Wirelessly-Powered Cage Designs for Supporting Long-Term Experiments on Small Freely Behaving Animals in a Large Experimental Arena
Abstract
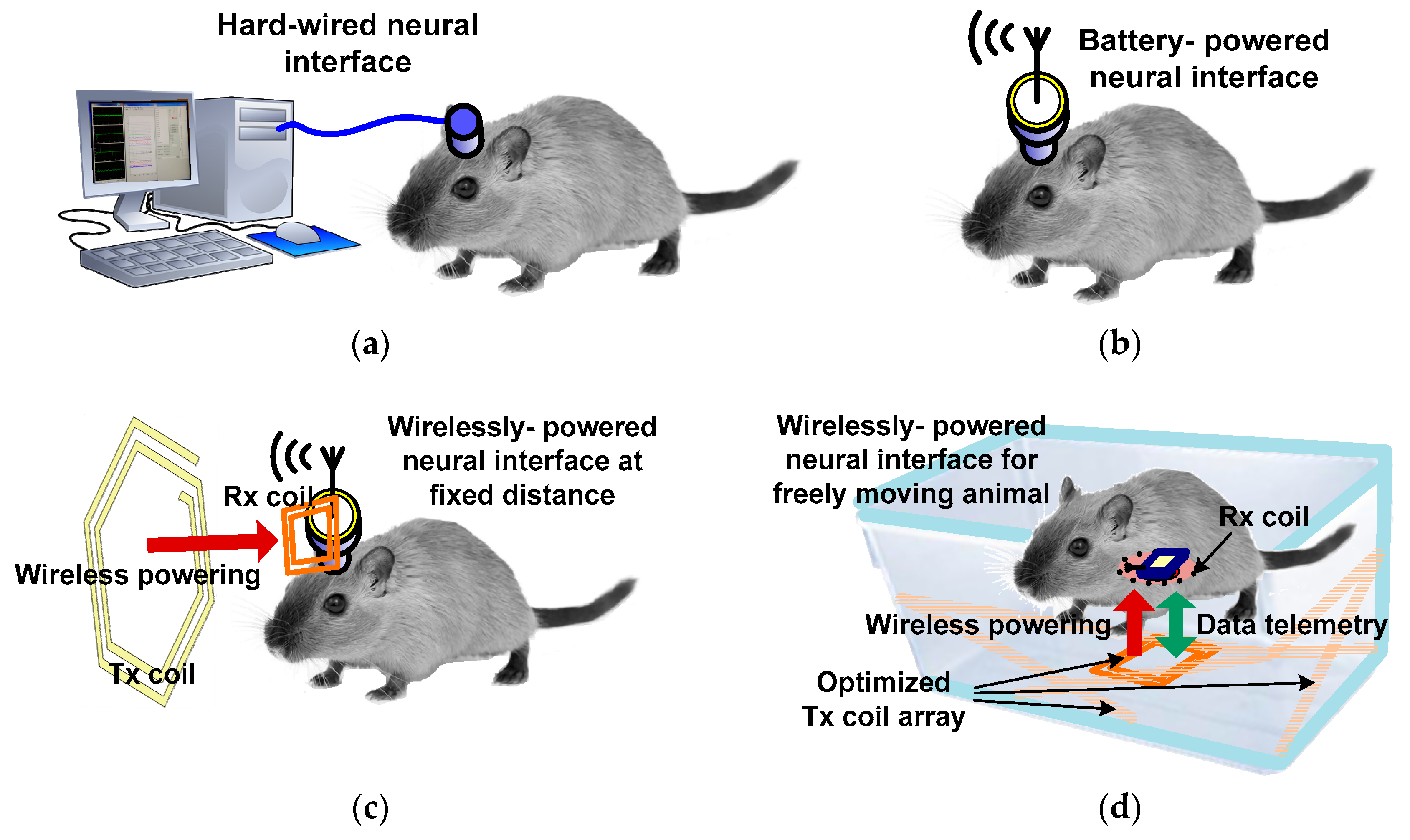
1. Introduction
2. Main Blocks for Wirelessly-Powered Cage System
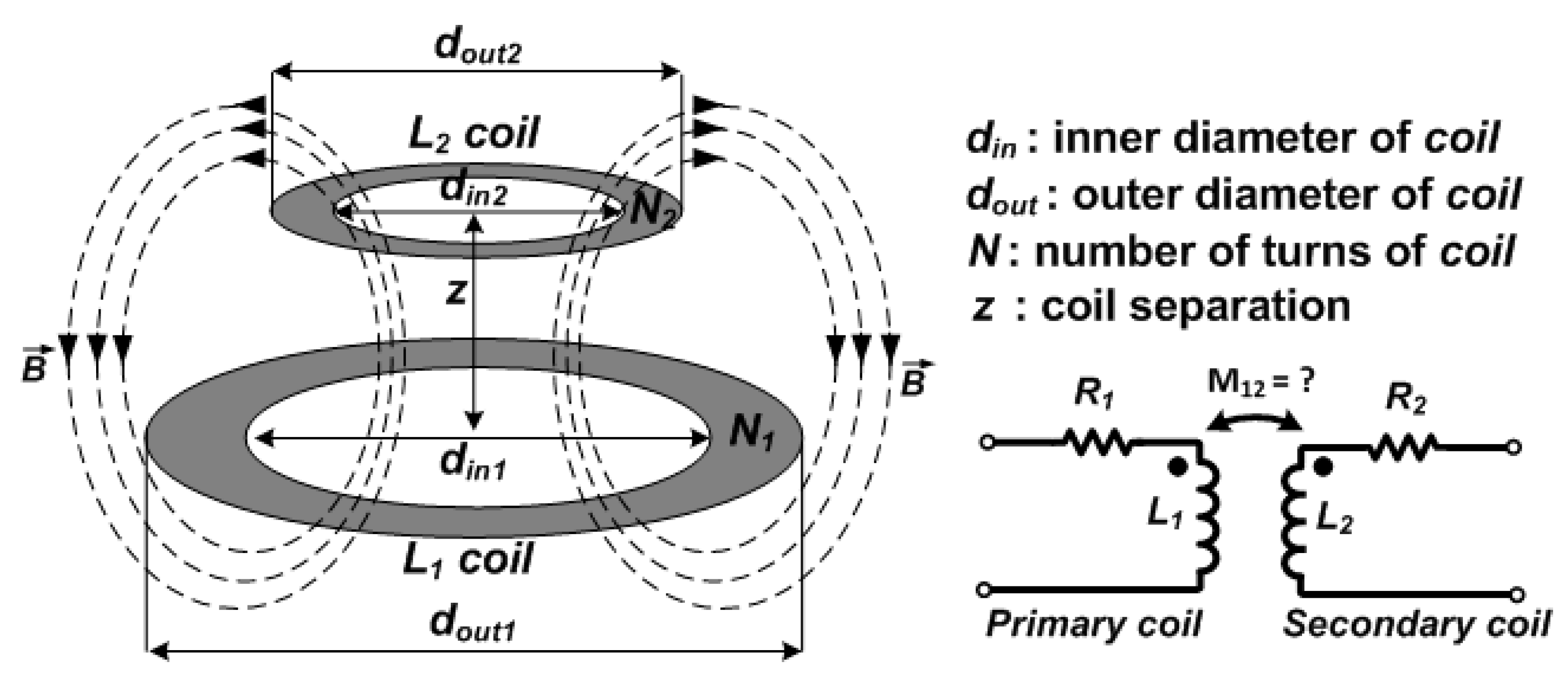
2.1. Coil Design and Optimization
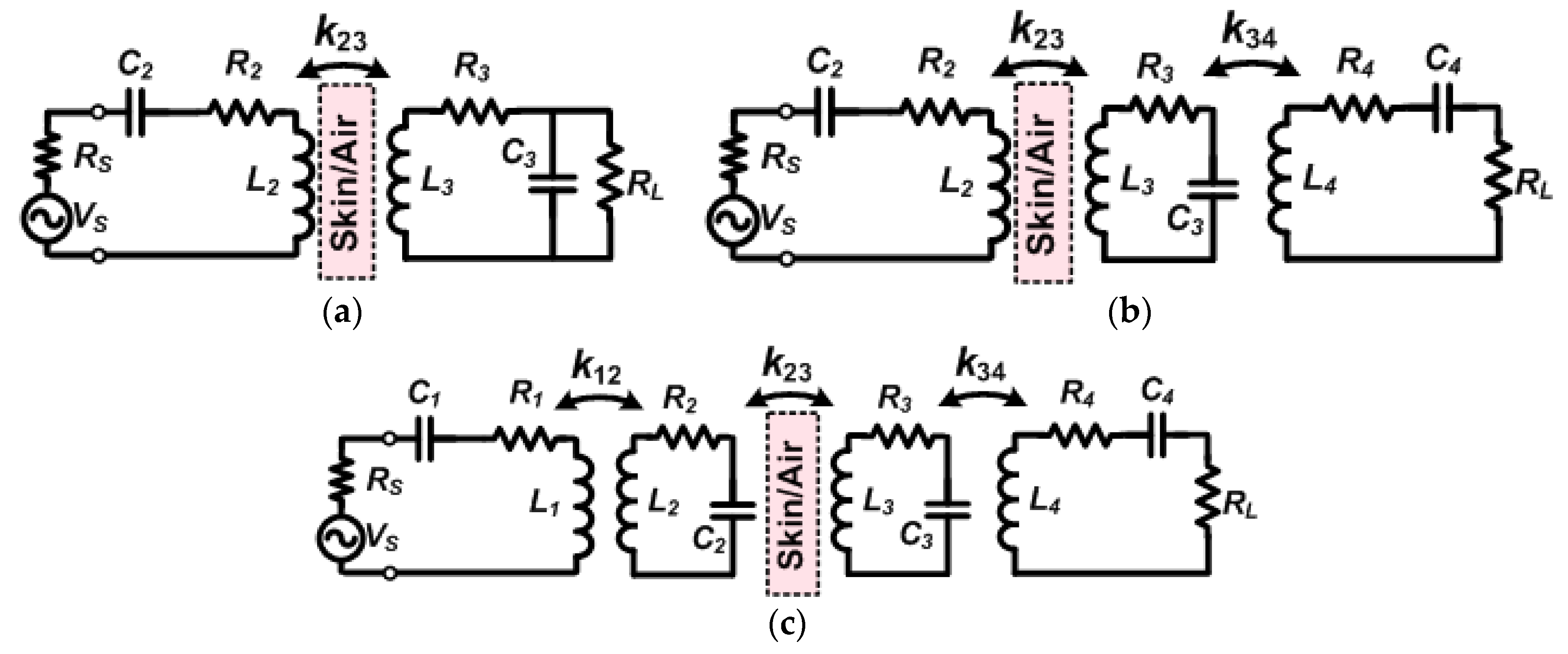
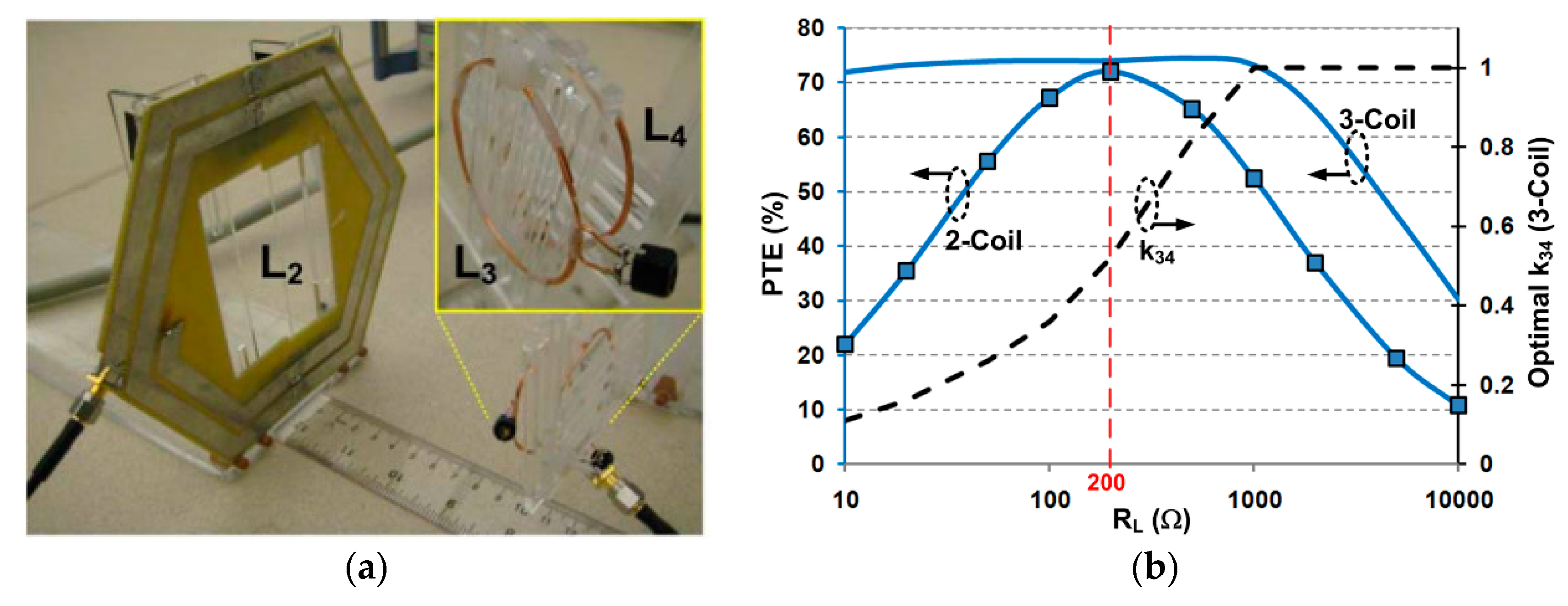
2.1.1. Coil Optimization for Conventional Two-/Three-/Four-Coil Inductive Links
2.1.2. Coil Optimization for Inductive Links Implemented in Wirelessly-Powered Cages
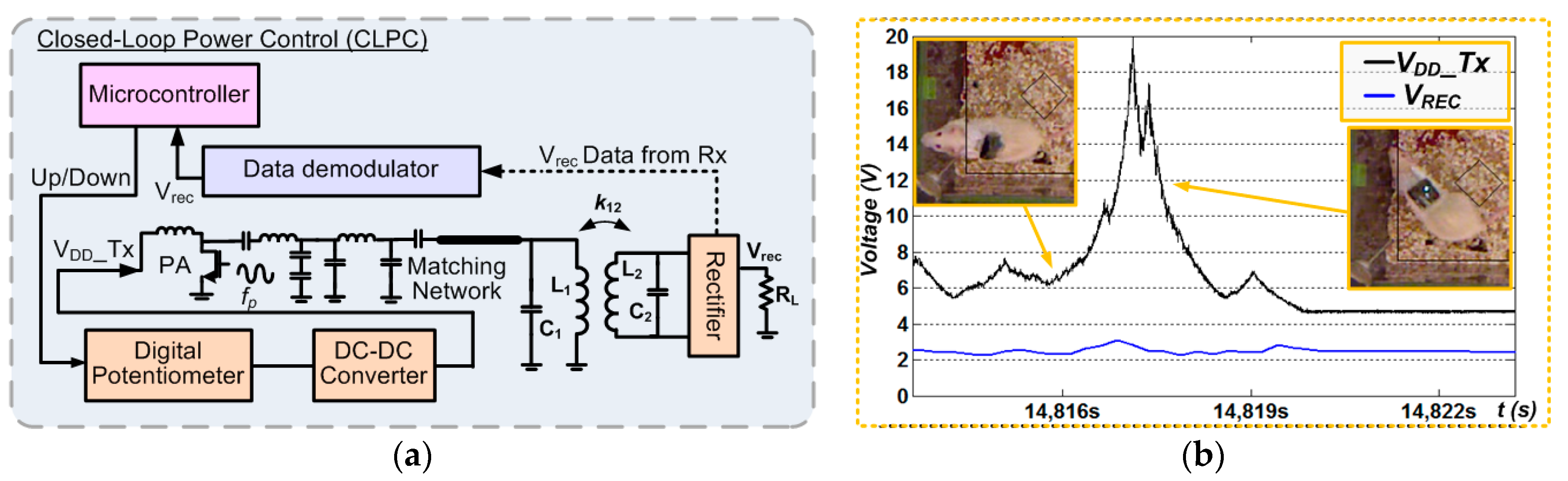
2.2. Closed-Loop Power Control (CLPC)
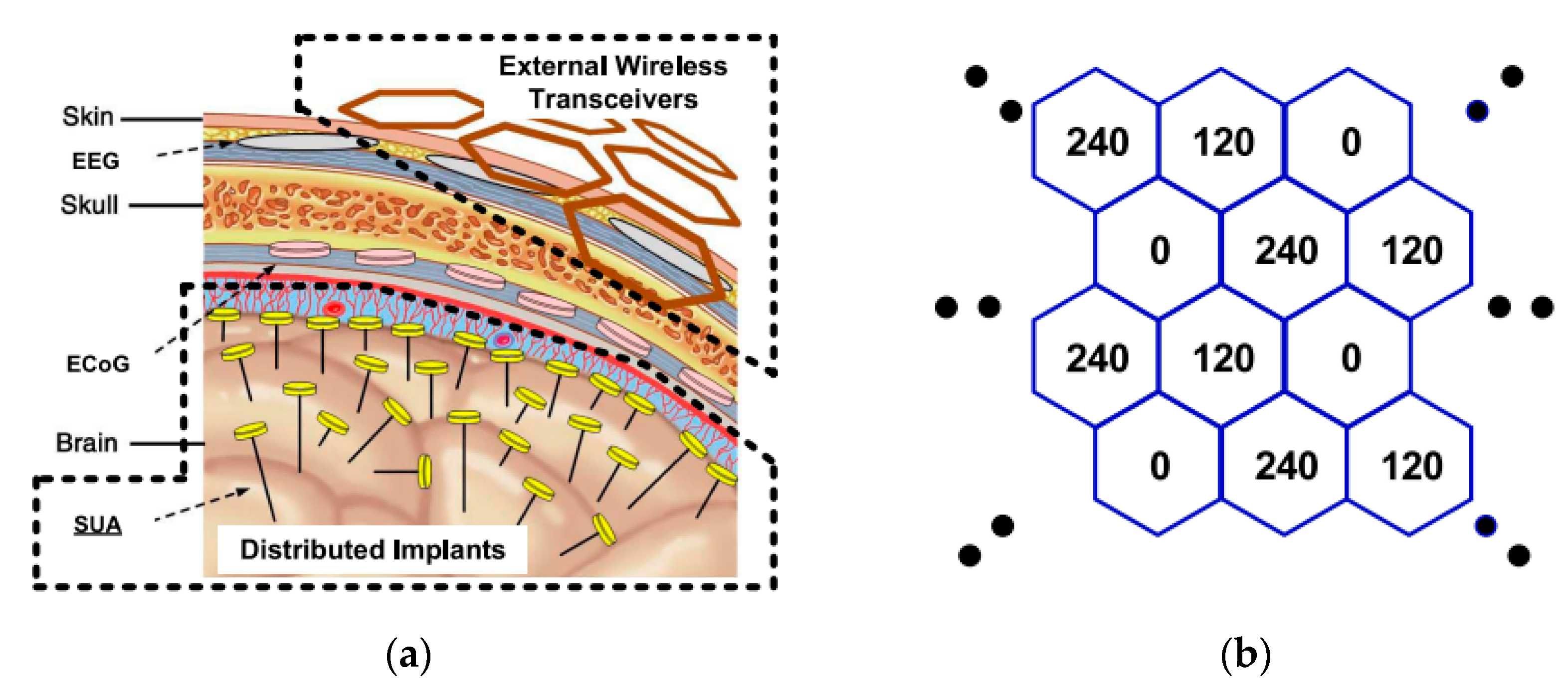
2.3. Scalability for Wireless Coverage
2.4. Spatial and Angular Misalignment between Tx and Rx Coils
2.5. Near-Field Data Telemetry
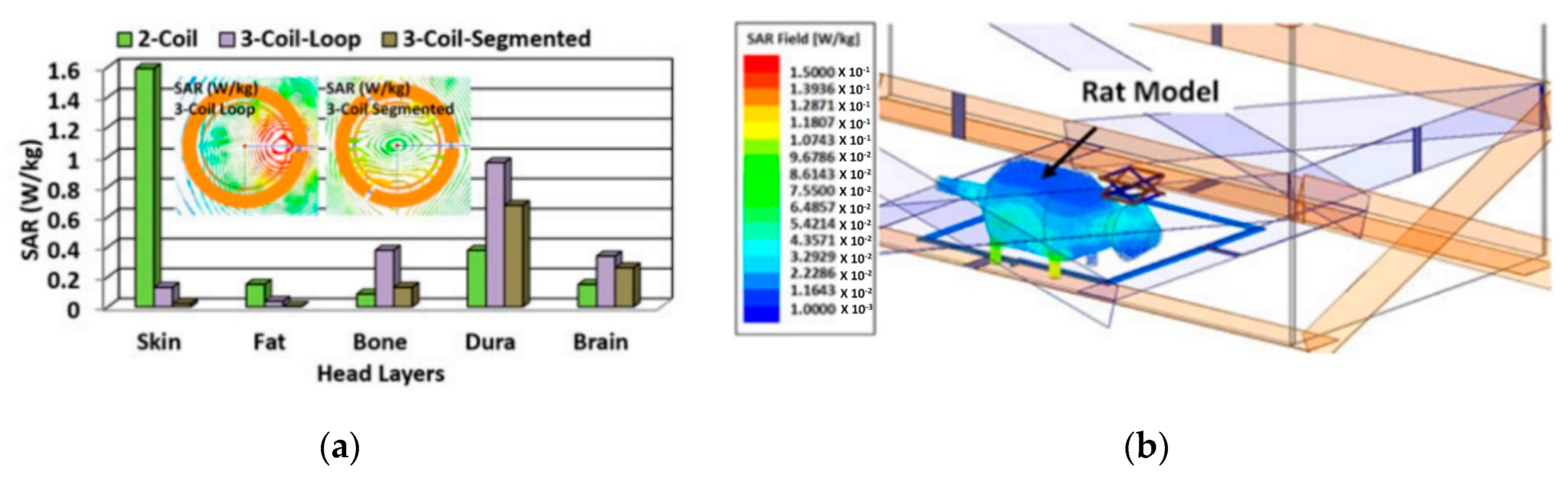
2.6. Specific Absorption Ratio (SAR) and Safety Issues
3. Designs of Wirelessly-Powered Cage Systems
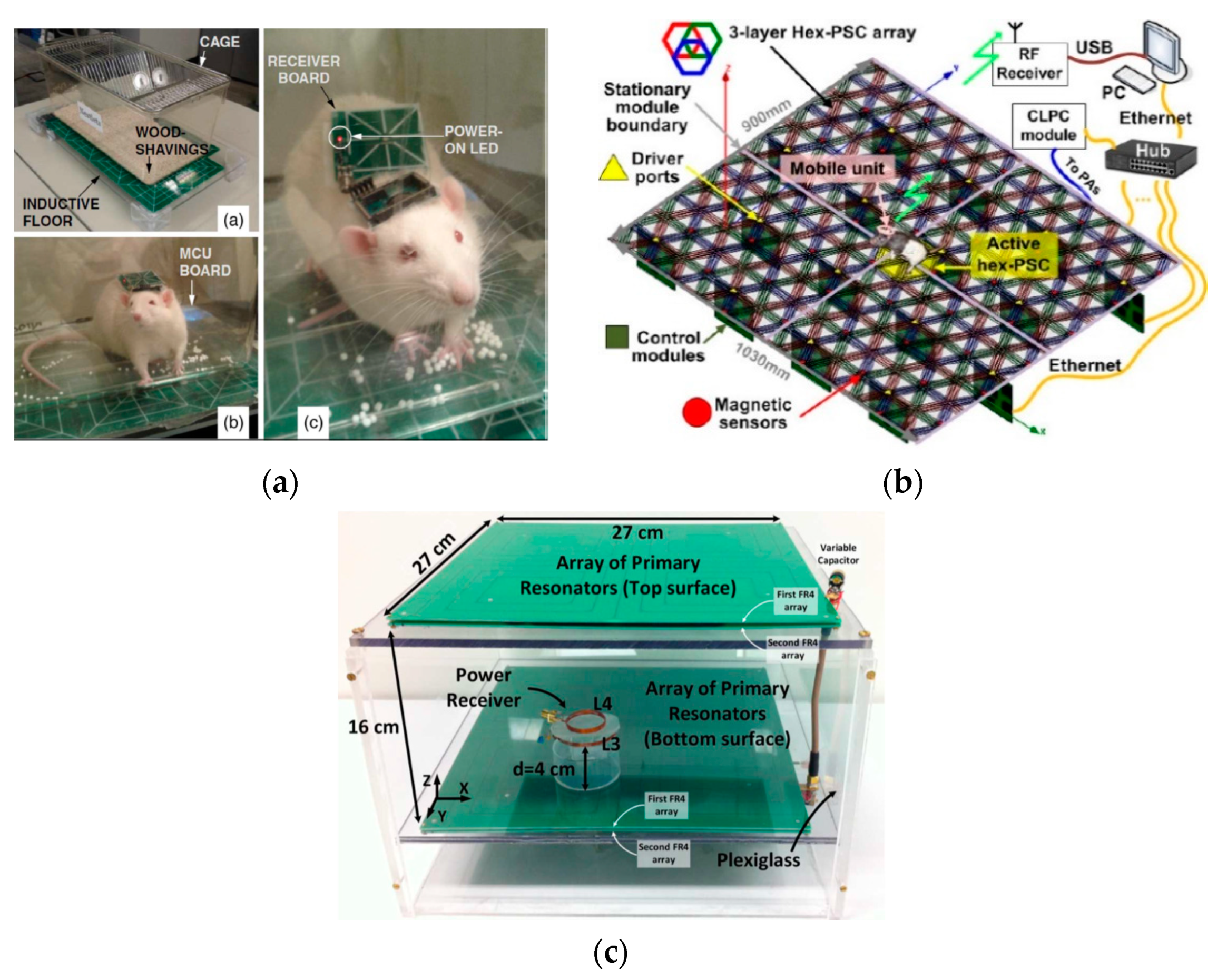
3.1. Wirelessly-Powered Cage Systems with Single Tx Coil Configuration
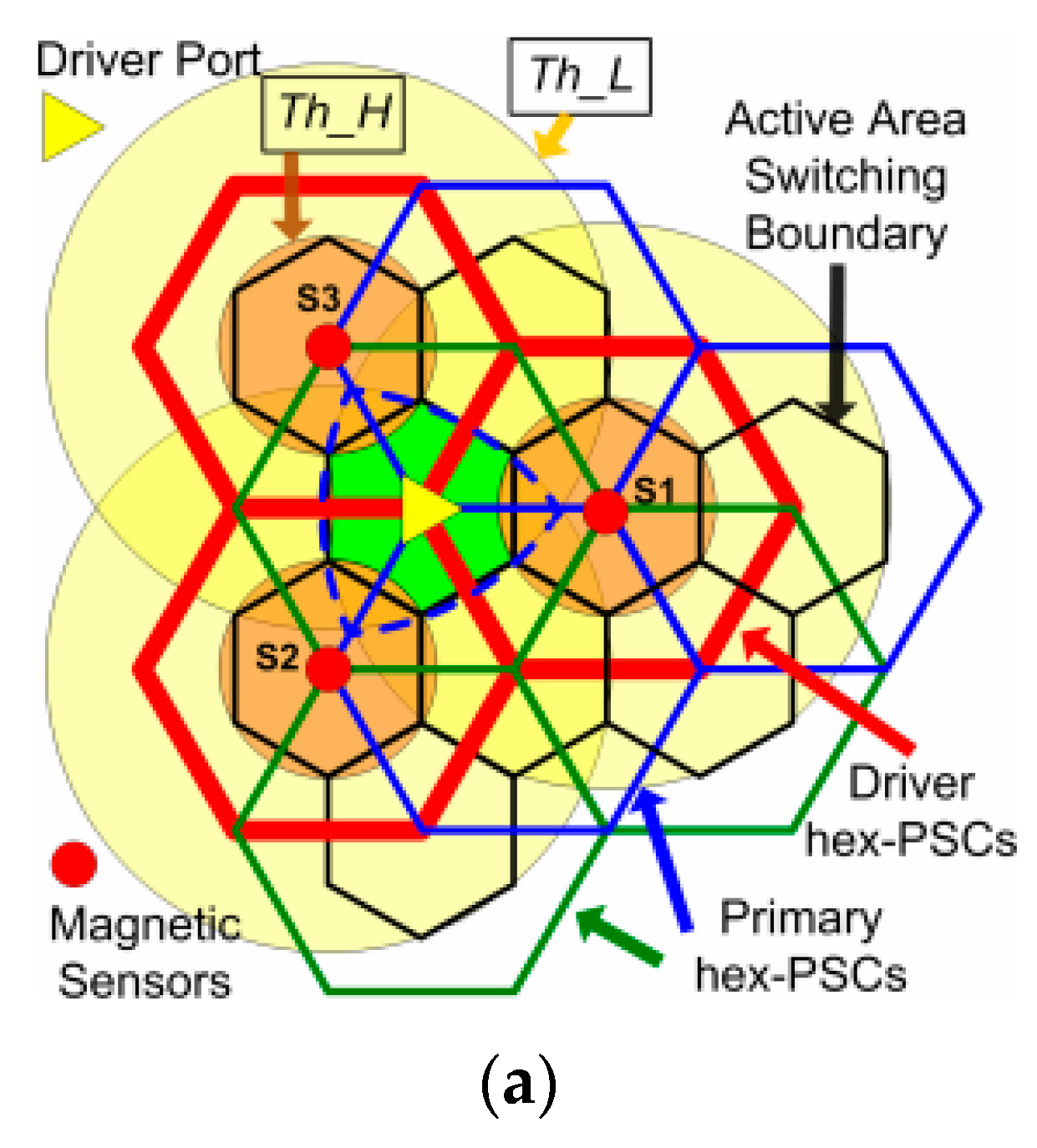
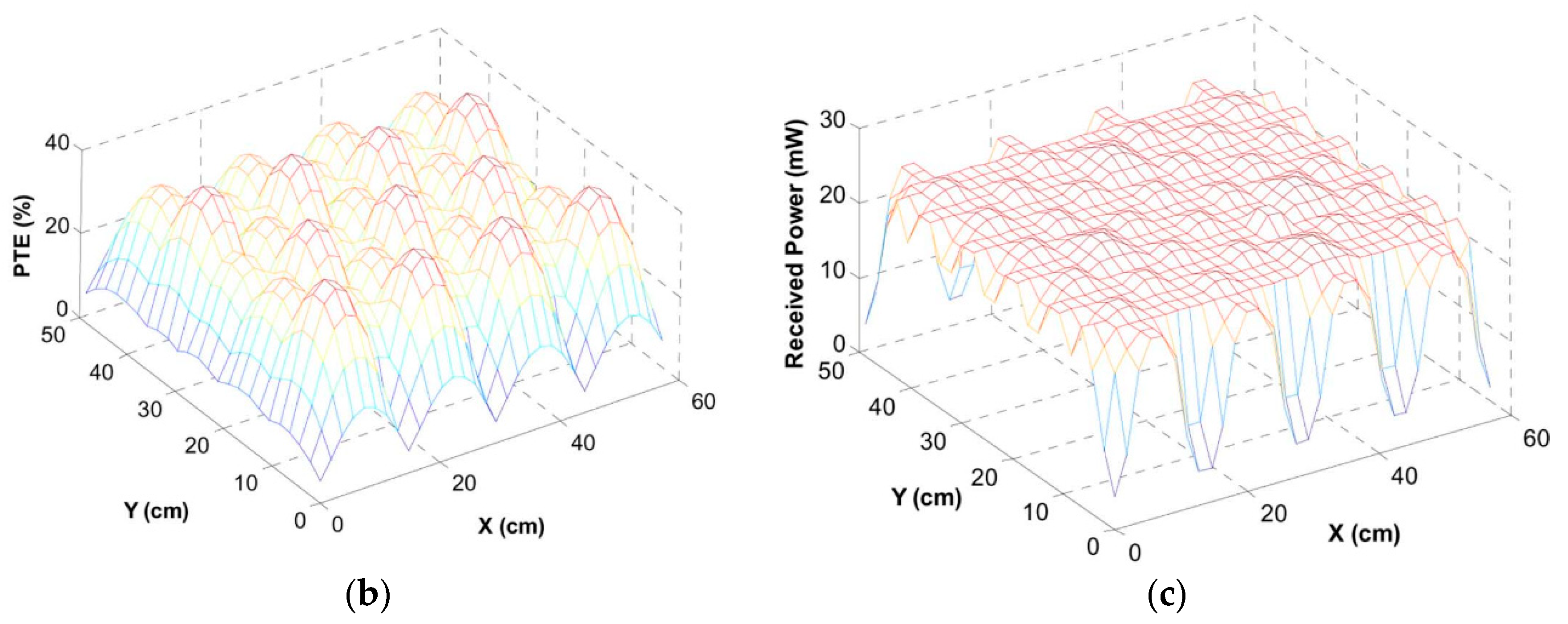
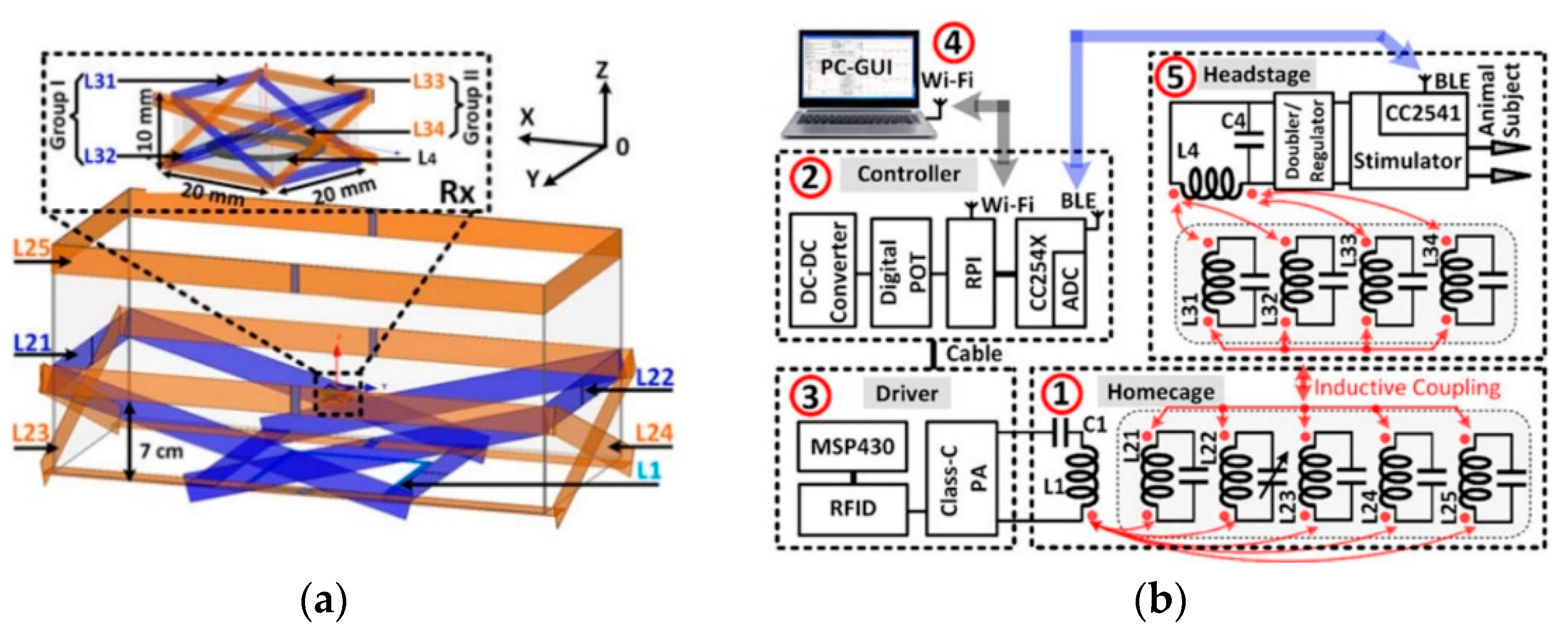
3.2. Wirelessly-Powered Cage Systems with a Scalable Tx Coil Array
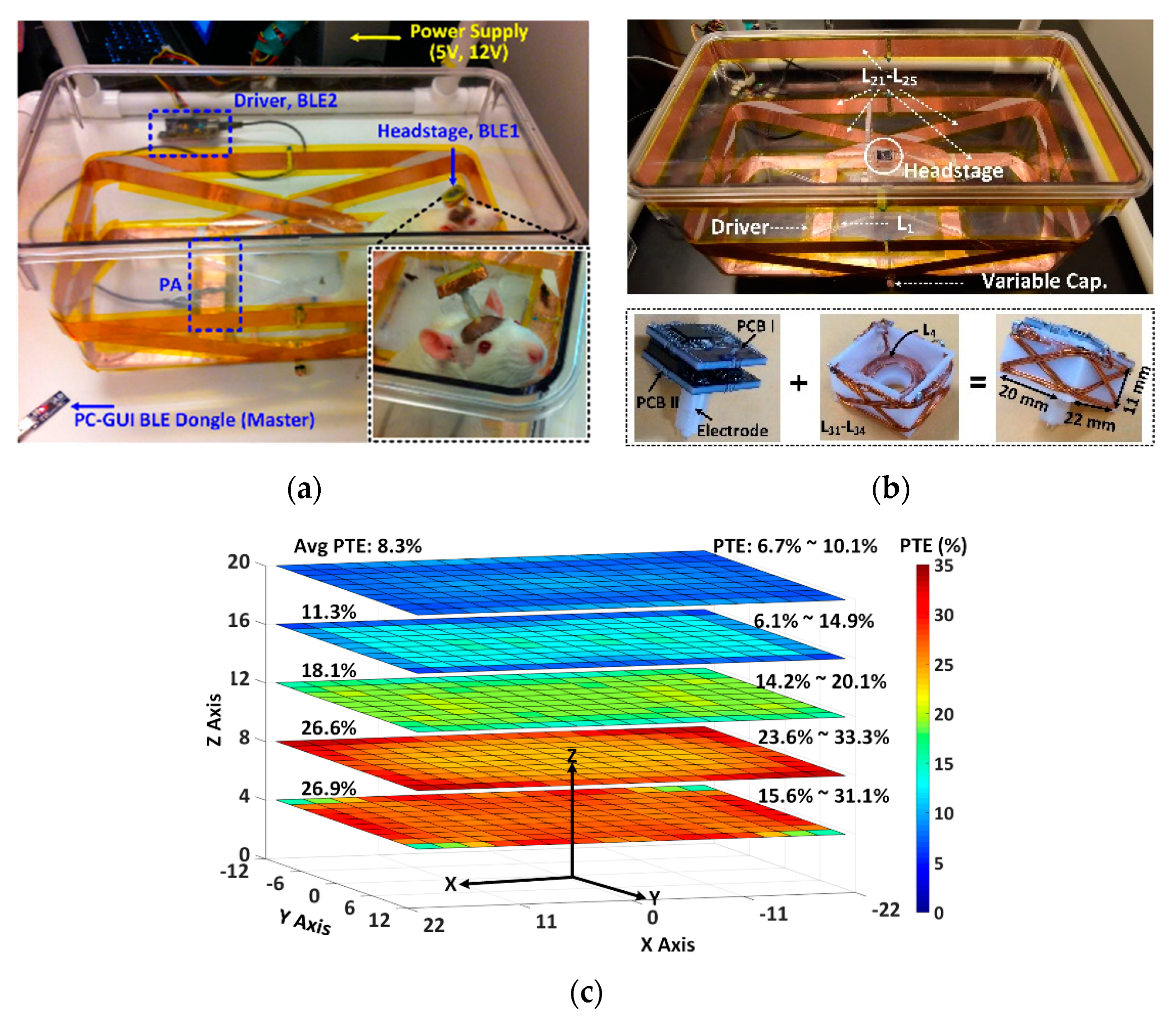
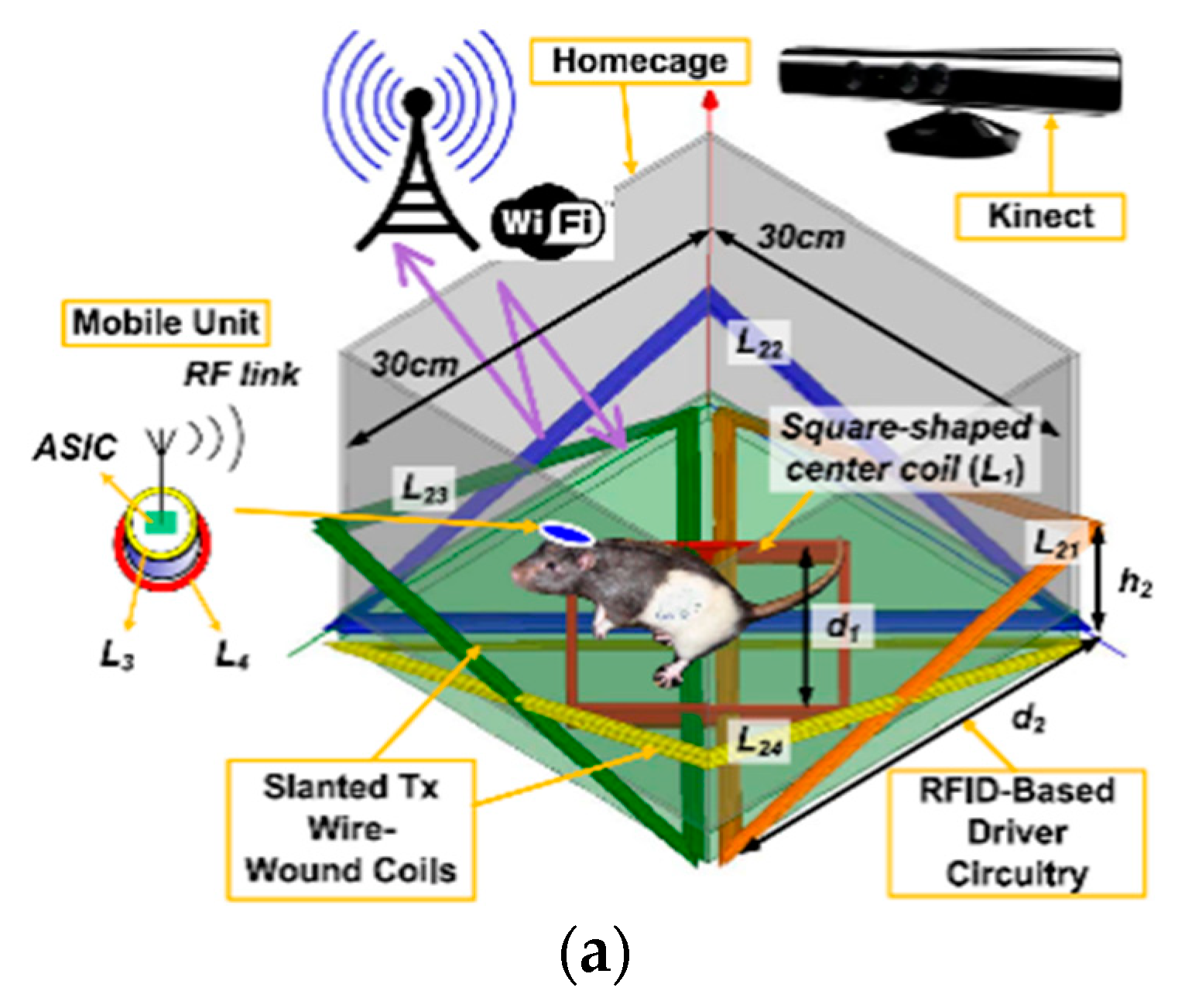
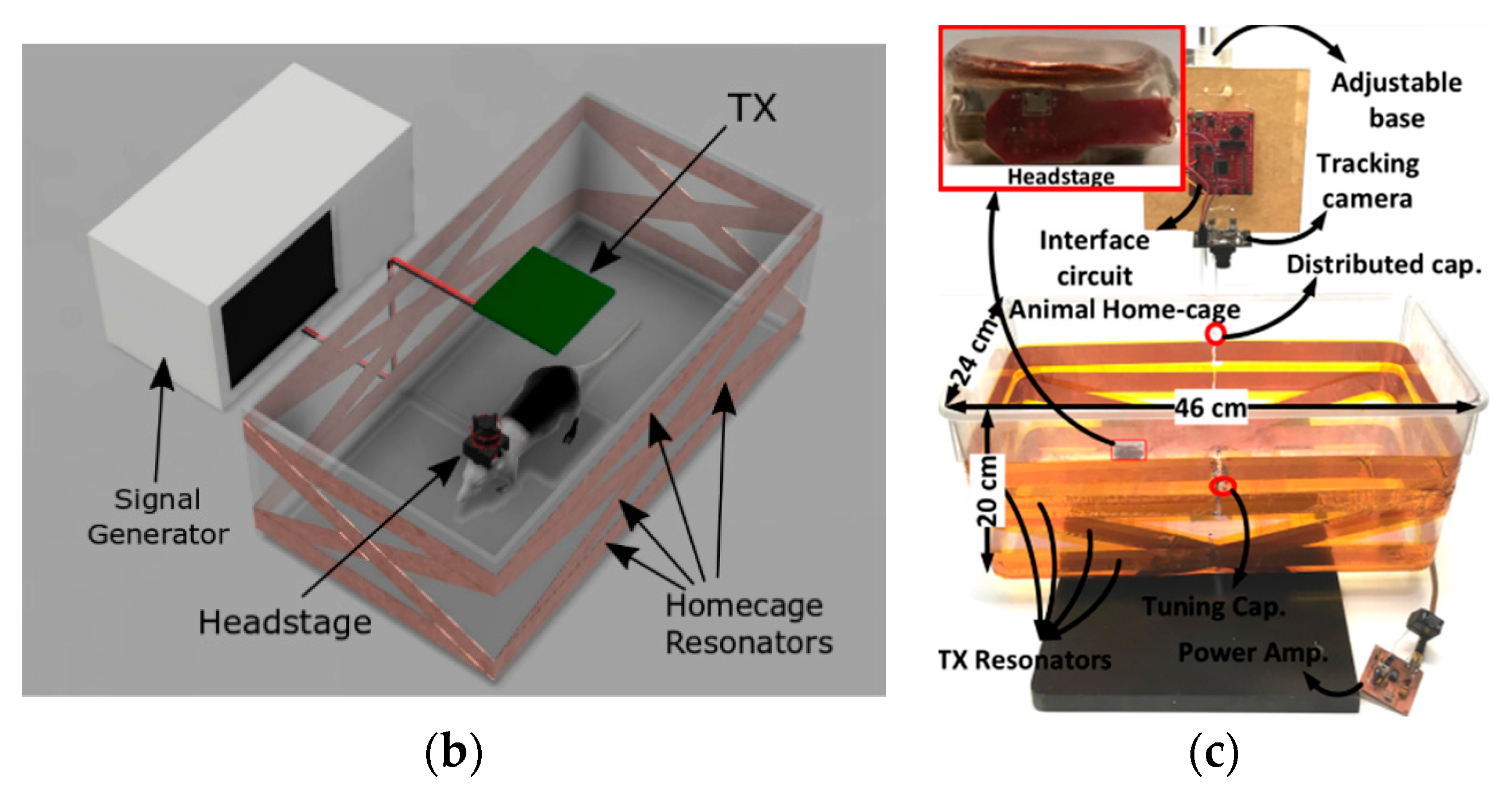
3.3. Wirelessly-Powered Cage Systems with Slanted Tx Resonators
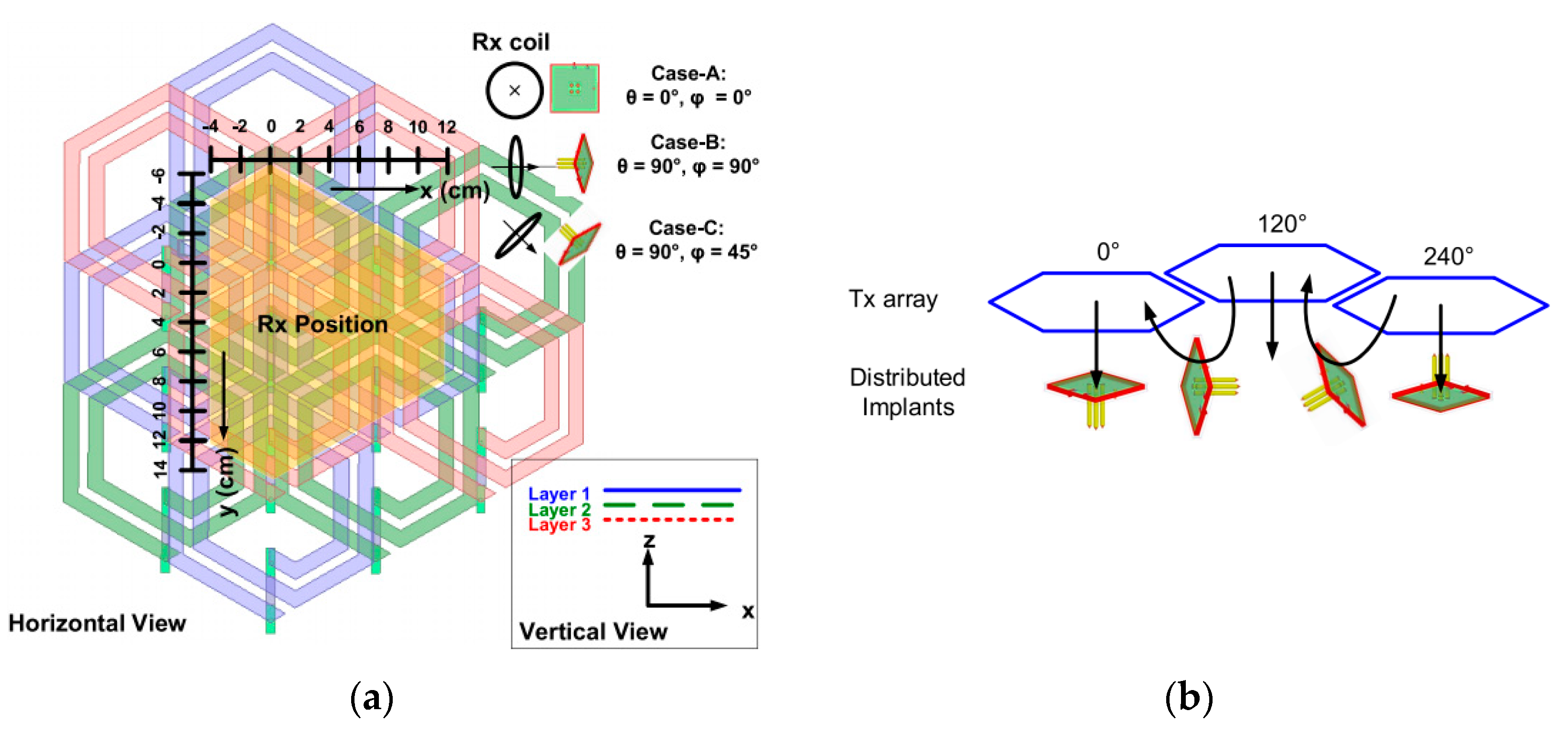
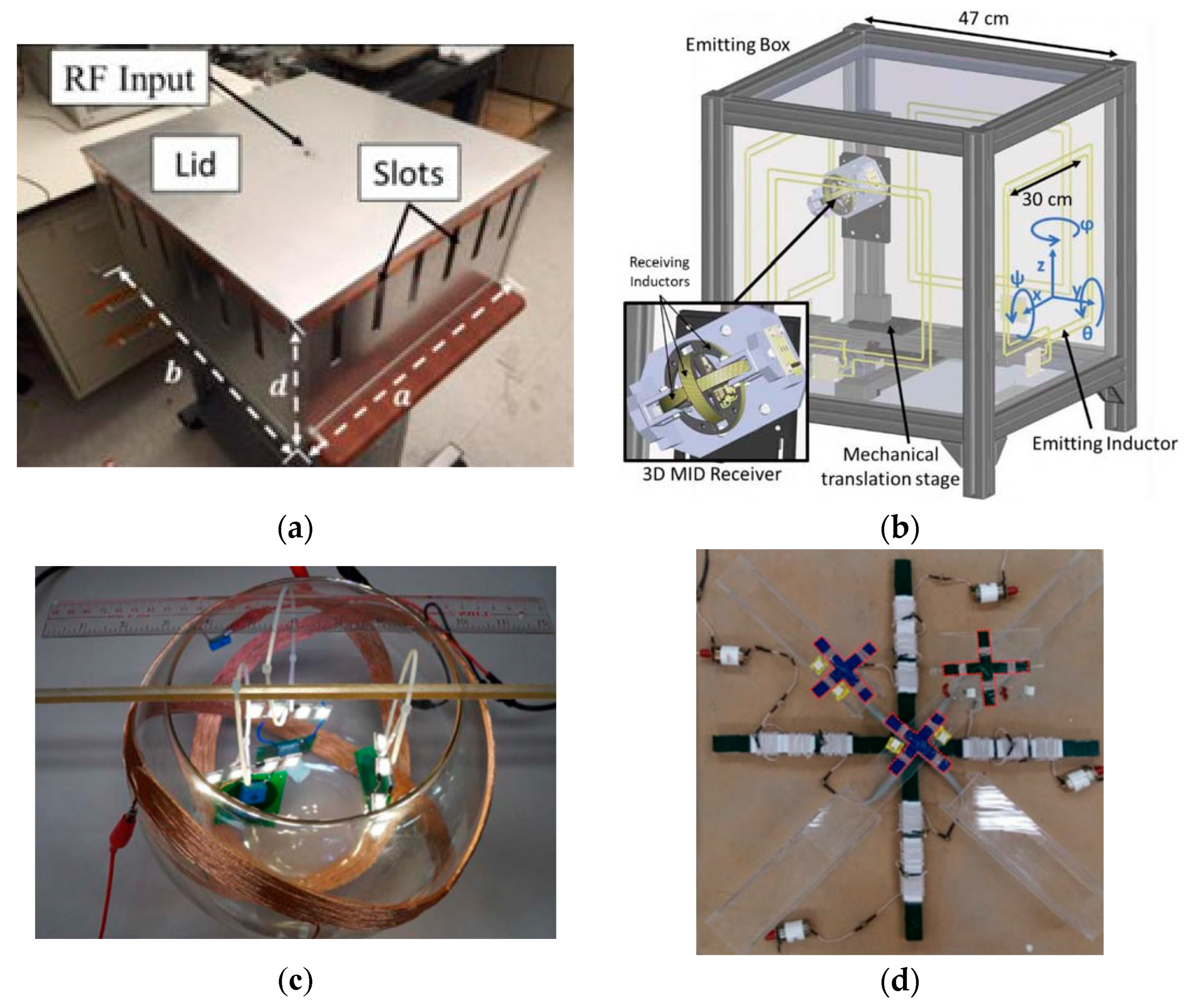
3.4. Wirelessly-Powered Cage Systems for Omnidirectional Power Transmission
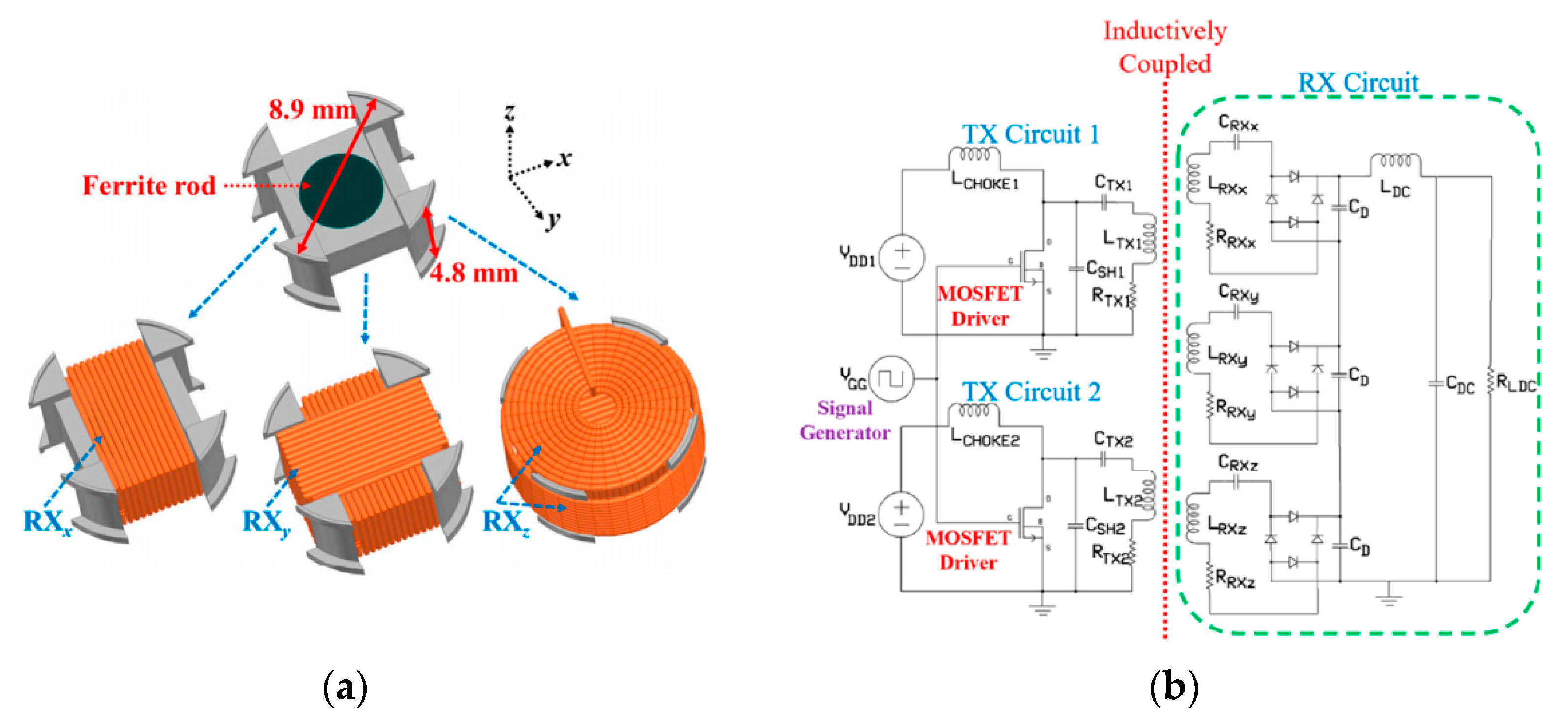
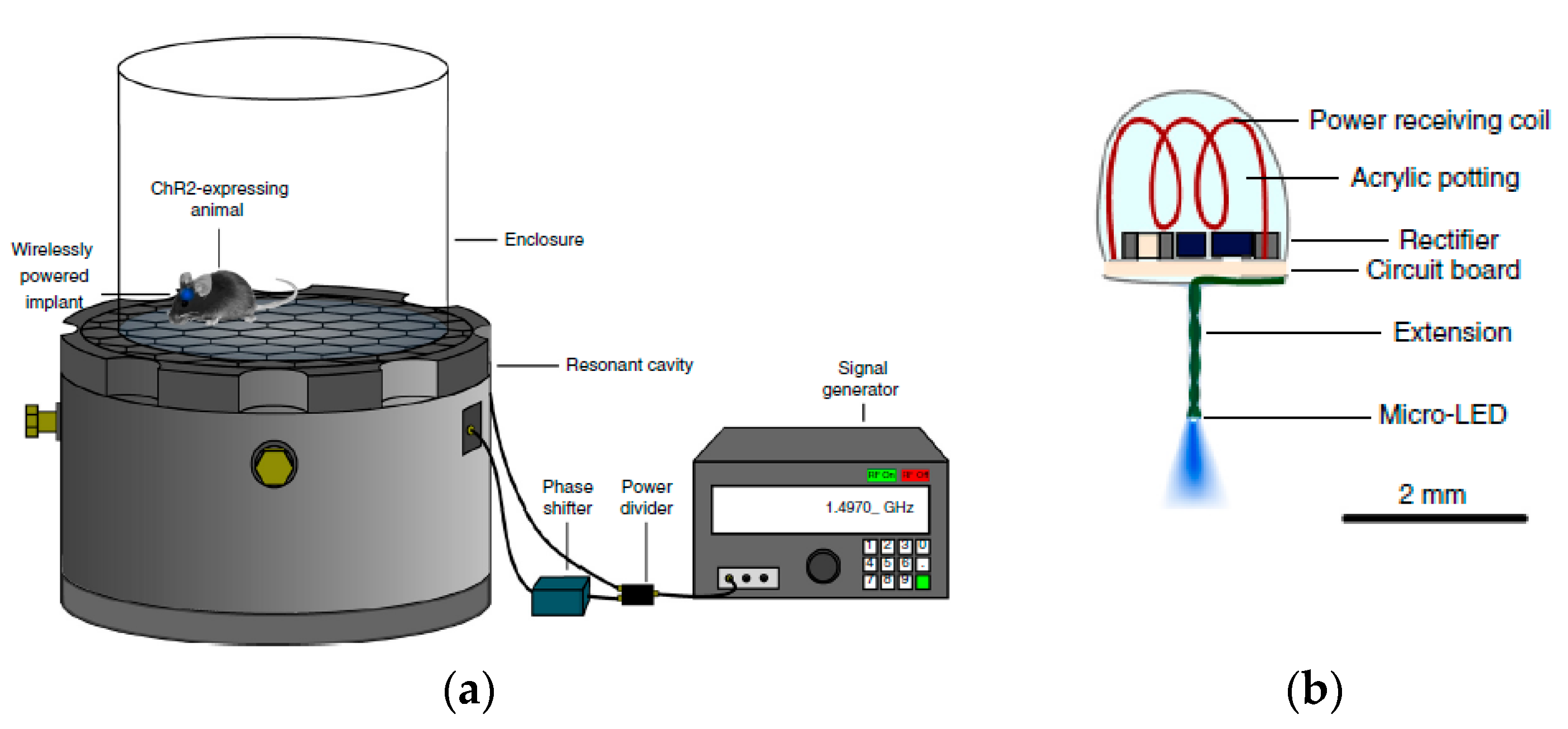
4. Designs of Wirelessly-Powered Cage for mm-Sized Implants
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Manns, J.R.; Eichenbaum, H. A congnitive map for object memory in the hippocampus. Learn. Mem. 2009, 16, 616–624. [Google Scholar] [CrossRef]
- Choi, Y.; Park, S.; Chung, Y.; Gore, R.K.; English, A.W. PDMS microchannel scaffolds for neural interfaces with the peripheral nervous system. In Proceedings of the 27th International Conference on Micro Electro Mechanical Systems (MEMS), San Francisco, CA, USA, 26–30 January 2014; pp. 873–876. [Google Scholar]
- Lee, S.B.; Yin, M.; Manns, J.R.; Ghovanloo, M. A wideband dual antenna receiver for wireless recording from animals behaving in large arenas. IEEE Trans. Biomed. Eng. 2013, 60, 1993–2004. [Google Scholar]
- Fan, D.; Rich, D.; Holtzman, T.; Ruther, P.; Dalley, J.W.; Lopez, A.; Rossi, M.A.; Barter, J.W.; Meza, D.S.; Herwik, S.; et al. A wireless multi-channel recording system for freely behaving mice and rats. PLoS ONE 2011, 6, e22033. [Google Scholar] [CrossRef]
- Lee, S.B.; Lee, H.M.; Kiani, M.; Jow, U.; Ghovanloo, M. An inductively powered scalable 32-channel wireless neural recording system-ona-chip for neuroscience applications. IEEE Trans. Biomed. Circuits Syst. 2010, 4, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Kiani, M.; Ghovanloo, M. A triple-loop inductive power transmission system for biomedical applications. IEEE Trans Biomed Circuits Syst. 2015, 10, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Ghovanloo, M. An overview of data telemetry in inductively powered implantable biomedical devices design and implementation of devices. IEEE Commun. Mag. 2019, 57, 74–80. [Google Scholar] [CrossRef]
- Harrison, R.R. Designing efficient inductive power links for implantable devices. In Proceedings of the 2007 International Symposium on Circuits and Systems, New Orleans, LA, USA, 27–30 May 2007; pp. 2080–2083. [Google Scholar]
- Kiani, M.; Jow, U.; Ghovanloo, M. Design and optimization of a 3-coil inductive link for efficient wireless power transmission. IEEE Trans. Biomed. Circuits Syst. 2011, 5, 579–591. [Google Scholar] [CrossRef]
- Mark, M.; Björninen, T.; Ukkonen, L.; Sydänheimo, L.; Rabaey, J.M. SAR reduction and link optimization for mm-size remotely powered wireless implants using segmented loop antennas. In Proceedings of the 2011 IEEE Topical Conference on Biomedical Wireless Technologies, Networks, and Sensing Systems, Phoenix, AZ, USA, 16–19 January 2011; pp. 7–10. [Google Scholar]
- Jow, U.E.; McMenamin, P.; Kiani, M.; Manns, J.R.; Ghovanloo, M. EnerCage: A smart experimental arena with scalable architecture for behavioral experiments. IEEE Trans. Biomed. Eng. 2014, 61, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Jow, U.E.; Kiani, M.; Huo, X.; Ghovanloo, M. Towards a Smart Experimental Arena for Long-Term Electrophysiology Experiments. IEEE Trans. Biomed. Circuits Syst. 2012, 6, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Mirbozorgi, S.A.; Jia, Y.; Canales, D.; Ghovanloo, M. A Wirelessly-Powered Homecage With Segmented Copper Foils and Closed-Loop Power Control. IEEE Trans. Biomed. Circuits Syst. 2016, 10, 979–989. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Mirbozorgi, S.A.; Wang, Z.; Hsu, C.H.; Madsen, T.E.; Rainnie, D.; Ghovanloo, M. Position and Orientation Insensitive Wireless Power Transmission for EnerCage-Homecage System. IEEE Trans. Biomed. Eng. 2017, 64, 2439–2449. [Google Scholar] [CrossRef] [PubMed]
- Mirbozorgi, S.A.; Jia, Y.; Zhang, P.; Ghovanloo, M. Towards a High-Throughput Wireless Smart Arena for Behavioral Experiments on Small Animals. IEEE Trans. Biomed. Circuits Syst. 2020, 67, 2359–2369. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Kiani, M.; Ghovanloo, M. A Smart Wirelessly Powered Homecage for Long-Term High-Throughput Behavioral Experiments. IEEE Sens. J. 2015, 15, 4905–4916. [Google Scholar] [CrossRef] [PubMed]
- Mirbozorgi, S.A.; Bahrami, H.; Sawan, M.; Gosselin, B. A Smart Cage with Uniform Wireless Power Distribution in 3D for Enabling Long-Term Experiments With Freely Moving Animals. IEEE Trans. Biomed. Circuits Syst. 2016, 10, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Kilinc, E.G.; Conus, G.; Weber, C.; Kawkabani, B.; Maloberti, F.; Dehollain, C. A System for Wireless Power Transfer of Micro-Systems In-Vivo Implantable in Freely Moving Animals. IEEE Sens. J. 2014, 14, 522–531. [Google Scholar] [CrossRef]
- Ou-Yang, T.H.; Tsai, M.L.; Yen, C.T.; Lin, T.T. An infrared range camera-based approach for three-dimensional locomotion tracking and pose reconstruction in a rodent. J. Neurosci. Methods 2011, 201, 116–123. [Google Scholar] [CrossRef]
- Khan, S.R.; Desmulliez, M.P.Y. Towards a Miniaturized 3D Receiver WPT System for Capsule Endoscopy. Micromachines 2019, 10, 545. [Google Scholar] [CrossRef]
- Lee, B.; Ahn, D.; Ghovanloo, M. Three-phase time-multiplexed planar power transmission to distributed implants. IEEE J. Emerg. Sel. Top. Power Electron. 2015, 4, 263–272. [Google Scholar] [CrossRef]
- Choi, B.H.; Lee, E.S.; Sohn, Y.H.; Jang, G.C.; Rim, C.T. Six Degrees of Freedom Mobile Inductive Power Transfer by Crossed Dipole Tx and Rx Coils. IEEE Trans. Power Electron. 2016, 31, 3252–3272. [Google Scholar] [CrossRef]
- IEEE Standard for Safety Levels with Respect to Human Exposure to Radio Frequency Electromagnetic Fields, 3 kHz to 300 GHz; IEEE Standard C95.1-2005; IEEE Standard: Piscataway, NJ, USA, 2005.
- McIntosh, R.L.; Anderson, V.; McKenzie, R.J. A numerical evaluation of SAR distribution and temperature changes around a metallic plate in the head of a RF exposed worker. Bioelectromagnetics 2005, 26, 377–388. [Google Scholar] [CrossRef]
- Mirbozorgi, S.A.; Yeon, P.; Ghovanloo, M. Robust wireless power transmission to mm-sized free-floating distributed implants. IEEE Trans. Biomed. Circuits Syst. 2017, 11, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Karipott, S.S.; Veetil, P.M.; Nelson, B.D.; Guldberg, R.E.; Ong, K.G. An Embedded Wireless Temperature Sensor for Orthopedic Implants. IEEE Sens. J. 2018, 18, 1265–1272. [Google Scholar] [CrossRef]
- Lu, D.; Yan, Y.; Avila, R.; Kandel, I.; Stepien, I.; Seo, M.H.; Bai, W.; Yang, Q.; Li, C.; Haney, C.R.; et al. Bioresorbable, wireless, passive sensors as temporary implants for monitoring regional body temperature. Adv. Healthc. Mater. 2020, 9, 2000942. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.P.-W.; Chung, H.S.-H.; Chan, L.L.-H.; Shen, R.; Tang, S.C. Optimal Design and Experimental Assessment of a Wireless Power Transfer System for Home-Cage Monitoring. IEEE Trans. Power Electron. 2019, 34, 9779–9793. [Google Scholar] [CrossRef]
- Nassirinia, F.; Straver, W.; Hoebeek, F.E.; Serdijn, W.A. Wireless power transfer and optogenetic stimulation of freely moving rodents. In Proceedings of the 2017 8th International IEEE/EMBS Conference on Neural Engineering (NER), Shanghai, China, 25–28 May 2017; pp. 456–460. [Google Scholar]
- Bernstein, J.G.; Boyden, E.S. Optogenetic tools for analyzing the neural circuits of behavior. Trends Cogn. Sci. 2011, 15, 592–600. [Google Scholar] [CrossRef]
- Soltani, N.; Aliroteh, M.S.; Salam, M.T.; Velazquez, J.L.P.; Genov, R. Low-Radiation Cellular Inductive Powering of Rodent Wireless Brain Interfaces: Methodology and Design Guide. IEEE Trans. Biomed. Circuits Syst. 2016, 10, 920–932. [Google Scholar] [CrossRef]
- Biswas, D.K.; Martinez, J.H.A.; Daniels, J.; Bendapudi, A.; Mahbub, I. A Novel 3-D Printed Headstage and Homecage based WPT System for Long-term Behavior Study of Freely Moving Animals. In Proceedings of the 2020 IEEE Radio and Wireless Symposium (RWS), San Antonio, TX, USA, 26–29 January 2020; pp. 268–271. [Google Scholar]
- Maghsoudloo, E.; Gagnon-Turcotte, G.; Rezaei, Z.; Gosselin, B. A Smart Neuroscience Platform with Wireless Power Transmission for Simultaneous Optogenetics and Electrophysiological Recording. In Proceedings of the 2018 IEEE International Symposium on Circuits and Systems (ISCAS), Florence, Italy, 27–30 May 2018; pp. 1–5. [Google Scholar]
- Mei, H.; Thackston, K.A.; Bercich, R.A.; Jefferys, J.G.R.; Irazoqui, P.P. Cavity resonator wireless power transfer system for freely moving animal experiments. IEEE Trans. Biomed. Eng. 2017, 64, 775–785. [Google Scholar] [CrossRef]
- Sergkei, K.; Lombard, P.; Semet, V.; Allard, B.; Moguedet, M.; Cabrera, M. The Potential of 3D-MID Technology for Omnidirectional Inductive Wireless Power Transfer. In Proceedings of the 2018 13th International Congress Molded Interconnect Devices (MID), Würzburg, Germany, 25–26 September 2018; pp. 1–6. [Google Scholar]
- Ng, W.M.; Zhang, C.; Lin, D.; Hui, S.Y.R. Two-and three-dimensional omnidirectional wireless power transfer. IEEE Trans. Power Electron. 2014, 29, 4470–4474. [Google Scholar] [CrossRef]
- Lee, B.; Koripalli, M.K.; Jia, Y.; Acosta, J.; Sendi, M.S.E.; Choi, Y.; Ghovanloo, M. An implantable peripheral nerve recording and stimulation system for experiments on freely moving animal subjects. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Lee, B.; Jia, Y.; Mirbozorgi, S.A.; Connolly, M.; Tong, X.; Zeng, Z.; Mahmoudi, B.; Ghovanloo, M. An inductively-powered wireless neural recording and stimulation system for freely-behaving animals. IEEE Trans. Biomed. Circuits Syst. 2019, 13, 413–424. [Google Scholar] [CrossRef]
- Ho, J.S. Fully internal, wirelessly powered systems for optogenetics. In Proceedings of the 2016 International Conference on Optical MEMS and Nanophotonics (OMN), Singapore, 31 July–4 August 2016; pp. 1–2. [Google Scholar]
- Amar, A.B.; Kouki, A.B.; Cao, H. Power approaches for implantable medical devices. Sensors 2015, 15, 28889–28914. [Google Scholar] [CrossRef] [PubMed]
- Shin, G.; Gomez, A.M.; Hasani, R.A.; Jeong, Y.R.; Kim, J.; Xie, Z.; Banks, A.; Lee, S.M.; Han, S.Y.; Yoo, C.J.; et al. Flexible Near-Field Wireless Optoelectronics as Subdermal Implants for Broad Applications in Optogenetics. Neuron 2017, 93, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Mirbozorgi, S.A.; Zhang, P.; Inan, O.T.; Li, W.; Ghovanloo, M. A dual-band wireless power transmission system for evaluating mm-sized implants. IEEE Trans. Biomed. Circuits Syst. 2019, 13, 595–607. [Google Scholar] [CrossRef] [PubMed]
- Park, S.I.; Brenner, D.S.; Shin, G.; Morgan, C.D.; Copits, B.A.; Chung, H.U.; Pullen, M.Y.; Noh, K.N.; Davidson, S.; Oh, S.J.; et al. Soft, Stretchable, Fully Implantable Miniaturized Optoelectronic Systems for Wireless Optogenetics. Nat. Biotechnol. 2015, 33, 1280–1286. [Google Scholar] [CrossRef]
- Piech, D.K.; Johnson, B.C.; Shen, K.; Ghanbari, M.M.; Li, K.Y.; Neely, R.M.; Kay, J.E.; Carmena, J.M.; Maharbiz, M.M.; Muller, R. A wireless millimeter-scale implantable neural stimulator with ultrasonically powered bidirectional communication. Nat. Biomed. Eng. 2020, 4, 207–222. [Google Scholar] [CrossRef]
- Liu, S.; Moncion, C.; Zhang, J.; Balachandar, L.; Kwaku, D.; Riera, J.J.; Volakis, J.L.; Chae, J. Fully Passive Flexible Wireless Neural Recorder for the Acquisition of Neuropotentials from a Rat Model. ACS Sens. 2019, 4, 3175–3185. [Google Scholar] [CrossRef]
- Yang, K.W.; Oh, K.; Ha, S. Challenges in Scaling Down of Free-Floating Implantable Neural Interfaces to Millimeter Scale. IEEE Access 2020, 8, 133295. [Google Scholar] [CrossRef]







| Inductive Link Configurations | ||||
|---|---|---|---|---|
| 2-Coil | 3-Coil | 4-Coil | ||
| Application Conditions | Size constrain | √ | × | × |
| Strong coupling (k) | √ | √ | × | |
| Weak coupling (k) | × | √ | √ | |
| Large PDL (small Rs) | √ | √ | × | |
| Small PDL (large Rs) | × | × | √ | |
| k variation w/small Rs | √ | √ | × | |
| k variation w/large Rs | × | × | √ | |
| Publications | [28] | [29] | [30] | [18] |
|---|---|---|---|---|
| Inductive link | 2-coil | 2-coil | 2-coil | 2-coil |
| Frequency | 6.78 MHz | 13.56 MHz | 120 kHz | 13.56 MHz |
| WPT coverage, length × width × height | 40 × 24 × 4 cm3 | 40 × 40 × 20 cm3 | diameter of 8 inch | 34 × 18 cm2 |
| Mobile device size, length × width × height | 24.5 × 13 × 16 mm3 | 10 × 10 × 10 mm3 | <1 cm3 | 12 × 12 mm2 |
| PTE | 3.8–7.5% | >0.56% | N/A | 17% |
| PDL | 100 mW | 8.5 mW | 2 W | 1.7 mW |
| CLPC | × | × | × | √ |
| Scalability | × | × | × | √ |
| Compatible to racks | √ | √ | √ | × |
| Coil size matching | × | × | × | √ |
| Vertical misalignment Resilience | √ | √ | × | × |
| Publications | [31] | [11] | [17] |
|---|---|---|---|
| Inductive link | 2-coil | 3/4-coil | 4-coil |
| Frequency | 1.5 MHz | 13.56 MHz | 13.56 MHz |
| WPT coverage, length × width × height | 42 × 18 × (8–11) cm3 | 3538 × 12 cm3 | 27 × 27 × 16 cm3 |
| Mobile device size, length × width × height | 40 × 40 mm2 | π × 202 × 20 mm3 | 42 mm diameter |
| PTE | 13–39% | 5.6–12.6% | 59% |
| PDL | 21–225 mW | 20 mW | 100 mW |
| CLPC | × | √ | × |
| Tx control simplicity | × | × | √ |
| Compatible to racks | √ | × | √ |
| Angular misalignment resilience | × | × | √ |
| No view-blocking | √ | √ | × |
| Publications | [16] | [13] | [32] | [33] |
|---|---|---|---|---|
| Inductive link | 3/4-coil | 4-coil | 4-coil | 42-coil |
| Frequency | 13.56 MHz | 13.56 MHz | 13.56 MHz | 13.56 MHz |
| WPT coverage, length × width × height | 30 × 30 × 17 cm3 | 46 × 24 × 20 cm3 | 28.5 × 18 × 13 cm3 | 46 × 24 × 20 cm3 |
| Mobile device size, length × width × height. | 40 × 40 × 20 mm3 | 20 × 22 × 5 mm3 | 26 × 26 × 35 mm3 | 15 × 15 × 10 mm2 |
| PTE | 16.1–36.3% | 14% | 34–42% | 17% |
| PDL | 24 mW | 42 mW | 13 mW | 62 mW |
| CLPC | √ | √ | × | √ |
| Tx control simplicity | × | √ | √ | √ |
| Compatible to racks | × | √ | √ | √ |
| No view blocking | √ | √ | × | √ |
| Publications | [34] | [14] | [35] | [36] | [22] |
|---|---|---|---|---|---|
| Inductive link | Cavity | 4-coil | 2-coil | 2-coil | 2-coil |
| Frequency | 346.6 MHz | 13.56 MHz | 6.78 MHz | 530 kHz | 280 kHz |
| WPT coverage, length × width × height | 61 × 61 × 30 cm3 | 46 × 24 × 20 cm3 | 30 × 30 × 30 cm3 | N/A | 1 m3 |
| Mobile device size, length × width × height | π × (7)2 × 25 mm3 | 20 × 22 × 11 mm3 | ~64 × 64 × 64 mm3 | N/A | 20 × 20 × 0.5 cm3 |
| PTE | 14.32% | 23.6–33.3% | 7.9% | N/A | 33.6% |
| PDL | 6.1–13 mW | 42 mW | 1.4 W | N/A | 10 W |
| CLPC | × | √ | × | × | × |
| Scalability | × | × | × | × | × |
| Tx control simplicity | √ | √ | √ | × | × |
| Compatible to racks | √ | √ | √ | √ | × |
| Blind spots | Center + 4 corners | Center lines | 2 corners | None | None |
| No view-blocking | × | √ | √ | × | √ |
| No Rx access blocking | √ | √ | × | √ | √ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, B.; Jia, Y. Wirelessly-Powered Cage Designs for Supporting Long-Term Experiments on Small Freely Behaving Animals in a Large Experimental Arena. Electronics 2020, 9, 1999. https://doi.org/10.3390/electronics9121999
Lee B, Jia Y. Wirelessly-Powered Cage Designs for Supporting Long-Term Experiments on Small Freely Behaving Animals in a Large Experimental Arena. Electronics. 2020; 9(12):1999. https://doi.org/10.3390/electronics9121999
Chicago/Turabian StyleLee, Byunghun, and Yaoyao Jia. 2020. "Wirelessly-Powered Cage Designs for Supporting Long-Term Experiments on Small Freely Behaving Animals in a Large Experimental Arena" Electronics 9, no. 12: 1999. https://doi.org/10.3390/electronics9121999
APA StyleLee, B., & Jia, Y. (2020). Wirelessly-Powered Cage Designs for Supporting Long-Term Experiments on Small Freely Behaving Animals in a Large Experimental Arena. Electronics, 9(12), 1999. https://doi.org/10.3390/electronics9121999






