Heat Transfer Study in Breast Tumor Phantom during Microwave Ablation: Modeling and Experimental Results for Three Different Antennas
Abstract
1. Introduction
2. Materials and Methods
2.1. Antenna Design
2.2. Computational Model
2.3. Phantom Preparation
2.4. Experimental Measurements
3. Results
3.1. FEM Thermal Distributions Results
3.2. Experimental Results: SWR Measurements.
3.3. Temperature Profiles
4. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ren, J.S.; Masuyer, E.; Ferlay, J. Global estimates of cancer prevalence for 27 sites in the adult population. Int. J. Cancer 2013, 132, 1133–1145. [Google Scholar] [CrossRef] [PubMed]
- Hung, W.K.; Ying, M.; Chan, C.M.; Lam, H.S.; Mak, L. Minimally invasive technology in the management of breast disease. Breast Cancer 2009, 16, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Pathak, S.; Jones, R.; Tang, J.M.F.; Parmar, C.; Fenwick, S.; Malik, H.; Poston, G. Ablative therapies for colorectal liver metastases: A systematic review. Colorectal Dis. 2011, 13, e252–e265. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.V.; Nagy, N.N. Naguib; Thomas, L.; Nour-Eldin, A.N.-E. Radiofrequency, microwave, and laser ablation of pulmonary neoplasms: Clinical studies and technical considerations. Eur. J. Radiol. 2009, 15, 290–296. [Google Scholar]
- Vogel, T.J.; Farshid, P.; Naguib, N.N.; Zangos, S. Thermal ablation therapies in patients with breast cancer liver metastases: A review. Eur. Radiol. 2013, 23, 797–804. [Google Scholar] [CrossRef]
- Carrafiello, G.; Fontana, F.; Cotta, E.; Petullà, M.; Brunese, L.; Mangini, M.; Fugazzola, C. Ultrasound-guided thermal radiofrequency ablation (RFA) as an adjunct to systemic chemotherapy for breast cancer liver metastases. Radiol. Med. 2011, 116, 1059–1066. [Google Scholar] [CrossRef]
- Simon, C.J.; Dupuy, D.E.; Mayo-Smith, W.W. Microwave ablation: Principles and applications. Radio Graph. 2005, 25, S69–S83. [Google Scholar] [CrossRef]
- Safarik, I.; Safarikova, M. Magnetic nanoparticles in biosciences. Monatschefte Chem. 2002, 133, 737–759. [Google Scholar]
- Saiyed, Z.M.; Telang, S.D.; Ramchand, C.N. Application of magnetic techniques in the field of drug discovery and biomedicine. BioMagn. Res. Technol. 2003, 1, 2. [Google Scholar] [CrossRef]
- Diederich, C.J. Thermal ablation and high-temperature thermal therapy: Overview of technology and clinical implementation. Int. J. Hyperth. 2005, 21, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Garrean, S.; Hering, J.; Saied, A.; Hoopes, P.J.; Helton, W.S.; Ryan, T.P.; Espat, N.J. Ultrasound monitoring of a novel microwave ablation MWA) device in porcine liver: Lessons learned and phenomena observed on ablative effects near major intrahepatic vessels. J. Gastrointest. Surg. 2009, 13, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Keangin, P.; Rattanadecho, P.; Wessapan, T. An analysis of heat transfer in liver tissue during microwave ablation using single and double slot antenna. Int. Commun. Heat Mass Transf. 2011, 38, 757–766. [Google Scholar] [CrossRef]
- Correa-Gallego, C.; Fong, Y.; Gonen, M.; D’Angelica, M.I.; Allen, P.J.; DeMatteo, R.P.; Jarnagin, W.R.; Kingham, T.P. A Retrospective Comparison of microwave Ablation vs. Radiofrequency Ablation for Colorectal Cancer Hepatic Metastases. Ann. Surg. Oncol. 2014, 21, 4278–4283. [Google Scholar] [CrossRef]
- Brace, C.L. Radiofrequency and microwave ablation of the liver, lung, kidney, and bone: What are the differences? Curr. Probl. Diagn. Radiol. 2009, 38, 135–143. [Google Scholar] [CrossRef]
- Laeseke, P.F.; Lee, F.T.; Sampson, L.A.; Van der Weide, D.W.; Brace, C.L. Microwave Ablation versus Radiofrequency Ablation in the kidney. J. Vasc. Interv. Radiol. 2009, 20, 1224–1229. [Google Scholar] [CrossRef]
- Martin, R.C.; Scoggins, C.R.; McMasters, K.M. Safety and efficacy of microwave ablation of hepatic tumors: A prospective review of a 5-year experience. Ann. Surg. Oncol. 2010, 17, 171–178. [Google Scholar] [CrossRef]
- Brace, C.L.; Hinshaw, J.L.; Laeseke, P.F.; Sampson, L.A.; Lee, F.T. Pulmonary thermal ablation: Comparison of radiofrequency and microwave devices by using gross pathologic and CT findings in a swine model. Radiology 2009, 251, 705–711. [Google Scholar] [CrossRef]
- Cavagnaro, M.; Amabile, C.; Bernardi, P.; Pisa, S.; Tosoratti, N. A minimally invasive antenna for microwave ablation therapies: Design, performances, and experimental assessment. IEEE Trans. Biomed. Eng. 2011, 58, 949–959. [Google Scholar] [CrossRef]
- Brace, C.L. Dual-slot antennas for microwave tissue heating: Parametric design analysis and experimental validation. Med. Phys. 2011, 38, 4232–4240. [Google Scholar] [CrossRef]
- Cavagnaro, M.; Amabile, C.; Bernardi, P.; Pisa, S.; Tosoratti, N. Design and realization of a new type of interstitial antenna for ablation therapies. In Proceedings of the IEEE 39th 2009 European Microwave Conference (EuMC), Rome, Italy, 29 September–1 October 2009; pp. 878–881. [Google Scholar]
- Bertram, J.M.; Yang, D.; Converse, M.C.; Webster, J.; Mahvi, D. Antenna design for microwave hepatic ablation using an axisymmetric electromagnetic model. BioMed. Eng. OnLine 2006, 5, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Yoshimura, H.; Ito, K.; Aoyagi, Y.; Horita, H. Clinical trials of interstitial microwave hyperthermia by use of coaxial slot antenna with two slots. IEEE Trans. Microw. Theory Tech. 2004, 52, 1987–1991. [Google Scholar] [CrossRef]
- Bernardi, P.; Cavagnaro, M.; Lin, J.C.; Pisa, S.; Piuzzi, E. Distribution of SAR and temperature elevation induced in a phantom by a microwave cardiac ablation catheter. IEEE Trans. Microw. Theory Tech. 2004, 52, 1978–1986. [Google Scholar] [CrossRef]
- Zhang, H.; Nan, Q.; Liu, Y. SAR distribution of microwave antenna for atrial fibrillation catheter ablation. In Proceedings of the 5th International Conference on Biomedical Engineering and Informatics (BMEI), Chongqing, China, 16–18 October 2012; pp. 664–667. [Google Scholar]
- Chaichanyut, M. Microwave ablation with cap-choke antenna: Result in computer simulation. In Proceedings of the ECTI International Conference on Electrical Engineering/Electronics, Computer, Telecommunications and Information Technology ECTI-CON2010, Chiang Mai, Thailand, 19–21 May 2010; pp. 679–682. [Google Scholar]
- Cepeda, M.F.; Vera, A.; Leija, L. Coaxial antenna for microwave coagulation therapy in ex vivo swine breast tissue. In Proceedings of the 7th International Conference on Electrical Engineering Computing Science and Automatic Control (CCE), Tuxtla Gutierrez, Mexico, 8–10 September 2010; pp. 268–273. [Google Scholar]
- Ortega-Palacios, R.; Vera, A.; Leija, L. Microwave Ablation Coaxial Antenna Computational Model Slot antenna comparison. In Proceedings of the 2012 Pan American Health Care Exchanges (PAHCE), Miami, FL, USA, 26–31 March 2012; pp. 112–115. [Google Scholar]
- Lopez Luna, J.F. Optimización de la Energia Entregada por un Aplicador de Doble Ranura Microaxial Para Tratamiento de Cancer de Mama Mediante Ablacion por Microondas Modelado FEM, Validacion en Phantom y Experimentacion In vitro e In vivo. Master’s Thesis, Cinvestav, Mexico City, Mexico, 2015. [Google Scholar]
- Lara, J.E.; Vera, A.; Leija, L. Proposal for the application of microwave ablation as a treatment for breast cancer using interstitial applicators: Antenna design and FEM modeling. In Proceedings of the 2016 Global Medical Engineering Physics Exchanges/Pan American Health Care Exchanges (GMEPE/PAHCE), Madrid, Spain, 4–9 April 2016; pp. 1–6. [Google Scholar]
- Manzanárez, A.; Lara, J.E.; Vera, A.; Leija, L.; Gutiérrez, M.I. Influence of the surrounding tissues in the radiation pattern of microcoaxial antenna for the treatment of breast tumors. In Proceedings of the 13th International Conference on Electrical Engineering, Computing Science and Automatic Control (CCE), Mexico City, Mexico, 26–30 September 2016; pp. 1–6. [Google Scholar]
- Ortega-Palacios, R.; Trujillo-Romero, C.J.; Cepeda Rubio, M.F.J.; Vera, A.; Leija, L.; Reyes, J.L.; Ramírez-Estudillo, M.C.; Morales-Alvarez, F.; Vega-López, M.A. Feasibility of Using a Novel 2.45GHz Double Short Distance Slot Coaxial Antenna for Minimally Invasive Cancer Breast Microwave Ablation Therapy: Computational Model, Phantom, and In Vivo Swine Experimentation. J. Healthc. Eng. 2018. [Google Scholar] [CrossRef]
- Nan, Q.; Guo, X.M.; Zhai, F. The Contrast of Two Kinds of Microwave Antenna SAR Simulation. Appl. Mech. Mater. 2014, 553, 379–383. [Google Scholar] [CrossRef]
- Cepeda Rubio, M.F.J.; Vera, A.; Leija Salas, L. Applicator Design for Microwave Ablation in Breast Cancer. In Proceedings of the International Microwave Proceedings of 43rd Annual Microwave Symposium, Washington, DC, USA, 8–10 July 2009; pp. 54–59. [Google Scholar]
- Cepeda Rubio, M.F.J.; Vera, A.; Leija Salas, L.; Ávila-Navarro, E.; Navarro, E.A. Coaxial Slot Antenna Design for Microwave Hyperthermia using Finite-Difference Time-Domain and Finite Element Method. Open Nanomed. J. 2011, 3, 2–9. [Google Scholar] [CrossRef]
- Navarro, E.A.; Wu, C.; Chung, P.Y.; Litva, J. Some considerations about the FDTD method in General Curvilinear Coordinates. IEEE Microw. Guided Wave Lett. 1994, 7, 396–398. [Google Scholar] [CrossRef]
- Reig, C.; Navarro, E.A.; Such, V. Calculation of the characteristic impedance of microstrips using a full-wave 2-D FDTD scheme. Microw. Opt. Technol. Lett. 1997, 16, 58–60. [Google Scholar] [CrossRef]
- Reig, C.; Navarro, E.A.; Such, V. Full-wave FDTD design and analysis of wideband microstrip-to-waveguide transitions. Microw. Opt. Technol. Lett. 2003, 38, 317–320. [Google Scholar] [CrossRef]
- Blanchard, C.; Portí, J.A.; Morente, J.A.; Salinas, A.; Navarro, E.A. Determination of the effective permittivity of dielectric mixtures with the transmission line matrix method. J. Appl. Phys. 2007, 102, 064101. [Google Scholar] [CrossRef]
- Sysoev, S.; Kislitsyn, A. Modeling of Microwave Heating and Oil Filtration in Stratum. In Numerical Simulations—Applications, Examples and Theory; Angermann, L., Ed.; InTech: London, UK, 2011; ISBN 978-953-307-440-5. [Google Scholar]
- Jin, J.-M.; Riley, D.J. Finite Element Analysis of Antennas and Arrays; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009. [Google Scholar]
- Jiao, T.; Wang, H.; Zhang, Y.; Yu, X.; Xue, H.; Lv, H.; Wang, J. A coaxial-slot antenna for invasive microwave hyperthermia therapy. J. Biomed. Sci. Eng. 2012, 5, 198–202. [Google Scholar] [CrossRef][Green Version]
- Gabriel, C. Compilation of the Dielectric Properties of Body Tissues at Rf and Microwave Frequencies; Technical Report No. AL/OETR-1996-0037; Brooks Air Force Base: San Antonio, TX, USA, 1996. [Google Scholar]
- García-Jimeno, S.; Ortega-Palacios, R.; Cepeda-Rubio, M.; Vera, A.; Leija, L.; Estelrich, J. Improved thermal ablation efficacy using magnetic nanoparticles: A study in tumor phantoms. Prog. Electromagn. Res. 2012, 128, 229–248. [Google Scholar] [CrossRef]
- Trujillo-Romero, C.J.; Leija, L.; Vera, A. FEM modeling for performance evaluation of an electromagnetic oncology deep hyperthermia applicator when using monopole, inverted T, and plate antennas. Prog. Electromagn. Res. 2011, 120, 99–125. [Google Scholar] [CrossRef][Green Version]
- Trujillo-Romero, C.J.; García-Jimeno, S.; Vera, A.; Leija, L.; Estelrich, J. Using nanoparticles for enhancing the focusing heating effect of an external waveguide applicator for oncology hyperthermia: Evaluation in muscle and tumor phantoms. Prog. Electromagn. Res. 2011, 121, 343363. [Google Scholar] [CrossRef]
- Bronzino, J.D. Medical Devices and Systems; The Biomedical Engineering Handbook; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Kapranov, S.; Kouzaev, G. Study of microwave heating of reference liquids in a coaxial waveguide reactor using the experimental, semi-analytical and numerical means. Int. J. Therm. Sci. 2019, 140, 505. [Google Scholar] [CrossRef]
- Pennes, H.H. Analysis of skin, muscle and brachial arterial blood temperatures in the resting normal human forearm. Am. J. Med. Sci. 1948, 215, 354. [Google Scholar]
- Ekstrand, V.; Wiksell, H.; Schultz, I.; Sandstedt, B.; Rotstein, S.; Eriksson, A. Influence of electrical and thermal properties on RF ablation of breast cancer: Is the tumour preferentially heated? BioMed. Eng. OnLine 2005, 4, 41. [Google Scholar] [CrossRef]
- Hernández, A.V.; Quero, J.E.C.; Salas, L.L.; Mier, Y.H.; Marchal, C. Hipertermia electromagnética, una alternativa para el tratamiento del cáncer: Antecedentes, aspectos físicos y biológicos. Rev. Mex. Ing. Bioméd. 2011, XXII, 78–88. [Google Scholar]
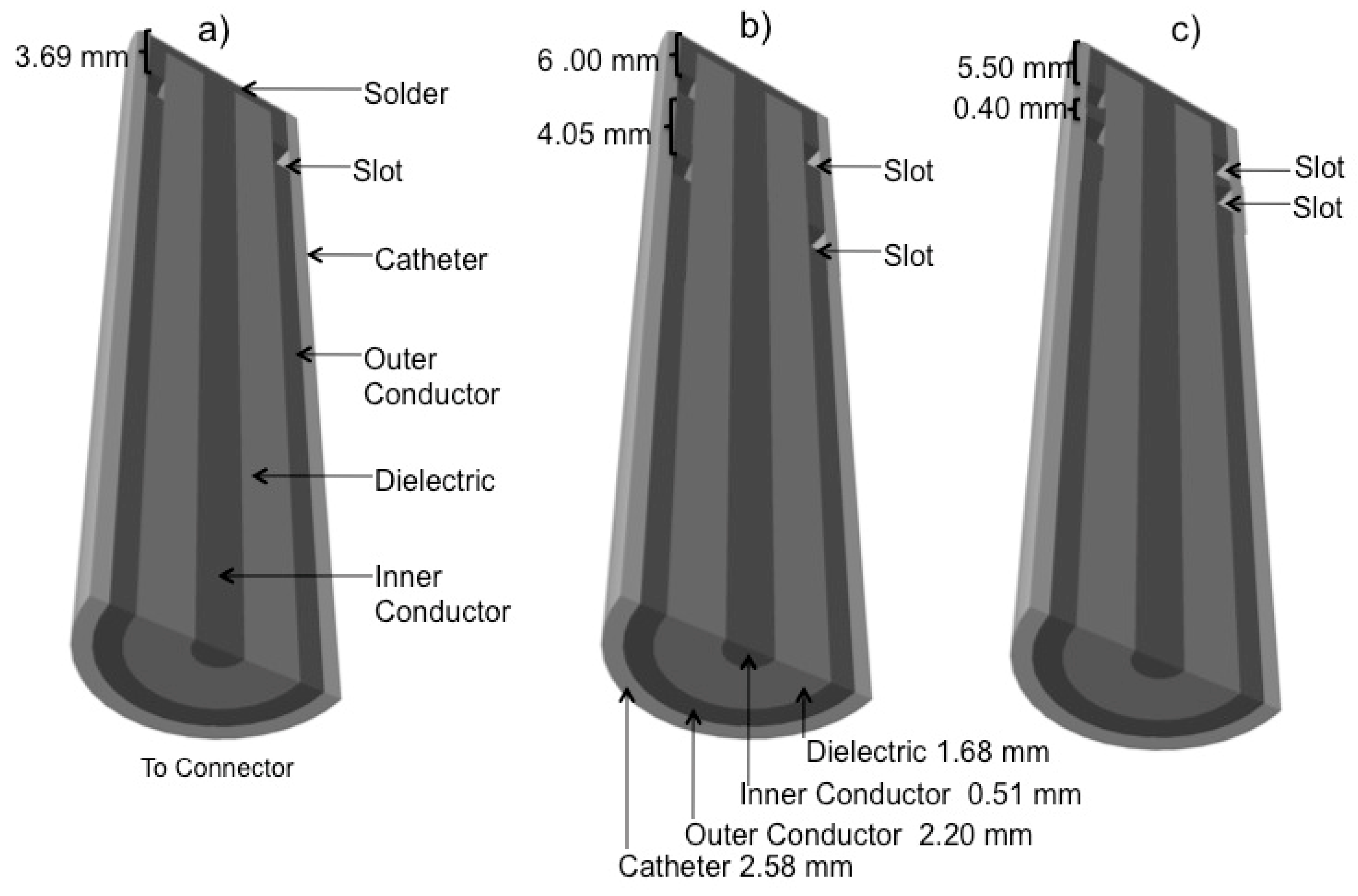
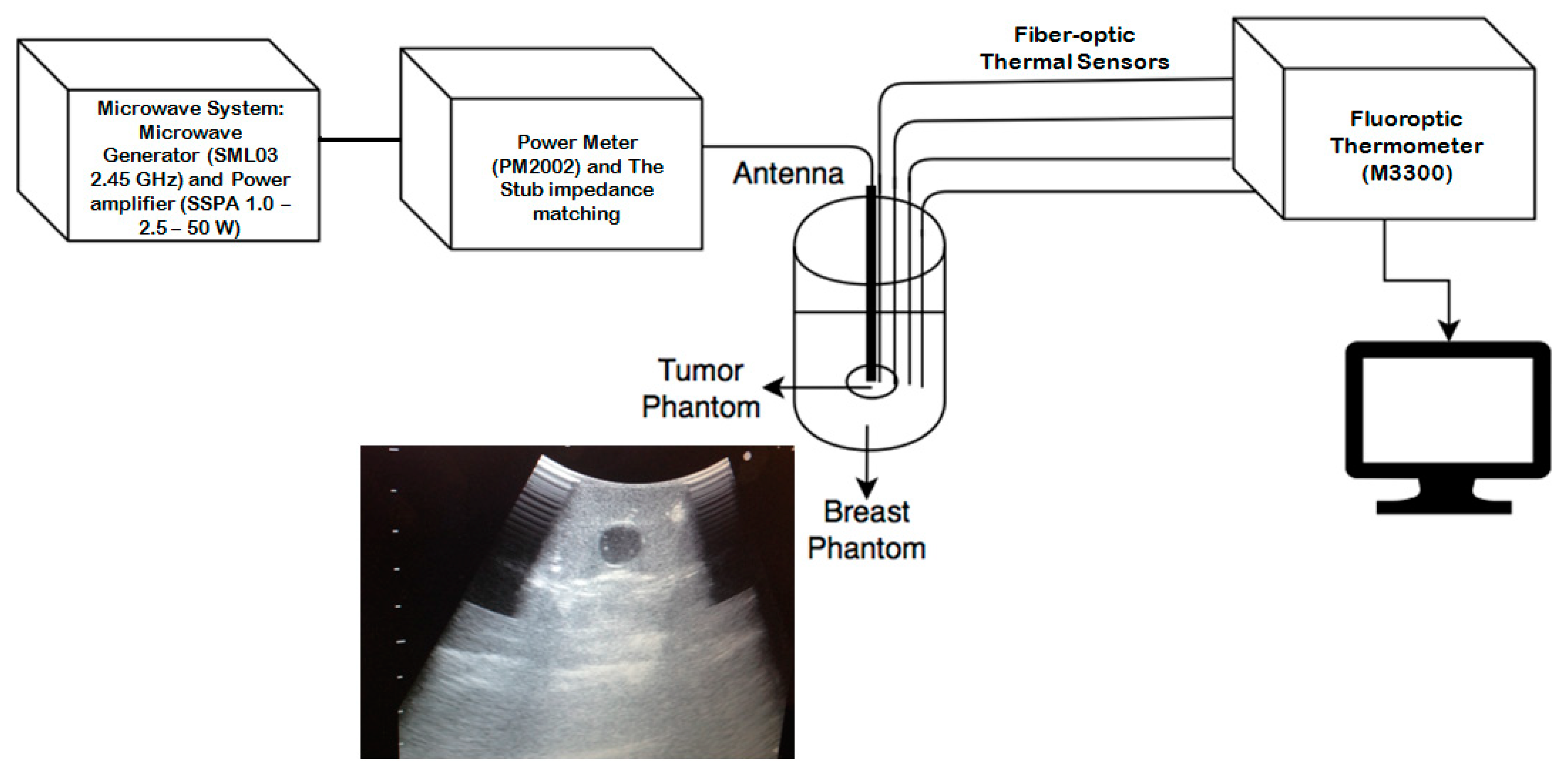
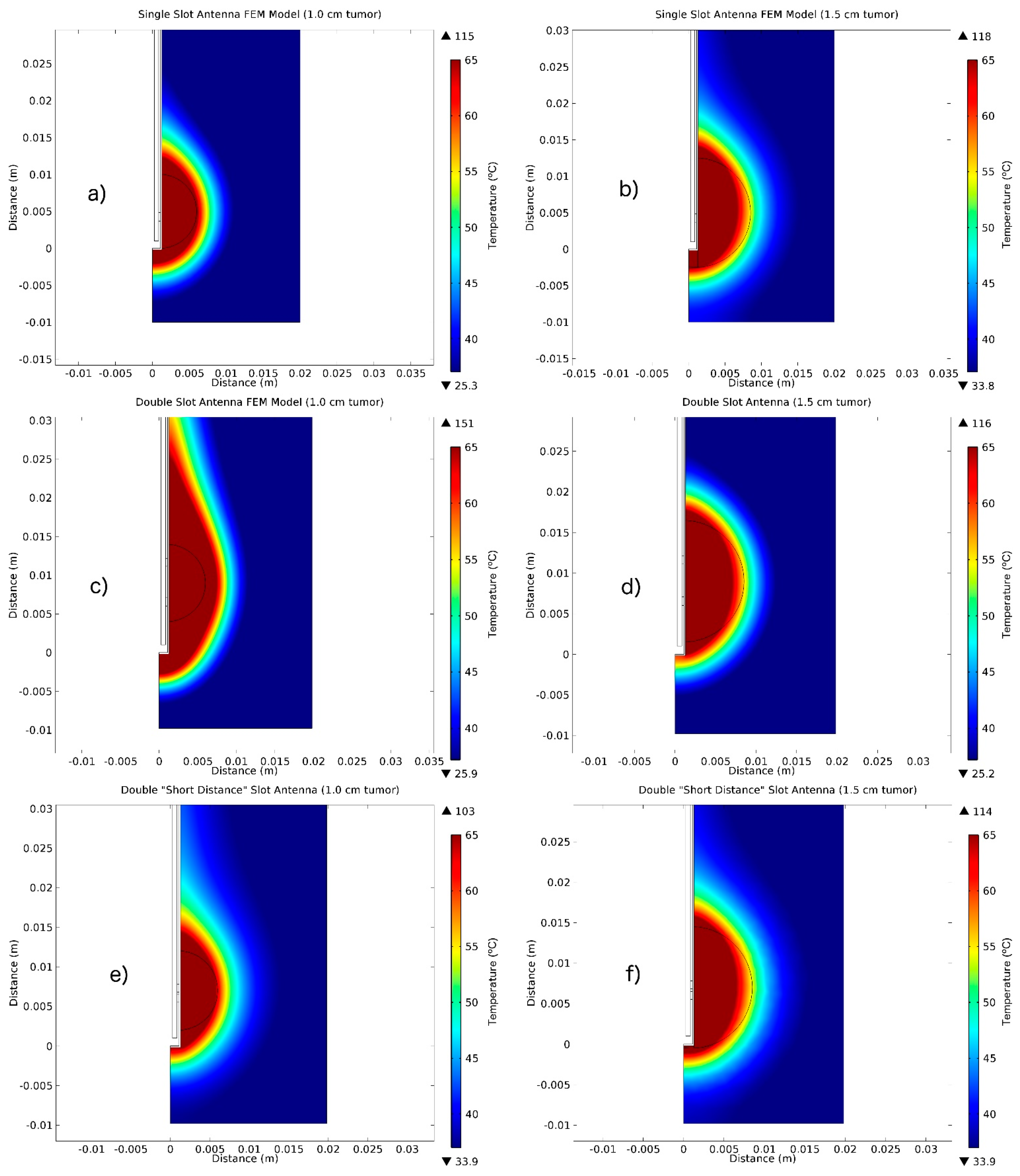
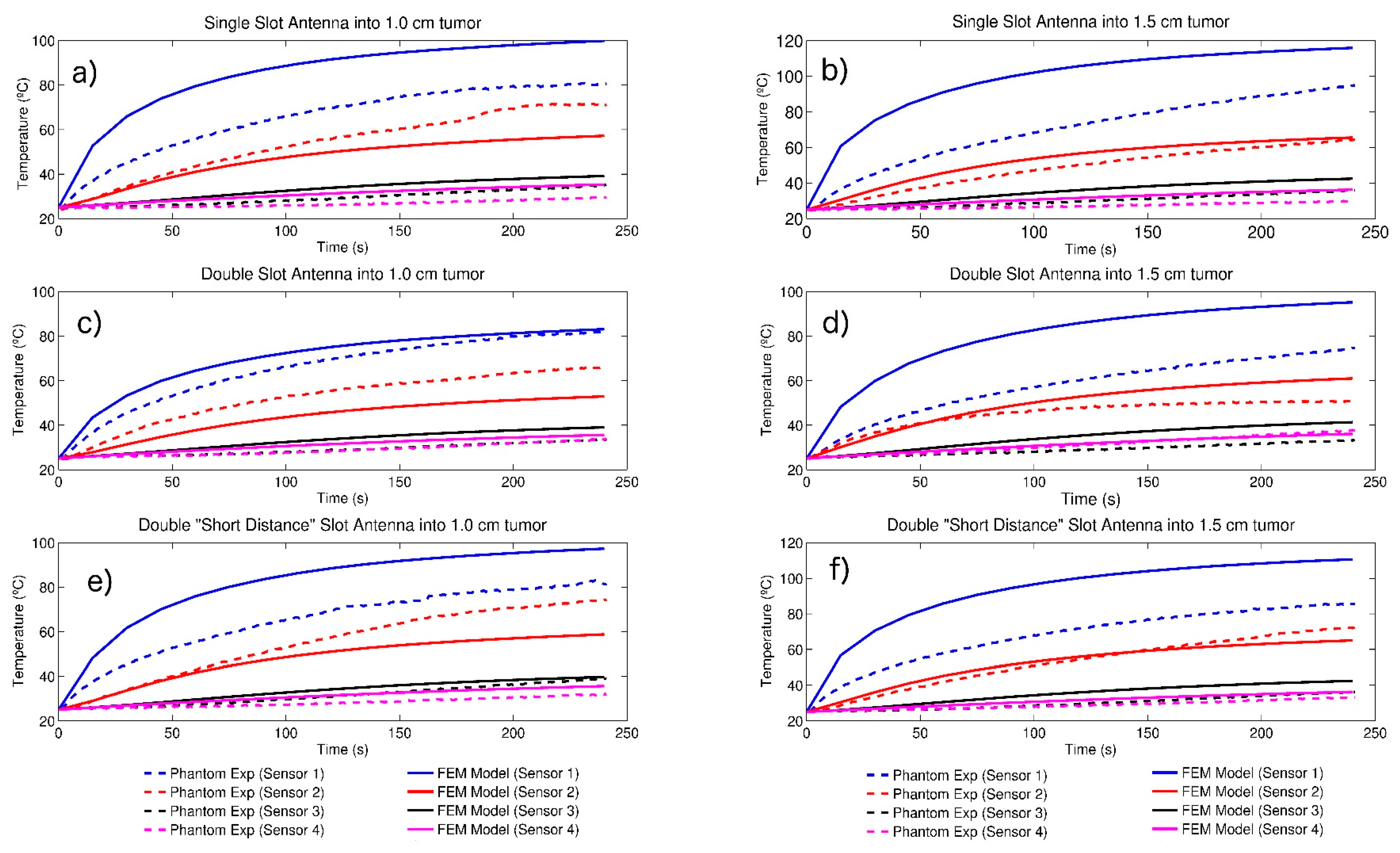
| Parameter | Value |
|---|---|
| Breast Electric Conductivity | 0.14 S/m |
| Breast Relative Permittivity | 5.14 |
| Breast Thermal Conductivity | 0.42 W/Mk |
| Breast Density | 1020.00 Kg/m3 |
| Tumor Electric Conductivity | 3.00 S/m |
| Tumor Relative Permittivity | 57.00 |
| Tumor Thermal Conductivity | 0.50 W/mK |
| Tumor Density | 1041.00 Kg/m3 |
| Blood Density | 1000 Kg/m3 |
| Blood Specific Heat | 3639 J/(Kg K) |
| Blood Perfusion Rate | 0.0036 1/s |
| Blood Temperature | 37 °C |
| Dielectric Relative Permittivity | 2.03 |
| Catheter Relative Permittivity | 2.60 |
| Microwave Frequency | 2.45 GHz |
| Input Microwave Power | 10 W |
| Antenna | SWR |
|---|---|
| Single Slot | 2.89 |
| Double Slot | 1.84 |
| Double “Short Distance” Slot | 1.07 |
| Tumor Dimension | Temperature (°C) at 240 s | ||||||||
| Single Slot Antenna | Computational Model | Phantom Experimentation | |||||||
| T1 | T2 | T3 | T4 | T1 | T2 | T3 | T4 | ||
| 1.0 cm | T ΔT | 99.88 74.88 | 57.20 32.2 | 39.16 14.16 | 35.32 10.32 | 80.89 55.89 | 71.62 46.62 | 35.12 10.12 | 29.72 4.72 |
| 1.5 cm | T ΔT | 115.87 90.87 | 65.55 40.55 | 42.45 17.45 | 36.25 11.25 | 94.87 69.87 | 64.46 39.46 | 35.95 10.95 | 29.95 4.95 |
| Double Slot Antenna | |||||||||
| 1.0 cm | T ΔT | 83.13 58.13 | 52.91 27.91 | 39.03 14.03 | 35.61 10.61 | 81.93 56.93 | 66.14 41.14 | 34.01 9.01 | 33.73 8.73 |
| 1.5 cm | T ΔT | 95.20 70.2 | 61.00 36.00 | 41.36 16.36 | 36.09 11.09 | 74.67 49.67 | 50.93 25.93 | 37.81 12.81 | 33.56 8.56 |
| Double “Short Distance” Slot Antenna | |||||||||
| 1.0 cm | T ΔT | 97.28 72.28 | 58.75 33.75 | 39.78 14.78 | 35.62 10.62 | 83.12 58.12 | 74.38 49.38 | 38.99 13.99 | 32.12 7.12 |
| 1.5 cm | T ΔT | 110.58 85.58 | 65.57 40.57 | 42.52 17.52 | 36.33 11.33 | 85.78 60.78 | 72.50 47.50 | 36.35 11.35 | 33.43 8.43 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega-Palacios, R.; Trujillo-Romero, C.J.; Cepeda-Rubio, M.F.J.; Leija, L.; Vera Hernández, A. Heat Transfer Study in Breast Tumor Phantom during Microwave Ablation: Modeling and Experimental Results for Three Different Antennas. Electronics 2020, 9, 535. https://doi.org/10.3390/electronics9030535
Ortega-Palacios R, Trujillo-Romero CJ, Cepeda-Rubio MFJ, Leija L, Vera Hernández A. Heat Transfer Study in Breast Tumor Phantom during Microwave Ablation: Modeling and Experimental Results for Three Different Antennas. Electronics. 2020; 9(3):535. https://doi.org/10.3390/electronics9030535
Chicago/Turabian StyleOrtega-Palacios, Rocío, Citlalli Jessica Trujillo-Romero, Mario Francisco Jesús Cepeda-Rubio, Lorenzo Leija, and Arturo Vera Hernández. 2020. "Heat Transfer Study in Breast Tumor Phantom during Microwave Ablation: Modeling and Experimental Results for Three Different Antennas" Electronics 9, no. 3: 535. https://doi.org/10.3390/electronics9030535
APA StyleOrtega-Palacios, R., Trujillo-Romero, C. J., Cepeda-Rubio, M. F. J., Leija, L., & Vera Hernández, A. (2020). Heat Transfer Study in Breast Tumor Phantom during Microwave Ablation: Modeling and Experimental Results for Three Different Antennas. Electronics, 9(3), 535. https://doi.org/10.3390/electronics9030535







