Improved Voltage Drop Compensation Method for Hybrid Fuel Cell Battery System
Abstract
1. Introduction
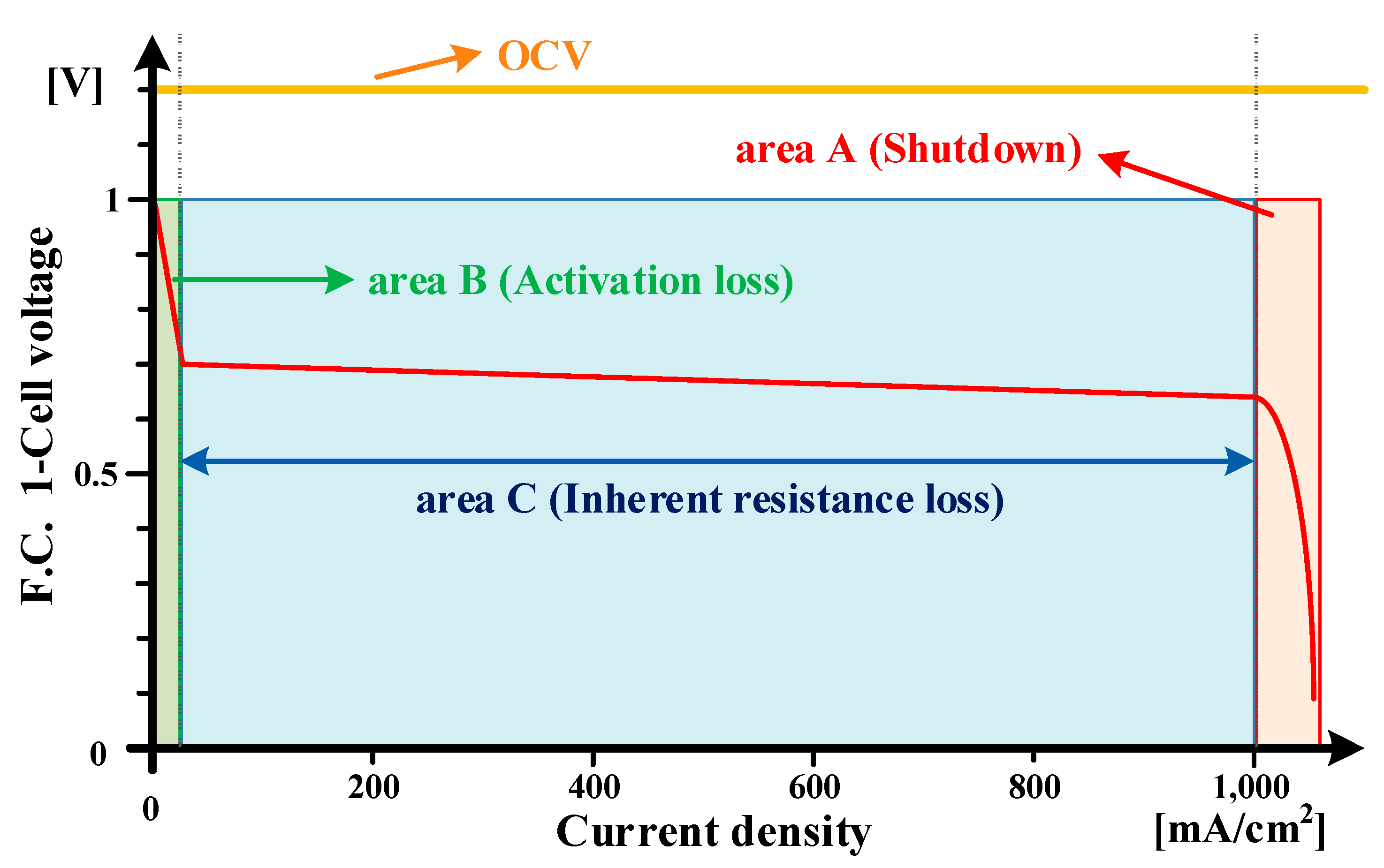
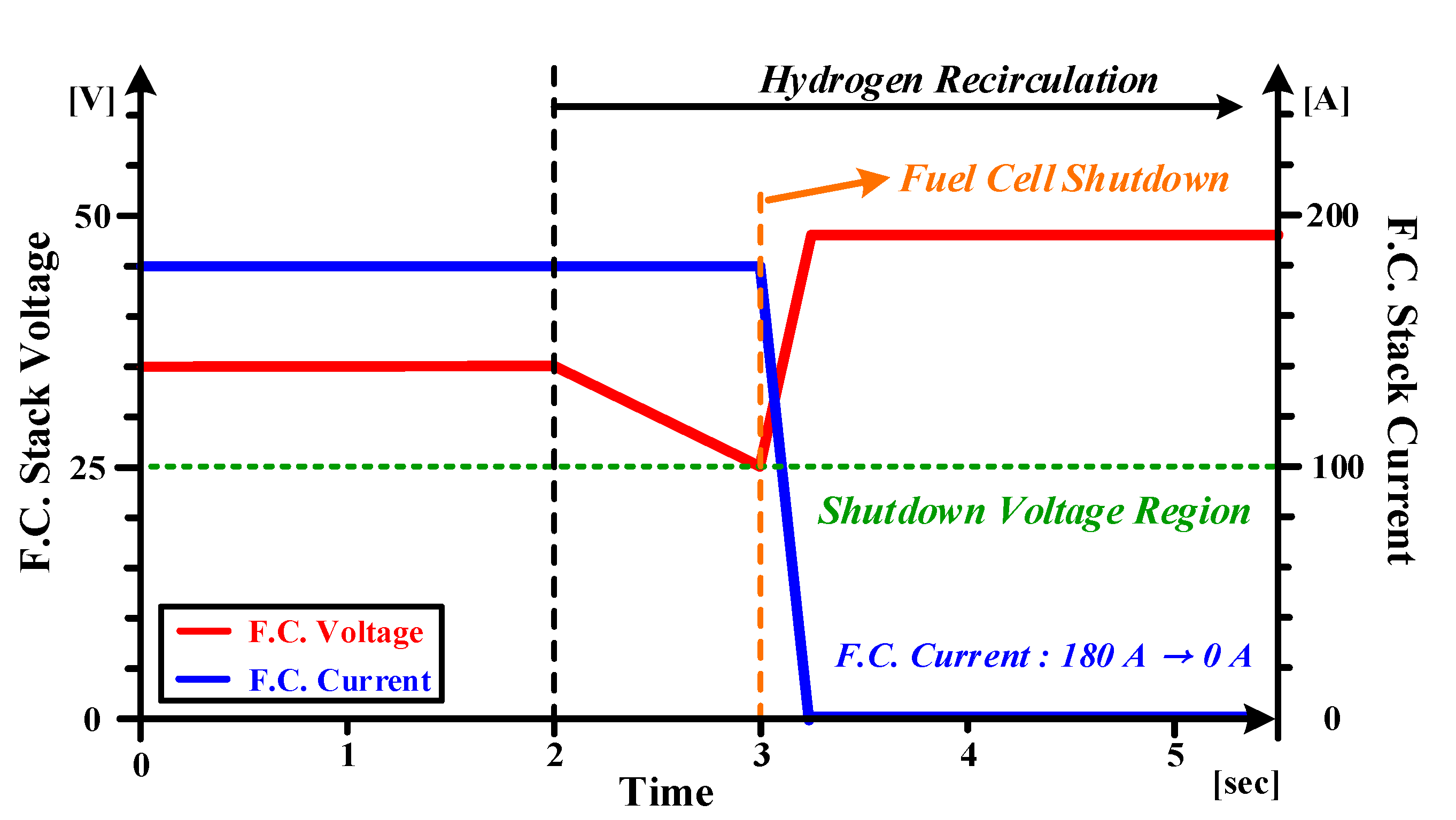
2. Analysis of Voltage Drop Characteristics of the Fuel Cell System during Hydrogen Recirculation
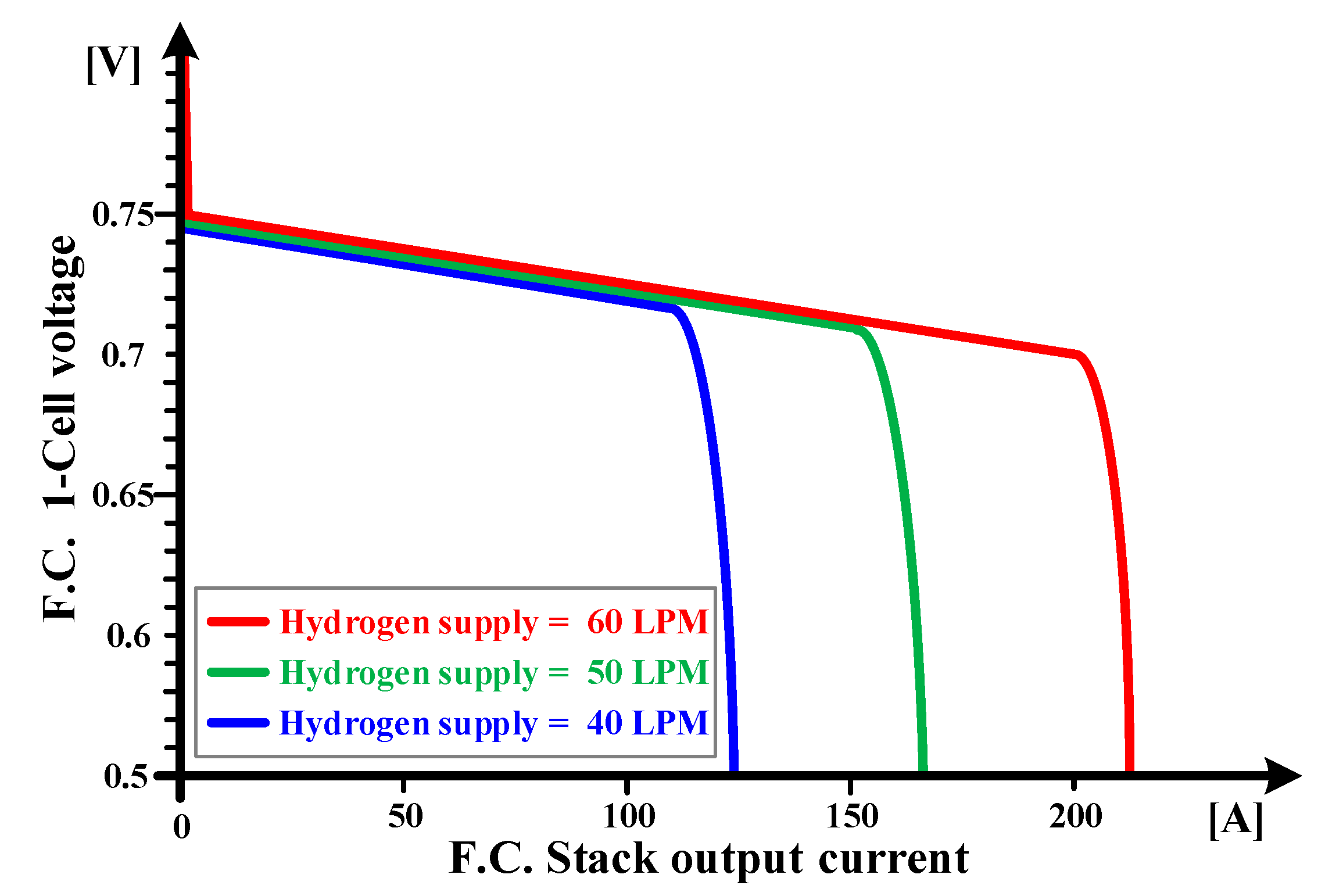
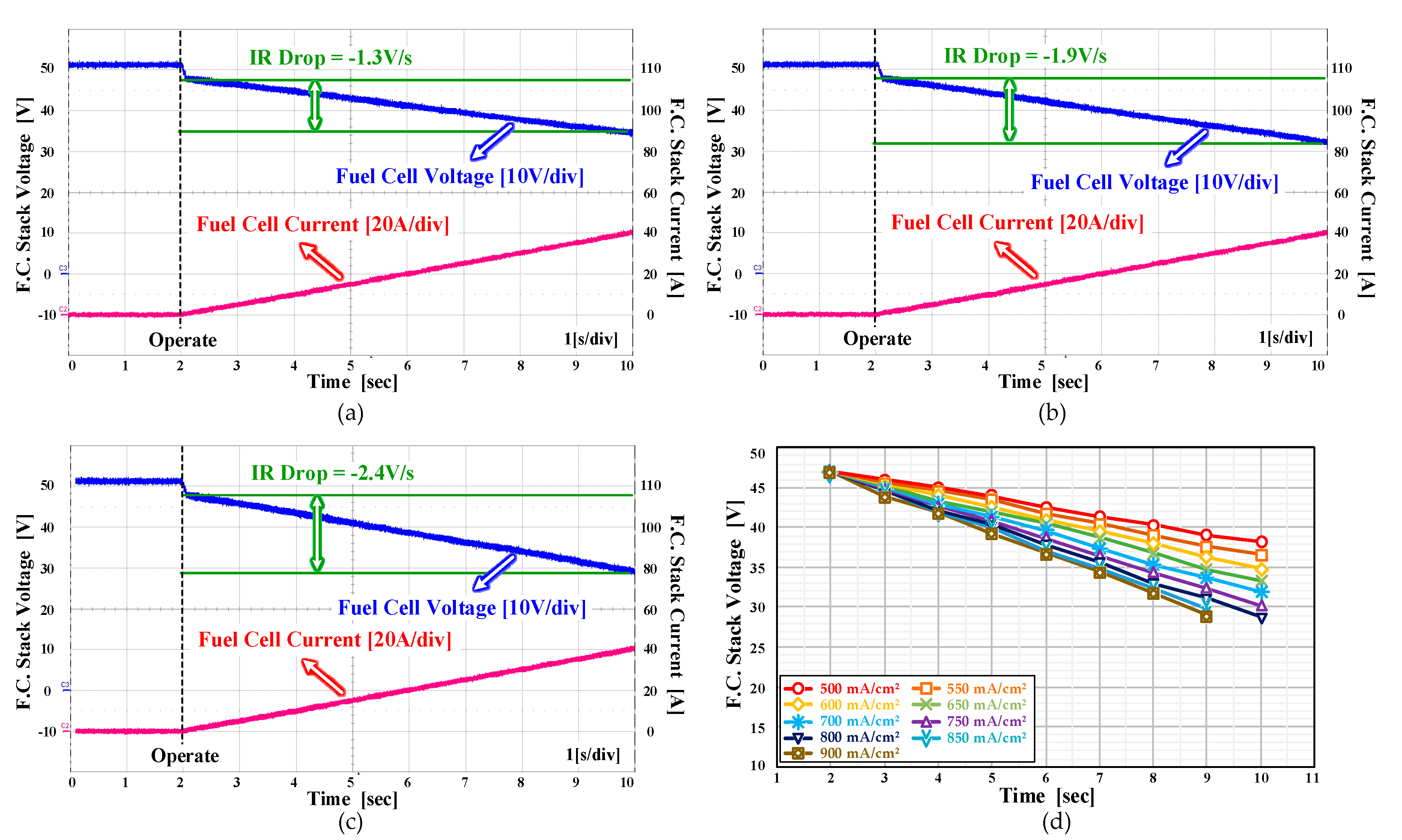
2.1. Voltage Drop Characteristics Depending on Hydrogen Supply
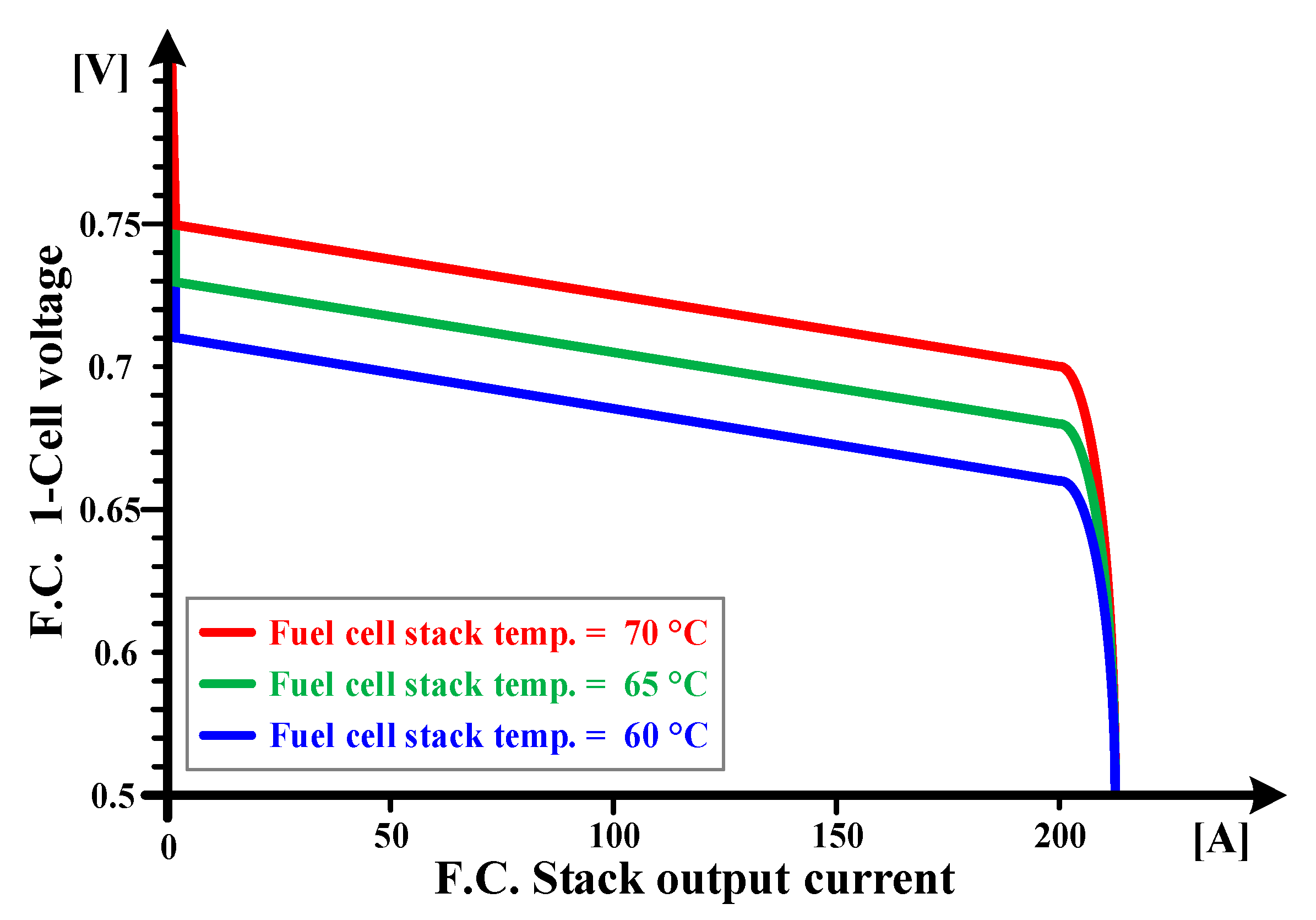
2.2. Voltage Drop Characteristics Depending on Temperature of the Fuel Cell Stack
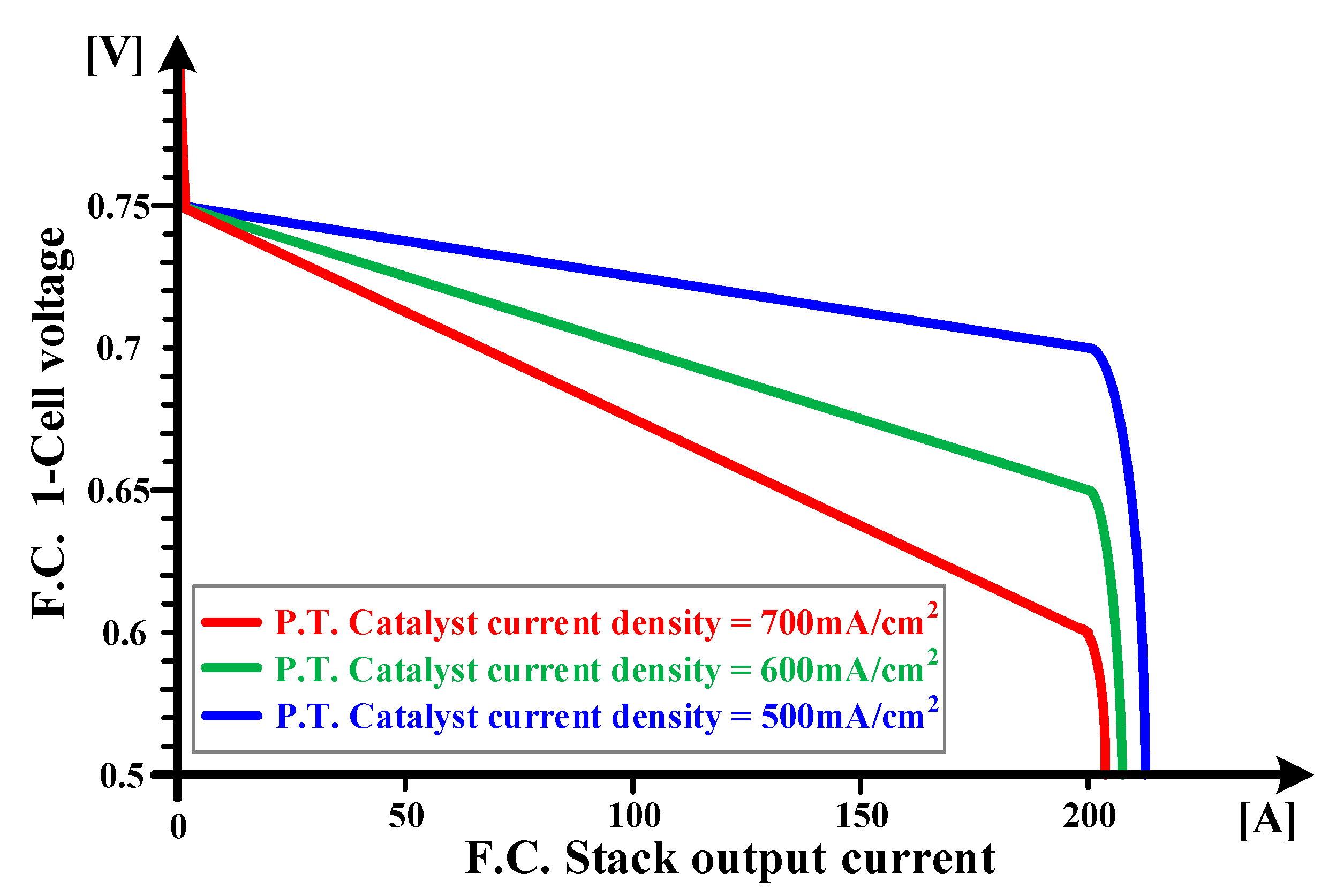
2.3. Voltage Drop Characteristics Depending on Current Density of Platinum Catalyst
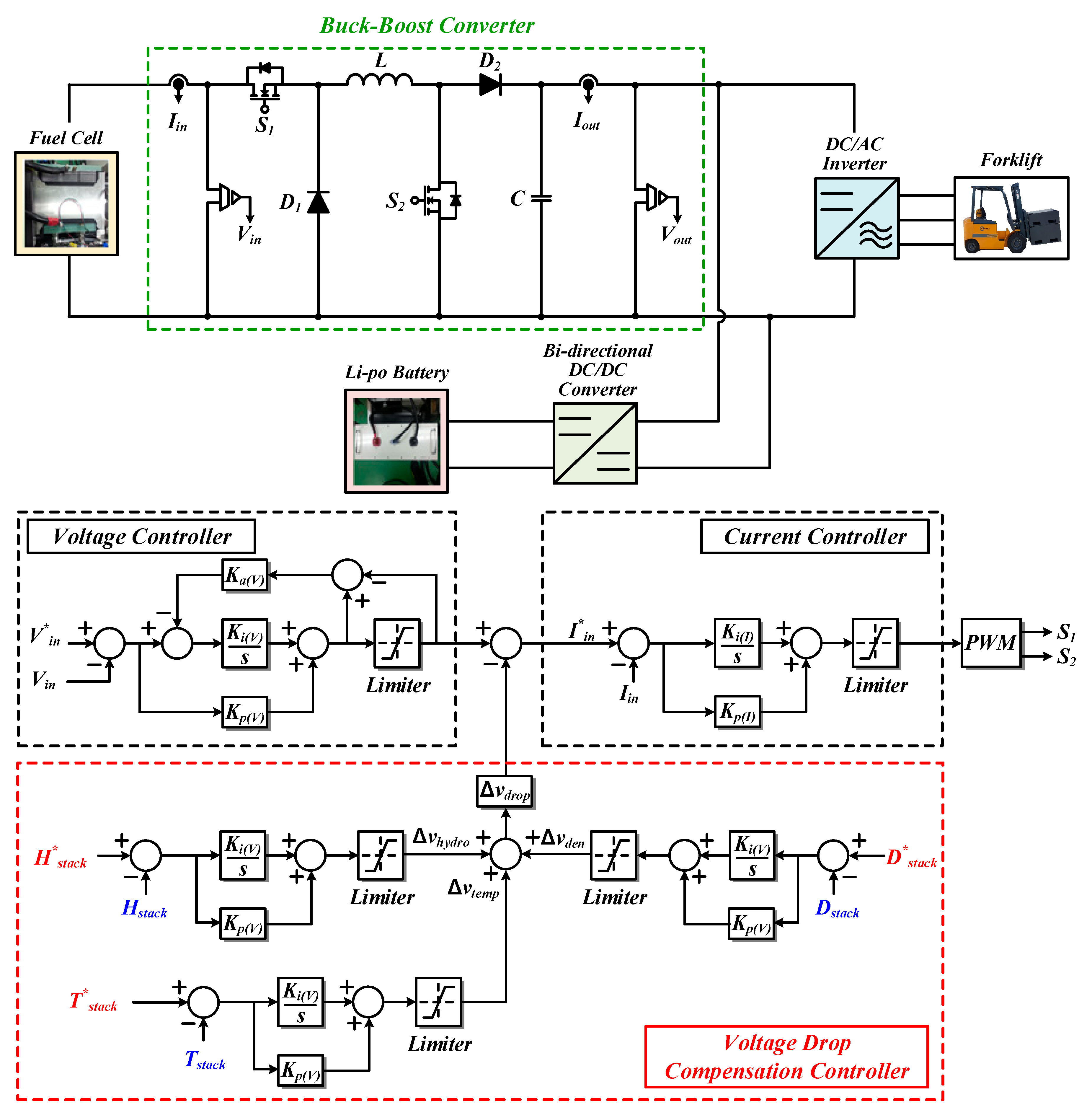
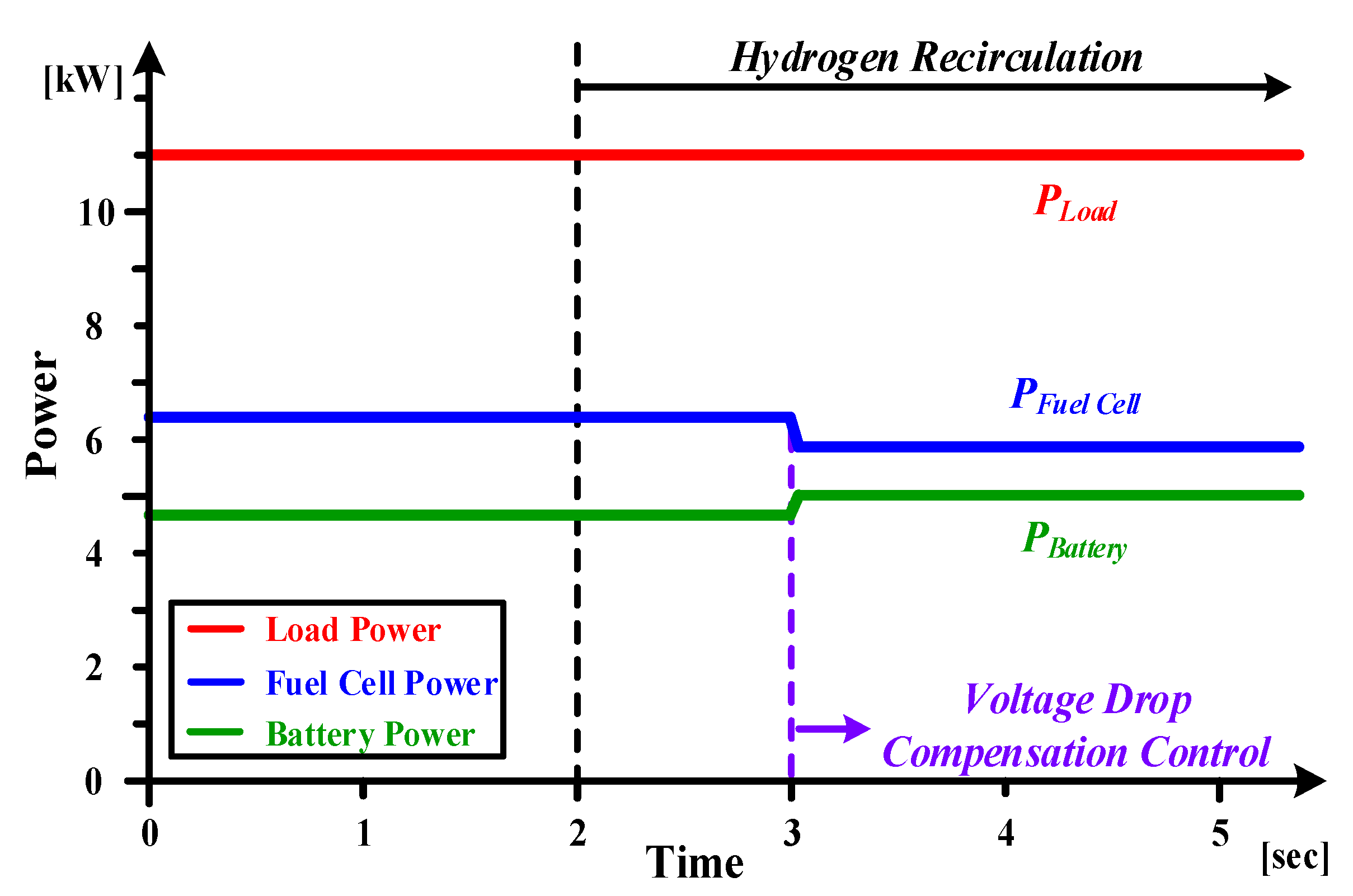
3. The Proposed Voltage Drop Compensation Method of Buck-Boost Converter Considering Voltage Drop during Hydrogen Recirculation
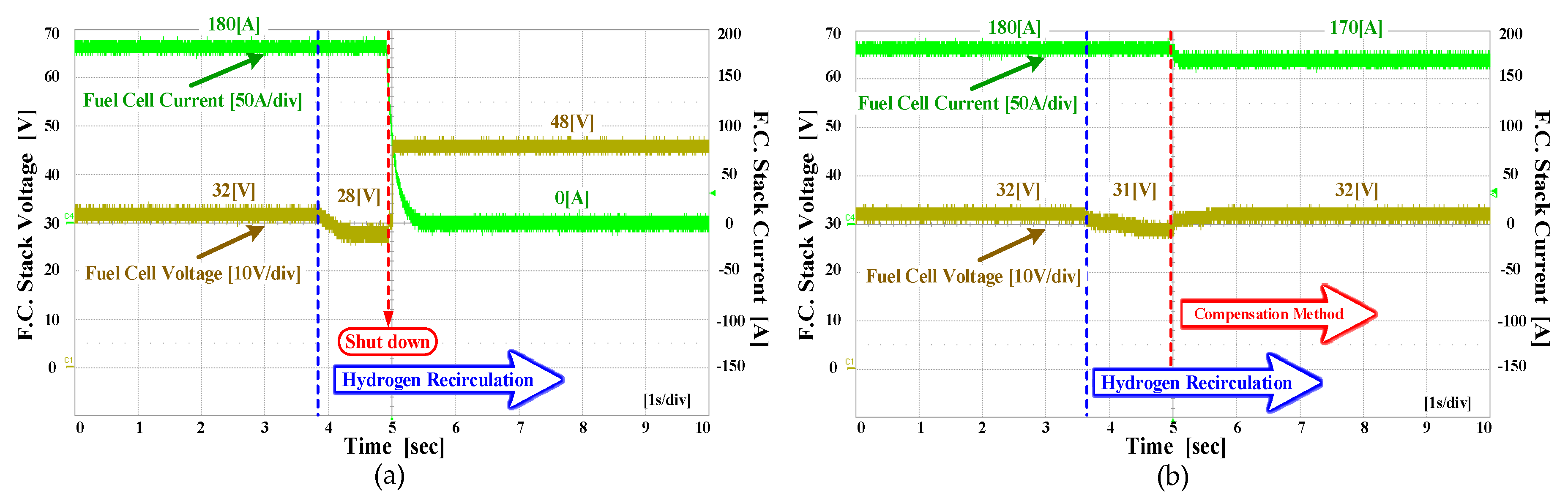
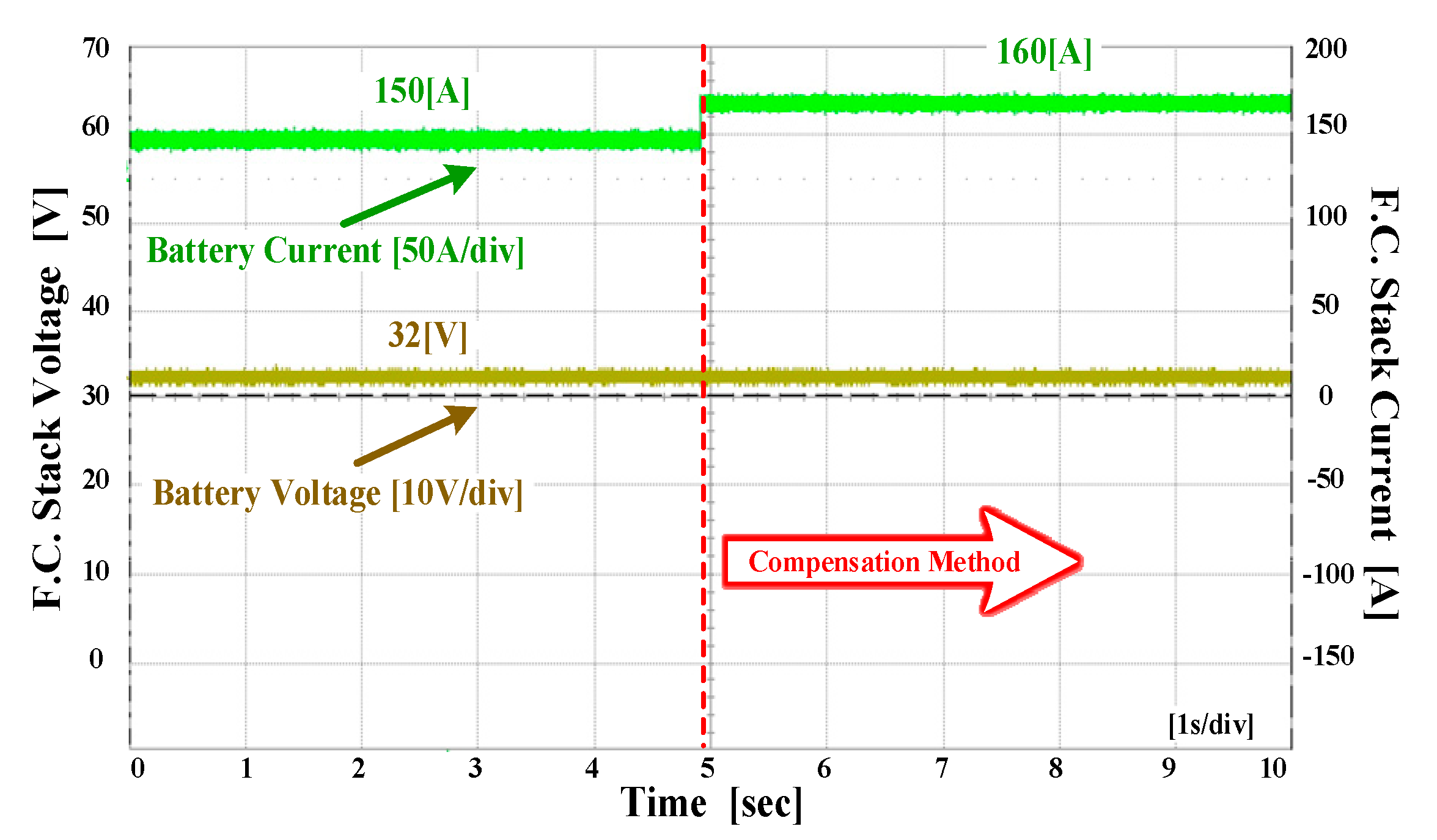
4. Experiment Results
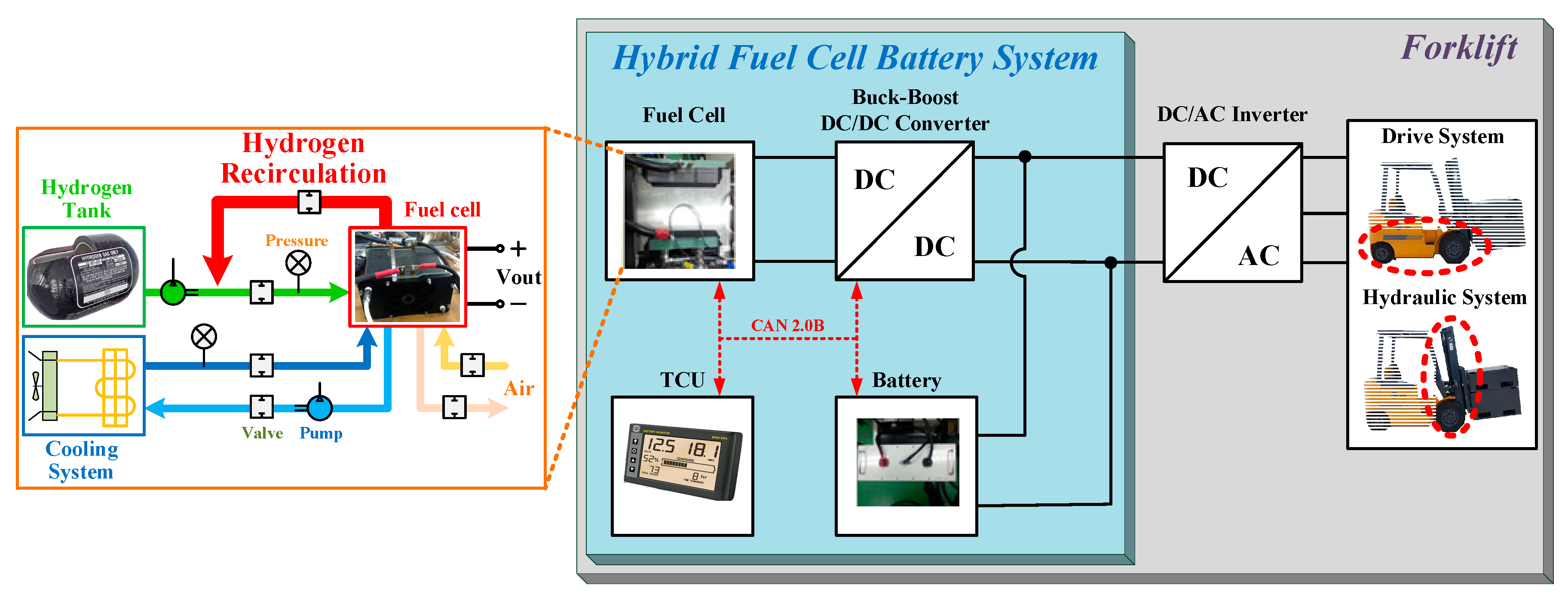
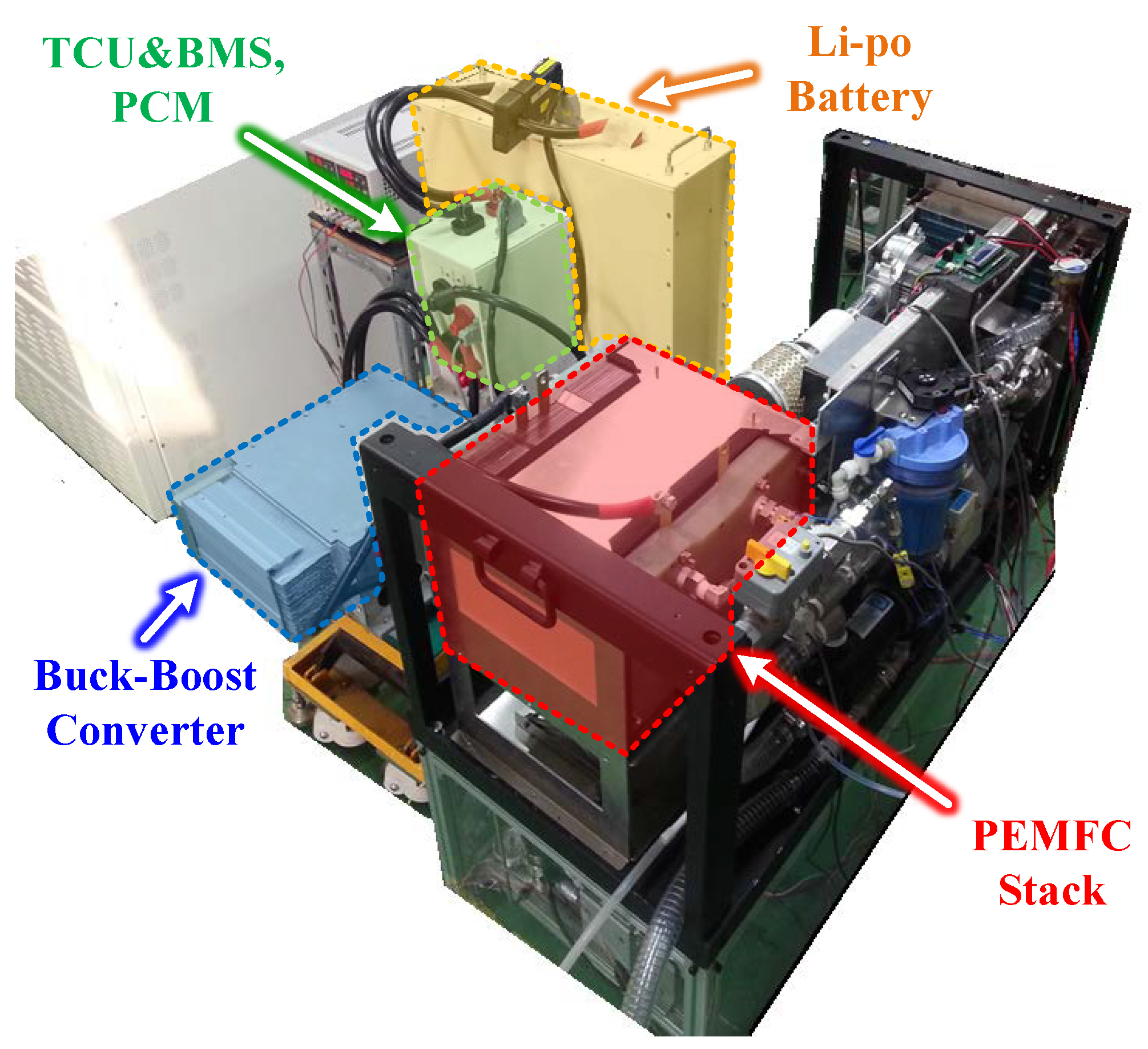
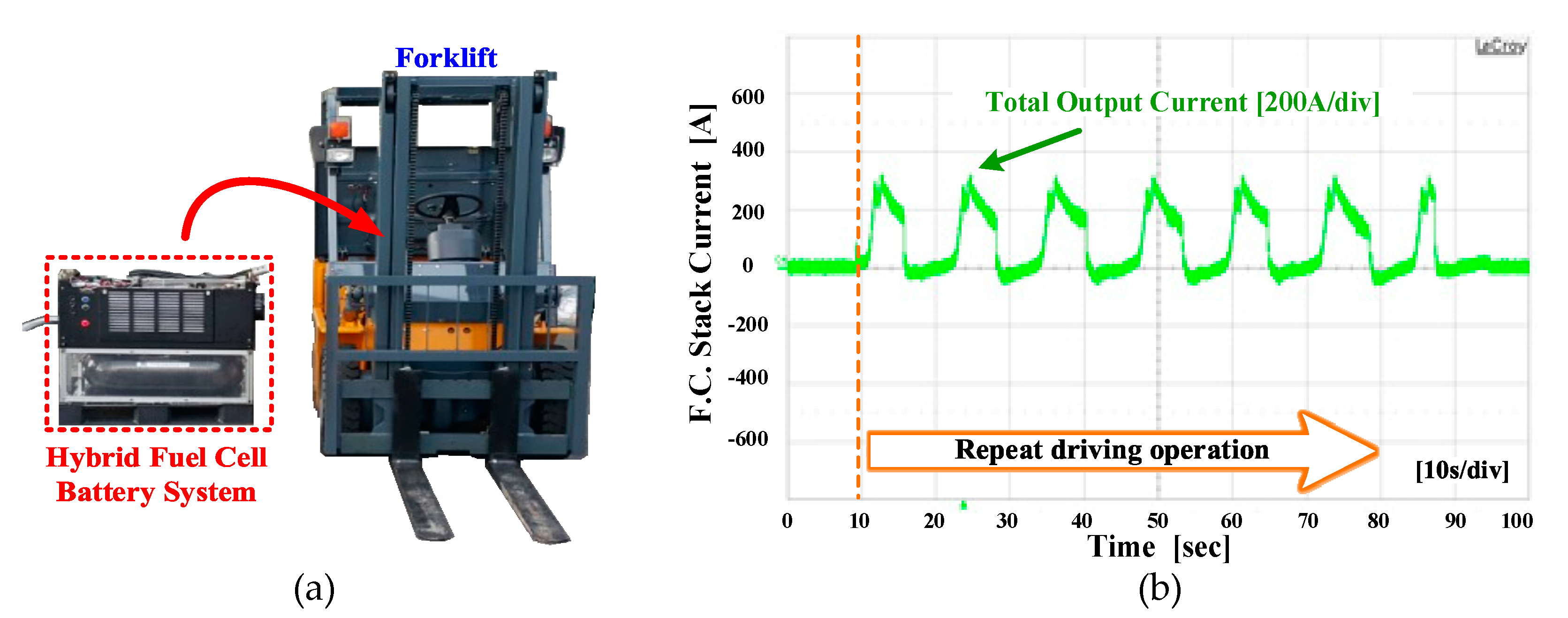
4.1. The Experiment Hardware Configuration
4.2. The Voltage Drop Characteristics of the Three Factors
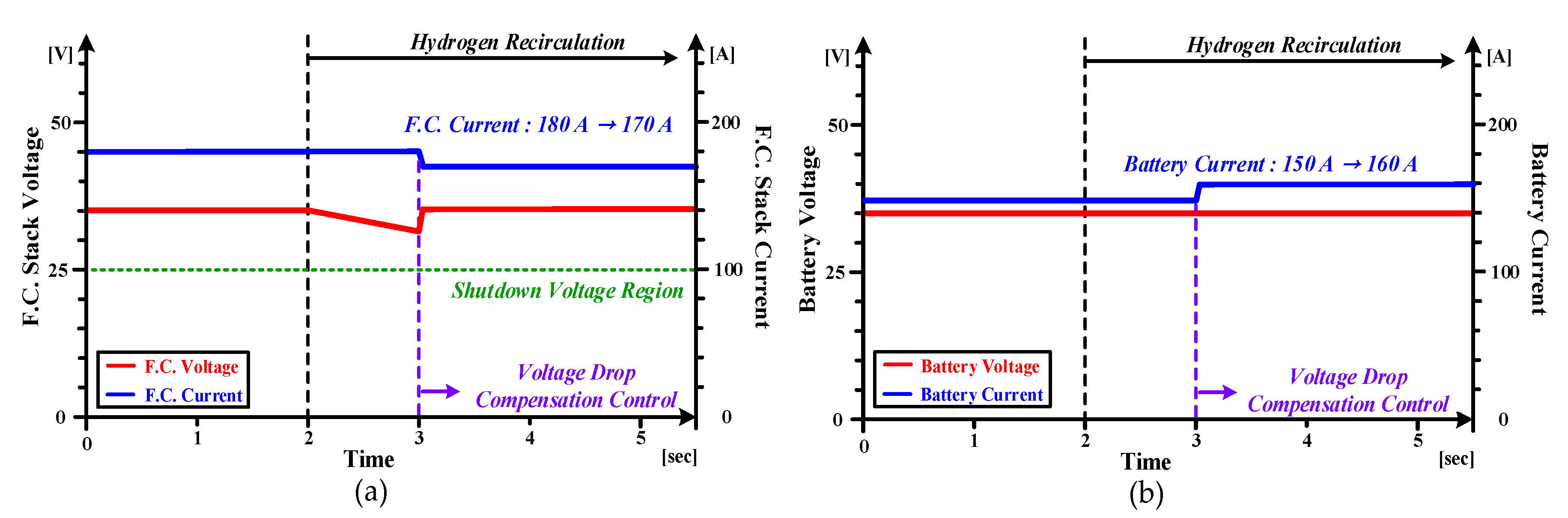
4.3. The Experiment Results for Hybrid Fuel Cell Battery System and Forklift Drive
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, S.; Guo, Y.; Dorrell, D. Analysis of rectangular EV inductive charging coupler. In Proceedings of the 2017 12th IEEE Conference on Industrial Electronics and Applications (ICIEA), Siem Reap, Cambodia, 18–20 June 2017; pp. 285–291. [Google Scholar]
- Zheng, J.; Wang, X.; Men, K.; Zhu, C.; Zhu, S. Aggregation Model-Based Optimization for Electric Vehicle Charging Strategy. IEEE Trans. Smart Grid 2013, 4, 1058–1066. [Google Scholar] [CrossRef]
- Abdulaal, A.; Cintuglu, M.H.; Asfour, S.; Mohammed, O.A. Solving the Multivariant EV Routing Problem Incorporating V2G and G2V Options. IEEE Trans. Transp. Electrif. 2017, 3, 238–248. [Google Scholar] [CrossRef]
- Repp, S.; Harputlu, E.; Gurgen, S.; Castellano, M.; Kremer, N.; Pompe, N.; Wörner, J.; Hoffmann, A.; Thomann, R.; Emen, F.M. Synergetic effects of Fe3+ doped spinel Li4Ti5O12 nanoparticles on reduced graphene oxide for high surface electrode hybrid supercapacitors. Nanoscale 2018, 10, 1877–1884. [Google Scholar] [CrossRef] [PubMed]
- Genc, R.; Alas, M.O.; Harputlu, E.; Repp, S.; Kremer, N.; Castellano, M.; Colak, S.G.; Ocakoglu, K.; Erdem, E. High-capacitance hybrid supercapacitor based on multi-colored fluorescent carbon-dots. Sci. Rep. 2017, 7, 11222. [Google Scholar] [CrossRef] [PubMed]
- Huffstutler, J.; Wasala, M.; Richie, J.; Barron, J.; Winchester, A.; Ghosh, S.; Yang, C.; Xu, W.; Song, L.; Kar, S.; et al. High Performance Graphene-Based Electrochemical Double Layer Capacitors Using 1-Butyl-1-methylpyrrolidinium tris (pentafluoroethyl) trifluorophosphate Ionic Liquid as an Electrolyte. Electronics 2018, 7, 229. [Google Scholar] [CrossRef]
- Kamal, T.; Karabacak, M.; Hassan, S.; Fernández-Ramírez, L.; Riaz, M.; Khan, M.; Khan, L. Energy Management and Switching Control of PHEV Charging Stations in a Hybrid Smart Micro-Grid System. Electronics 2018, 7, 156. [Google Scholar] [CrossRef]
- Kuehne, P.; Wenske, M.; Heuer, M.; Wolter, M. Unitized reversible PEM fuel cells for flexible electrical energy storage. In Proceedings of the International ETG Congress 2017, Bonn, Germany, 28–29 November 2017; pp. 1–6. [Google Scholar]
- Lai, J.S.; Ellis, M.W. Fuel Cell Power Systems and Applications. Proc. IEEE 2017, 105, 2166–2190. [Google Scholar] [CrossRef]
- Zhang, Z.; Mortensen, H.H.; Jensen, J.V.; Andersen, M.A.E. Fuel Cell and Battery Powered Forklifts. In Proceedings of the 2013 IEEE Vehicle Power and Propulsion Conference (VPPC), Beijing, China, 15–18 October 2013; pp. 1–5. [Google Scholar]
- Shin, M.H.; Eom, T.H.; Park, Y.H.; Won, C.Y. Design and control of fuel cell-battery hybrid system for forklift. In Proceedings of the 2016 IEEE Transportation Electrification Conference and Expo, Asia-Pacific (ITEC Asia-Pacific), Busan, Korea, 1–4 June 2016; pp. 584–589. [Google Scholar]
- Graber, G.; Galdi, V.; Calderaro, V.; Piccolo, A. A method to size the stack and the battery of a fuel cell vehicle reducing the fuel consumption. In Proceedings of the 2017 AEIT International Annual Conference, Cagliari, Italy, 20–22 September 2017; pp. 1–6. [Google Scholar]
- Lewis, M.; Hearn, C.; Feng, X.; Hanlin, J.; Levin, J.; Ambrosio, J.; Guggenheim, P.; Walker, C. Design and modeling for hydrogen fuel cell conversion of parcel delivery trucks. In Proceedings of the 2017 IEEE Transportation Electrification Conference and Expo (ITEC), Chicago, IL, USA, 26–28 June 2017; pp. 674–678. [Google Scholar]
- D’Ovidio, G.; Masciovecchio, C.; Rotondale, A. Hydrogen fuel cell and kinetic energy recover systems technologies for powering urban bus with zero emission energy cycle. IET Intell. Transp. Syst. 2016, 10, 573–578. [Google Scholar] [CrossRef]
- Vasallo, M.J.; Andujar, J.M.; Garcia, C.; Brey, J.J. A Methodology for Sizing Backup Fuel-Cell/Battery Hybrid Power Systems. IEEE Trans. Ind. Electron. 2010, 57, 1964–1975. [Google Scholar] [CrossRef]
- Khaligh, A.; Rahimi, A.M.; Lee, Y.J.; Cao, J.; Emadi, A.; Andrews, S.D.; Robinson, C.; Finnerty, C. Digital Control of an Isolated Active Hybrid Fuel Cell/Li-Ion Battery Power Supply. IEEE Trans. Veh. Technol. 2007, 56, 3709–3721. [Google Scholar] [CrossRef]
- Zaidi, S.J. Research trends in polymer electrolyte membranes for PEMFC. In Polymer Membranes for Fuel Cells; Springer: Boston, MA, USA, 2009; pp. 7–25. [Google Scholar]
- Owejan, J.P.; Gagliardo, J.J.; Sergi, J.M.; Kandlikar, S.G.; Trabold, T.A. Water management studies in PEM fuel cells, Part I: Fuel cell design and in situ water distributions. Int. J. Hydrogen Energy 2009, 34, 3436–3444. [Google Scholar] [CrossRef]
- Pandiyan, S.; Jayakumar, K.; Rajalakshmi, N.; Dhathathreyan, K.S. Thermal and electrical energy management in a PEMFC stack–An analytical approach. Int. J. Heat Mass Transf. 2008, 51, 469–473. [Google Scholar] [CrossRef]
- Hussain, M.M.; Baschuk, J.J.; Li, X.; Dincer, I. Thermodynamic analysis of a PEM fuel cell power system. Int. J. Therm. Sci. 2005, 44, 903–911. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, B.; Kim, Y. An experimental study on water transport through the membrane of a PEFC operating in the dead-end mode. Int. J. Hydrogen Energy 2009, 34, 7768–7779. [Google Scholar] [CrossRef]
- Chen, J.; Siegel, J.B.; Stefanopoulou, A.G.; Waldecker, J.R. Optimization of purge cycle for dead-ended anode fuel cell operation. Int. J. Hydrogen Energy 2013, 38, 5092–5105. [Google Scholar] [CrossRef]
- Spernjak, D.; Prasad, A.K.; Advani, S.G. Experimental investigation of liquid water formation and transport in a transparent single-serpentine PEM fuel cell. J. Power Sources 2007, 170, 334–344. [Google Scholar] [CrossRef]
- Bradley, T.H.; Moffitt, B.A.; Mavris, D.N.; Parekh, D.E. Development and experimental characterization of a fuel cell powered aircraft. J. Power Sources 2007, 171, 793–801. [Google Scholar] [CrossRef]
- Siegel, J.B.; McKay, D.A.; Stefanopoulou, A.G.; Hussey, D.S.; Jacobson, D.L. Measurement of liquid water accumulation in a PEMFC with dead-ended anode. J. Electrochem. Soc. 2008, 155, B1168–B1178. [Google Scholar] [CrossRef]
- Matsuura, T.; Chen, J.; Siegel, J.B.; Stefanopoulou, A.G. Degradation phenomena in PEM fuel cell with dead-ended anode. Int. J. Hydrogen Energy 2013, 38, 11346–11356. [Google Scholar] [CrossRef]
- Han, I.; Jeong, J.; Shin, H.K. PEM fuel-cell stack design for improved fuel utilization. Int. J. Hydrogen Energy 2013, 38, 11996–12006. [Google Scholar] [CrossRef]
- Wei, Y.C.; Chen, Y. A mathematical model to study the performance of a proton exchange membrane fuel cell in a dead-ended anode mode. Appl. Energy 2014, 130, 113–121. [Google Scholar]
- Siegel, J.B. Experiments and Modeling of PEM Fuel Cells for Dead-Ended Anode Operation. Ph.D. Thesis, University of Michigan, Ann Arbor, MI, USA, 2010. [Google Scholar]
- Tüber, K.; Pócza, D.; Hebling, C. Visualization of water buildup in the cathode of a transparent PEM fuel cell. J. Power Sources 2003, 124, 403–414. [Google Scholar] [CrossRef]
- Gomez, A.; Raj, A.; Sasmito, A.P.; Shamim, T. Effect of operating parameters on the transient performance of a polymer electrolyte membrane fuel cell stack with a dead-end anode. Appl. Energy 2014, 130, 692–701. [Google Scholar] [CrossRef]
- Ebadighajari, A.; Homayouni, H.; DeVaal, J.; Golnaraghi, F. Model Predictive Control of Polymer Electrolyte Membrane fuel cell with dead-end anode and periodic purging. In Proceedings of the 2016 IEEE Conference on Control Applications (CCA), Nice, France, 8–10 October 2016. [Google Scholar]
- Chen, J.; Siegel, J.B.; Stefanopoulou, A.G. Nitrogen blanketing front equilibria in dead end anode fuel cell operation. In Proceedings of the American Control Conference (ACC), San Francisco, CA, USA, 29 June–1 July 2011. [Google Scholar]
- Himanen, O.; Hottinen, T.; Tuurala, S. Operation of a planar free-breathing PEMFC in a dead-end mode. Electrochem. Commun. 2007, 9, 891–894. [Google Scholar] [CrossRef]
- Hearn, C.S.; Lewis, M.C.; Thompson, R.T.; Chen, D.; Hanlm, J.; Zuckerman, D.; Lindsay, T. Design and demonstration of an extended range hydrogen fuel cell utility vehicle. In Proceedings of the 2011 IEEE Vehicle Power and Propulsion Conference, Chicago, IL, USA, 6–9 September 2011; pp. 1–5. [Google Scholar]
- Dallago, E.; Danioni, A.; Passoni, M.; Venchi, G. Comparison of transient performance in a peak current-mode flyback converter. In Proceedings of the 2002 IEEE International Symposium on Industrial Electronics, ISIE 2002, L’Aquila, Italy, 8–11 July 2002; Volume 4, pp. 1049–1052. [Google Scholar]
- Chen, T.H.; Lin, W.L.; Liaw, C.M. Dynamic modeling and controller design of flyback converter. IEEE Trans. Aerosp. Electron. Syst. 1999, 35, 1230–1239. [Google Scholar] [CrossRef]
- Kleebchampee, W.; Bunlaksananusorn, C. Modeling and Control Design of a Current-Mode Controlled Flyback Converter with Optocoupler Feedback. In Proceedings of the 2005 International Conference on Power Electronics and Drives Systems, Kuala Lumpur, Malaysia, 28 November–1 December 2005; pp. 787–792. [Google Scholar]
- Shen, C.L.; Chiu, P.C. Buck-boost-flyback integrated converter with single switch to achieve high voltage gain for PV or fuel-cell applications. IET Power Electron. 2016, 9, 1228–1237. [Google Scholar] [CrossRef]
- Ebadighajari, A.; DeVaal, J.; Golnaraghi, F. Reduction of hydrogen transfer by constrained control of anode hydrogen recirculation in a polymer electrolyte membrane fuel cell. In Proceedings of the 2017 IEEE 56th Annual Conference on Decision and Control (CDC), Melbourne, VIC, Australia, 12–15 December 2017; pp. 1440–1445. [Google Scholar]
- Ross, D.K. Hydrogen storage: The major technological barrier to the development of hydrogen fuel cell cars. Vacuum 2006, 80, 1084–1089. [Google Scholar] [CrossRef]
- Li, X.; Xiao, M.; Choe, S.-Y. Reduced order of electrochemical model for a pouch type high power Li-polymer battery. In Proceedings of the 2011 International Conference on Clean Electrical Power (ICCEP), Ischia, Italy, 14–16 June 2011. [Google Scholar]
- Zhang, S.; Yuan, X.Z.; Hin, J.N.; Wang, H.; Friedrich, K.A.; Schulze, M. A review of platinum-based catalyst layer degradation in proton exchange membrane fuel cells. J. Power Sources 2009, 194, 588–600. [Google Scholar] [CrossRef]
- Shao, Y.; Yin, G.; Gao, Y. Understanding and approaches for the durability issues of Pt-based catalysts for PEM fuel cell. J. Power Sources 2007, 171, 558–566. [Google Scholar] [CrossRef]
- Cho, Y.H.; Park, H.S.; Cho, Y.H.; Jung, D.S.; Park, H.Y.; Sung, Y.E. Effect of platinum amount in carbon supported platinum catalyst on performance of polymer electrolyte membrane fuel cell. J. Power Sources 2007, 172, 89–93. [Google Scholar] [CrossRef]



| Parameters | Values | Units |
|---|---|---|
| Rated power | 10 | [kW] |
| Input voltage | 30~50 | [VDC] |
| Input current | 200 | [ADC] |
| Output voltage | 42~54 | [VDC] |
| Output current | 200 | [ADC] |
| Output ripple | ±2 | [%] |
| Switching frequency | 25 | [kHz] |
| Boost inductor | 200 | [μH] |
| Output capacitor | 50 | [μF] |
| Parameters | Values | Units |
|---|---|---|
| Hydrogen supply | 50–70 | [LPM] |
| Stack temperature | 60 | [°C] |
| Current density | 700 | [mA/cm2] |
| Parameters | Values | Units |
|---|---|---|
| Hydrogen supply | 70 | [LPM] |
| Stack temperature | 40–90 | [°C] |
| Current density | 700 | [mA/cm2] |
| Parameters | Values | Units |
|---|---|---|
| Hydrogen supply | 70 | [LPM] |
| Stack temperature | 60 | [°C] |
| Current density | 500–900 | [mA/cm2] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eom, T.-H.; Kang, J.-W.; Kim, J.; Shin, M.-H.; Lee, J.-H.; Won, C.-Y. Improved Voltage Drop Compensation Method for Hybrid Fuel Cell Battery System. Electronics 2018, 7, 331. https://doi.org/10.3390/electronics7110331
Eom T-H, Kang J-W, Kim J, Shin M-H, Lee J-H, Won C-Y. Improved Voltage Drop Compensation Method for Hybrid Fuel Cell Battery System. Electronics. 2018; 7(11):331. https://doi.org/10.3390/electronics7110331
Chicago/Turabian StyleEom, Tae-Ho, Jin-Wook Kang, Jintae Kim, Min-Ho Shin, Jung-Hyo Lee, and Chung-Yuen Won. 2018. "Improved Voltage Drop Compensation Method for Hybrid Fuel Cell Battery System" Electronics 7, no. 11: 331. https://doi.org/10.3390/electronics7110331
APA StyleEom, T.-H., Kang, J.-W., Kim, J., Shin, M.-H., Lee, J.-H., & Won, C.-Y. (2018). Improved Voltage Drop Compensation Method for Hybrid Fuel Cell Battery System. Electronics, 7(11), 331. https://doi.org/10.3390/electronics7110331




