Myoelectric Control for Upper Limb Prostheses
Abstract
1. Introduction
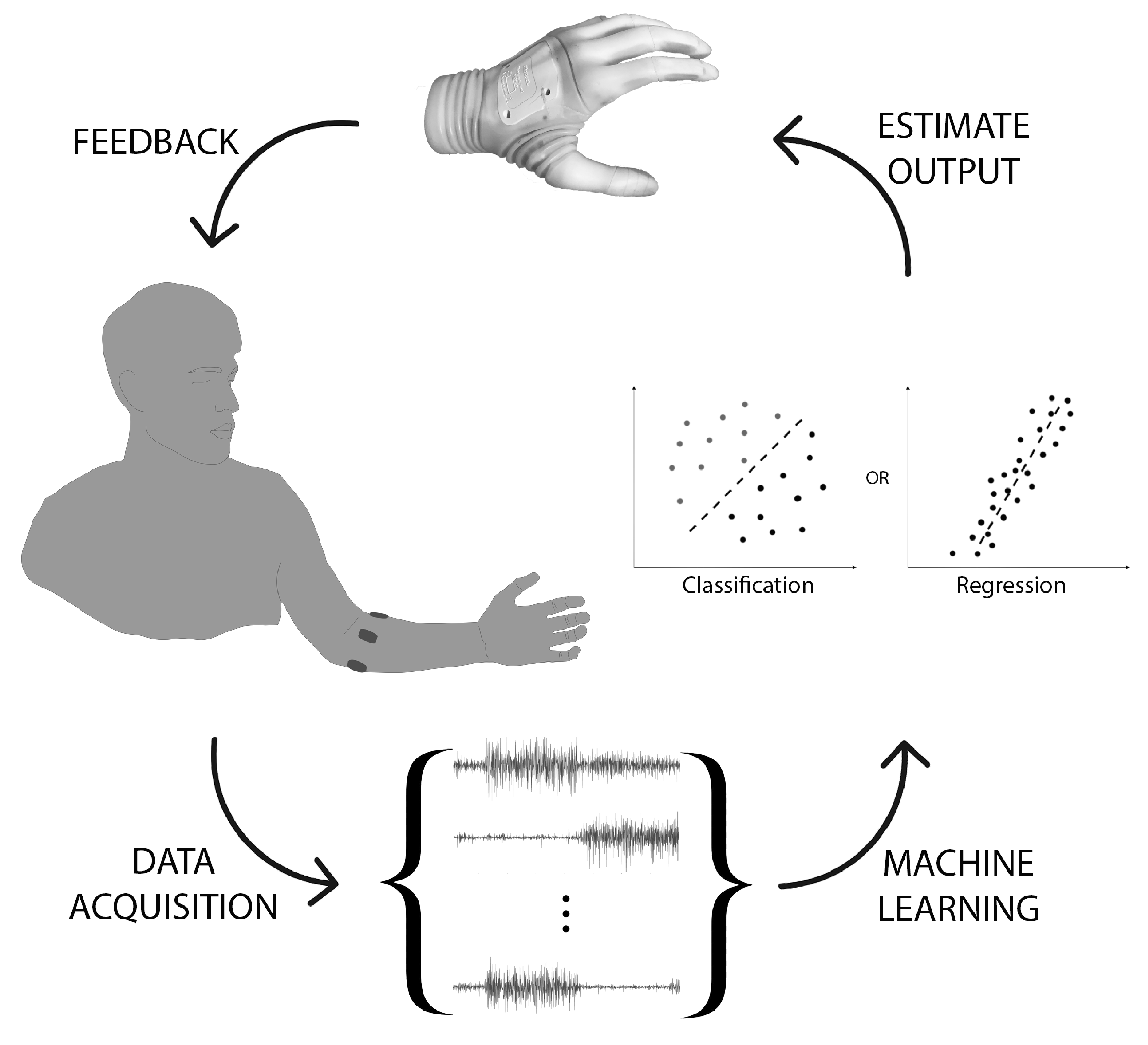
2. Data Acquisition
2.1. Input Source
2.2. Data Amount: Number of Channels and Sampling Frequency
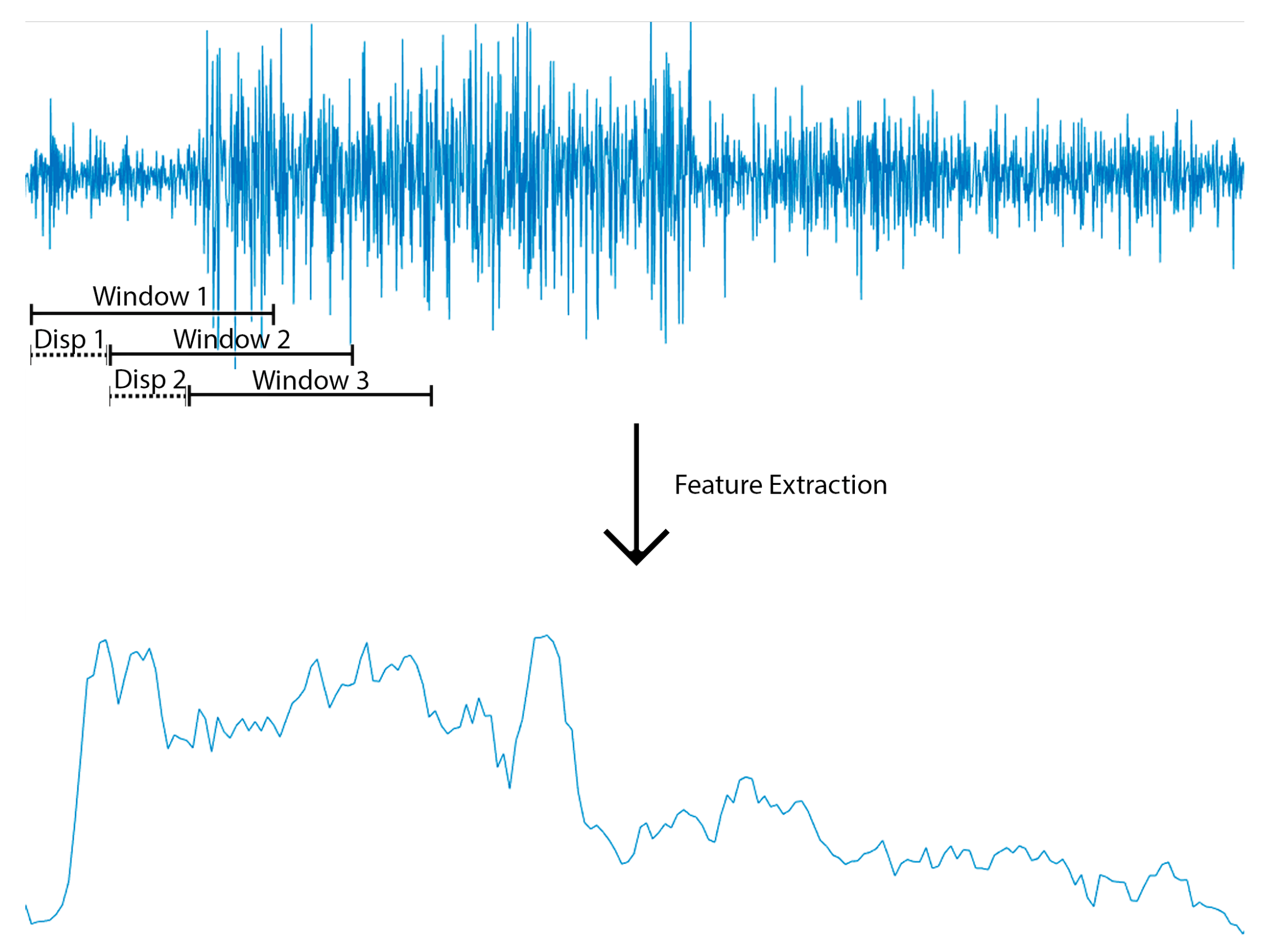
2.3. Data Segmentation: Sample Size for Feature Extraction
2.4. Feature Extraction
3. Learning
3.1. Classification
3.2. Regression
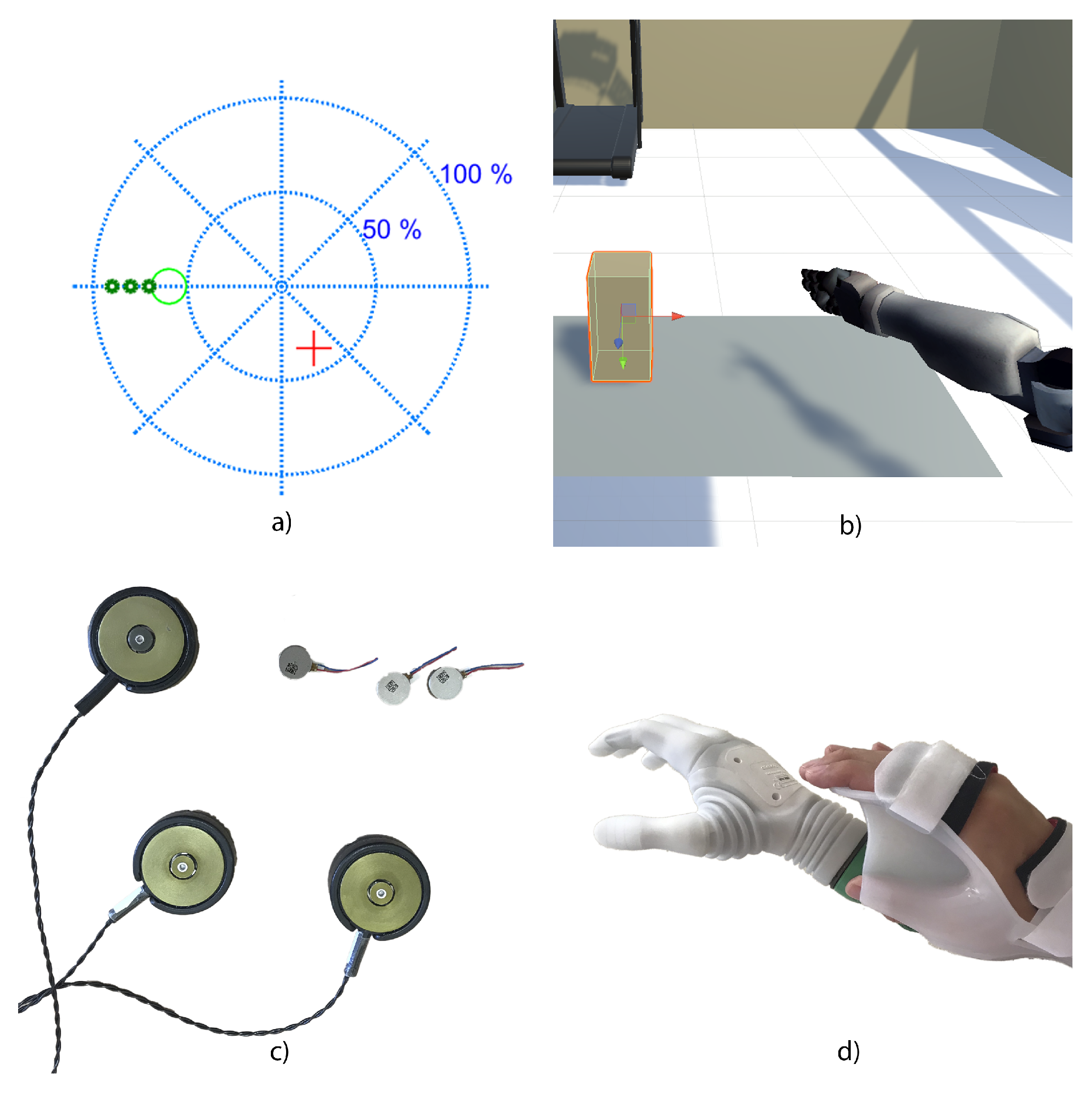
3.3. Feedback
3.4. Human Adaptation
3.5. Co-Adaptation
4. Usability
5. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dillingham, T.R.; Pezzin, L.E.; MacKenzie, E.J. Limb amputation and limb deficiency: Epidemiology and recent trends in the United States. South. Med. J. 2002, 95, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Ziegler-Graham, K.; MacKenzie, E.J.; Ephraim, P.L.; Travison, T.G.; Brookmeyer, R. Estimating the Prevalence of Limb Loss in the United States: 2005 to 2050. Arch. Phys. Med. Rehabil. 2008, 89, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Biddiss, E.; Chau, T. Upper-limb prosthetics: Critical factors in device abandonment. Am. J. Phys. Med. Rehabil. 2007, 86, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Datta, D.; Selvarajah, K.; Davey, N. Functional outcome of patients with proximal upper limb deficiency–acquired and congenital. Clin. Rehabil. 2004, 18, 172–177. [Google Scholar] [CrossRef]
- Davidson, J. A survey of the satisfaction of upper limb amputees with their prostheses, their lifestyles, and their abilities. J. Hand Ther. 2002, 15, 62–70. [Google Scholar] [CrossRef]
- Scheme, E.; Englehart, K. Electromyogram pattern recognition for control of powered upper-limb prostheses: State of the art and challenges for clinical use. J. Rehabil. Res. Dev. 2011, 48, 643–659. [Google Scholar] [CrossRef]
- Scheme, E.; Fougner, A.; Stavdahl, O.; Chan, A.D.C.; Englehart, K. Examining the adverse effects of limb position on pattern recognition based myoelectric control. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 6337–6340. [Google Scholar] [CrossRef]
- Dohnálek, P.; Gajdoš, P.; Peterek, T. Human activity recognition on raw sensor data via sparse approximation. In Proceedings of the 2013 36th International Conference on Telecommunications and Signal Processing (TSP), Rome, Italy, 2–4 July 2013; pp. 700–703. [Google Scholar] [CrossRef]
- Marasco, P.D.; Hebert, J.S.; Sensinger, J.W.; Shell, C.E.; Schofield, J.S.; Thumser, Z.C.; Nataraj, R.; Beckler, D.T.; Dawson, M.R.; Blustein, D.H.; et al. Illusory movement perception improves motor control for prosthetic hands. Sci. Transl. Med. 2018, 10. [Google Scholar] [CrossRef]
- Biddiss, E.A.; Chau, T.T. Upper limb prosthesis use and abandonment: A survey of the last 25 years. Prosthet. Orthot. Int. 2007, 31, 236–257. [Google Scholar] [CrossRef]
- Vujaklija, I.; Farina, D.; Aszmann, O.C. New developments in prosthetic arm systems. Orthop. Res. Rev. 2016, 8, 31–39. [Google Scholar] [CrossRef]
- Jiang, N.; Dosen, S.; Muller, K.; Farina, D. Myoelectric Control of Artificial Limbs—Is There a Need to Change Focus? IEEE Signal Process. Mag. 2012, 29, 150–152. [Google Scholar] [CrossRef]
- Ison, M.; Vujaklija, I.; Whitsell, B.; Farina, D.; Artemiadis, P. High-Density Electromyograph and Motor Skill Learning for Robust Long-Term Control of a 7-DoF Robot Arm. IEEE Trans. Neural Syst. Rehabil. Eng. 2016, 24, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Hahne, J.M.; Dähne, S.; Hwang, H.J.; Müller, K.R.; Parra, L.C. Concurrent adaptation of human and machine improves simultaneous and proportional myoelectric control. IEEE Trans. Neural Syst. Rehabil. Eng. 2015, 23, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Ameri, A.; Akhaee, M.A.; Scheme, E.; Englehart, K. Regression convolutional neural network for improved simultaneous EMG control. J. Neural Eng. 2019, 16, 036015. [Google Scholar] [CrossRef] [PubMed]
- Scheme, E.J.; Englehart, K.B.; Hudgins, B.S. Selective classification for improved robustness of myoelectric control under nonideal conditions. IEEE Trans. Biomed. Eng. 2011, 58, 1698–1705. [Google Scholar] [CrossRef]
- Jiang, N.; Farina, D. Myoelectric control of upper limb prosthesis: Current status, challenges and recent advances. Front. Neuroeng. 2014. [Google Scholar] [CrossRef]
- Fougner, A.; Scheme, E.; Chan, A.D.C.; Englehart, K.; Stavdahl, O. Resolving the Limb Position Effect in Myoelectric Pattern Recognition. IEEE Trans. Neural Syst. Rehabil. Eng. 2011, 19, 644–651. [Google Scholar] [CrossRef]
- Young, A.J.; Hargrove, L.J.; Kuiken, T.A. The effects of electrode size and orientation on the sensitivity of myoelectric pattern recognition systems to electrode shift. IEEE Trans. Biomed. Eng. 2011, 58, 2537–2544. [Google Scholar] [CrossRef]
- Cipriani, C.; Sassu, R.; Controzzi, M.; Carrozza, M.C. Influence of the weight actions of the hand prosthesis on the performance of pattern recognition based myoelectric control: Preliminary study. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 1620–1623. [Google Scholar] [CrossRef]
- Amsuess, S.; Paredes, L.P.; Rudigkeit, N.; Graimann, B.; Herrmann, M.J.; Farina, D. Long term stability of surface EMG pattern classification for prosthetic control. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 3622–3625. [Google Scholar] [CrossRef]
- Vujaklija, I.; Roche, A.D.; Hasenoehrl, T.; Sturma, A.; Amsuess, S.; Farina, D.; Aszmann, O.C. Translating Research on Myoelectric Control into Clinics—Are the Performance Assessment Methods Adequate? Front. Neurorobot. 2017, 11, 7. [Google Scholar] [CrossRef]
- Hargrove, L.J.; Miller, L.A.; Turner, K.; Kuiken, T.A. Myoelectric Pattern Recognition Outperforms Direct Control for Transhumeral Amputees with Targeted Muscle Reinnervation: A Randomized Clinical Trial. Sci. Rep. 2017, 7, 2045–2322. [Google Scholar] [CrossRef]
- Hochberg, L.R.; Serruya, M.D.; Friehs, G.M.; Mukand, J.A.; Saleh, M.; Caplan, A.H.; Branner, A.; Chen, D.; Penn, R.D.; Donoghue, J.P. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature 2006, 442, 164–171. [Google Scholar] [CrossRef]
- Velliste, M.; Perel, S.; Spalding, M.C.; Whitford, A.S.; Schwartz, A.B. Cortical control of a prosthetic arm for self-feeding. Nature 2008, 453, 1098–1101. [Google Scholar] [CrossRef] [PubMed]
- McMullen, D.P.; Hotson, G.; Katyal, K.D.; Wester, B.A.; Fifer, M.S.; McGee, T.G.; Harris, A.; Johannes, M.S.; Vogelstein, R.J.; Ravitz, A.D.; et al. Demonstration of a Semi-Autonomous Hybrid Brain–Machine Interface Using Human Intracranial EEG, Eye Tracking, and Computer Vision to Control a Robotic Upper Limb Prosthetic. IEEE Trans. Neural Syst. Rehabil. Eng. 2014, 22, 784–796. [Google Scholar] [CrossRef] [PubMed]
- Frisoli, A.; Loconsole, C.; Leonardis, D.; Banno, F.; Barsotti, M.; Chisari, C.; Bergamasco, M. A New Gaze-BCI-Driven Control of an Upper Limb Exoskeleton for Rehabilitation in Real-World Tasks. IEEE Trans. Syst. Man Cybern. Part C (Appl. Rev.) 2012, 42, 1169–1179. [Google Scholar] [CrossRef]
- Ganguly, K.; Carmena, J.M. Emergence of a Stable Cortical Map for Neuroprosthetic Control. PLoS Biol. 2009, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Galán, F.; Nuttin, M.; Lew, E.; Ferrez, P.; Vanacker, G.; Philips, J.; del R. Millán, J. A brain-actuated wheelchair: Asynchronous and non-invasive Brain–computer interfaces for continuous control of robots. Clin. Neurophysiol. 2008, 119, 2159–2169. [Google Scholar] [CrossRef] [PubMed]
- Sherman, E.D. A Russian bioelectric-controlled prosthesis. Can. Med. Assoc. J. 1964, 91, 1268–1270. [Google Scholar] [PubMed]
- Childress, D.S. Historical aspects of powered limb prostheses. Clin. Prosthet. Orthot. 1985, 9, 2–13. [Google Scholar]
- Marquardt, E. The Heidelberg pneumatic arm prosthesis. J. Bone Jt. 1965, 47, 425–434. [Google Scholar] [CrossRef]
- Englehart, K.; Hudgins, B. A robust, real-time control scheme for multifunction myoelectric control. IEEE Trans. Biomed. Eng. 2003, 50, 848–854. [Google Scholar] [CrossRef]
- Parker, P.; Englehart, K.; Hudgins, B. Myoelectric signal processing for control of powered limb prostheses. J. Electromyogr. Kinesiol. 2006, 16, 541–548. [Google Scholar] [CrossRef]
- Fougner, A.; Stavdahl, Ø.; Kyberd, P.J.; Losier, Y.G.; Parker, P.A. Control of upper limb prostheses: Terminology and proportional myoelectric control—A review. IEEE Trans. Neural Syst. Rehabil. Eng. 2012, 20, 663–677. [Google Scholar] [CrossRef] [PubMed]
- Resnik, L.; Huang, H.H.; Winslow, A.; Crouch, D.L.; Zhang, F.; Wolk, N. Evaluation of EMG pattern recognition for upper limb prosthesis control: A case study in comparison with direct myoelectric control. J. Neuroeng. Rehabil. 2018, 15, 23. [Google Scholar] [CrossRef] [PubMed]
- Sartori, M.; Durandau, G.; Došen, S.; Farina, D. Robust simultaneous myoelectric control of multiple degrees of freedom in wrist-hand prostheses by real-time neuromusculoskeletal modeling. J. Neural Eng. 2018, 15, 066026. [Google Scholar] [CrossRef] [PubMed]
- Kuiken, T.A.; Li, G.; Lock, B.A.; Lipschutz, R.D.; Miller, L.A.; Stubblefield, K.A.; Englehart, K.B. Targeted muscle reinnervation for real-time myoelectric control of multifunction artificial arms. JAMA 2009, 301, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zhou, P.; Li, G.; Kuiken, T.A. An Analysis of EMG Electrode Configuration for Targeted Muscle Reinnervation Based Neural Machine Interface. IEEE Trans. Neural Syst. Rehabil. Eng. 2008, 16, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.M.; Cheesborough, J.E.; Ko, J.H.; Cho, M.S.; Kuiken, T.A.; Dumanian, G.A. Targeted Muscle Reinnervation: A Novel Approach to Postamputation Neuroma Pain. Clin. Orthop. Relat. Res. 2014, 472, 2984–2990. [Google Scholar] [CrossRef]
- Miller, L.A.; Lipschutz, R.D.; Stubblefield, K.A.; Lock, B.A.; Huang, H.; Williams, T.W.; Weir, R.F.; Kuiken, T.A. Control of a Six Degree of Freedom Prosthetic Arm After Targeted Muscle Reinnervation Surgery. Arch. Phys. Med. Rehabil. 2008, 89, 2057–2065. [Google Scholar] [CrossRef]
- Kuiken, T.A.; Dumanian, G.A.; Lipschutz, R.D.; Miller, L.A.; Stubblefield, K.A. The use of targeted muscle reinnervation for improved myoelectric prosthesis control in a bilateral shoulder disarticulation amputee. Prosthet. Orthot. Int. 2004, 28, 245–253. [Google Scholar] [CrossRef]
- Dumanian, G.A.; Potter, B.K.; Mioton, L.M.; Ko, J.H.; Cheesborough, J.E.; Souza, J.M.; Ertl, W.J.; Tintle, S.M.; Nanos, G.P.; Valerio, I.L.; et al. Targeted muscle reinnervation treats neuroma and phantom pain in major limb amputees: A randomized clinical trial. Ann. Surg. 2019, 270, 238–246. [Google Scholar] [CrossRef]
- Mioton, L.M.; Dumanian, G.A. Targeted muscle reinnervation and prosthetic rehabilitation after limb loss. J. Surg. Oncol. 2018, 118, 807–814. [Google Scholar] [CrossRef]
- Young, A.; Smith, L.; Rouse, E.; Hargrove, L. Classification of Simultaneous Movements using Surface EMG Pattern Recognition. IEEE Trans. Biomed. Eng. 2013, 60, 1250–1258. [Google Scholar] [CrossRef] [PubMed]
- Hahne, J.M.; Rehbaum, H.; Biessmann, F.; Meinecke, F.C.; Müller, K.R.; Jiang, N.; Farina, D.; Parra, L.C. Simultaneous and proportional control of 2D wrist movements with myoelectric signals. In Proceedings of the 2012 IEEE International Workshop on Machine Learning for Signal Processing, Santander, Spain, 23–26 September 2012; pp. 1–6. [Google Scholar] [CrossRef]
- Hahne, J.; Biessmann, F.; Jiang, N.; Rehbaum, H.; Farina, D.; Meinecke, F.; Muller, K.R.; Parra, L. Linear and nonlinear regression techniques for simultaneous and proportional myoelectric control. IEEE Trans. Neural Syst. Rehabil. Eng. 2014, 22, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Oskoei, M.A.; Hu, H. Myoelectric control systems—A survey. Biomed. Signal Process. Control 2007, 2, 275–294. [Google Scholar] [CrossRef]
- Peerdeman, B.; Boere, D.; Witteveen, H.; Huis in `tVeld, R.; Hermens, H.; Stramigioli, S.; Rietman, H.; Veltink, P.; Misra, S. Myoelectric forearm prostheses: State of the art from a user-centered perspective. J. Rehabil. Res. Dev. 2011, 48, 719–737. [Google Scholar] [CrossRef]
- Sensinger, J.W.; Lock, B.A.; Kuiken, T.A. Adaptive Pattern Recognition of Myoelectric Signals: Exploration of Conceptual Framework and Practical Algorithms. IEEE Trans. Neural Syst. Rehabil. Eng. 2009, 17, 270–278. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, D.; Zhu, X. Application of a self-enhancing classification method to electromyography pattern recognition for multifunctional prosthesis control. J. NeuroEng. Rehabil. 2013, 10, 44. [Google Scholar] [CrossRef]
- Pilarski, P.; Dawson, M.; Degris, T.; Fahimi, F.; Carey, J.; Sutton, R. Online human training of a myoelectric prosthesis controller via actor-critic reinforcement learning. In Proceedings of the 2011 IEEE International Conference on Rehabilitation Robotics (ICORR), Zurich, Switzerland, 29 June–1 July 2011. [Google Scholar]
- Clancy, E.A.; Morin, E.L.; Merletti, R. Sampling, noise-reduction and amplitude estimation issues in surface electromyography. J. Electromyogr. Kinesiol. 2002, 12, 1–16. [Google Scholar] [CrossRef]
- Ajiboye, A.B.; Weir, R.F. A heuristic fuzzy logic approach to EMG pattern recognition for multifunctional prosthesis control. IEEE Trans. Neural Syst. Rehabil. Eng. 2005, 13, 280–291. [Google Scholar] [CrossRef]
- Chu, J.U.; Moon, I.; Mun, M.S. A real-time EMG pattern recognition system based on linear-nonlinear feature projection for a multifunction myoelectric hand. IEEE Trans. Biomed. Eng. 2006, 53, 2232–2239. [Google Scholar]
- Farina, D.; Merletti, R.; Enoka, R.M. The extraction of neural strategies from the surface EMG. J. Appl. Physiol. 2004, 96, 1486–1495. [Google Scholar] [CrossRef]
- Li, G.; Li, Y.; Yu, L.; Geng, Y. Conditioning and Sampling Issues of EMG Signals in Motion Recognition of Multifunctional Myoelectric Prostheses. Ann. Biomed. Eng. 2011, 39, 1779–1787. [Google Scholar] [CrossRef] [PubMed]
- Phinyomark, A.; Khushaba, R.N.; Scheme, E. Feature Extraction and Selection for Myoelectric Control Based on Wearable EMG Sensors. Sensors 2018, 18, 1615. [Google Scholar] [CrossRef] [PubMed]
- Farrell, T.R.; Weir, R.F. The Optimal Controller Delay for Myoelectric Prostheses. IEEE Trans. Neural Syst. Rehabil. Eng. 2007, 15, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.L.; Holmgaard, S.; Jiang, N.; Englehart, K.B.; Farina, D.; Parker, P.A. Simultaneous and Proportional Force Estimation for Multifunction Myoelectric Prostheses Using Mirrored Bilateral Training. IEEE Trans. Biomed. Eng. 2011, 58, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Phinyomark, A.; Quaine, F.; Charbonnier, S.; Serviere, C.; Tarpin-Bernard, F.; Laurillau, Y. EMG feature evaluation for improving myoelectric pattern recognition robustness. Expert Syst. Appl. 2013, 40, 4832–4840. [Google Scholar] [CrossRef]
- Graupe, D.; Cline, W.K. Functional separation of EMG signals via ARMA identification methods for prosthesis control purposes. IEEE Trans. Syst. Man, Cybern. 1975, 1, 252–259. [Google Scholar] [CrossRef]
- Kelly, M.F.; Parker, P.A.; Scott, R.N. The application of neural networks to myoelectric signal analysis: A preliminary study. IEEE Trans. Biomed. Eng. 1990, 37, 221–230. [Google Scholar] [CrossRef]
- Spanias, J.A.; Perreault, E.J.; Hargrove, L.J. Detection of and compensation for EMG disturbances for powered lower limb prosthesis control. IEEE Trans. Neural Syst. Rehabil. Eng. 2015, 24, 226–234. [Google Scholar] [CrossRef]
- Huang, Y.; Englehart, K.B.; Hudgins, B.; Chan, A.D. A Gaussian mixture model based classification scheme for myoelectric control of powered upper limb prostheses. IEEE Trans. Biomed. Eng. 2005, 52, 1801–1811. [Google Scholar] [CrossRef]
- Castellini, C.; van der Smagt, P. Surface EMG in advanced hand prosthetics. Biol. Cybern. 2009, 100, 35–47. [Google Scholar] [CrossRef]
- Alkan, A.; Günay, M. Identification of EMG signals using discriminant analysis and SVM classifier. Expert Syst. Appl. 2012, 39, 44–47. [Google Scholar] [CrossRef]
- Al-Timemy, A.H.; Bugmann, G.; Escudero, J.; Outram, N. Classification of finger movements for the dexterous hand prosthesis control with surface electromyography. IEEE J. Biomed. Health Informat. 2013, 17, 608–618. [Google Scholar] [CrossRef]
- Chan, A.D.; Englehart, K.B. Continuous myoelectric control for powered prostheses using hidden Markov models. IEEE Trans. Biomed. Eng. 2004, 52, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Zardoshti-Kermani, M.; Wheeler, B.C.; Badie, K.; Hashemi, R.M. EMG feature evaluation for movement control of upper extremity prostheses. IEEE Trans. Rehabil. Eng. 1995, 3, 324–333. [Google Scholar] [CrossRef]
- Karlik, B.; Tokhi, M.O.; Alci, M. A fuzzy clustering neural network architecture for multifunction upper-limb prosthesis. IEEE Trans. Biomed. Eng. 2003, 50, 1255–1261. [Google Scholar] [CrossRef]
- Rahimi, A.; Benatti, S.; Kanerva, P.; Benini, L.; Rabaey, J.M. Hyperdimensional biosignal processing: A case study for EMG-based hand gesture recognition. In Proceedings of the 2016 IEEE International Conference on Rebooting Computing (ICRC), San Diego, CA, USA, 17–19 October 2016; pp. 1–8. [Google Scholar]
- Hudgins, B.; Parker, P.; Scott, R.N. A new strategy for multifunction myoelectric control. IEEE Trans. Biomed. Eng. 1993, 40, 82–94. [Google Scholar] [CrossRef]
- Hargrove, L.J.; Li, G.; Englehart, K.B.; Hudgins, B.S. Principal components analysis preprocessing for improved classification accuracies in pattern-recognition-based myoelectric control. IEEE Trans. Biomed. Eng. 2008, 56, 1407–1414. [Google Scholar] [CrossRef]
- Li, X.; Samuel, O.W.; Zhang, X.; Wang, H.; Fang, P.; Li, G. A motion-classification strategy based on sEMG-EEG signal combination for upper-limb amputees. J. Neuroeng. Rehabil. 2017, 14, 2. [Google Scholar] [CrossRef]
- Peleg, D.; Braiman, E.; Yom-Tov, E.; Inbar, G.F. Classification of finger activation for use in a robotic prosthesis arm. IEEE Trans. Neural Syst. Rehabil. Eng. 2002, 10, 290–293. [Google Scholar] [CrossRef]
- Oskoei, M.A.; Hu, H. GA-based feature subset selection for myoelectric classification. In Proceedings of the 2006 IEEE International Conference on Robotics and Biomimetics, Kunming, China, 17–20 December 2006; pp. 1465–1470. [Google Scholar]
- Huang, H.P.; Liu, Y.H.; Liu, L.W.; Wong, C.S. EMG classification for prehensile postures using cascaded architecture of neural networks with self-organizing maps. In Proceedings of the 2003 IEEE International Conference on Robotics and Automation (Cat. No. 03CH37422), Taipei, Taiwan, 14–19 September 2003; Volume 1, pp. 1497–1502. [Google Scholar]
- Khushaba, R.N.; Al-Jumaily, A. Channel and feature selection in multifunction myoelectric control. In Proceedings of the 2007 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Lyon, France, 22–26 August 2007; pp. 5182–5185. [Google Scholar]
- Hahne, J.M.; Graimann, B.; Muller, K.R. Spatial filtering for robust myoelectric control. IEEE Trans. Biomed. Eng. 2012, 59, 1436–1443. [Google Scholar] [CrossRef]
- Almström, C.; Herberts, P.; Körner, L. Experience with Swedish multifunctional prosthetic hands controlled by pattern recognition of multiple myoelectric signals. Int. Orthop. 1981, 5, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Lorrain, T.; Jiang, N.; Farina, D. Influence of the training set on the accuracy of surface EMG classification in dynamic contractions for the control of multifunction prostheses. J. Neuroeng. Rehabil. 2011, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Farina, D.; Jiang, N.; Rehbaum, H.; Holobar, A.; Graimann, B.; Dietl, H.; Aszmann, O.C. The extraction of neural information from the surface EMG for the control of upper-limb prostheses: Emerging avenues and challenges. IEEE Trans. Neural Syst. Rehabil. Eng. 2014, 22, 797–809. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhang, D.; Jiang, N.; Sheng, X.; Farina, D.; Zhu, X. User adaptation in long-term, open-loop myoelectric training: Implications for EMG pattern recognition in prosthesis control. J. Neural Eng. 2015, 12, 046005. [Google Scholar] [CrossRef]
- Vidovic, M.M.; Hwang, H.J.; Amsuess, S.; Hahne, J.M.; Farina, D.; Muller, K.R. Improving the robustness of myoelectric pattern recognition for upper limb prostheses by covariate shift adaptation. IEEE Trans. Neural Syst. Rehabil. Eng. 2016, 24, 961–970. [Google Scholar] [CrossRef]
- Von Bünau, P.; Meinecke, F.C.; Király, F.C.; Müller, K.R. Finding stationary subspaces in multivariate time series. Phys. Rev. Lett. 2009, 103, 214101. [Google Scholar] [CrossRef]
- Samek, W.; Vidaurre, C.; Müller, K.R.; Kawanabe, M. Stationary common spatial patterns for brain–computer interfacing. J. Neural Eng. 2012, 9, 026013. [Google Scholar] [CrossRef]
- Radmand, A.; Scheme, E.; Englehart, K. A characterization of the effect of limb position on EMG features to guide the development of effective prosthetic control schemes. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 662–667. [Google Scholar]
- Roy, S.H.; De Luca, G.; Cheng, M.S.; Johansson, A.; Gilmore, L.D.; De Luca, C.J. Electro-mechanical stability of surface EMG sensors. Med. Biol. Eng. Comput. 2007, 45, 447–457. [Google Scholar] [CrossRef]
- Hargrove, L.; Englehart, K.; Hudgins, B. A training strategy to reduce classification degradation due to electrode displacements in pattern recognition based myoelectric control. Biomed. Signal Process. Control 2008, 3, 175–180. [Google Scholar] [CrossRef]
- Hahne, J.M.; Markovic, M.; Farina, D. User adaptation in Myoelectric Man-Machine Interfaces. Sci. Rep. 2017, 7, 4437. [Google Scholar] [CrossRef]
- Jiang, N.; Englehart, K.B.; Parker, P.A. Extracting simultaneous and proportional neural control information for multiple-DOF prostheses from the surface electromyographic signal. IEEE Trans. Bio-Med. Eng. 2009, 56, 1070–1080. [Google Scholar] [CrossRef] [PubMed]
- Ameri, A.; Kamavuako, E.N.; Scheme, E.J.; Englehart, K.B.; Parker, P.A. Support Vector Regression for Improved Real-Time, Simultaneous Myoelectric Control. IEEE Trans. Neural Syst. Rehabil. Eng. 2014, 22, 1198–1209. [Google Scholar] [CrossRef] [PubMed]
- Ameri, A.; Scheme, E.; Kamavuako, E.; Englehart, K.; Parker, P. Real-Time, Simultaneous Myoelectric Control Using Force and Position-Based Training Paradigms. Biomed. Eng. IEEE Trans. 2014, 61, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Ameri, A.; Kamavuako, E.N.; Scheme, E.J.; Englehart, K.B.; Parker, P.A. Real-time, simultaneous myoelectric control using visual target-based training paradigm. Biomed. Signal Process. Control 2014, 13, 8–14. [Google Scholar] [CrossRef]
- Igual, C.; Igual, J.; Hahne, J.M.; Parra, L.C. Adaptive Auto-Regressive Proportional Myoelectric Control. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.J.; Hahne, J.; Mueller, K.R. Real-time robustness evaluation of regression based myoelectric control against arm position change and donning/doffing. PLoS ONE 2017, 12, e0186318. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Yang, D.; Jiang, L.; Zhang, H.; Liu, H.; Kotani, K.; Huang, Q.; Yang, D.; Jiang, L.; Zhang, H.; et al. A Novel Unsupervised Adaptive Learning Method for Long-Term Electromyography (EMG) Pattern Recognition. Sensors 2017, 17, 1370. [Google Scholar] [CrossRef] [PubMed]
- Strazzulla, I.; Nowak, M.; Controzzi, M.; Cipriani, C.; Castellini, C. Online Bimanual Manipulation Using Surface Electromyography and Incremental Learning. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 25, 227–234. [Google Scholar] [CrossRef]
- Smith, L.H.; Kuiken, T.A.; Hargrove, L.J. Evaluation of Linear Regression Simultaneous Myoelectric Control Using Intramuscular EMG. IEEE Trans. Biomed. Eng. 2016, 63, 737–746. [Google Scholar] [CrossRef]
- Engeberg, E.D.; Meek, S.G.; Minor, M.A. Hybrid force–velocity sliding mode control of a prosthetic hand. IEEE Trans. Biomed. Eng. 2008, 55, 1572–1581. [Google Scholar] [CrossRef]
- Muceli, S.; Farina, D. Simultaneous and Proportional Estimation of HandKinematics From EMG During Mirrored Movements at MultipleDegrees-of-Freedom. Neural Syst. Rehabil. Eng. IEEE Trans. 2012, 20, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Ameri, A.; Englehart, K.B.; Parker, P.A. A comparison between force and position control strategies in myoelectric prostheses. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; pp. 1342–1345. [Google Scholar] [CrossRef]
- Fang, Y.; Zhou, D.; Li, K.; Liu, H. Interface Prostheses With Classifier-Feedback-Based User Training. IEEE Trans. Biomed. Eng. 2017, 64, 2575–2583. [Google Scholar] [CrossRef] [PubMed]
- Powell, M.; Kaliki, R.; Thakor, N. User training for pattern recognition-based myoelectric prostheses: Improving phantom limb movement consistency and distinguishability. IEEE Trans. Neural Syst. Rehabil. Eng. 2014, 22, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.; Ung, G.; McGarvey, C.; Brown, J.D. Comparison of vibrotactile and joint-torque feedback in a myoelectric upper-limb prosthesis. J. Neuroeng. Rehabil. 2019, 16, 70. [Google Scholar] [CrossRef]
- Guémann, M.; Bouvier, S.; Halgand, C.; Borrini, L.; Paclet, F.; Lapeyre, E.; Ricard, D.; Cattaert, D.; de Rugy, A. Sensory and motor parameter estimation for elbow myoelectric control with vibrotactile feedback. Ann. Phys. Rehabil. Med. 2018, 61, e467. [Google Scholar] [CrossRef]
- Markovic, M.; Schweisfurth, M.A.; Engels, L.F.; Farina, D.; Dosen, S. Myocontrol is closed-loop control: Incidental feedback is sufficient for scaling the prosthesis force in routine grasping. J. Neuroeng. Rehabil. 2018, 15, 81. [Google Scholar] [CrossRef]
- Radhakrishnan, S.M.; Baker, S.N.; Jackson, A. Learning a Novel Myoelectric-Controlled Interface Task. J. Neurophysiol. 2008, 100, 2397–2408. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Liu, J.; Zhang, D.; Sheng, X.; Jiang, N. Cascaded Adaptation Framework for Fast Calibration of Myoelectric Control. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 25, 254–264. [Google Scholar] [CrossRef]
- Nishikawa, D.; Yu, W.; Yokoi, H.; Kakazu, Y. On-line learning method for EMG prosthetic hand control. Electron. Commun. Jpn. (Part III Fundam. Electron. Sci.) 2001, 84, 35–46. [Google Scholar] [CrossRef]
- Betthauser, J.L.; Hunt, C.L.; Osborn, L.E.; Masters, M.R.; Lévay, G.; Kaliki, R.R.; Thakor, N.V. Limb Position Tolerant Pattern Recognition for Myoelectric Prosthesis Control with Adaptive Sparse Representations From Extreme Learning. IEEE Trans. Biomed. Eng. 2018, 65, 770–778. [Google Scholar] [CrossRef]
- Müller, J.S.; Vidaurre, C.; Schreuder, M.; Meinecke, F.C.; Von Bünau, P.; Müller, K.R. A mathematical model for the two-learners problem. J. Neural Eng. 2017, 14, 036005. [Google Scholar] [CrossRef] [PubMed]
- Couraud, M.; Cattaert, D.; Paclet, F.; Oudeyer, P.Y.; Rugy, A.d. Model and experiments to optimize co-adaptation in a simplified myoelectric control system. J. Neural Eng. 2018, 15, 026006. [Google Scholar] [CrossRef] [PubMed]
- Ottobock Website. Available online: https://www.ottobock.de (accessed on 15 September 2019).
- Coapt Engineering Website. Available online: https://www.coaptengineering.com (accessed on 15 September 2019).
- Yeung, D.; Farina, D.; Vujaklija, I. Directional Forgetting for Stable Co-Adaptation in Myoelectric Control. Sensors 2019, 19, 2203. [Google Scholar] [CrossRef] [PubMed]
- Hahne, J.M.; Schweisfurth, M.A.; Koppe, M.; Farina, D. Simultaneous control of multiple functions of bionic hand prostheses: Performance and robustness in end users. Sci. Robot. 2018, 3. [Google Scholar] [CrossRef]
- Paaßen, B.; Schulz, A.; Hahne, J.; Hammer, B. Expectation maximization transfer learning and its application for bionic hand prostheses. Neurocomputing 2018, 298, 122–133. [Google Scholar] [CrossRef]
- Braun, D.A.; Waldert, S.; Aertsen, A.; Wolpert, D.M.; Mehring, C. Structure Learning in a Sensorimotor Association Task. PLoS ONE 2010, 5, 1–8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wheaton, L.A. Neurorehabilitation in upper limb amputation: Understanding how neurophysiological changes can affect functional rehabilitation. J. Neuroeng. Rehabil. 2017, 14, 41. [Google Scholar] [CrossRef]
- Amsuess, S.; Vujaklija, I.; Goebel, P.; Roche, A.D.; Graimann, B.; Aszmann, O.C.; Farina, D. Context-Dependent Upper Limb Prosthesis Control for Natural and Robust Use. IEEE Trans. Neural Syst. Rehabil. Eng. 2016, 24, 744–753. [Google Scholar] [CrossRef]
- Kuiken, T.A.; Miller, L.A.; Turner, K.; Hargrove, L.J. A comparison of pattern recognition control and direct control of a multiple degree-of-freedom transradial prosthesis. IEEE J. Transl. Eng. Health Med. 2016, 4, 1–8. [Google Scholar] [CrossRef]
- Markovic, M.; Schweisfurth, M.A.; Engels, L.F.; Bentz, T.; Wüstefeld, D.; Farina, D.; Dosen, S. The clinical relevance of advanced artificial feedback in the control of a multi-functional myoelectric prosthesis. J. Neuroeng. Rehabil. 2018, 15, 28. [Google Scholar] [CrossRef]
- Dargazany, A.R.; Abtahi, M.; Mankodiya, K. An end-to-end (deep) neural network applied to raw EEG, fNIRs and body motion data for data fusion and BCI classification task without any pre-/post-processing. arXiv 2019, arXiv:1907.09523. [Google Scholar]
- Silva, K.; Rand, S.; Cancel, D.; Chen, Y.; Kathirithamby, R.; Stern, M. Three-dimensional (3-D) printing: A cost-effective solution for improving global accessibility to prostheses. PM&R 2015, 7, 1312–1314. [Google Scholar]
- Farahani, B.; Firouzi, F.; Chang, V.; Badaroglu, M.; Constant, N.; Mankodiya, K. Towards fog-driven IoT eHealth: Promises and challenges of IoT in medicine and healthcare. Future Gener. Comput. Syst. 2018, 78, 659–676. [Google Scholar] [CrossRef]
- Hiremath, S.; Yang, G.; Mankodiya, K. Wearable Internet of Things: Concept, architectural components and promises for person-centered healthcare. In Proceedings of the 2014 4th International Conference on Wireless Mobile Communication and Healthcare-Transforming Healthcare Through Innovations in Mobile and Wireless Technologies (MOBIHEALTH), Athens, Greece, 3–5 November 2014; pp. 304–307. [Google Scholar]
- LeMoyne, R. Future and advanced concepts for the powered prosthesis. In Advances for Prosthetic Technology; Springer: Tokyo, Japan, 2016; pp. 127–130. [Google Scholar]
- Li, G.; Zhang, L.; Sun, Y.; Kong, J. Towards the sEMG hand: Internet of things sensors and haptic feedback application. Multimed. Tools Appl. 2018, 1–18. [Google Scholar] [CrossRef]
- Wubben, D.; Rost, P.; Bartelt, J.S.; Lalam, M.; Savin, V.; Gorgoglione, M.; Dekorsy, A.; Fettweis, G. Benefits and impact of cloud computing on 5G signal processing: Flexible centralization through cloud-RAN. IEEE Signal Process. Mag. 2014, 31, 35–44. [Google Scholar] [CrossRef]
- Cordella, F.; Ciancio, A.L.; Sacchetti, R.; Davalli, A.; Cutti, A.G.; Guglielmelli, E.; Zollo, L. Literature Review on Needs of Upper Limb Prosthesis Users. Front. Neurosci. 2016, 10, 209. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Igual, C.; Pardo, L.A., Jr.; Hahne, J.M.; Igual, J. Myoelectric Control for Upper Limb Prostheses. Electronics 2019, 8, 1244. https://doi.org/10.3390/electronics8111244
Igual C, Pardo LA Jr., Hahne JM, Igual J. Myoelectric Control for Upper Limb Prostheses. Electronics. 2019; 8(11):1244. https://doi.org/10.3390/electronics8111244
Chicago/Turabian StyleIgual, Carles, Luis A. Pardo, Jr., Janne M. Hahne, and Jorge Igual. 2019. "Myoelectric Control for Upper Limb Prostheses" Electronics 8, no. 11: 1244. https://doi.org/10.3390/electronics8111244
APA StyleIgual, C., Pardo, L. A., Jr., Hahne, J. M., & Igual, J. (2019). Myoelectric Control for Upper Limb Prostheses. Electronics, 8(11), 1244. https://doi.org/10.3390/electronics8111244





