts of newly identified patients should be screened to identify C. auris colonization [116]. Common disinfectants such as quaternary ammonia compounds have proven ineffective, but surface disinfectants such as chlorine and hydrogen peroxide have good efficacy against C. auris [112]. With the implementation of C.D.C.’s recommendations for infection control, it is possible to prevent the spread of C. auris in hospital care settings, as shown in a brief report by Reimer-McAtee et al. They report the success of transmission control in in-patient rehabilitation and intensive care settings [109].
12. Conclusions
With the increasing cases of COVID-19 exhausting healthcare facilities around the globe, any additional outbreaks, local or widespread, will undoubtedly be a challenge to manage and resolve. Given that the breeding grounds and risk factors of Candida transmission are present in hospitals, especially in COVID-19 patients, it is crucial to highlight and recognize the possibility of C. auris outbreaks among these patients. The multisystem involvement, rapid progression, and failure associated with the Candida species require additional medical intervention and must be given priority. In addition, necessary preventive measures should be considered in all medical setups worldwide. It is also worth noting that due to the increased antifungal use and eventual resistance, the development and evolution of medical therapy for fungal infections should be given due importance. Such advancements in health care will help decrease the spread of Candida and the eventual mortality of patients. The healthcare sector must watch for co-morbidities, and care and devise long-term policies that facilitate patient-specific care to curb another epidemic.
Author Contributions
Writing—review and editing: H.N., S.A.S., Z.A., S.H.A., S.U.R.U., F.J. and H.N.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing does not apply to this article as no datasets were generated or analyzed during the current study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Meis, J.F.; Chowdhary, A. Candida auris: A global fungal public health threat. Lancet Infect. Dis. 2018, 18, 1298–1299. [Google Scholar] [CrossRef]
- Superinfections and Coinfections in COVID-19. Available online: https://www.medpagetoday.com/infectiousdisease/covid19/86192 (accessed on 24 May 2022).
- Chowdhary, A.; Sharma, A. The lurking scourge of multi-drug resistant Candida auris in times of COVID-19 pandemic. J. Glob. Antimicrob. Resist. 2020, 22, 175. [Google Scholar] [CrossRef]
- Chowdhary, A.; Tarai, B.; Singh, A.; Sharma, A. Multidrug-resistant Candida auris infections in critically ill coronavirus disease patients, India, April–July 2020. Emerg. Infect. Dis. 2020, 26, 2694. [Google Scholar] [CrossRef] [PubMed]
- Ostrowsky, B.; Greenko, J.; Adams, E.; Quinn, M.; O’Brien, B.; Chaturvedi, V.; Berkow, E.; Vallabhaneni, S.; Forsberg, K.; Chaturvedi, S.; et al. Candida auris isolates resistant to three classes of antifungal medications—New York, 2019. Morb. Mortal. Wkly. Rep. 2020, 69, 6. [Google Scholar] [CrossRef]
- Rodríguez, J.Y.; Morales-López, S.E.; Rodríguez, G.J.; Álvarez-Moreno, C.A.; Esquea, K.; Pinzon, H.; Ramirez, L.R.; Moreno, L.; Ocampo, W.; Cepeda, M.L. Case series study of melioidosis, Colombia. Emerg. Infect. Dis. 2019, 25, 1531. [Google Scholar] [CrossRef]
- Schauwvlieghe, A.F.; Rijnders, B.J.; Philips, N.; Verwijs, R.; Vanderbeke, L.; Van Tienen, C.; Lagrou, K.; Verweij, P.E.; Van de Veerdonk, F.L.; Gommers, D.; et al. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: A retrospective cohort study. Lancet Respir. Med. 2018, 6, 782–792. [Google Scholar] [CrossRef]
- Villanueva-Lozano, H.; Treviño-Rangel, R.D.J.; González, G.M.; Ramírez-Elizondo, M.T.; Lara-Medrano, R.; Aleman-Bocanegra, M.C.; Guajardo-Lara, C.E.; Gaona-Chávez, N.; Castilleja-Leal, F.; Torre-Amione, G.; et al. Outbreak of Candida auris infection in a COVID-19 hospital in Mexico. Clin. Microbiol. Infect. 2021, 27, 813–816. [Google Scholar] [CrossRef]
- Alvarado, M.; Bartolomé Álvarez, J.; Lockhart, S.R.; Valentín, E.; Ruiz-Gaitán, A.C.; Eraso, E.; de Groot, P.W. Identification of Candida auris and related species by multiplex PCR based on unique GPI protein-encoding genes. Mycoses 2021, 64, 194–202. [Google Scholar] [CrossRef]
- Hernando-Ortiz, A.; Mateo, E.; Perez-Rodriguez, A.; De Groot, P.W.; Quindós, G.; Eraso, E. Virulence of Candida auris from different clinical origins in Caenorhabditis elegans and Galleria mellonella host models. Virulence 2021, 12, 1063–1075. [Google Scholar] [CrossRef]
- Welsh, R.M.; Bentz, M.L.; Shams, A.; Houston, H.; Lyons, A.; Rose, L.J.; Litvintseva, A.P. Survival, persistence, and isolation of the emerging multidrug-resistant pathogenic yeast Candida auris on a plastic health care surface. J. Clin. Microbiol. 2017, 55, 2996–3005. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Khan, Z.; Al-Sweih, N.; Alfouzan, W.; Joseph, L. Candida auris in various hospitals across Kuwait and their susceptibility and molecular basis of resistance to antifungal drugs. Mycoses 2020, 63, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 21, 339. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, R.; Goodarzi, P.; Asadi, M.; Soltani, A.; Aljanabi, H.A.; Jeda, A.S.; Dashtbin, S.; Jalalifar, S.; Mohammadzadeh, R.; Teimoori, A.; et al. Bacterial co-infections with SARS-CoV-2. IUBMB Life 2020, 72, 2097–2111. [Google Scholar] [CrossRef] [PubMed]
- Lansbury, L.; Lim, B.; Baskaran, V.; Lim, W.S. Co-infections in people with COVID-19: A systematic review and meta-analysis. J. Infect. 2020, 81, 266–275. [Google Scholar] [CrossRef]
- Garcia-Vidal, C.; Sanjuan, G.; Moreno-García, E.; Puerta-Alcalde, P.; Garcia-Pouton, N.; Chumbita, M.; Fernandez-Pittol, M.; Pitart, C.; Inciarte, A.; Bodro, M.; et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: A retrospective cohort study. Clin. Microbiol. Infect. 2021, 27, 83–88. [Google Scholar] [CrossRef]
- Parrill, A.; Tsao, T.; Dong, V.; Huy, N.T. SARS-CoV-2-induced immunodysregulation and the need for higher clinical suspicion for co-infection and secondary infection in COVID-19 patients. J. Microbiol. Immunol. Infect. 2021, 54, 105–108. [Google Scholar] [CrossRef]
- Chen, X.; Liao, B.; Cheng, L.; Peng, X.; Xu, X.; Li, Y.; Hu, T.; Li, J.; Zhou, X.; Ren, B. The microbial co-infection in COVID-19. Appl. Microbiol. Biotechnol. 2020, 11, 7777–7785. [Google Scholar] [CrossRef]
- Saeed, N.K.; Al-Khawaja, S.; Alsalman, J.; Almusawi, S.; Albalooshi, N.A.; Al-Biltagi, M. Bacterial co-infection in patients with SARS-CoV-2 in the Kingdom of Bahrain. World J. Virol. 2021, 10, 168. [Google Scholar] [CrossRef]
- Ramos-Martinez, A.; Fernández-Cruz, A.; Domínguez, F.; Forteza, A.; Cobo, M.; Sánchez-Romero, I.; Asensio, A. Hospital-acquired infective endocarditis during COVID-19 pandemic. Infect. Prev. Pract. 2020, 2, 100080. [Google Scholar] [CrossRef]
- Lima, W.G.; Brito, J.C.; da Cruz Nizer, W.S. Ventilator-associated pneumonia (V.A.P.) caused by carbapenem-resistant Acinetobacter baumannii in patients with COVID-19: Two problems, one solution? Med. Hypotheses 2020, 144, 110139. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.A.; Kaur, J.; Wani, F.; Kichloo, A.; Bhanot, R. Renal transplant recipient with concurrent COVID-19 and Stenotrophomonas maltophilia pneumonia treated with trimethoprim/sulfamethoxazole leading to acute kidney injury: A therapeutic dilemma. Am. J. Case Rep. 2020, 21, e926464-1. [Google Scholar] [CrossRef]
- Nori, P.; Cowman, K.; Chen, V.; Bartash, R.; Szymczak, W.; Madaline, T.; Katiyar, C.P.; Jain, R.; Aldrich, M.; Weston, G.; et al. Bacterial and fungal co-infections in COVID-19 patients hospitalized during the New York City pandemic surge. Infect. Control Hosp. Epidemiol. 2021, 42, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Senok, A.; Alfaresi, M.; Khansaheb, H.; Nassar, R.; Hachim, M.; Al Suwaidi, H.; Almansoori, M.; Alqaydi, F.; Afaneh, Z.; Mohamed, A.; et al. Coinfections in Patients Hospitalized with COVID-19: A Descriptive Study from the United Arab Emirates. Infect. Drug Resist. 2021, 14, 2289–2296. [Google Scholar] [CrossRef] [PubMed]
- Peci, A.; Tran, V.; Guthrie, J.L.; Li, Y.; Nelson, P.; Schwartz, K.L.; Eshaghi, A.; Buchan, S.A.; Gubbay, J.B. Prevalence of co-infections with respiratory viruses in individuals investigated for SARS-CoV-2 in Ontario, Canada. Viruses 2021, 13, 130. [Google Scholar] [CrossRef]
- Chen, T.; Song, J.; Liu, H.; Zheng, H.; Chen, C. Positive Epstein–Barr virus detection in coronavirus disease 2019 (COVID-19) patients. Sci. Rep. 2021, 11, 10902. [Google Scholar] [CrossRef] [PubMed]
- Kula, B.E.; Clancy, C.J.; Nguyen, M.H.; Schwartz, I.S. Invasive mould disease in fatal COVID-19: A systematic review of autopsies. Lancet Microbe 2021, 2, e405–e414. [Google Scholar] [CrossRef]
- Musuuza, J.S.; Watson, L.; Parmasad, V.; Putman-Buehler, N.; Christensen, L.; Safdar, N. Prevalence and outcomes of co-infection and superinfection with SARS-CoV-2 and other pathogens: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0251170. [Google Scholar] [CrossRef]
- Hashemi, S.A.; Safamanesh, S.; Ghasemzadeh-moghaddam, H.; Ghafouri, M.; Azimian, A. High prevalence of SARS-CoV-2 and influenza A virus (H1N1) co-infection in dead patients in Northeastern Iran. J. Med Virol. 2021, 93, 1008–1012. [Google Scholar] [CrossRef]
- Salehi, M.; Ahmadikia, K.; Mahmoudi, S.; Kalantari, S.; Jamalimoghadamsiahkali, S.; Izadi, A.; Kord, M.; Dehghan Manshadi, S.A.; Seifi, A.; Ghiasvand, F.; et al. Oropharyngeal candidiasis in hospitalized COVID-19 patients from Iran: Species identification and antifungal susceptibility pattern. Mycoses 2020, 63, 771–778. [Google Scholar] [CrossRef]
- Mulet Bayona, J.V.; Tormo Palop, N.; Salvador García, C.; Fuster Escrivá, B.; Chanzá Aviñó, M.; Ortega García, P.; Gimeno Cardona, C. Impact of the SARS-CoV-2 Pandemic in Candidaemia, Invasive Aspergillosis and Antifungal Consumption in a Tertiary Hospital. J. Fungi 2021, 7, 440. [Google Scholar] [CrossRef] [PubMed]
- Wee, L.E.; Ko, K.K.; Ho, W.Q.; Kwek, G.T.; Tan, T.T.; Wijaya, L. Community-acquired viral respiratory infections amongst hospitalized inpatients during a COVID-19 outbreak in Singapore: Co-infection and clinical outcomes. J. Clin. Virol. 2020, 128, 104436. [Google Scholar] [CrossRef] [PubMed]
- Hazra, A.; Collison, M.; Pisano, J.; Kumar, M.; Oehler, C.; Ridgway, J.P. Co-infections with SARS-CoV-2 and other respiratory pathogens. Infect. Control Hosp. Epidemiol. 2020, 41, 1228–1229. [Google Scholar] [CrossRef] [PubMed]
- Tadolini, M.; Codecasa, L.R.; García-García, J.M.; Blanc, F.X.; Borisov, S.; Alffenaar, J.W.; Andréjak, C.; Bachez, P.; Bart, P.A.; Belilovski, E.; et al. Active tuberculosis, sequelae and COVID-19 co-infection: First cohort of 49 cases. Eur. Respir. J. 2020, 56, 2001398. [Google Scholar] [CrossRef]
- Pemán, J.; Ruiz-Gaitán, A.; García-Vidal, C.; Salavert, M.; Ramírez, P.; Puchades, F.; García-Hita, M.; Alastruey-Izquierdo, A.; Quindós, G. Fungal co-infection in COVID-19 patients: Should we be concerned? Rev. Iberoam. De Micol. 2020, 37, 41–46. [Google Scholar] [CrossRef]
- Song, G.; Liang, G.; Liu, W. Fungal co-infections associated with global COVID-19 pandemic: A clinical and diagnostic perspective from China. Mycopathologia 2020, 31, 1–8. [Google Scholar] [CrossRef]
- Fungal Diseases and COVID-19|CDC, Cdc.gov. 2021. Available online: https://www.cdc.gov/fungal/covid-fungal.html (accessed on 26 August 2021).
- Singh, A.K.; Singh, R.; Joshi, S.R.; Misra, A. Mucormycosis in COVID-19: A systematic review of cases reported worldwide and in India. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 102146. [Google Scholar] [CrossRef]
- White, P.L.; Dhillon, R.; Cordey, A.; Hughes, H.; Faggian, F.; Soni, S.; Pandey, M.; Whitaker, H.; May, A.; Morgan, M.; et al. A national strategy to diagnose coronavirus disease 2019–associated invasive fungal disease in the intensive care unit. Clin. Infect. Dis. 2020, 73, e1634–e1644. [Google Scholar] [CrossRef]
- Machado, M.; Valerio, M.; Álvarez-Uría, A.; Olmedo, M.; Veintimilla, C.; Padilla, B.; De la Villa, S.; Guinea, J.; Escribano, P.; Ruiz-Serrano, M.J.; et al. Invasive pulmonary aspergillosis in the COVID-19 era: An expected new entity. Mycoses 2021, 64, 132–143. [Google Scholar] [CrossRef]
- Rabagliati, R.; Rodríguez, N.; Núñez, C.; Huete, A.; Bravo, S.; Garcia, P. COVID-19–Associated Mold Infection in Critically Ill Patients, Chile. Emerg. Infect. Dis. 2021, 27, 1454. [Google Scholar] [CrossRef]
- Martins, A.C.; Psaltikidis, E.M.; de Lima, T.C.; Fagnani, R.; Schreiber, A.Z.; de Oliveira Conterno, L.; Kamei, K.; Watanabe, A.; Trabasso, P.; Resende, M.R.; et al. COVID-19 and invasive fungal co-infections: A case series at a Brazilian referral hospital. J. Med Mycol. 2021, 31, 101175. [Google Scholar] [CrossRef] [PubMed]
- Cafardi, J.; Haas, D.; Lamarre, T.; Feinberg, J. Opportunistic fungal infection associated with COVID-19. In InOpen Forum Infectious Diseases; Oxford University Press: Oxford, UK, 2021. [Google Scholar]
- Satoh, K.; Makimura, K.; Hasumi, Y.; Nishiyama, Y.; Uchida, K.; Yamaguchi, H. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol. Immunol. 2009, 53, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Bing, J.; Hu, T.; Ennis, C.L.; Nobile, C.J.; Huang, G. Candida auris: Epidemiology, biology, antifungal resistance, and virulence. PLoS Pathog. 2020, 16, e1008921. [Google Scholar] [CrossRef] [PubMed]
- De Cássia Orlandi Sardi, J.; Silva, D.R.; Soares Mendes Giannini, M.J.; Rosalen, P.L. Candida auris: Epidemiology, risk factors, virulence, resistance, and therapeutic options. Microb. Pathog. 2018, 125, 116–121. [Google Scholar] [CrossRef]
- Lockhart, S.R.; Etienne, K.A.; Vallabhaneni, S.; Farooqi, J.; Chowdhary, A.; Govender, N.P. Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin. Infect. Dis. 2017, 64, 134–140. [Google Scholar] [CrossRef]
- Mathur, P.; Hasan, F.; Singh, P.K.; Malhotra, R.; Walia, K.; Chowdhary, A. Five-year profile of candidaemia at an Indian trauma centre: High rates of Candida auris blood stream infections. Mycoses 2018, 61, 674–680. [Google Scholar] [CrossRef]
- Eyre, D.W.; Sheppard, A.E.; Madder, H.; Moir, I.; Moroney, R.; Quan, T.P.; Jeffery, K.J. A Candida auris outbreak and its control in an intensive care setting. N. Engl. J. Med. 2018, 379, 1322–1331. [Google Scholar] [CrossRef]
- Allaw, F.; Kara Zahreddine, N.; Ibrahim, A.; Tannous, J.; Taleb, H.; Bizri, A.R.; Dbaibo, G.; Kanj, S.S. First Candida auris Outbreak during a COVID-19 Pandemic in a Tertiary-Care Center in Lebanon. Pathogens 2021, 10, 157. [Google Scholar] [CrossRef]
- Mason, R.J. Pathogenesis of COVID-19 from a Cell Biology Perspective. Eur. Respir. J. 2020, 55, 2000607. [Google Scholar] [CrossRef]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N. Eng. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef]
- Zaim, S.; Chong, J.H.; Sankaranarayanan, V.; Harky, A. COVID-19 and multiorgan response. Curr. Probl. Cardiol. 2020, 45, 100618. [Google Scholar] [CrossRef] [PubMed]
- Leisman, D.E.; Deutschman, C.S.; Legrand, M. Facing COVID-19 in the I.C.U.: Vascular dysfunction, thrombosis, and dysregulated inflammation. Intensive Care Med. 2020, 46, 1105–1108. [Google Scholar] [CrossRef] [PubMed]
- Teuwen, L.A.; Geldhof, V.; Pasut, A.; Carmeliet, P. Author Correction: COVID-19: The vasculature unleashed. Nat. Rev. Immunol. 2020, 20, 389–391. [Google Scholar] [CrossRef] [PubMed]
- Jeffery-Smith, A.; Taori, S.K.; Schelenz, S.; Jeffery, K.; Johnson, E.M.; Borman, A. Candida auris Incident Management Team; Manuel, R.; Brown CS. Candida auris: A review of the literature. Clin. Microbiol. Rev. 2018, 31, e00029-17. [Google Scholar] [CrossRef]
- Day, A.M.; McNiff, M.M.; da Silva Dantas, A.; Gow, N.A.; Quinn, J. Hog1 regulates stress tolerance and virulence in the emerging fungal pathogen Candida auris. MSphere 2018, 3, e00506-18. [Google Scholar] [CrossRef]
- Pathirana, R.U.; Friedman, J.; Norris, H.L.; Salvatori, O.; McCall, A.D.; Kay, J.; Edgerton, M. Fluconazole-resistant Candida auris is susceptible to salivary histatin 5 killing and to intrinsic host defenses. Antimicrob. Agents Chemother. 2018, 62, e01872-17. [Google Scholar] [CrossRef]
- Spivak, E.S.; Hanson, K.E. Candida auris: An emerging fungal pathogen. J. Clin. Microbiol. 2018, 56, e01588-17. [Google Scholar] [CrossRef]
- Abastabar, M.; Haghani, I.; Ahangarkani, F.; Rezai, M.S.; Taghizadeh Armaki, M.; Roodgari, S.; Kiakojuri, K.; Al-Hatmi, A.M.S.; Meis, J.F.; Badali, H. Candida auris otomycosis in Iran and review of recent literature. Mycoses 2019, 62, 101–105. [Google Scholar] [CrossRef]
- Mirhendi, H.; Charsizadeh, A.; Aboutalebian, S.; Mohammadpour, M.; Nikmanesh, B.; de Groot, T.; Meis, J.F.; Badali, H. South Asian (Clade I) Candida auris meningitis in a pediatric patient in Iran with a review of the literature. Mycoses 2022, 65, 134–139. [Google Scholar] [CrossRef]
- Schelenz, S.; Hagen, F.; Rhodes, J.L.; Abdolrasouli, A.; Chowdhary, A.; Hall, A.; Ryan, L.; Shackleton, J.; Trimlett, R.; Meis, J.F.; et al. First hospital outbreak of the globally emerging Candida auris in a European hospital. Antimicrob. Resist. Infect. Control 2016, 5, 35. [Google Scholar] [CrossRef] [Green Version]
- Fakhim, H.; Vaezi, A.; Dannaoui, E.; Chowdhary, A.; Nasiry, D.; Faeli, L.; Meis, J.F.; Badali, H. Comparative virulence of Candida auris with Candida haemulonii, Candida glabrata and Candida albicans in a murine model. Mycoses 2018, 61, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Alampalli, S.V.; Nageshan, R.K.; Chettiar, S.T.; Joshi, S.; Tatu, U.S. Draft genome of a commonly misdiagnosed multi-drug resistant pathogen Candida auris. BMC Genom. 2015, 16, 686. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Banerjee, T.; Pratap, C.B.; Tilak, R. Itraconazole-resistant Candida auris with phospholipase, proteinase and hemolysin activity from a case of vulvovaginitis. J. Infect. Dev. Ctries. 2015, 9, 435–437. [Google Scholar] [CrossRef] [PubMed]
- Larkin, E.; Hager, C.; Chandra, J.; Mukherjee, P.K.; Retuerto, M.; Salem, I.; Long, L.; Isham, N.; Kovanda, L.; Borroto-Esoda, K.; et al. The emerging pathogen Candida auris: Growth phenotype, virulence factors, activity of antifungals, and effect of SCY-078, a novel glucan synthesis inhibitor, on growth morphology and biofilm formation. Antimicrob. Agents Chemother. 2017, 61, e02396-16. [Google Scholar] [CrossRef]
- Sherry, L.; Ramage, G.; Kean, R.; Borman, A.; Johnson, E.M.; Richardson, M.D.; Rautemaa-Richardson, R. Biofilm-forming capability of highly virulent, multidrug-resistant Candida auris. Emerg. Infect. Dis. 2017, 23, 328. [Google Scholar] [CrossRef]
- Borman, A.M.; Szekely, A.; Johnson, E.M. Comparative pathogenicity of United Kingdom isolates of the emerging pathogen Candida auris and other key pathogenic Candida species. MSphere 2016, 1, e00189-16. [Google Scholar] [CrossRef]
- Samudrala, P.K.; Kumar, P.; Choudhary, K.; Thakur, N.; Wadekar, G.S.; Dayaramani, R.; Agrawal, M.; Alexander, A. Virology, pathogenesis, diagnosis and in-line treatment of COVID-19. Eur. J. Pharmacol. 2020, 883, 173375. [Google Scholar] [CrossRef]
- Nucci, M.; Barreiros, G.; Guimarães, L.F.; Deriquehem, V.A.; Castiñeiras, A.C.; Nouér, S.A. Increased incidence of candidemia in a tertiary care hospital with the COVID-19 pandemic. Mycoses 2021, 64, 152–156. Available online: https://pubmed.ncbi.nlm.nih.gov/33275821/ (accessed on 12 July 2021). [CrossRef]
- Liu, J.; Li, S.; Liu, J.; Liang, B.; Wang, X.; Wang, H.; Li, W.; Tong, Q.; Yi, J.; Zhao, L.; et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine 2020, 55, 102763. [Google Scholar] [CrossRef]
- Xu, X.; Ong, Y.K.; Wang, Y. Role of adjunctive treatment strategies in COVID-19 and a review of international and national clinical guidelines. Mil. Med. Res. 2020, 7, 22. [Google Scholar] [CrossRef]
- Xu, X.; Han, M.; Li, T.; Sun, W.; Wang, D.; Fu, B.; Zhou, Y.; Zheng, X.; Yang, Y.; Li, X.; et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc. Natl. Acad. Sci. USA 2020, 117, 10970–10975. [Google Scholar] [CrossRef] [PubMed]
- Antinori, S.; Bonazzetti, C.; Gubertini, G.; Capetti, A.; Pagani, C.; Morena, V.; Rimoldi, S.; Galimberti, L.; Sarzi-Puttini, P.; Ridolfo, A.L. Tocilizumab for cytokine storm syndrome in COVID-19 pneumonia: An increased risk for candidemia? Autoimmun. Rev. 2020, 19, 102564. [Google Scholar] [CrossRef] [PubMed]
- Salehi, M.; Ahmadikia, K.; Badali, H.; Khodavaisy, S. Opportunistic Fungal Infections in the Epidemic Area of COVID-19: A Clinical and Diagnostic Perspective from Iran. Mycopathologia 2020, 185, 607–611. Available online: /pmc/articles/PMC7393345/ (accessed on 7 June 2021). [CrossRef]
- Medeiros, M.A.P.; Melo, A.P.V.; Bento, A.O.; Souza, L.B.F.C.; Neto, F.A.B.; Garcia, J.B.; Zuza-Alves, D.L.; Francisco, E.C.; Melo, A.S.A.; Chaves, G.M. Epidemiology and prognostic factors of nosocomial candidemia in Northeast Brazil: A six-year retrospective study. PLoS ONE 2019, 14, e0221033. [Google Scholar] [CrossRef] [PubMed]
- Kenters, N.; Kiernan, M.; Chowdhary, A.; Denning, D.W.; Pemán, J.; Saris, K.; Schelenz, S.; Tartari, E.; Widmer, A.; Meis, J.F.; et al. Control of Candida auris in healthcare institutions: Outcome of an International Society for Antimicrobial Chemotherapy expert meeting. Int. J. Antimicrob. Agents 2019, 54, 400–406. [Google Scholar] [CrossRef]
- Görkem, A.; Hafize, S.A.V.; Özge, K.A.A.N.; Esma, E.R.E.N. Coronavirus disease and candidemia infection: A case report. J. Mycol. Med. 2021, 31. Available online: https://pubmed.ncbi.nlm.nih.gov/34146997/ (accessed on 12 July 2021). [CrossRef] [PubMed]
- Rees, E.M.; Nightingale, E.S.; Jafari, Y.; Waterlow, N.R.; Clifford, S.; Pearson, C.A.B.; CMMID Working Group; Jombart, T.; Procter, S.R.; Knight, G. COVID-19 length of hospital stay: A systematic review and data synthesis. BMC Med. 2020, 18, 270. [Google Scholar] [CrossRef]
- Nobrega de Almeida, J., Jr.; Brandão, I.B.; Francisco, E.C.; de Almeida, S.L.R.; de Oliveira Dias, P.; Pereira, F.M.; Santos Ferreira, F.; de Andrade, T.S.; de Miranda Costa, M.M.; de Souza Jordão, R.T.; et al. Axillary Digital Thermometers uplifted a multidrug-susceptible Candida auris outbreak among COVID-19 patients in Brazil. Mycoses 2021, 35, e00094-21. [Google Scholar] [CrossRef]
- Rodriguez, J.Y.; Le Pape, P.; Lopez, O.; Esquea, K.; Labiosa, A.L.; Alvarez-Moreno, C. Candida auris: A latent threat to critically ill patients with COVID-19. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2020, 73, ciaa1595, Advance online publication. [Google Scholar] [CrossRef]
- Baccolini, V.; Migliara, G.; Isonne, C.; Dorelli, B.; Barone, L.C.; Giannini, D.; Marotta, D.; Marte, M.; Mazzalai, E.; Alessandri, F.; et al. The impact of the COVID-19 pandemic on healthcare-associated infections in intensive care unit patients: A retrospective cohort study. Antimicrob. Resist. Infect. Control 2021, 10, 87. [Google Scholar] [CrossRef]
- Gorospe-Sarasúa, L.; Gallego-Rivera, J.I.; Muñoz-Molina, G.M.; Mirambeaux-Villalona, R.M.; Ajuria-Illarramendi, O.; González-García, A.; Barbolla-Díaz, I. Delayed Candida Costochondritis and Spondylitis in a Post-COVID-19 Patient Previously Treated With Corticosteroids, Antibiotics, and Tocilizumab. Costocondritis y espondilitis diferidas por Candida en paciente post-COVID-19 tratado previamente con corticoides, antibióticos y tocilizumab. Arch. Bronconeumol. 2021, 57 (Suppl. S2), 48–50. [Google Scholar] [CrossRef] [PubMed]
- Di Pilato, V.; Codda, G.; Ball, L.; Giacobbe, D.R.; Willison, E.; Mikulska, M.; Magnasco, L.; Crea, F.; Vena, A.; Pelosi, P.; et al. Molecular Epidemiological Investigation of a Nosocomial Cluster of C. auris: Evidence of Recent Emergence in Italy and Ease of Transmission during the COVID-19 Pandemic. J. Fungi 2021, 7, 140. [Google Scholar] [CrossRef] [PubMed]
- Majumder, J.; Minko, T. Recent Developments on Therapeutic and Diagnostic Approaches for COVID-19. AAPS J. 2021, 23, 14. [Google Scholar] [CrossRef]
- Bhatt, K.; Agolli, A.; Patel, M.H.; Garimella, R.; Devi, M.; Garcia, E.; Amin, H.; Domingue, C.; Del Castillo, R.G.; Sanchez-Gonzalez, M. High mortality co-infections of COVID-19 patients: Mucormycosis and other fungal infections. Discoveries 2021, 9, e126. [Google Scholar] [CrossRef] [PubMed]
- Arastehfar, A.; Shaban, T.; Zarrinfar, H.; Roudbary, M.; Ghazanfari, M.; Hedayati, M.T.; Sedaghat, A.; Ilkit, M.; Najafzadeh, M.J.; Perlin, D.S. Candidemia among iranian patients with severe COVID-19 admitted to I.C.U.s. J. Fungi 2021, 7, 280. [Google Scholar] [CrossRef]
- Alashqar, M.B.; Alabdan, L.; Khan, M.; Almakadma, A.H.; Almustanyir, S. A Case Report of a Candida auris Infection in Saudi Arabia. Cureus 2021, 13, 15240. [Google Scholar] [CrossRef]
- Almeida, J.N.; Francisco, E.C.; Hagen, F.; Brandão, I.B.; Pereira, F.M.; Presta Dias, P.H.; de Miranda Costa, M.M.; de Souza Jordão, R.T.; de Groot, T.; Colombo, A.L. Emergence of Candida auris in Brazil in a COVID-19 intensive care unit. J. Fungi 2021, 7, 220. [Google Scholar] [CrossRef]
- Invasive Candidiasis|Candidiasis|Types of Fungal Diseases|Fungal Diseases|CDC [Internet]. Available online: https://www.cdc.gov/fungal/diseases/candidiasis/invasive/index.html (accessed on 12 July 2021).
- Candida Auris: Symptoms, Causes, Diagnosis, Treatment. Available online: https://www.verywellhealth.com/candida-auris-4692475 (accessed on 12 July 2021).
- Breazzano, M.P.; Tooley, A.A.; Godfrey, K.J.; Iacob, C.E.; Yannuzzi, N.A.; Flynn, H.W. Candida auris and endogenous panophthalmitis: Clinical and histopathological features. Am. J. Ophthalmol. Case Rep. 2020, 19, 100738. [Google Scholar] [CrossRef]
- Shenoy, V.; Ballenberger, M.; Prince, A.; Maslak, S. Panophthalmitis from Candida auris. Ann. Intern. Med. 2019, 171, 941–943. [Google Scholar] [CrossRef]
- Supreeth, S.; Al Ghafri, K.A.; Jayachandra, R.K.; Al Balushi, Z.Y. First Report of Candida auris Spondylodiscitis in Oman: A Rare Presentation. World Neurosurg. 2020, 135, 335–338. [Google Scholar] [CrossRef]
- Choi, H.I.; An, J.; Hwang, J.J.; Moon, S.Y.; Son, J.S. Otomastoiditis caused by Candida auris: Case report and literature review. Mycoses 2017, 60, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Dewaele, K.; Frans, J.; Smismans, A.; Ho, E.; Tollens, T.; Lagrou, K. First case of Candida auris infection in Belgium in a surgical patient from Kuwait. Acta Clin. Belg. 2018, 73, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Sarma, S.; Upadhyay, S. Current perspective on emergence, diagnosis and drug resistance in Candida auris. Infect. Drug Resist. 2017, 10, 155. [Google Scholar] [CrossRef] [PubMed]
- Procedure for collection of patient swabs for Candida auris. Available online: https://www.cdc.gov/fungal/candida-auris/c-auris-patient-swab.html (accessed on 12 July 2021).
- Arastehfar, A.; Carvalho, A.; Nguyen, M.H.; Hedayati, M.T.; Netea, M.G.; Perlin, D.S.; Hoenigl, M. COVID-19-associated candidiasis (C.A.C.): An underestimated complication in the absence of immunological predispositions? J. Fungi 2020, 6, 211. [Google Scholar] [CrossRef] [PubMed]
- Osei Sekyere, J. Candida auris: A systematic review and meta-analysis of current updates on an emerging multidrug-resistant pathogen. Microbiologyopen 2018, 7, e00578. [Google Scholar] [CrossRef]
- Identification of Candida auris|Candida auris|Fungal Diseases|CDC [Internet]. Available online: https://www.cdc.gov/fungal/candida-auris/identification.html (accessed on 12 July 2021).
- Fasciana, T.; Cortegiani, A.; Ippolito, M.; Giarratano, A.; Di Quattro, O.; Lipari, D.; Graceffa, D.; Giammanco, A. Candida auris: An overview of how to screen, detect, test and control this emerging pathogen. Antibiotics 2020, 9, 778. [Google Scholar] [CrossRef]
- Rudramurthy, S.M.; Chakrabarti, A.; Paul, R.A.; Sood, P.; Kaur, H.; Capoor, M.R.; Kindo, A.J.; Marak, R.S.; Arora, A.; Sardana, R.; et al. Candida auris candidaemia in Indian I.C.U.s: Analysis of risk factors. J. Antimicrob. Chemother. 2017, 72, 1794–1801. [Google Scholar] [CrossRef]
- Lai, C.C.; Wang, C.Y.; Hsueh, P.R. Co-infections among patients with COVID-19: The need for combination therapy with non-anti-SARS-CoV-2 agents? J. Microbiol. Immunol. Infect. 2020, 53, 505–512. [Google Scholar] [CrossRef]
- Zuo, T.; Zhan, H.; Zhang, F.; Liu, Q.; Tso, E.Y.; Lui, G.C.; Chen, N.; Li, A.; Lu, W.; Chan, F.K.; et al. Alterations in fecal fungal microbiome of patients with COVID-19 during time of hospitalization until discharge. Gastroenterology 2020, 159, 1302–1310. [Google Scholar] [CrossRef]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2015, 62, e1–e50. [Google Scholar] [CrossRef] [Green Version]
- Treatment and Management of Infections and Colonization|Candida auris|Fungal Diseases|CDC, Cdc.gov. 2021. Available online: https://www.cdc.gov/fungal/candida-Auris/c-Auris-treatment.html (accessed on 27 August 2021).
- Ademe, M.; Girma, F. Candida auris: From Multidrug Resistance to Pan-Resistant Strains. Infect. Drug Resist. 2020, 13, 1287–1294. [Google Scholar] [CrossRef] [PubMed]
- Magnasco, L.; Mikulska, M.; Giacobbe, D.; Taramasso, L.; Vena, A.; Dentone, C.; Dettori, S.; Tutino, S.; Labate, L.; Di Pilato, V.; et al. Spread of Carbapenem-Resistant Gram-Negatives and Candida auris during the COVID-19 Pandemic in Critically Ill Patients: One Step Back in Antimicrobial Stewardship? Microorganisms 2021, 9, 95. [Google Scholar] [CrossRef] [PubMed]
- Pristov, K.; Ghannoum, M. Resistance of Candida to azoles and echinocandins worldwide. Clin. Microbiol. Infect. 2019, 25, 792–798. [Google Scholar] [CrossRef]
- Chow, N.A.; Muñoz, J.F.; Gade, L.; Berkow, E.L.; Li, X.; Welsh, R.M.; Forsberg, K.; Lockhart, S.R.; Adam, R.; Alanio, A.; et al. Tracing the Evolutionary History and Global Expansion of Candida auris Using Population Genomic Analyses. mBio 2020, 11, e03364-19. [Google Scholar] [CrossRef] [PubMed]
- Gebremariam, T.; Alkhazraji, S.; Alqarihi, A.; Jeon, H.H.; Gu, Y.; Kapoor, M.; Shaw, K.J.; Ibrahim, A.S. APX001 Is Effective in the Treatment of Murine Invasive Pulmonary Aspergillosis. Antimicrob. Agents Chemother. 2019, 63, e01715-18. [Google Scholar] [CrossRef]
- Wring, S.; Borroto-Esoda, K.; Solon, E.; Angulo, D. SCY-078, a Novel Fungicidal Agent, Demonstrates Distribution to Tissues Associated with Fungal Infections during Mass Balance Studies with Intravenous and Oral [14C] SCY-078 in Albino and Pigmented Rats. Antimicrob. Agents Chemother. 2019, 63, e02119-18. [Google Scholar] [CrossRef]
- Ham, Y.; Lewis, J.; Thompson, G. Rezafungin: A novel antifungal for the treatment of invasive candidiasis. Future Microbiol. 2021, 16, 27–36. [Google Scholar] [CrossRef]
- Infection Prevention and Control for Candida auris|Candida auris|Fungal Diseases|CDC, Cdc.gov. 2021. Available online: https://www.cdc.gov/fungal/candida-Auris/c-Auris-infection-control.html (accessed on 27 August 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).