Motor-Imagery Classification Using Riemannian Geometry with Median Absolute Deviation
Abstract
1. Introduction
2. Background
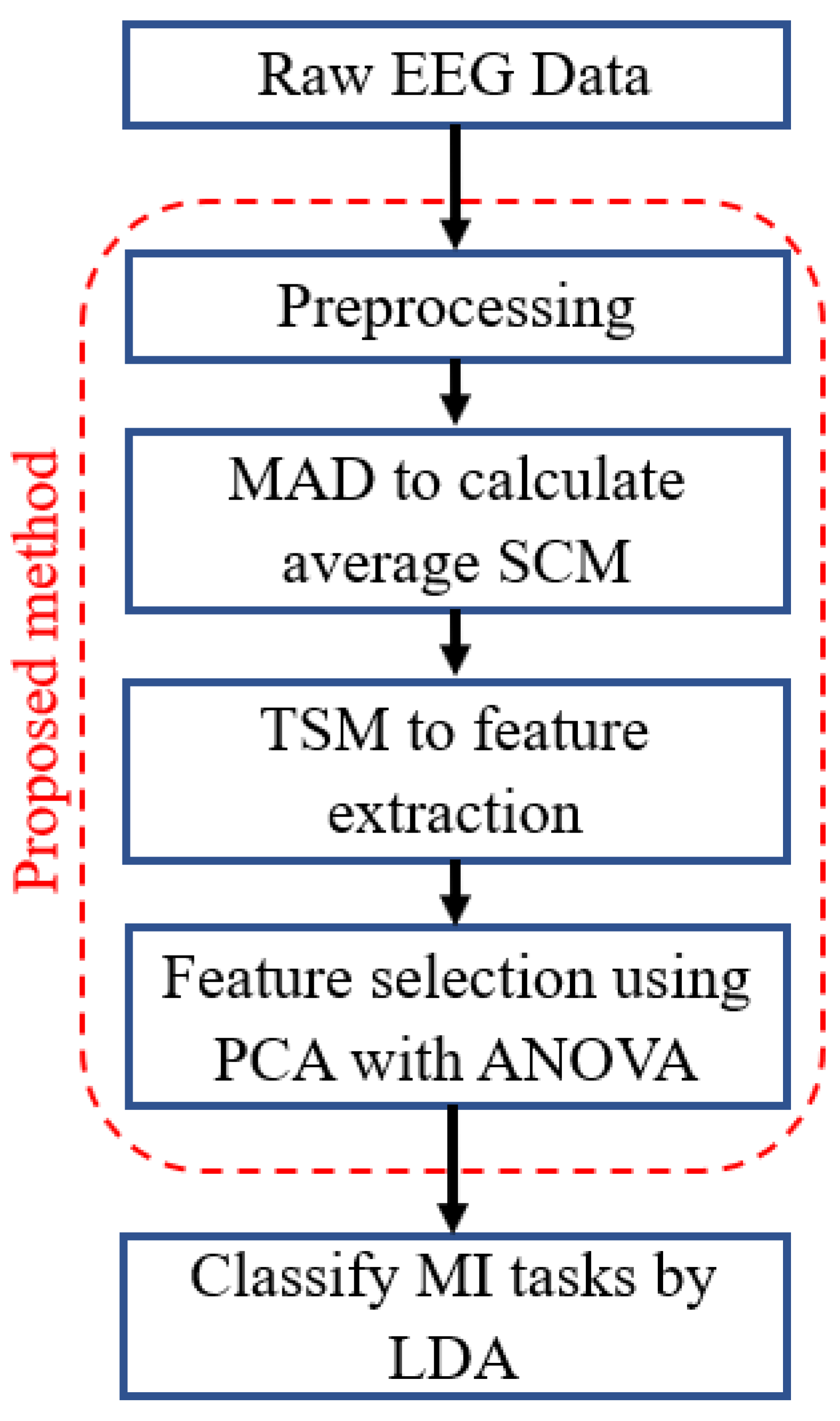
3. Proposed Methodology
3.1. Use of Covariance Matrices in BCI
3.2. Reference Matrices Calculation from Covariance Matrices
- Step 1:
- Sorted data values in ascending order. Replace the same or repeated varieties with different varieties as necessary within the given knowledge set.
- Step 2:
- If the number of observations is odd, calculate the median of the given data by dividing it by two; otherwise, it express the two midmost numbers as normal.
- Step 3:
- Calculate the deviation of each value from the median by subtracting every median value.
- Step 4:
- Then, calculate the absolute value of each deviation.
- Step 5:
- Select all perfect deviations in ascending order and calculate the median of these deviations according to step 2. These median values are known as MAD.
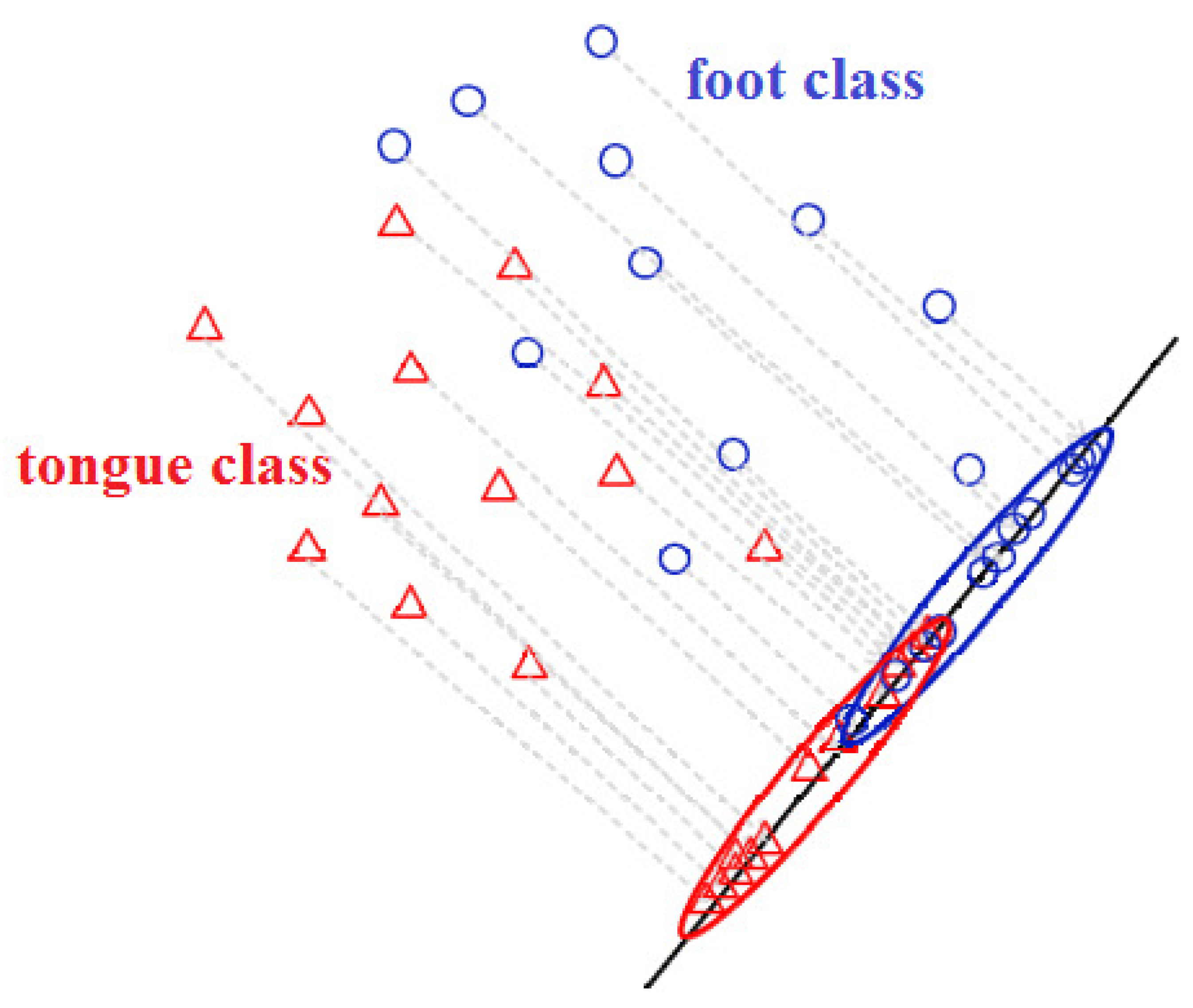
3.3. Feature Extraction, Feature Selection, and Classification
3.3.1. Feature Extraction
| Algorithm 1: Tangent space mapping (TSM). |
| Input: SPD matrices set I with |
| Output: a set of I vector |
|
3.3.2. Feature Selection
3.3.3. Classification
4. Experimental Dataset, Results, and Discussion
4.1. Dataset Descriptions
- BCI III-IIIa (binary-class)
- BCI IV- IIa (Two-classes)
4.2. Experimental Evaluation, Results, and Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ang, K.K.; Guan, C. EEG-based strategies to detect motor imagery for control and rehabilitation. IEEE Trans. Neural Syst. Rehabil. Eng. 2016, 25, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Li, W.; He, X.; Wei, Z.; Zhang, D.; Wu, C.; Song, A. Motor Imagery Based Continuous Teleoperation Robot Control with Tactile Feedback. Electronics 2020, 9, 174. [Google Scholar] [CrossRef]
- Zhang, X.; Yong, X.; Menon, C. Evaluating the versatility of EEG models generated from motor imagery tasks: An exploratory investigation on upper-limb elbow-centered motor imagery tasks. PLoS ONE 2017, 12, e0188293. [Google Scholar]
- Yuan, H.; He, B. Brain—Computer interfaces using sensorimotor rhythms: Current state and future perspectives. IEEE Trans. Biomed. Eng. 2014, 61, 1425–1435. [Google Scholar] [CrossRef]
- Ehrsson, H.H.; Geyer, S.; Naito, E. Imagery of voluntary movement of fingers, toes, and tongue activates corresponding body-part-specific motor representations. J. Neurophysiol. 2003, 90, 3304–3316. [Google Scholar] [CrossRef]
- Islam, M.R.; Tanaka, T.; Molla, M.K. Multiband tangent space mapping and feature selection for classification of EEG during motor imagery. J. Neural Eng. 2018, 15, 046021. [Google Scholar] [CrossRef]
- Ramoser, H.; Muller-Gerking, J.; Pfurtscheller, G. Optimal spatial filtering of single trial EEG during imagined hand movement. IEEE Trans. Rehabil. Eng. 2000, 8, 441–446. [Google Scholar] [CrossRef]
- Xygonakis, I.; Athanasiou, A.; Pandria, N.; Kugiumtzis, D.; Bamidis, P.D. Decoding motor imagery through common spatial pattern filters at the EEG source space. Comput. Intell. Neurosci. 2018. [Google Scholar] [CrossRef]
- Samek, W.; Kawanabe, M. Robust common spatial patterns by minimum divergence covariance estimator. In Proceedings of the 2014 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), Florence, Italy, 4–9 May 2014; pp. 2040–2043. [Google Scholar]
- Tang, Z.; Sun, S.; Zhang, S.; Chen, Y.; Li, C.; Chen, S. A brain-machine interface based on ERD/ERS for an upper-limb exoskeleton control. Sensors 2016, 16, 2050. [Google Scholar] [CrossRef]
- Lotte, F.; Guan, C. Regularizing common spatial patterns to improve BCI designs: Unified theory and new algorithms. IEEE Trans. Biomed. Eng. 2010, 58, 355–362. [Google Scholar]
- Zhang, Y.; Nam, C.S.; Zhou, G.; Jin, J.; Wang, X.; Cichocki, A. Temporally constrained sparse group spatial patterns for motor imagery BCI. IEEE Trans. Cybern. 2018, 49, 3322–3332. [Google Scholar] [CrossRef] [PubMed]
- Nicolas-Alonso, L.F.; Gomez-Gil, J. Brain computer interfaces: A review. Sensors 2012, 12, 1211–1279. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.C.; Li, C.; Wu, J.F.; Liu, P.C.; Cheng, S.W. Classification of EEG-based single-trial motor imagery tasks using a B-CSP method for BCI. Front. Inf. Technol. Electron. Eng. 2019, 20, 1087–1098. [Google Scholar] [CrossRef]
- Novi, Q.; Guan, C.; Dat, T.H.; Xue, P. Sub-band common spatial pattern (SBCSP) for brain-computer interface. In Proceedings of the 2007 3rd International IEEE/EMBS Conference on Neural Engineering, Kohala Coast, HI, USA, 2–5 May 2007; pp. 204–207. [Google Scholar]
- Khan, J.; Bhatti, M.H.; Khan, U.G.; Iqbal, R. Multiclass EEG motor-imagery classification with sub-band common spatial patterns. EURASIP J. Wirel. Commun. Netw. 2019, 2019, 174. [Google Scholar] [CrossRef]
- Joy, M.M.; Hasan, M.; Miah, A.S.; Ahmed, A.; Tohfa, S.A.; Bhuaiyan, M.F.; Zannat, A.; Rashid, M.M. Multiclass MI-Task Classification Using Logistic Regression and Filter Bank Common Spatial Patterns. In Proceedings of the International Conference on Computing Science, Communication and Security, Mehsana, India, 26–28 March 2020; Springer: Berlin/Heidelberg, Germany, 2020; pp. 160–170. [Google Scholar]
- Barbaresco, F. Innovative tools for radar signal processing based on Cartan’s geometry of SPD matrices & information geometry. In Proceedings of the 2008 IEEE Radar Conference, Rome, Italy, 26–30 May 2008; pp. 1–6. [Google Scholar]
- Barachant, A.; Bonnet, S.; Congedo, M.; Jutten, C. Multiclass brain—Computer interface classification by Riemannian geometry. IEEE Trans. Biomed. Eng. 2011, 59, 920–928. [Google Scholar] [CrossRef]
- Barachant, A.; Bonnet, S.; Congedo, M.; Jutten, C. Classification of covariance matrices using a Riemannian-based kernel for BCI applications. Neurocomputing 2013, 112, 172–178. [Google Scholar] [CrossRef]
- Uehara, T.; Tanaka, T.; Fiori, S. Robust averaging of covariance matrices by Riemannian geometry for motor-imagery brain–computer interfacing. In Advances in Cognitive Neurodynamics (V); Springer: Berlin/Heidelberg, Germany, 2016; pp. 347–353. [Google Scholar]
- Miah, A.S.; Ahmed, S.R.; Ahmed, M.R.; Bayat, O.; Duru, A.D.; Molla, M.K. Motor-imagery BCI task classification using Riemannian geometry and averaging with mean absolute deviation. In Proceedings of the 2019 Scientific Meeting on Electrical-Electronics & Biomedical Engineering and Computer Science (EBBT), Istanbul, Turkey, 24–26 April 2019; pp. 1–7. [Google Scholar]
- Horev, I.; Yger, F.; Sugiyama, M. Geometry-aware principal component analysis for symmetric positive definite matrices. In Asian Conference on Machine Learning; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–16. [Google Scholar]
- Fletcher, P.T.; Joshi, S. Riemannian geometry for the statistical analysis of diffusion tensor data. Signal Process. 2007, 87, 250–262. [Google Scholar] [CrossRef]
- Boldi, P.; Vigna, S. Axioms for centrality. Internet Math. 2014, 10, 222–262. [Google Scholar] [CrossRef]
- Arsigny, V.; Fillard, P.; Pennec, X.; Ayache, N. Geometric means in a novel vector space structure on symmetric positive-definite matrices. SIAM J. Matrix Anal. Appl. 2007, 29, 328–347. [Google Scholar] [CrossRef]
- Fletcher, P.T.; Venkatasubramanian, S.; Joshi, S. The geometric median on Riemannian manifolds with application to robust atlas estimation. NeuroImage 2009, 45, S143–S152. [Google Scholar] [CrossRef]
- Fu, R.; Tian, Y.; Bao, T.; Meng, Z.; Shi, P. Improvement motor imagery EEG classification based on regularized linear discriminant analysis. J. Med Syst. 2019, 43, 169. [Google Scholar] [CrossRef] [PubMed]
- Subasi, A.; Gursoy, M.I. EEG signal classification using PCA, ICA, LDA and support vector machines. Expert Syst. Appl. 2010, 37, 8659–8666. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, G.; Jin, J.; Wang, X.; Cichocki, A. Optimizing spatial patterns with sparse filter bands for motor-imagery based brain–computer interface. J. Neurosci. Methods 2015, 255, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.D.; Feng, D.Z.; Shi, Y.; Liu, W.J. Dimensionality reduction by collaborative preserving Fisher discriminant analysis. Neurocomputing 2019, 356, 228–243. [Google Scholar] [CrossRef]
- Blankertz, B.; Muller, K.R.; Krusienski, D.J.; Schalk, G.; Wolpaw, J.R.; Schlogl, A.; Pfurtscheller, G.; Millan, J.R.; Schroder, M.; Birbaumer, N. The BCI competition III: Validating alternative approaches to actual BCI problems. IEEE Trans. Neural Syst. Rehabil. Eng. 2006, 14, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Tangermann, M.; Müller, K.R.; Aertsen, A.; Birbaumer, N.; Braun, C.; Brunner, C.; Leeb, R.; Mehring, C.; Miller, K.J.; Mueller-Putz, G.; et al. Review of the BCI competition IV. Front. Neurosci. 2012, 6, 55. [Google Scholar] [CrossRef]
- Kumar, S.; Mamun, K.; Sharma, A. CSP-TSM: Optimizing the performance of Riemannian tangent space mapping using common spatial pattern for MI-BCI. Comput. Biol. Med. 2017, 91, 231–242. [Google Scholar] [CrossRef]
- Belwafi, K.; Romain, O.; Gannouni, S.; Ghaffari, F.; Djemal, R.; Ouni, B. An embedded implementation based on adaptive filter bank for brain—Computer interface systems. J. Neurosci. Methods 2018, 305, 1–6. [Google Scholar] [CrossRef]
- Raza, H.; Cecotti, H.; Li, Y.; Prasad, G. Adaptive learning with covariate shift-detection for motor imagery-based brain—Computer interface. Soft Comput. 2016, 20, 3085–3096. [Google Scholar] [CrossRef]

| Functions | Equation |
|---|---|
| Euclidean distance | |
| Riemannian geodesic distance | |
| Log Euclidean distance | |
| Harmonic mean | |
| Resolvent mean | |
| Euclidean geometric median | |
| Riemannian geometric median | |
| Log-Euclidean geometric median |
| Method | K3b | K6b | L1b | Average |
|---|---|---|---|---|
| Arithmetic mean | 91.11 | 91.66 | 73.33 | 85.37 |
| Riemannian geometric mean | 90 | 95 | 68.33 | 84.44 |
| Log Euclidean mean | 88.88 | 93.33 | 66.66 | 82.96 |
| Harmonic mean | 91.11 | 91.66 | 70 | 84.26 |
| Resolvent mean | 91.11 | 95 | 71.66 | 85.92 |
| Euclidean geometric median | 91.11 | 93.33 | 68.33 | 84.26 |
| Riemannian geometric median | 82.22 | 95 | 68.33 | 81.85 |
| Log-Euclidean geometric median | 88.88 | 93.33 | 65 | 82.4 |
| MAD (Proposed) | 93.33 | 95.83 | 75 | 88.05 |
| Subject | Feet | Tongue | Average |
|---|---|---|---|
| K3b | 97.77 | 88.88 | 93.33 |
| K6b | 95.83 | 95.83 | 95.83 |
| L1b | 66.66 | 83.33 | 75.00 |
| Method | A01T | A03T | A07T | A08T | A09T | Average |
|---|---|---|---|---|---|---|
| Arithmetic mean | 84.02 | 85.41 | 89.58 | 92.36 | 76.38 | 85.55 |
| Riemannian geometric mean | 82.63 | 87.5 | 88.19 | 93.75 | 73.61 | 85.14 |
| Log Euclidean mean | 86.8 | 86.8 | 89.58 | 93.75 | 75 | 86.39 |
| Harmonic mean | 83.33 | 84.02 | 89.58 | 93.05 | 81.25 | 86.25 |
| Resolvent mean | 84.02 | 88.88 | 89.58 | 93.75 | 82.63 | 87.77 |
| Euclidean geometric median | 79.86 | 86.11 | 90.72 | 93.05 | 79.16 | 85.78 |
| Riemannian geometric median | 73.61 | 52.08 | 88.88 | 57.63 | 54.86 | 65.41 |
| Log-Euclidean geometric median | 84.72 | 85.41 | 89.58 | 93.05 | 74.3 | 85.41 |
| MAD (Proposed) | 87.5 | 89.17 | 91.67 | 95.83 | 87.5 | 90.33 |
| Subject | Feet | Tongue | Average |
|---|---|---|---|
| A01 | 91.66 | 83.33 | 87.5 |
| A03 | 83.33 | 95 | 89.17 |
| A07 | 100 | 83.33 | 91.67 |
| A08 | 91.66 | 100 | 95.83 |
| A09 | 91.66 | 83.33 | 87.5 |
| Methods | K3b | K6b | L1b | Average |
|---|---|---|---|---|
| SRCSP [11] | 96.67 | 53.33 | 93.33 | 81.11 |
| TSGSP [12] | 99.2 | 67.2 | 96.5 | 87.63 |
| MDRM [19] | 96.66 | 60 | 88.33 | 81.66 |
| HOREV MDRM [23] | 95.56 | 68.33 | 85 | 82.96 |
| WOLA CSP [35] | 97.77 | 61.66 | 93.33 | 84.25 |
| CSP [11] | 95.56 | 61.67 | 93.33 | 83.52 |
| Proposed method | 93.33 | 95.83 | 75.00 | 88.05 |
| Methods | A01 | A03 | A07 | A08 | A09 | Average |
|---|---|---|---|---|---|---|
| CSP+LDA [19] | 78.3 | 82.2 | 81 | 68.5 | 77.4 | 77.48 |
| TLCSP1 [36] | 90.28 | 93.75 | 62.5 | 90.97 | 85.42 | 84.58 |
| FBCSP with LR [17] | 88.26 | 83.88 | 89.17 | 88.93 | 83.33 | 86.71 |
| WOLA CSP [35] | 86.81 | 94.44 | 78.47 | 97.91 | 93.75 | 90.28 |
| TSLDA [19] | 80.5 | 87.5 | 82.1 | 84.8 | 86.1 | 84.2 |
| Proposed method | 87.50 | 89.17 | 91.67 | 95.83 | 87.50 | 90.33 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miah, A.S.M.; Rahim, M.A.; Shin, J. Motor-Imagery Classification Using Riemannian Geometry with Median Absolute Deviation. Electronics 2020, 9, 1584. https://doi.org/10.3390/electronics9101584
Miah ASM, Rahim MA, Shin J. Motor-Imagery Classification Using Riemannian Geometry with Median Absolute Deviation. Electronics. 2020; 9(10):1584. https://doi.org/10.3390/electronics9101584
Chicago/Turabian StyleMiah, Abu Saleh Musa, Md Abdur Rahim, and Jungpil Shin. 2020. "Motor-Imagery Classification Using Riemannian Geometry with Median Absolute Deviation" Electronics 9, no. 10: 1584. https://doi.org/10.3390/electronics9101584
APA StyleMiah, A. S. M., Rahim, M. A., & Shin, J. (2020). Motor-Imagery Classification Using Riemannian Geometry with Median Absolute Deviation. Electronics, 9(10), 1584. https://doi.org/10.3390/electronics9101584







