Wearable Photoplethysmographic Sensors—Past and Present
Abstract
:1. Introduction
2. Photoplethysmography (PPG)
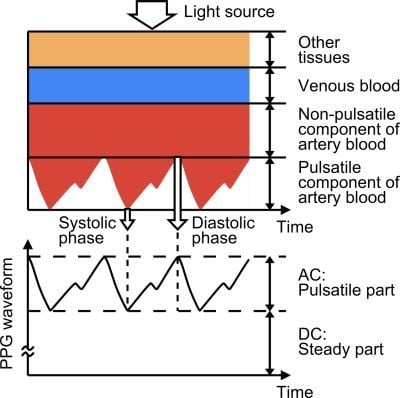
2.1. Principle of PPG
2.2. Light Wavelength
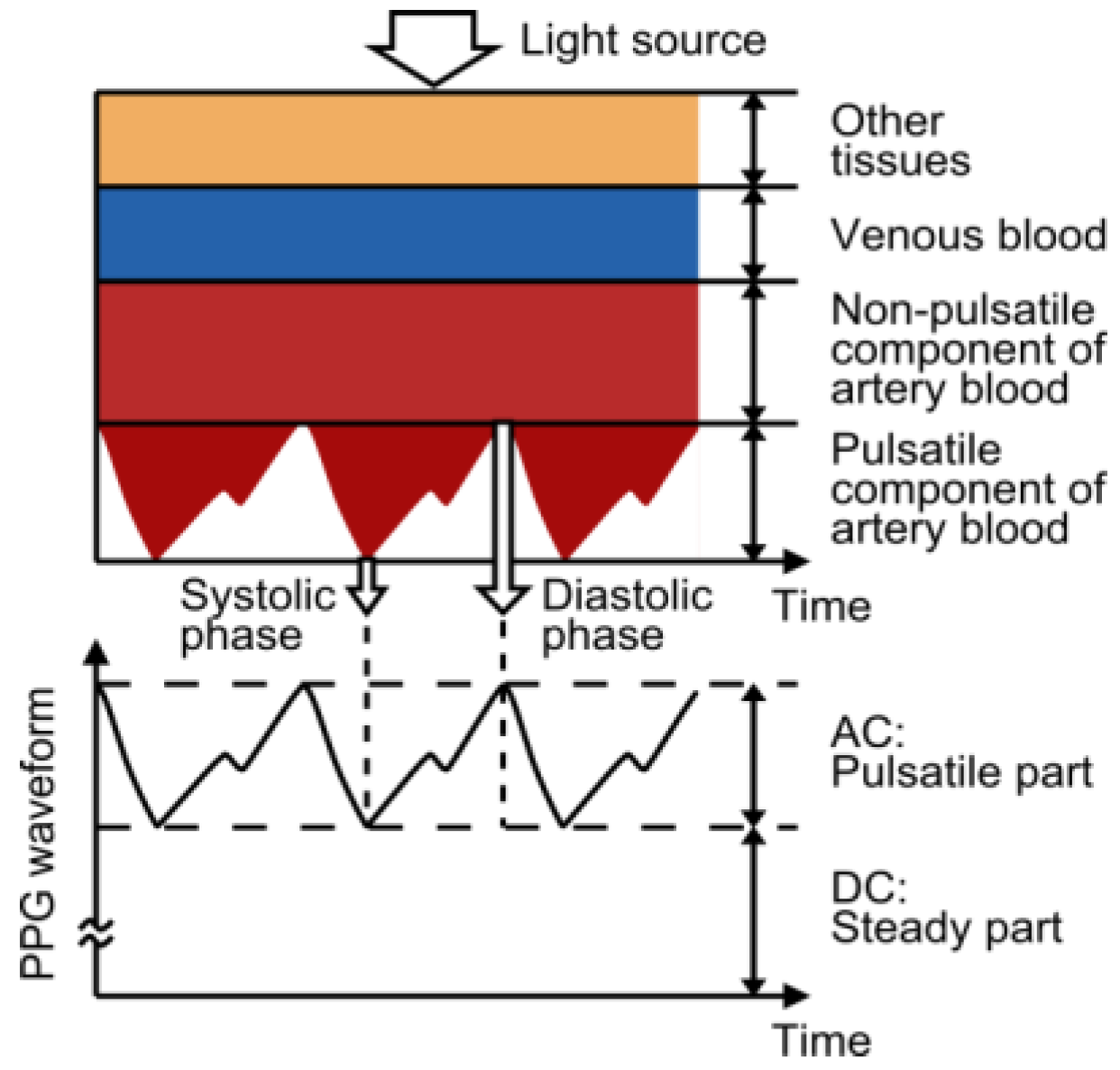
2.3. Reflected and Transmitted Signals
3. Factors Affecting PPG Recordings
3.1. Measurement Site of Probe
| Features | In-mount | CUHK | e-AR | Imperial | MIT | MIT | Pulsear | Samsung |
|---|---|---|---|---|---|---|---|---|
| Year published | 2009 [20] | 2008 [26] | 2007 [19] | 2009 [27] | 2010 [22] | 2012 [24] | 2004 [23] | 2009 [21] |
| Sensing site | Auditory canal | Inferior auricle | Superior auricle | Superior auricle | Earlobe | External ear cartilage | External ear cartilage | Earlobe |
| Probe attachment | Otoplastic insertion | Earhook | Earhook | Tape | Magnetic earring | Earphone | Earcup headphones | Spring-loaded clip |
| Wireless communication | Yes | Yes | No | No | Yes | Yes | No | No |
| Motion cancellation | None | None | Passive motion cancellation | None | Automatic noise cancellation | Automatic noise cancellation | PCA | Automatic noise cancellation |

3.2. Probe Contact Force
3.3. Video Plethysmography
3.4. Signal Processing
3.4.1. Introduction
3.4.2. Moving Average Filter
3.4.3. Fourier Analysis
3.4.4. Adaptive Filter
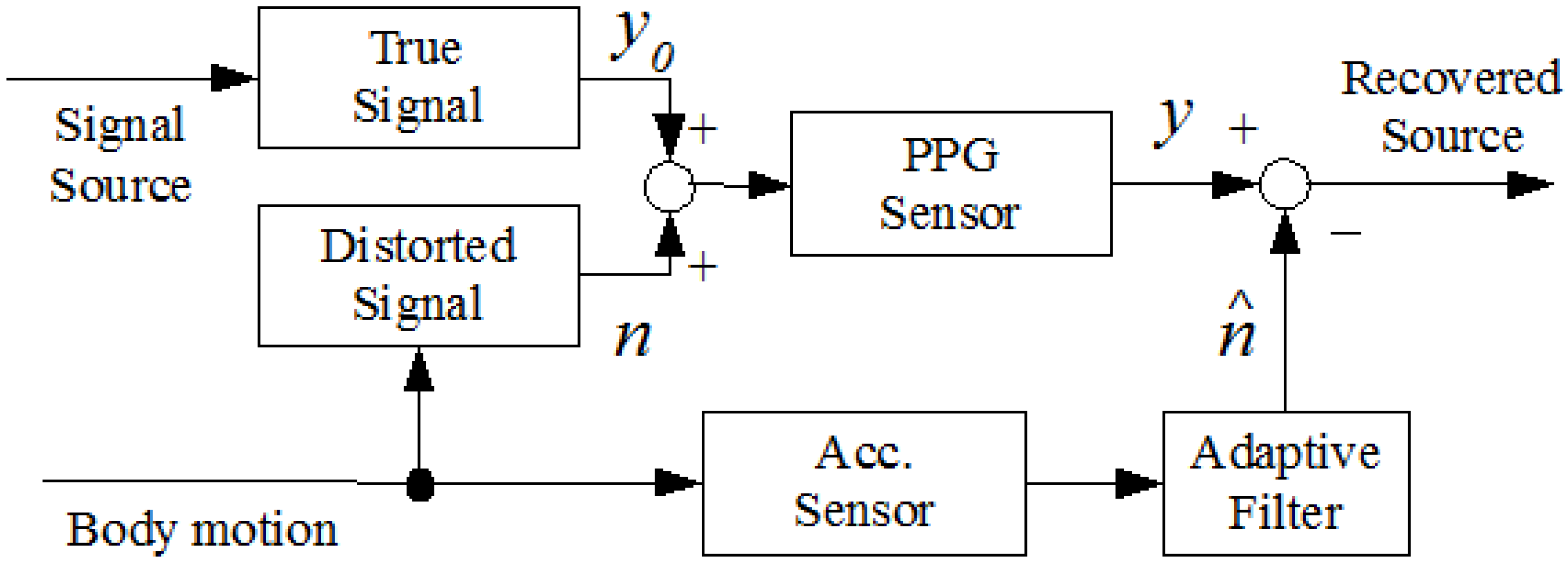
3.4.4.1. Least Mean Square Adaptive Algorithm
3.4.4.2. Kalman Filter
3.4.4.3. Time-Frequency Methods and Wavelet Transformation
3.4.4.4. Principle Component Analysis (PCA)
3.4.4.5. Independent Component Analysis (ICA)
3.4.4.6. Widrow’s Adaptive Noise Cancellation (ANC)
3.4.4.7. Laguerre Expansion
3.5. Model-Based Algorithm
3.6. Passive Motion Cancellation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Togawa, T.; Tamura, T.; Öberg, P.Å. Biomedical Sensors and Instruments, 2nd ed.; CRC Press: New York, NY, USA, 2011; pp. 19–190. [Google Scholar]
- Challoner, A.V.J. Photoelectric plethysmography for estimating cutaneous blood flow. In Non-invasive Physiological Measurement; Rolfe, P., Ed.; Academic Press: Oxford, UK, 1979; Volume 1, pp. 127–151. [Google Scholar]
- Kamal, A.A.R.; Harness, J.B.; Irving, G.; Mearns, A.J. Skin photoplethysmography—A review. Comput. Methods Programs Biomed. 1989, 28, 257–269. [Google Scholar] [CrossRef]
- Alen, J. Photoplethysmography and its application in clinical physiological measurement. Physiol. Meas. 2007, 28, R1–R39. [Google Scholar] [CrossRef]
- Anderson, R.R.; Parris, E.D. The optics of human skin. J. Invest. Dermatol. 1981, 77, 13–19. [Google Scholar]
- Giltvedt, J.; Sita, A.; Helme, P. Pulsed multifrequency photoplethysmograph. Med. Biol. Eng. Comput. 1984, 22, 212–215. [Google Scholar] [CrossRef]
- Cui, W.; Ostrander, L.E.; Lee, B.Y. In vivo reflectance of blood and tissue as a function of light wavelength. IEEE Trans. Biomed. Eng. 1990, 37, 632–639. [Google Scholar] [CrossRef]
- Zijlstra, W.G.; Buursma, A.; Meeuwsen-van der Roest, W.P. Absorption spectra of human fetal and adult oxyhemoglobin, de-oxyhemoglobin, carboxyhemoglobin, and Methemoglobin. Clin. Chem. 1991, 37, 1633–1638. [Google Scholar]
- Meada, Y.; Sekine, M.; Tamura, T. The advantage of green reflected photoplethysmograph. J. Med. Syst. 2011, 35, 829–834. [Google Scholar] [CrossRef]
- Mio Train with Heart. Available online: http://www.mioglobal.com/explore-mio-products.htm (accessed on 10 April 2014).
- Verkruysse, W.; Svaasand, L.O.; Nelson, J.S. Remote plethysmographic imaging using ambient light. Opt. Express 2008, 16, 21434–21445. [Google Scholar] [CrossRef]
- Tarassenko, L.; Villarroel, M.; Guazzi, A.; Jorge, J.; Clifton, D.A.; Pugh, C. Non-contact video-based vital sign monitoring using ambient light and auto-regressive models. Physiol. Meas. 2014, 35, 807–831. [Google Scholar] [CrossRef]
- Hertzman, A.B. The blood supply of various skin areas as estimated by the photoelectric plethysmograph. Amer. J. Physiol. 1938, 124, 328–340. [Google Scholar]
- Rhee, S.; Yang, B.-H.; Asada, H.H. Artifact-resistant, power-efficient design of finger-ring plethysmographic sensors. IEEE Trans. Biomed. Eng. 2001, 48, 795–805. [Google Scholar] [CrossRef]
- Jung, J.Y.; Lee, L.W. Zigbee device access control and reliable data transmission in Zigbee based health monitoring. Proc. Int. Conf. Adv. Commun. Technol. (ICACT 2008) 2008, 1, 795–797. [Google Scholar] [CrossRef]
- Lee, Y.; Shin, H.; Jo, J.; Lee, Y. Development of a wristwatch-type PPG array sensor module. In Proceedings of the IEEE International Conference on Consumer Electronics, Berlin, Germany, 6–8 September 2011; pp. 168–171.
- Maguire, M.; Ward, T. The design and clinical use of a reflective brachial photoplethysmograph. In Technical Report; National University of Ireland: Maynooth, Ireland, 2002; pp. 1–13. [Google Scholar]
- Maeda, Y.; Sekine, M.; Tamura, T. Relationship between measurement site and motion artifacts in wearable reflected photoplethysmography. J. Med. Syst. 2011, 35, 969–976. [Google Scholar] [CrossRef]
- Vogel, S.; Hülsbusch, M.; Henning, T.; Blazek, V.; Leonhardt, S. In-ear vital signs monitoring using a novel microoptic reflective sensor. IEEE Trans. Inform. Tech. Biomed. 2009, 13, 882–889. [Google Scholar] [CrossRef]
- Shin, K.; Kim, Y.; Bae, S.; Park, K.; Kim, S. A novel headset with a transmissive PPG sensor for heart rate measurement. Proc. Int. Conf. Biomed. Eng. IFMBE 2009, 23, 519–522. [Google Scholar] [CrossRef]
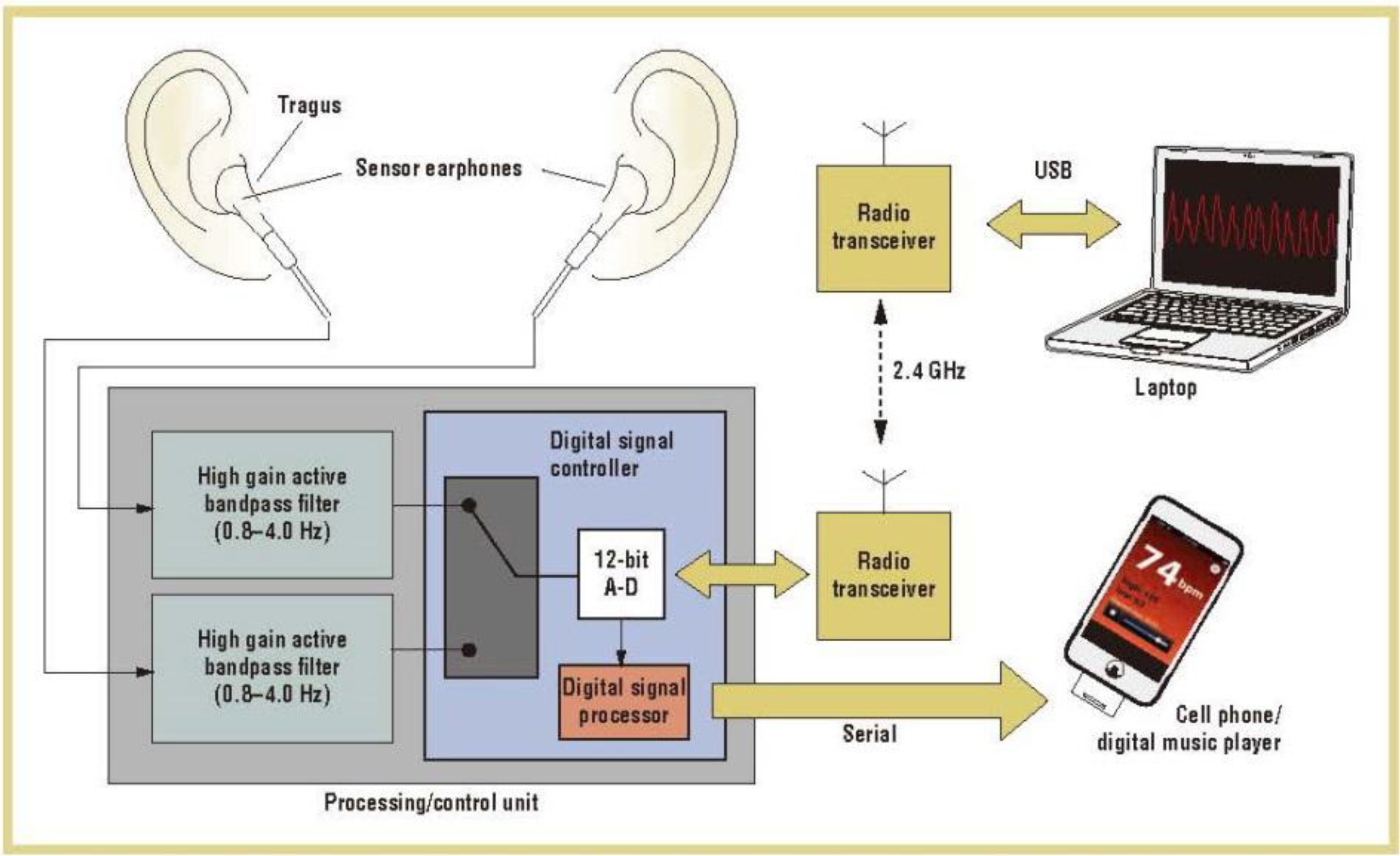
- Poh, M.-Z.; Swenson, N.C.; Picard, R.W. Motion-tolerant magnetic earring sensor and wireless earpiece for wearable photoplethysmography. IEEE Trans. Inform. Tech. Biomed. 2010, 14, 786–794. [Google Scholar] [CrossRef]
- Celka, P.; Verjus, C.; Vetter, R. Motion resistant earphone located infrared based heart rate measurement device. In Proceedings of the 2nd International Conference on Biomedical Engineering, Innsbruck, Austria, 16–18 February 2004; pp. 582–585.
- Poh, M.-Z.; Kim, K.; Goessling, A.; Swenson, N.; Picard, R. Cardiovascular monitoring using earphones and a mobile device. Pervasive Comput. 2012, 11, 18–26. [Google Scholar]
- Budidha, K.; Kyriacou, P.A. The human ear canal: Investigation of its suitability for monitoring photoplethysmographs and arterial oxygen saturation. Phys. Meas. 2014, 35, 111–128. [Google Scholar] [CrossRef]
- Wang, L.; Lo, B.; Yang, G.Z. Multichannel reflective PPG earpiece sensor with passive motion cancellation. IEEE Trans. Biomed. Circuits Syst. 2007, 1, 235–241. [Google Scholar] [CrossRef]
- Wang, C.Z.; Zheng, Y.P. Home-telecare of the elderly living alone using a new designed ear-wearable sensor. In Proceedings of the 5th International Workshop Wearable Implantable Body Sensor Networks, Hong Kong, China, 1–3 June 2008; pp. 71–74.
- Patterson, J.A.C.; McIlwraith, D.G.; Yang, G.-Z. A flexible, low noise reflectiveG sensor platform for ear-worn heart rate monitoring. In Proceedings of 6th International Workshop Wearable Implantable Body Sensor Networks, Berkeley, CA, USA, 3–5 June 2009; pp. 286–291.
- Kyriacou, P.A.; Moye, A.R.; Gregg, R.M.; Choi, D.M.A.; Langford, R.M.; Jones, D.P. A system for investigating oesophageal photoplethysmographic signals in anaesthetised patients. Med. Biol. Eng. Comput. 1999, 37, 639–643. [Google Scholar] [CrossRef]
- Kyriacou, P.A.; Powell, S.; Langford, R.M.; Jones, D.P. Esophageal pulse oximetry utilizing reflectance photoplethysmography. IEEE Trans. Biomed. Eng. 2002, 49, 1360–1368. [Google Scholar] [CrossRef]
- Kyriacou, P.A. Direct pulse oximetry within the esophagus, on the surface of abdominal viscera, and on free flaps. Anesth. Analg. 2013, 117, 824–833. [Google Scholar] [CrossRef]
- Mendelson, Y.; Pujary, C. Measurement site and photodetector size considerations in optimizing power consumption of a wearable reflectance pulse oximeter. Conf. Proc. IEEE EMBS 2003, 4, 3016–3019. [Google Scholar]
- Lee, E.M.; Shin, J.Y.; Hong, J.H.; Cha, E.J.; Lee, T.S. Glass-type wireless PPG measuring system. Conf. Proc. IEEE EMBS. 2010, 1, 1433–1436. [Google Scholar]
- Tur, E.; Tur, M.; Maibach, H.I.; Guy, R.H. Basal perfusion of the cutaneous microcirculation: Measurements as a function of anatomic position. J. Invest. Dermatol. 1983, 81, 442–446. [Google Scholar]
- Asada, H.H.; Shaltis, P.; Reisner, A.; Rhee, S.; Hutchinson, R.C. Mobile monitoring with wearable photoplethysmographic biosensors. IEEE Eng. Med. Biol. Mag. 2003, 22, 28–40. [Google Scholar]
- Teng, X.F.; Zhang, Y.T. The effect of contacting force on photoplethysmographic signals. Physiol. Meas. 2004, 25, 1323–1335. [Google Scholar] [CrossRef]
- Spigulis, J. Optical non-invasive monitoring of skin blood pulsations. Appl. Opt. 2005, 44, 1850–1857. [Google Scholar] [CrossRef]
- Maeda, Y.; Sekine, M.; Tamura, T.; Mizutani, K. The effect of contact pressure to the photoplethysmographic sensor during walking. Conf. Proc. EMBS. 2013. Available online: http://embc.embs.org/files/2013/3170_FI.pdf (accessed on 22 April 2014).
- Dresher, R.; Mendelson, Y. Attachment of a wearable skin reflectance pulse oximeter. In Proceedings of the 2005 BMES Annual Fall Meeting, Baltimore, MD, USA, 28 September–1 October 2005.
- Dassel, A.C.M.; Graaff, R.; Sikkema, M.; Meijer, A.; Zijlstra, W.G.; Aamoudse, J.G. Reflectance pulse oximetry at the forehead improves by pressure on the probe. J. Clin. Monit. 1995, 11, 237–244. [Google Scholar] [CrossRef]
- Dassel, A.C.M.; Graaff, R.; Meijer, A.; Zijlstra, W.G.; Aarnoudse, J.G. Reflectance pulse oximetry at the forehead of newborns. The influence of varying pressure on the probe. J. Clin. Monit. 1996, 12, 421–428. [Google Scholar] [CrossRef]
- Dassel, A.C.M.; Graaff, R.; Aardema, M.; Zijlstra, W.G.; Aarnoudse, J.G. Effect of location of the sensor on reflectance pulse oximetry. Br. J. Obstet. Gynaecol. 1997, 104, 910–916. [Google Scholar] [CrossRef]
- Hsiu, H.; Hsu, C.L.; Wu, T.L. Effects of different contacting pressure on the transfer function between finger photoplethysmographic and radial blood pressure waveforms. J. Eng. Med. 2011, 225, 575–583. [Google Scholar] [CrossRef]
- Grabovskis, A.; Marcinkevics, Z.; Rubins, U.; Kviesis-Kipge, E. Effect of probe contact pressure on the photoplethysmographic assessment of conduit artery stiffness. J. Biomed. Opt. 2013, 18. [Google Scholar] [CrossRef]
- Hu, S.; Zheng, J.; Chouliaras, V.; Summers, R. Feasibility of imaging photoplethysmography. In Proceedings of the International Conference on Biomedical Engineering and Informatics, Sanya, Hainan, China, 27–30 May 2008.
- Poh, M.Z.; McDuff, D.J.; Picard, R.W. Non-contact, automated cardiac pulse measurements using video imaging and blind source separation. Opt. Express 2010, 18, 10762–10774. [Google Scholar] [CrossRef]
- Poh, M.Z.; McDuff, D.J.; Picard, R.W. Advancements in noncontact, multiparameter physiological measurements using a webcam. IEEE Trans. Biomed. Eng. 2011, 58, 7–11. [Google Scholar] [CrossRef]
- Jonathan, E.; Leahy, M. Investigating a smartphone imaging unit for photoplethysmography. Physiol. Meas. 2010, 31, N79–N83. [Google Scholar] [CrossRef]
- Instant Hear Rate. Available online: http://www.azumio.com/apps/heart-rate/ (accessed on 10 April 2014).
- Lee, H.-W.; Lee, J.-W.; Jung, W.-C.; Lee, G.-K. The periodic moving average filter for removing motion artifacts from PPG signals. Int. J. Ctrl. Autom. Syst. 2007, 5, 701–706. [Google Scholar]
- Lee, J.; Jung, W.; Kang, I.T.; Kim, Y.; Lee, G. Design of filter to reject motion artifact of pulse oximetry. Comput. Stand. Interfaces 2004, 26, 241–249. [Google Scholar] [CrossRef]
- Reddy, K.A.; George, B.; Kumar, V.J. Use of Fourier series analysis for motion artifact reduction and data compression of photoplethysmographic signals. IEEE Trans. Instrum. Meas. 2009, 58, 1706–1711. [Google Scholar] [CrossRef]
- Asada, H.H.; Jiang, H.H.; Gibbs, P. Active noise cancellation using MEMS accelerometers for motion-tolerant wearable bio-sensors. Conf. Proc. IEEE EMBS 2004, 3, 2157–2160. [Google Scholar]
- Gibbs, P.T.; Wood, L.B.; Asada, H.H. Active motion artifact cancellation for wearable health monitoring sensors using collocated MEMS accelerometers. Proc. SPIE Smart Struct. Mater. Sens. Smart Struct. Technol. Civil Mech. Aerosp. Syst. 2005. [Google Scholar] [CrossRef]
- Chan, K.W.; Zhang, Y.T. Adaptive reduction of motion artifact from photoplethysmographic recordings using a variable step size LMS filter. Proc. IEEE Sens. 2002, 2, 1343–1346. [Google Scholar] [CrossRef]
- Han, H.; Kim, M.-J.; Kim, J. Development of real-time motion artifact reduction algorithm for a wearable photoplethysmography. Conf. Proc. IEEE EMBS 2007, 3, 1538–1541. [Google Scholar]
- Wei, P.; Guo, R.; Zhang, J.; Zhang, Y.T. A new wristband wearable sensor using adaptive reduction filter to reduce motion artifact. In Proceedings of the 2008 International Conference on Information Technology and Applications in Biomedicine (ITAB 2008), Shenzhen, China, 30–31 May 2008; pp. 278–281.
- Wood, L.B. Motion artifact reduction for wearable photoplethysmogram sensors using micro accelerometers and Laguerre series adaptive filters. MIT MS Thesis, Degree-Granting University, Cambridge, MA, USA, 2008; pp. 1–74. [Google Scholar]
- Han, H.; Kim, J. Artifacts in wearable photoplethysmographs during daily life motions and their reduction with least mean square based active noise cancellation method. Comput. Biol. Med. 2012, 42, 387–393. [Google Scholar] [CrossRef]
- Ram, M.R.; Madhav, K.V.; Krishna, E.H.; Komalla, N.R.; Reddy, K.A. A novel approach for artifact reduction in PPG signals based on AS-LMS adaptive filter. IEEE Instrum. Meas. 2012, 61, 1445–1457. [Google Scholar] [CrossRef]
- Nehorai, A.; Porat, B. Adaptive comb filtering for harmonic signal enhancement. IEEE Trans. Acoust. Speech Signal Proc. 1986, 34, 1124–1138. [Google Scholar] [CrossRef]
- Lee, B.; Kee, Y.; Han, J.; Yi, W.-J. Adaptive comb filtering for motion artifact reduction from PPG with a structure of adaptive lattice IIR notch filter. Conf. Proc. EMBS 2011, 2011, 7937–7940. [Google Scholar]
- Kim, S.C.; Hwang, E.J.; Kim, D.W. Noise reduction of PPG signal during free movements using adaptive SFLC (scaled Fourier liner combiner). IFMBE Proc. 2007, 14, 1191–1194. [Google Scholar] [CrossRef]
- Lee, B.; Han, J.; Baek, H.-J.; Shin, J.-H.; Park, K.-W.; Yi, W.-J. Improved estimation of motion artifacts from a photoplethysmographic signal using a Kalman smoother with simultaneous accelerometry. Physiol. Meas. 2010, 31, 1585–1603. [Google Scholar] [CrossRef]
- Seyeditabaii, S.; Seyedtabaii, L. Kalman filter based adaptive reduction of motion artifact from photoplethysmographic signal. Proc. World Acad. Sci. Eng. Technol. 2008, 39, 173–176. [Google Scholar]
- Lee, Y.-K.; Jo, J.; Lee, Y.; Shin, H.-S.; Kwon, O.-W. Particle filter-based noise reduction of PPG signals for robust emotion recognition. In Proceedings of the IEEE International Conference on Consumer Electronics (ICCE), Las Vegas, NV, USA, 13–16 January 2012; pp. 598–599.
- Yousefi, R.; Nourani, M.; Ostadabbas, S.; Panashi, I. A motin-tolenrat adaptive algorithm for wearable photoplethymographic biosesnors. IEEE J. Biomed. Health Inform. 2013, 18, 670–681. [Google Scholar]
- Lee, S.-M.; Zhang, Y.-T. Reduction of motion artifacts from photoplethysmographic recordings using a wavelet denoising approach. In Proceedings of the IEEE EMBS Asian-Pacific Conference on Biomedical Engineering, Kyoto-Osaka-Nara, Japan, 20–22 October 2003; pp. 194–195.
- Yan, Y.; Poon, C.C.Y.; Zhang, Y.T. Reduction of motion artifact in pulse oximetry by smoothed pseudo-Wigner-Ville distribution. J. Neuroeng. Rehabil. 2005, 2, 3. [Google Scholar] [CrossRef]
- Lee, S.; Ibey, B.L.; Xu, W.; Wilson, M.A.; Ericson, M.N.; Coté, G.L. Processing of pulse oximeter data using discrete wavelet analysis. IEEE Trans. Biomed. Eng. 2005, 52, 1350–1352. [Google Scholar] [CrossRef]
- Foo, J.Y.A. Comparison of wavelet transformation and adaptive filtering in restoring artifact-induced time-related measurement. Biomed. Signal Process. Control 2006, 1, 93–98. [Google Scholar] [CrossRef]
- Yao, J.; Warren, S. A short study to assess the potential of independent component analysis for motion artifact separation in wearable pulse oximeter signals. Conf. Proc. IEEE EMBS 2005, 4, 3585–3588. [Google Scholar]
- Kim, B.S.; Yoo, S.K. Motion artifact reduction in photoplethysmography using independent component analysis. IEEE Trans. Biomed. Eng. 2006, 53, 566–568. [Google Scholar] [CrossRef]
- Widraw, B.; Glover, J.R., Jr.; McCool, J.M.; Kaunitz, J.; Williams, C.S.; Hearn, R.H.; Zeidler, J.R.; Dong, E., Jr.; Goodlin, R.C. Adaptive noise cancellation: Principles and applications. Proc. IEEE 1975, 63, 1692–1716. [Google Scholar] [CrossRef]
- Wood, L.B.; Asada, H.H. Noise cancellation model validation for reduced motion artifact wearable PPG sensors using MEMS accelerometers. In Proceedings of the IEEE 28th Annual International Conference on Engineering in Medicine and Biology Society, New York, NY, USA, 30 August–3 September 2006; pp. 3525–3528.
- Wood, L.B.; Asada, H.H. Low variance adaptive filter for cancelling motion artifact in wearable photoplethysmogram sensor signals. In Proceedings of the IEEE 29th Annual International Conference on Engineering in Medicine and Biology Society (EMBS 2007), Lyon, France, 22–26 August 2007; pp. 652–655.
- Wood, L.B.; Asada, H.H. Active motion artifact reduction for wearable sensors using Laguerre expansion and signal separation. In Proceedings IEEE Conference on EMBS, Shanghai, China, 17–18 January 2005; pp. 652–655.
- Hayes, M.J.; Smith, P.R. Artifact reduction in photoplethysmography. Appl. Opt. 1998, 37, 7437–7446. [Google Scholar] [CrossRef]
- Hayes, M.J.; Smith, P.R. A new method for pulse oximetry processing inherent insensitivity to artifact. IEEE Trans. Biomed. Eng. 2001, 48, 452–461. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tamura, T.; Maeda, Y.; Sekine, M.; Yoshida, M. Wearable Photoplethysmographic Sensors—Past and Present. Electronics 2014, 3, 282-302. https://doi.org/10.3390/electronics3020282
Tamura T, Maeda Y, Sekine M, Yoshida M. Wearable Photoplethysmographic Sensors—Past and Present. Electronics. 2014; 3(2):282-302. https://doi.org/10.3390/electronics3020282
Chicago/Turabian StyleTamura, Toshiyo, Yuka Maeda, Masaki Sekine, and Masaki Yoshida. 2014. "Wearable Photoplethysmographic Sensors—Past and Present" Electronics 3, no. 2: 282-302. https://doi.org/10.3390/electronics3020282
APA StyleTamura, T., Maeda, Y., Sekine, M., & Yoshida, M. (2014). Wearable Photoplethysmographic Sensors—Past and Present. Electronics, 3(2), 282-302. https://doi.org/10.3390/electronics3020282