Recent Advances and Applications of Odor Biosensors
Abstract
1. Introduction
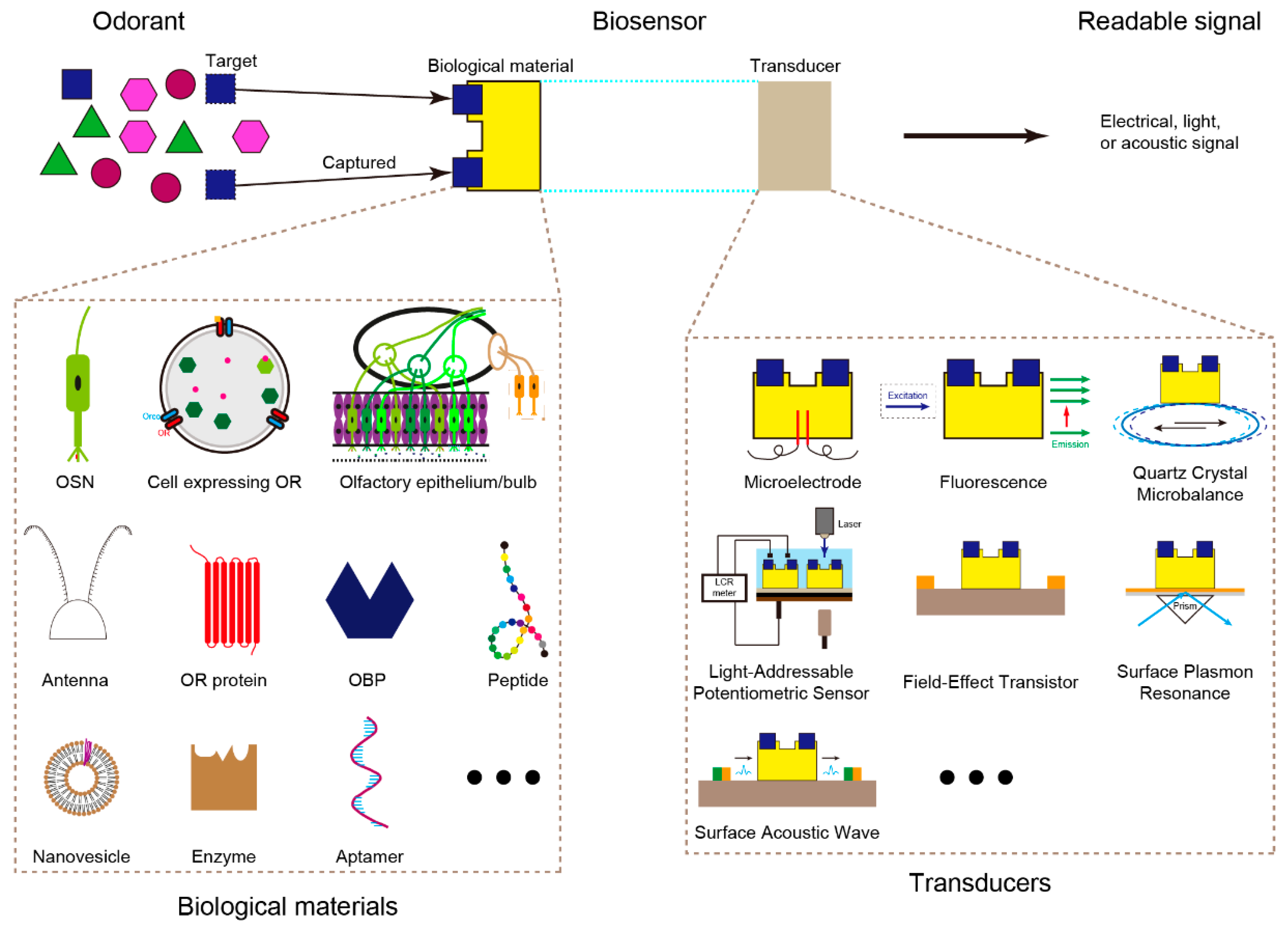
2. Odor Biosensors with Different Transducers
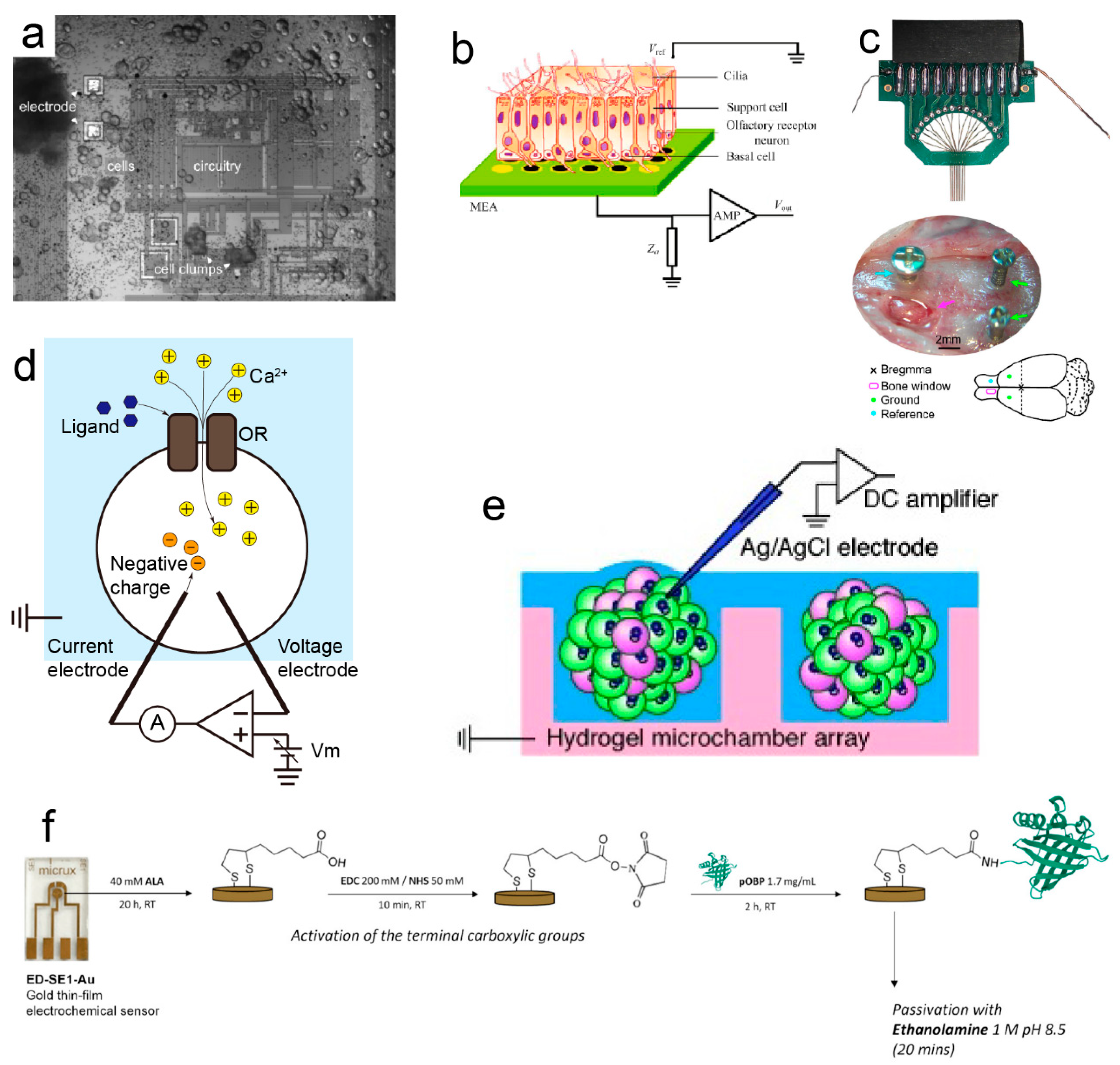
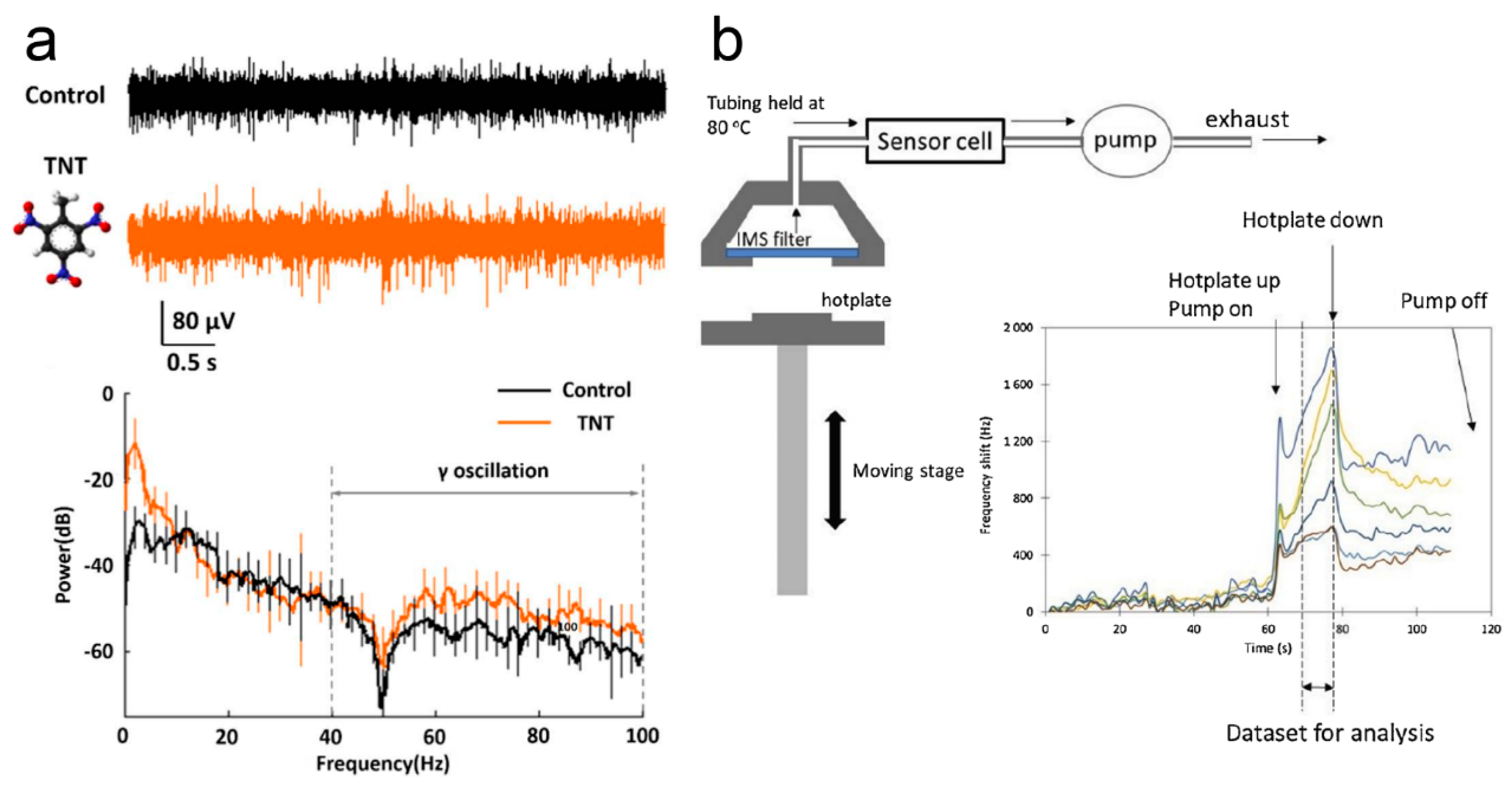
2.1. Microelectrode-Based Odor Biosensors
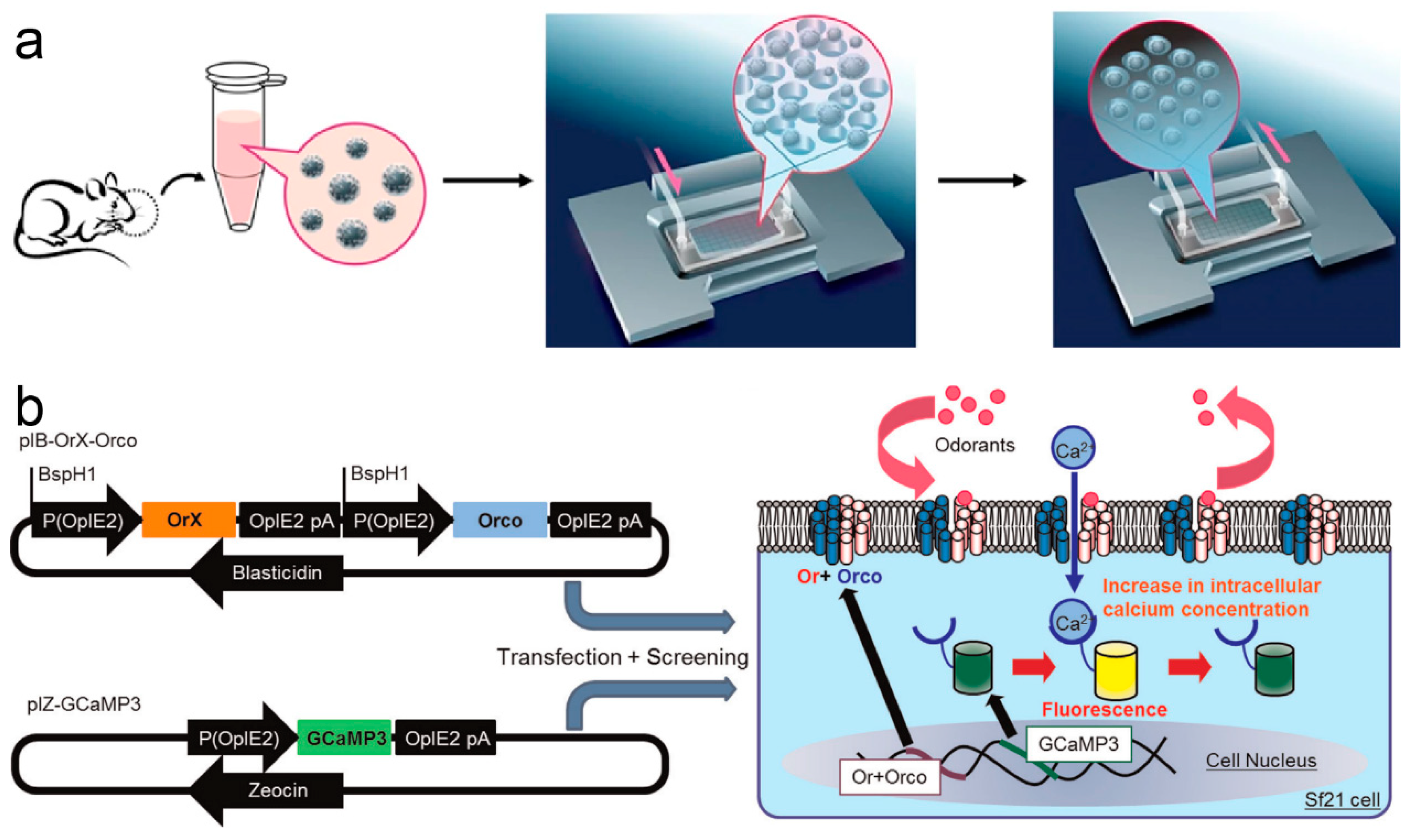
2.2. Fluorescence-Based Odor Biosensors
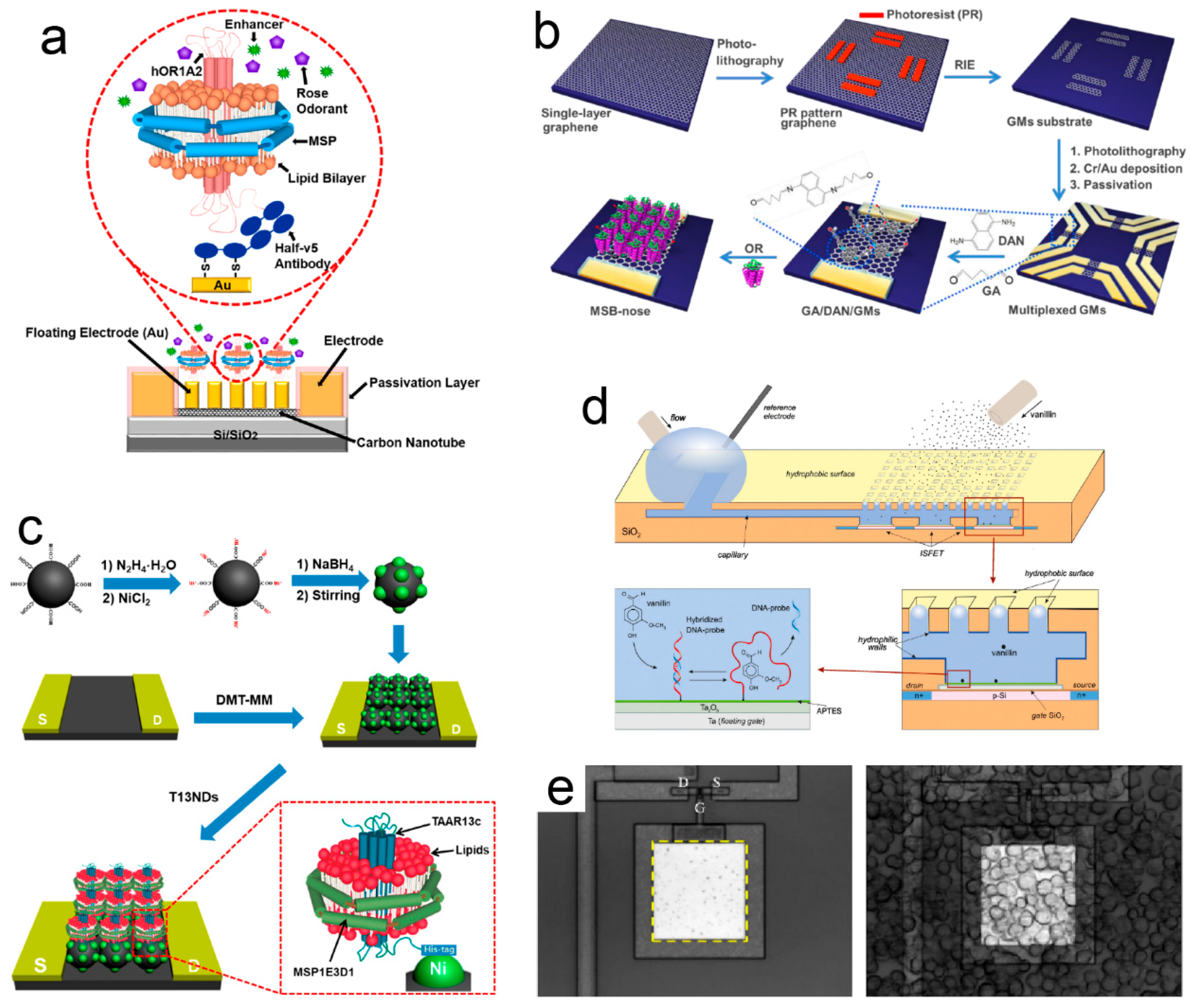
2.3. SPR-Based Odor Biosensors
2.4. FET-Based Odor Biosensors
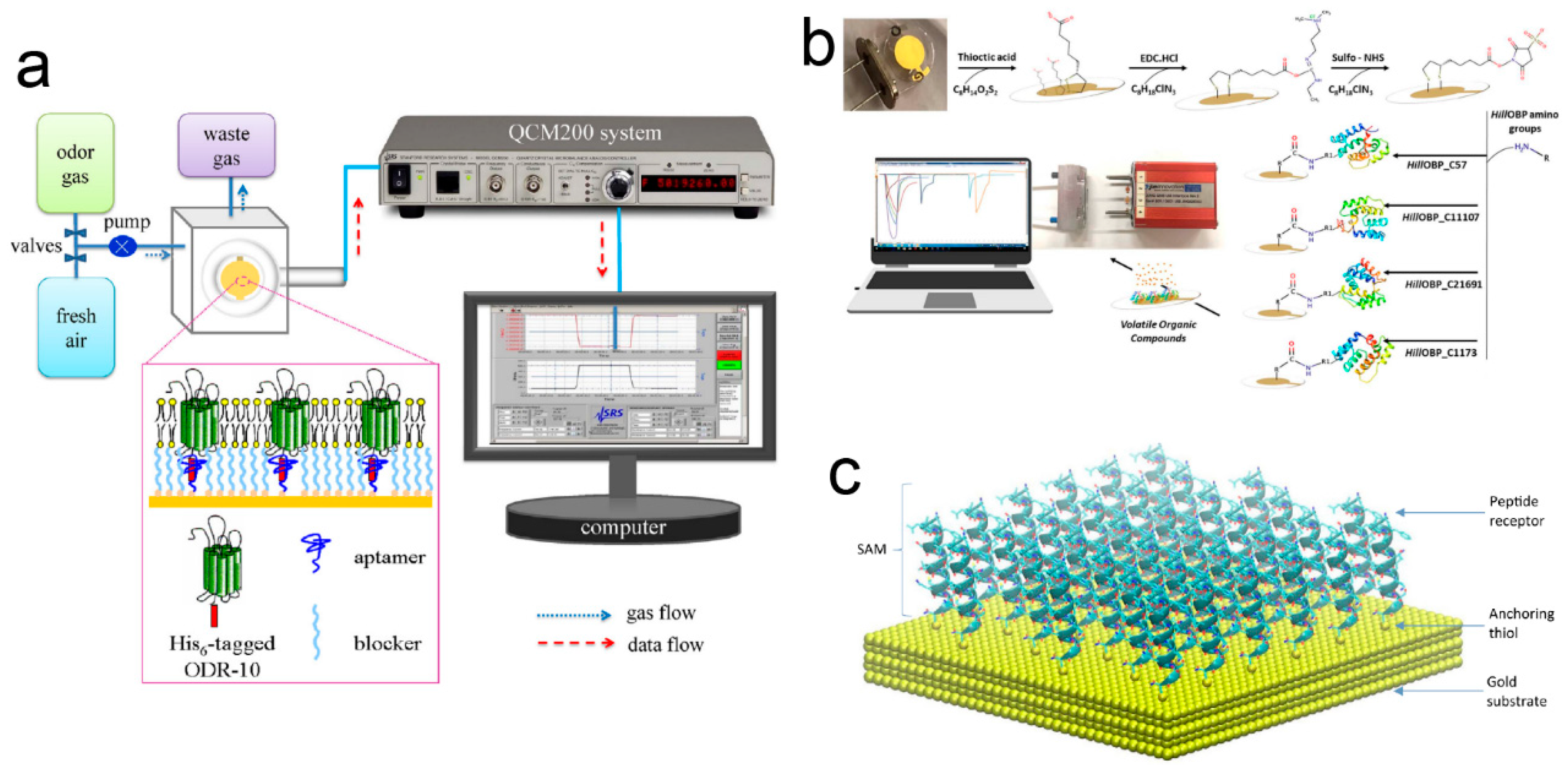
2.5. QCM-Based Odor Biosensors
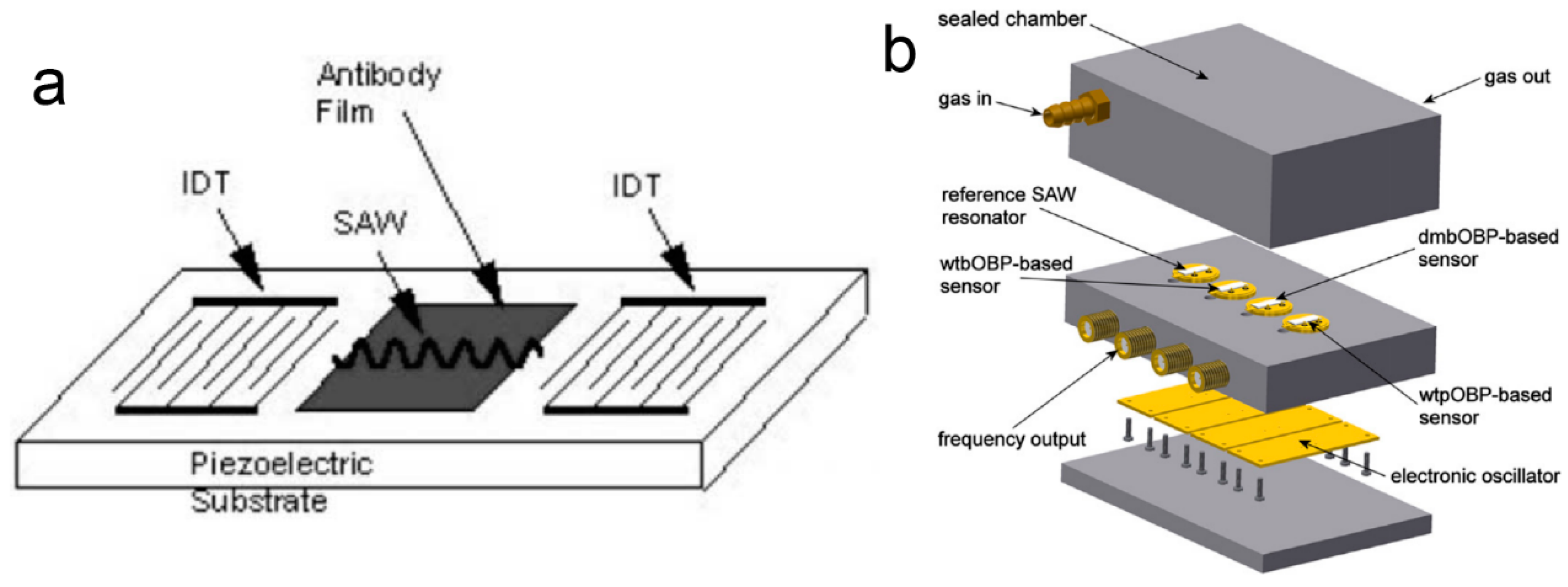
2.6. SAW-Based Odor Biosensors
2.7. Other Odor Biosensors
3. Odor Biosensor Application Fields
3.1. Environmental Monitoring

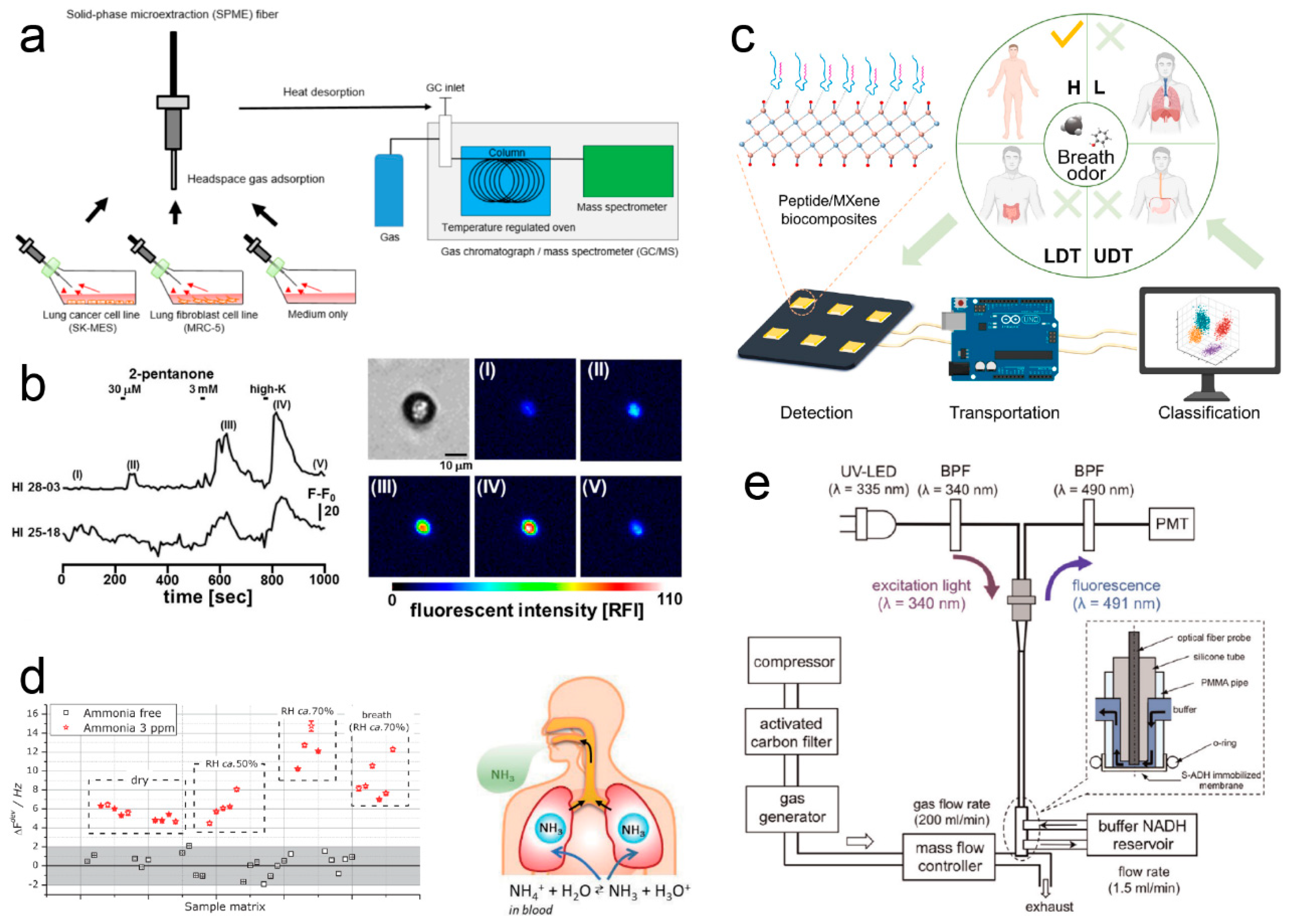
3.2. Disease Diagnosis
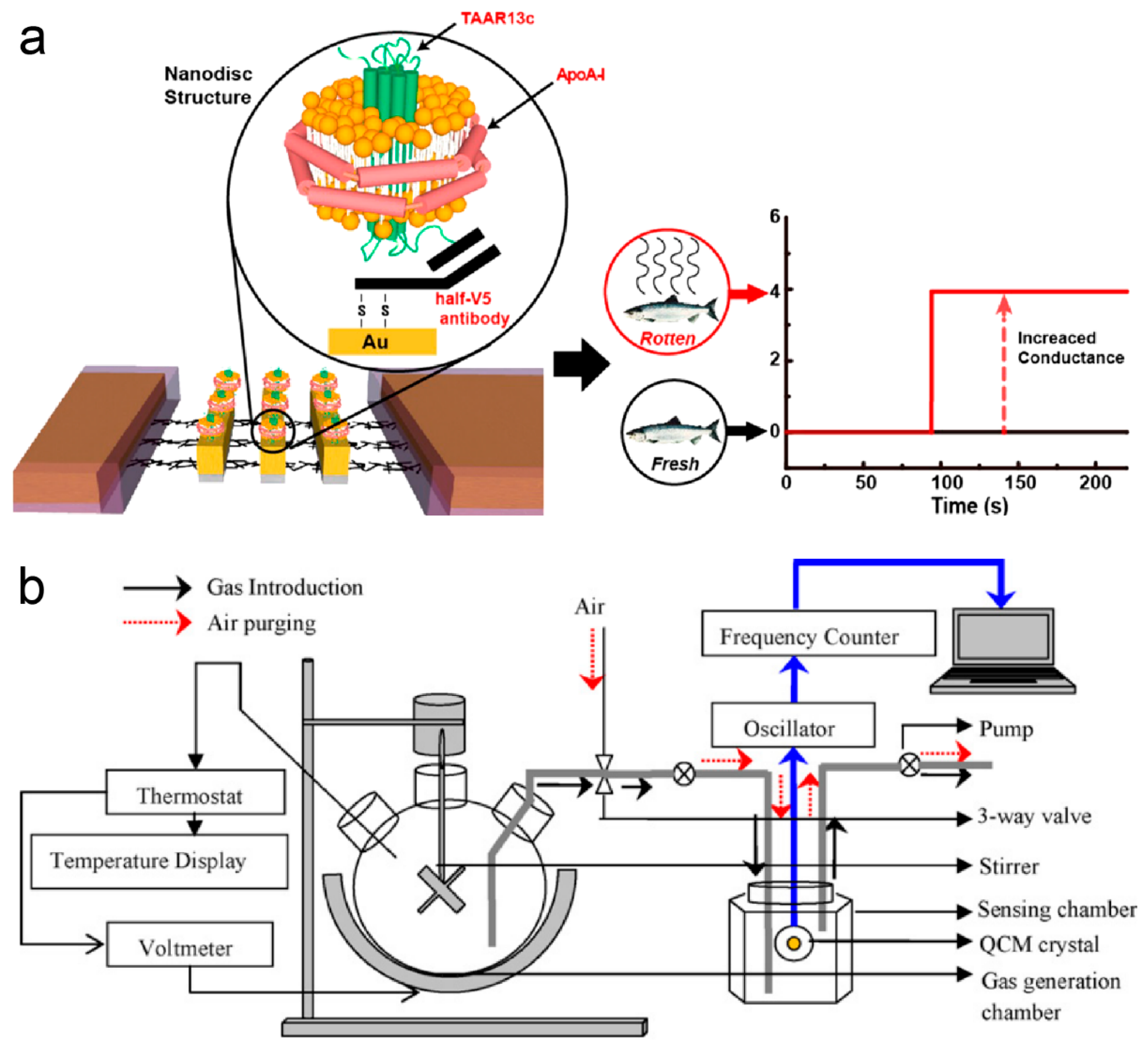
3.3. Food Safety
3.4. Security
4. Future Prospects
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bushdid, C.; Magnasco, M.O.; Vosshall, L.B.; Keller, A. Humans can discriminate more than 1 trillion olfactory stimuli. Science 2014, 343, 1370–1372. [Google Scholar] [CrossRef] [PubMed]
- Pevsner, J.; Reed, R.R.; Feinstein, P.G.; Snyder, S.H. Molecular cloning of odorant-binding protein: Member of a ligand carrier family. Science 1988, 241, 336–339. [Google Scholar] [CrossRef]
- Franco, R.; Garrigós, C.; Capó, T.; Serrano-Marín, J.; Rivas-Santisteban, R.; Lillo, J. Olfactory receptors in neural regeneration in the central nervous system. Neural Regen. Res. 2025, 20, 2480–2494. [Google Scholar] [CrossRef] [PubMed]
- Preuschhof, C.; Heekeren, H.R.; Taskin, B.; Schubert, T.; Villringer, A. Neural correlates of olfactory working memory in the human brain. J. Neurosci. 2006, 26, 13231–13239. [Google Scholar] [CrossRef] [PubMed]
- Naffaa, M.M. Neurogenesis dynamics in the olfactory bulb: Deciphering circuitry organization, function, and adaptive plasticity. Neural Regen. Res. 2025, 20, 1565–1581. [Google Scholar] [CrossRef]
- Keller, A.; Gerkin, R.C.; Guan, Y.; Dhurandhar, A.; Turu, G.; Szalai, B.; Mainland, J.D.; Ihara, Y.; Yu, C.W.; Meyer, P.; et al. Predicting human olfactory perception from chemical features of odor molecules. Science 2017, 355, 820–826. [Google Scholar] [CrossRef]
- Johnson, D.C. Airway mucus function and dysfunction. N. Engl. J. Med. 2011, 364, 978. [Google Scholar] [CrossRef]
- Schiefner, A.; Freier, R.; Eichinger, A.; Skerra, A. Crystal structure of the human odorant binding protein, OBPIIa. Proteins Struct. Funct. Bioinforma. 2015, 83, 1180–1184. [Google Scholar] [CrossRef]
- Nielsen, B.L.; Rampin, O.; Meunier, N.; Bombail, V. Behavioral responses to odors from other species: Introducing a complementary model of allelochemics involving vertebrates. Front. Neurosci. 2015, 9, 226. [Google Scholar] [CrossRef]
- German, P.F.; van der Poel, S.; Carraher, C.; Kralicek, A.V.; Newcomb, R.D. Insights into subunit interactions within the insect olfactory receptor complex using FRET. Insect Biochem. Mol. Biol. 2013, 43, 138–145. [Google Scholar] [CrossRef]
- Lee, M.; Kim, Y.S. Analysis of volatile and odor-active compounds in charcoal-grilled marinated beef using gas chromatography–mass spectrometry and gas chromatography–olfactometry. Food Sci. Biotechnol. 2025, 34, 1339–1349. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Li, J.; Liu, J.; Tan, R. Examining the effects of processing techniques on the quality of hawk tea through liquid chromatography–tandem mass spectrometry and two-dimensional gas chromatography–time-of-flight mass spectrometry. Food Chem. 2025, 465, 142012. [Google Scholar] [CrossRef]
- Corvino, A.; Khomenko, I.; Betta, E.; Brigante, F.I.; Bontempo, L.; Biasioli, F.; Capozzi, V. Rapid Profiling of Volatile Organic Compounds Associated with Plant-Based Milks Versus Bovine Milk Using an Integrated PTR-ToF-MS and GC-MS Approach. Molecules 2025, 30, 761. [Google Scholar] [CrossRef]
- Fernández-Lodeiro, A.; Constantinou, M.; Panteli, C.; Agapiou, A.; Andreou, C. Breath Analysis via Surface Enhanced Raman Spectroscopy. ACS Sens. 2025, 10, 602–621. [Google Scholar] [CrossRef] [PubMed]
- Sobotka, A.; Krueger, T.D.; Solaris, J.; Chen, C.; Feng, Y.; Mayer, S.G.; Qian, Y.P.; Fang, C.; Qian, M.C. Developing nondestructive sensitive detection for smoke-exposure assessment in wine via advanced Raman spectroscopy. Food Chem. 2025, 475, 143327. [Google Scholar] [CrossRef]
- Ju, J.; Cong, S.; Hu, Y.; Xu, Z.; Ji, Z.; Xu, R.; Zhang, Z.; Cao, X. Rapid detection of nitenpyram in fruits using molecularly imprinted polymers coupled with SERS technology. J. Food Compos. Anal. 2025, 139, 107170. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Z.; Chang, R.; Zhang, Z.; Xu, X.; Xu, Y.; Mao, J. New Method of Direct Sampling Gas Chromatography-Ion Mobility Spectrometry to Identify the Dynamic Retronasal Volatile Compounds in the Alcoholic Beverage and Their Release Behaviors: A Case Study on Huangjiu. J. Agric. Food Chem. 2025, 73, 4331–4341. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, C.; Xie, Y.; Xu, P.; Xu, X.; Pang, L.; Lv, Z.; Lu, G. Physiological characteristics and volatile organic compound dynamics in bruising sweetpotatoes during short-term storage: Insights from GC–MS and GC-IMS. Food Chem. X 2025, 27, 102418. [Google Scholar] [CrossRef]
- Zhu, G.; Yang, Y.; Zhang, F.; Wei, J.; Tian, X.; Liu, L.; Ma, Z.; Zhang, G. Non-Invasive Monitoring and Differentiation of Aging Mice Treated with Goat Whey Powder by an Electronic Nose Coupled with Chemometric Methods. Sensors 2025, 25, 1496. [Google Scholar] [CrossRef]
- Frischmon, C.; Crosslin, J.; Burks, L.; Weckesser, B.; Hannigan, M.; Duderstadt, K. Detecting air pollution episodes and exploring their impacts using low-cost sensor data and simultaneous community symptom and odor reports. Environ. Res. Lett. 2025, 20, 044043. [Google Scholar] [CrossRef]
- Kong, R.; Shen, W.; Gao, Y.; Lv, D.; Ai, L.; Song, W.; Tan, R. Enhancing E-Nose Performance via Metal-Oxide Based MEMS Sensor Arrays Optimization and Feature Alignment for Drug Classification. Sensors 2025, 25, 1480. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhou, L.; Wang, X. Surface acoustic wave sensor for formaldehyde gas detection using the multi-source spray-deposited graphene/PMMA composite film. Front. Mater. 2023, 9, 1025903. [Google Scholar] [CrossRef]
- Arasaradnam, R.P.; Krishnamoorthy, A.; Hull, M.A.; Wheatstone, P.; Kvasnik, F.; Persaud, K.C. The Development and Optimisation of a Urinary Volatile Organic Compound Analytical Platform Using Gas Sensor Arrays for the Detection of Colorectal Cancer. Sensors 2025, 25, 599. [Google Scholar] [CrossRef] [PubMed]
- Eaton, J.R.; Christian, J.E.; Campbell, J.A. Surface phenomena related to odor measurements. Ann. N. Y. Acad. Sci. 1954, 58, 239–249. [Google Scholar] [CrossRef]
- Moncrieff, R.W. An instrument for measuring and classifying odors. J. Appl. Physiol. 1961, 16, 742–749. [Google Scholar] [CrossRef]
- Persaud, K.; Dodd, G. Analysis of discrimination mechanisms in the mammalian olfactory system using a model nose. Nature 1982, 299, 352–355. [Google Scholar] [CrossRef]
- Hatfield, J.V.; Neaves, P.; Hicks, P.J.; Persaud, K.; Travers, P. Towards an integrated electronic nose using conducting polymer sensors. Sens. Actuators B Chem. 1994, 18, 221–228. [Google Scholar] [CrossRef]
- Vlasov, Y.; Legin, A. Non-selective chemical sensors in analytical chemistry: From ‘electronic nose’ to ‘electronic tongue’, Fresenius. J. Anal. Chem. 1998, 361, 255–260. [Google Scholar] [CrossRef]
- Deng, H.; Mitsuno, H.; Kuroda, E.; Niki, S.; Kanzaki, R.; Nakamoto, T. Gas-phase odor mixture quantification based on relative comparison method using multiple olfactory receptors. Sens. Actuators B Chem. 2024, 401, 134995. [Google Scholar] [CrossRef]
- Mitsubayashi, K.; Toma, K.; Iitani, K.; Arakawa, T. Gas-phase biosensors: A review. Sens. Actuators B Chem. 2022, 367, 132053. [Google Scholar] [CrossRef]
- Hirata, Y.; Oda, H.; Osaki, T.; Takeuchi, S. Biohybrid sensor for odor detection. Lab A Chip 2021, 21, 2643–2657. [Google Scholar] [CrossRef]
- Hurot, C.; Scaramozzino, N.; Buhot, A.; Hou, Y. Bio-inspired strategies for improving the selectivity and sensitivity of artificial noses: A review. Sensors 2020, 20, 1803. [Google Scholar] [CrossRef]
- Deng, H.; Nakamoto, T. Odor Biosensors Based on Cell Expressing Olfactory Receptor: Recent Advances. Anal. Sens. 2024, 4, e202400006. [Google Scholar] [CrossRef]
- Xu, S.; Tian, M.; Li, C.; Liu, G.; Wang, J.; Yan, T.; Li, Z. Using a crumpled graphene field-effect transistor for ultrasensitive SARS-CoV-2 detection via its papain-like protease. Chem. Eng. J. 2025, 505, 159386. [Google Scholar] [CrossRef]
- Oprea, A.; Weimar, U. Gas sensors based on mass-sensitive transducers. Part 2: Improving the sensors towards practical application. Anal. Bioanal. Chem. 2020, 412, 6707–6776. [Google Scholar] [CrossRef] [PubMed]
- Bohbot, J.D.; Vernick, S. The emergence of insect odorant receptor-based biosensors. Biosensors 2020, 10, 26. [Google Scholar] [CrossRef]
- Rashed, M.S.; Abdelkarim, E.A.; Elsamahy, T.; Sobhy, M.; El-Mesery, H.S.; Salem, A. Advances in cell-based biosensors: Transforming food flavor evaluation with novel approaches. Food Chem. X 2025, 26, 102336. [Google Scholar] [CrossRef]
- Qin, C.; Wang, Y.; Hu, J.; Wang, T.; Liu, D.; Dong, J.; Lu, Y. Artificial Olfactory Biohybrid System: An Evolving Sense of Smell. Adv. Sci. 2023, 10, 2204726. [Google Scholar] [CrossRef]
- Misawa, N.; Mitsuno, H.; Kanzaki, R.; Takeuchi, S. Highly sensitive and selective odorant sensor using living cells expressing insect olfactory receptors. Proc. Natl. Acad. Sci. USA 2010, 107, 15340–15344. [Google Scholar] [CrossRef]
- Pawson, S.M.; Kerr, J.L.; O’Connor, B.C.; Lucas, P.; Strand, T.M. Light-Weight Portable Electroantennography Device as a Future Field-Based Tool for Applied Chemical Ecology. J. Chem. Ecol. 2020, 46, 557–566. [Google Scholar] [CrossRef]
- Lagunas, A.; Belloir, C.; Lalis, M.; Briand, L.; Topin, J.; Gorostiza, P.; Samitier, J. Ligand discrimination in hOR1A1 based on the capacitive response. Biosens. Bioelectron. 2025, 271, 117000. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Z.; Liu, T.; Zhang, L. An implantable ionic liquid-gel microelectrode for in vivo monitoring of K+ levels in the living rat brain. Chem. Sci. 2025. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Adib, M.R.; Barrett, C.; O’Sullivan, S.; Flynn, A.; McFadden, M.; Kennedy, E.; O’Riordan, A. In situ pH-Controlled electrochemical sensors for glucose and pH detection in calf saliva. Biosens. Bioelectron. 2025, 275, 117234. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Ye, W.; Xiao, L.; Du, L.; Hu, N.; Wang, P. Extracellular potentials recording in intact olfactory epithelium by microelectrode array for a bioelectronic nose. Biosens. Bioelectron. 2010, 25, 2212–2217. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Hu, N.; Ye, W.; Cai, H.; Zhang, F.; Wang, P. Extracellular recording of spatiotemporal patterning in response to odors in the olfactory epithelium by microelectrode arrays. Biosens. Bioelectron. 2011, 27, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, F.; Hu, N.; Wang, H.; Hsia, K.J.; Wang, P. Microelectrode Recording of Tissue Neural Oscillations for a Bionic Olfactory Biosensor. J. Bionic Eng. 2012, 9, 494–500. [Google Scholar] [CrossRef]
- Liu, Q.; Hu, N.; Zhang, F.; Zhang, D.; Hsia, K.J.; Wang, P. Olfactory epithelium biosensor: Odor discrimination of receptor neurons from a bio-hybrid sensing system. Biomed. Microdevices 2012, 14, 1055–1061. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, F.; Zhang, D.; Hu, N.; Hsia, K.J.; Wang, P. Extracellular potentials recording in intact taste epithelium by microelectrode array for a taste sensor. Biosens. Bioelectron. 2013, 43, 186–192. [Google Scholar] [CrossRef]
- Zhuang, L.; Hu, N.; Dong, Q.; Liu, Q.; Wang, P. A high sensitive in vivo biosensing detection for odors by multiunit in rat olfactory bulb. Sens. Actuators B Chem. 2013, 186, 308–314. [Google Scholar] [CrossRef]
- Zhuang, L.; Hu, N.; Tian, F.; Dong, Q.; Hu, L.; Li, R. A high-sensitive detection method for carvone odor by implanted electrodes in rat olfactory bulb. Chin. Sci. Bull. 2014, 59, 29–37. [Google Scholar] [CrossRef]
- Gao, K.; Gao, F.; Du, L.; He, C.; Wan, H.; Wang, P. Integrated olfaction, gustation and toxicity detection by a versatile bioengineered cell-based biomimetic sensor. Bioelectrochemistry 2019, 128, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Gao, F.; Li, J.; He, C.; Liu, M.; Zhu, Q.; Qian, Z.; Ma, T.; Wang, P. Biomimetic integrated olfactory sensory and olfactory bulb systems in vitro based on a chip. Biosens. Bioelectron. 2021, 171, 112739. [Google Scholar] [CrossRef]
- Gao, F.; Gao, K.; Zhang, P.; Fu, Y.; Liu, X.; Bai, S.; Li, W.; Qian, Z. A biomimetic sensor using neurotransmitter detection to decode odor perception by an olfactory network. Biosens. Bioelectron. 2022, 211, 114391. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Zhuang, L.; Qin, Z.; Zhang, B.; Hu, N.; Wang, P. Multi-odor discrimination by a novel bio-hybrid sensing preserving rat’s intact smell perception in vivo. Sens. Actuators B Chem. 2016, 225, 34–41. [Google Scholar] [CrossRef]
- Sato, K.; Takeuchi, S. Chemical vapor detection using a reconstituted insect olfactory receptor complex. Angew. Chem.—Int. Ed. 2014, 53, 11798–11802. [Google Scholar] [CrossRef]
- Micholt, E.; Jans, D.; Callewaert, G.; Bartic, C.; Lammertyn, J.; Nicolaï, B. Extracellular recordings from rat olfactory epithelium slices using micro electrode arrays. Sens. Actuators B Chem. 2013, 184, 40–47. [Google Scholar] [CrossRef]
- Liu, P.; Cao, T.; Xu, J.; Mao, X.; Wang, D.; Li, A. Plasticity of Sniffing Pattern and Neural Activity in the Olfactory Bulb of Behaving Mice During Odor Sampling, Anticipation, and Reward. Neurosci. Bull. 2020, 36, 598–610. [Google Scholar] [CrossRef]
- Gao, K.; Li, S.; Zhuang, L.; Qin, Z.; Zhang, B.; Huang, L.; Wang, P. In vivo bioelectronic nose using transgenic mice for specific odor detection. Biosens. Bioelectron. 2018, 102, 150–156. [Google Scholar] [CrossRef]
- Zhu, P.; Liu, S.; Tian, Y.; Chen, Y.; Chen, W.; Wang, P.; Du, L.; Wu, C. In Vivo Bioelectronic Nose Based on a Bioengineered Rat Realizes the Detection and Classification of Multiodorants. ACS Chem. Neurosci. 2022, 13, 1727–1737. [Google Scholar] [CrossRef]
- Yuan, Q.; Qin, C.; Duan, Y.; Jiang, N.; Liu, M.; Wan, H.; Zhuang, L.; Wang, P. An: In vivo bioelectronic nose for possible quantitative evaluation of odor masking using M/T cell spatial response patterns. Analyst 2022, 147, 178–186. [Google Scholar] [CrossRef]
- Khadka, R.; Aydemir, N.; Carraher, C.; Hamiaux, C.; Colbert, D.; Cheema, J.; Malmstrm, J.; Kralicek, A.; Travas-Sejdic, J. An ultrasensitive electrochemical impedance-based biosensor using insect odorant receptors to detect odorants. Biosens. Bioelectron. 2019, 126, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Jaffrezic-Renault, N.; Martelet, C.; Zhang, A.; Errachid, A. A novel detection strategy for odorant molecules based on controlled bioengineering of rat olfactory receptor I7. Biosens. Bioelectron. 2006, 22, 1550–1555. [Google Scholar] [CrossRef] [PubMed]
- Khadka, R.; Aydemir, N.; Carraher, C.; Hamiaux, C.; Baek, P.; Cheema, J.; Kralicek, A.; Travas-Sejdic, J. Investigating Electrochemical Stability and Reliability of Gold Electrode-electrolyte Systems to Develop Bioelectronic Nose Using Insect Olfactory Receptor. Electroanalysis 2019, 31, 726–738. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, H.; Li, H.; Zhang, J.; Zhuang, S.; Zhang, F.; Hsia, K.J.; Ping, W. Impedance sensing and molecular modeling of an olfactory biosensor based on chemosensory proteins of honeybee. Biosens. Bioelectron. 2013, 40, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, D.; Zhang, Q.; Huang, Y.; Luo, S.; Yao, Y.; Li, S.; Liu, Q. Impedance spectroscopy analysis of human odorant binding proteins immobilized on nanopore arrays for biochemical detection. Biosens. Bioelectron. 2016, 79, 251–257. [Google Scholar] [CrossRef]
- Calabrese, A.; Battistoni, P.; Ceylan, S.; Zeni, L.; Capo, A.; Varriale, A.; D’Auria, S.; Staiano, M. An Impedimetric Biosensor for Detection of Volatile Organic Compounds in Food. Biosensors 2023, 13, 341. [Google Scholar] [CrossRef]
- Khadka, R.; Carraher, C.; Hamiaux, C.; Travas-Sejdic, J.; Kralicek, A. Synergistic improvement in the performance of insect odorant receptor based biosensors in the presence of Orco. Biosens. Bioelectron. 2020, 153, 112040. [Google Scholar] [CrossRef]
- Peng, C.; Sui, Y.; Fang, C.; Sun, H.; Liu, W.; Li, X.; Qu, C.; Li, W.; Liu, J.; Wu, C. Highly sensitive and selective electrochemical biosensor using odorant-binding protein to detect aldehydes. Anal. Chim. Acta 2024, 1318, 342932. [Google Scholar] [CrossRef]
- Minic, J.; Persuy, M.A.; Godel, E.; Aioun, J.; Connerton, I.; Salesse, R.; Pajot-Augy, E. Functional expression of olfactory receptors in yeast and development of a bioassay for odorant screening. FEBS J. 2004, 272, 524–537. [Google Scholar] [CrossRef]
- Sung, J.H.; Ko, H.J.; Park, T.H. Piezoelectric biosensor using olfactory receptor protein expressed in Escherichia coli. Biosens. Bioelectron. 2005, 21, 1981–1986. [Google Scholar] [CrossRef]
- Ko, H.J.; Park, T.H. Piezoelectric olfactory biosensor: Ligand specificity and dose-dependence of an olfactory receptor expressed in a heterologous cell system. Biosens. Bioelectron. 2005, 20, 1327–1332. [Google Scholar] [CrossRef] [PubMed]
- Homma, C.; Tsukiiwa, M.; Noguchi, H.; Tanaka, M.; Okochi, M.; Tomizawa, H.; Sugizaki, Y.; Isobayashi, A.; Hayamizu, Y. Designable peptides on graphene field-effect transistors for selective detection of odor molecules. Biosens. Bioelectron. 2022, 224, 115047. [Google Scholar] [CrossRef] [PubMed]
- Datta-Chaudhuri, T.; Araneda, R.C.; Abshire, P.; Smela, E. Olfaction on a chip. Sens. Actuators B Chem. 2016, 235, 74–78. [Google Scholar] [CrossRef]
- Ozdemir, A.; Algül, Y.; Tirgil, N.Y. Reusable Turn-On Fluorescent Biosensor for Cardiac Biomarker Troponin I Detection Using QDs-SPION-Aptamer. J. Fluoresc. 2025. ahead of print. [Google Scholar] [CrossRef]
- Huang, Q.; Han, L.; Ma, H.; Lan, W.; Tu, K.; Peng, J.; Su, J.; Pan, L. An Aptamer Sensor Based on Alendronic Acid-Modified Upconversion Nanoparticles Combined with Magnetic Separation for Rapid and Sensitive Detection of Thiamethoxam. Foods 2025, 14, 182. [Google Scholar] [CrossRef]
- Park, S.Y.; Tan, J.K.S.; Mo, X.; Song, Y.; Lim, J.; Liew, X.R.; Chung, H.; Kim, S. Carbon Quantum Dots with Tunable Size and Fluorescence Intensity for Development of a Nano-biosensor. Small 2025, 21, e2404524. [Google Scholar] [CrossRef]
- Figueroa, X.A.; Cooksey, G.A.; Votaw, S.V.; Horowitz, L.F.; Folch, A. Large-scale investigation of the olfactory receptor space using a microfluidic microwell array. Lab a Chip 2010, 10, 1120–1127. [Google Scholar] [CrossRef]
- Fukutani, Y.; Koshizawa, T.; Yohda, M. Application of Vapor Phase Stimulation Method for Screening of Human Odorant Receptors Responding to Cinnamaldehyde. Sens. Mater. 2021, 33, 4319–4329. [Google Scholar] [CrossRef]
- de March, C.A.; Fukutani, Y.; Vihani, A.; Kida, H.; Matsunami, H. Real-time in vitro monitoring of odorant receptor activation by an odorant in the vapor phase. J. Vis. Exp. 2019, 146, e59446. [Google Scholar] [CrossRef]
- Hirata, Y.; Morimoto, Y.; Nam, E.; Takeuchi, S. Portable biohybrid odorant sensors using cell-laden collagen micropillars. Lab a Chip 2019, 19, 1971–1976. [Google Scholar] [CrossRef]
- Lee, S.H.; Oh, E.H.; Park, T.H. Cell-based microfluidic platform for mimicking human olfactory system. Biosens. Bioelectron. 2015, 74, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Yoshimoto, N.; Shimono, K.; Kuroda, S. Deciphering the Receptor Repertoire Encoding Specific Odorants by Time-Lapse Single-Cell Array Cytometry. Sci. Rep. 2016, 6, 19934. [Google Scholar] [CrossRef]
- Auer, T.O.; Khallaf, M.A.; Silbering, A.F.; Zappia, G.; Benton, R. Olfactory receptor and circuit evolution promote host specialization. Nature 2020, 579, 402–408. [Google Scholar] [CrossRef]
- Pfister, P.; Smith, B.C.; Evans, B.J.; Brann, J.H.; Rogers, M.E. Odorant Receptor Inhibition Is Fundamental to Odor Encoding. Curr. Biol. 2020, 30, 2574–2587.e6. [Google Scholar] [CrossRef] [PubMed]
- Si, G.; Kanwal, J.K.; Hu, Y.; Tabone, C.J.; Baron, J.; Berck, M.; Vignoud, G.; Samuel, A.D.T. Structured Odorant Response Patterns across a Complete Olfactory Receptor Neuron Population. Neuron 2019, 101, 950–962.e7. [Google Scholar] [CrossRef] [PubMed]
- Mitsuno, H.; Sakurai, T.; Namiki, S.; Mitsuhashi, H.; Kanzaki, R. Novel cell-based odorant sensor elements based on insect odorant receptors. Biosens. Bioelectron. 2015, 65, 287–294. [Google Scholar] [CrossRef]
- Kida, H.; Fukutani, Y.; Mainland, J.D.; De March, C.A.; Vihani, A.; Li, Y.R.; Chi, Q.; Toyama, A.; Liu, L.; Kameda, M. Vapor detection and discrimination with a panel of odorant receptors. Nat. Commun. 2018, 9, 4556. [Google Scholar] [CrossRef] [PubMed]
- Sukekawa, Y.; Mitsuno, H.; Kanzaki, R.; Nakamoto, T. Odor discrimination using cell-based odor biosensor system with fluorescent image processing. IEEE Sens. J. 2019, 19, 7192–7200. [Google Scholar] [CrossRef]
- Deng, H.; Mitsuno, H.; Kanzaki, R.; Nakamoto, T. Gas Phase Odorant Detection by Insect Olfactory Receptor. IEEE Sens. J. 2021, 21, 21184–21191. [Google Scholar] [CrossRef]
- Park, T.H.; Lee, S.H.; Oh, E.H.; Lee, S.H.; Ko, H.J. Cell-based high-throughput odorant screening system through visualization on a microwell array. Biosens. Bioelectron. 2013, 53, 18–25. [Google Scholar] [CrossRef]
- Deng, H.; Mitsuno, H.; Kanzaki, R.; Nakamoto, T. Study of Liquid Film Thickness for Gas Phase Odor Biosensor. IEEE Sens. J. 2022, 22, 16785–16793. [Google Scholar] [CrossRef]
- Chen, T.W.; Wardill, T.J.; Sun, Y.; Pulver, S.R.; Renninger, S.L.; Baohan, A.; Schreiter, E.R.; Kerr, R.A.; Orger, M.B.; Jayaraman, V.; et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 2013, 499, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.W.; Ko, H.J.; Park, T.H. Identification of a Lung Cancer Biomarker Using a Cancer Cell Line and Screening of Olfactory Receptors for Biomarker Detection. Biotechnol. Bioprocess Eng. 2021, 26, 55–62. [Google Scholar] [CrossRef]
- El Kazzy, M.; Weerakkody, J.S.; Hurot, C.; Mathey, R.; Hou, Y. An overview of artificial olfaction systems with a focus on surface plasmon resonance for the analysis of volatile organic compounds. Biosensors 2021, 11, 244. [Google Scholar] [CrossRef]
- Woyessa, G.; Bang, O. Sensitivity Enhancement of Polymer Optical Fiber Surface Plasmon Resonance Sensor Utilizing ITO Overlayer. Sensors 2025, 25, 1863. [Google Scholar] [CrossRef] [PubMed]
- Ashrafian, M.; Olyaee, S.; Seifouri, M. Highly sensitive cancer detection using an open D-channel PCF-based SPR biosensor. Sci. Rep. 2025, 15, 10168. [Google Scholar] [CrossRef]
- Xu, Q.; Yin, H.; Cui, M.; Huang, R.; Su, R. An Enhanced Bimetallic Optical Fiber SPR Biosensor Using Graphene Oxide for the Label-Free and Sensitive Detection of Human IgG. Sensors 2025, 25, 1630. [Google Scholar] [CrossRef]
- Quintanilla-Villanueva, G.E.; Rodríguez-Quiroz, O.; Sánchez-Álvarez, A.; Rodríguez-Delgado, J.M.; Villarreal-Chiu, J.F.; Luna-Moreno, D.; Rodríguez-Delgado, M.M. An Innovative Enzymatic Surface Plasmon Resonance-Based Biosensor Designed for Precise Detection of Glycine Amino Acid. Biosensors 2025, 15, 81. [Google Scholar] [CrossRef]
- Rossi, C.; Homand, J.; Bauche, C.; Hamdi, H.; Ladant, D.; Chopineau, J. Differential Mechanisms for Calcium-Dependent Protein/Membrane Association as Evidenced from SPR-Binding Studies on Supported Biomimetic Membranes. Biochemistry 2003, 42, 15273–15283. [Google Scholar] [CrossRef]
- Lee, J.Y.; Ko, H.J.; Lee, S.H.; Park, T.H. Cell-based measurement of odorant molecules using surface plasmon resonance. Enzym. Microb. Technol. 2006, 39, 375–380. [Google Scholar] [CrossRef]
- Cennamo, N.; Stefano, D.G.; Antonio, V.; Maria, S.; Fabio, D.P.; Andrea, N.; Luigi, Z.; Sabato, D.; Karl-Wilhelm, K. Easy to use plastic optical fiber-based biosensor for detection of butanal. PLoS ONE 2015, 10, e0116770. [Google Scholar] [CrossRef]
- Zhang, D.; Lu, Y.; Zhang, Q.; Yao, Y.; Li, S.; Li, H.; Zhuang, S.; Jing, J.; Gang, L.L.; Liu, Q. Nanoplasmonic monitoring of odorants binding to olfactory proteins from honeybee as biosensor for chemical detection. Sens. Actuators B Chem. 2015, 221, 341–349. [Google Scholar] [CrossRef]
- Choi, Y.R.; Shim, J.; Park, J.H.; Kim, Y.S.; Kim, M.J. Discovery of orphan olfactory receptor 6m1 as a new anticancer target in mcf-7 cells by a combination of surface plasmon resonance-based and cell-based systems. Sensors 2021, 21, 3468. [Google Scholar] [CrossRef]
- Benilova, I.; Chegel, V.I.; Ushenin, Y.V.; Vidic, J.; Soldatkin, A.P.; Martelet, C.; Pajot, E.; Jaffrezic-Renault, N. Stimulation of human olfactory receptor 17–40 with odorants probed by surface plasmon resonance. Eur. Biophys. J. 2008, 37, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.H.; Lee, S.H.; Ko, H.J.; Park, T.H. Odorant detection using liposome containing olfactory receptor in the SPR system. Sens. Actuators B Chem. 2014, 198, 188–193. [Google Scholar] [CrossRef]
- Lee, S.H.; Ko, H.J.; Park, T.H. Real-time monitoring of odorant-induced cellular reactions using surface plasmon resonance. Biosens. Bioelectron. 2009, 25, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Vidic, J.; Grosclaude, J.; Monnerie, R.; Persuy, M.A.; Badonnel, K.; Baly, C.; Caillol, M.; Briand, L.; Salesse, R.; Pajot-Augy, E.; et al. On a chip demonstration of a functional role for odorant binding protein in the preservation of olfactory receptor activity at high odorant concentration. Lab a Chip 2008, 8, 678–688. [Google Scholar] [CrossRef]
- Lee, S.; Park, J.; Song, J.; Lee, J.J.; Kim, J. The Effect of GO Flake Size on Field-Effect Transistor (FET)-Based Biosensor Performance for Detection of Ions and PACAP 38. Biosensors 2025, 15, 86. [Google Scholar] [CrossRef]
- Hadded, A.; Ayed, M.B.; Alshaya, S.A. An FPGA-Based SiNW-FET Biosensing System for Real-Time Viral Detection: Hardware Amplification and 1D CNN for Adaptive Noise Reduction. Sensors 2025, 25, 236. [Google Scholar] [CrossRef]
- Nandanwar, S.; Lee, S.; Park, M.; Kim, H.J. Label-Free Extended Gate Field-Effect Transistor for Sensing Microcystin-LR in Freshwater Samples. Sensors 2025, 25, 1587. [Google Scholar] [CrossRef]
- Das, D.; Dhar, R.S.; Ghosh, P.K.; Sharma, Y.; Banerjee, A. Sensitivity Analysis of Biosensor based SiGe Source Dual Gate Tunnel FET Having Negative Capacitance. IEEE Access 2025, 13, 7426–7436. [Google Scholar] [CrossRef]
- Hao, R.; Liu, L.; Yuan, J.; Wu, L.; Lei, S. Recent Advances in Field Effect Transistor Biosensors: Designing Strategies and Applications for Sensitive Assay. Biosensors 2023, 13, 426. [Google Scholar] [CrossRef] [PubMed]
- Murugathas, T.; Zheng, H.Y.; Colbert, D.; Kralicek, A.V.; Carraher, C.; Plank, N.O.V. Biosensing with Insect Odorant Receptor Nanodiscs and Carbon Nanotube Field-Effect Transistors. ACS Appl. Mater. Interfaces 2019, 11, 9530–9538. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Yang, H.; Kim, D.; Yang, M.; Park, T.H.; Hong, S. Human-like smelling of a rose scent using an olfactory receptor nanodisc-based bioelectronic nose. Sci. Rep. 2018, 8, 13945. [Google Scholar] [CrossRef]
- Yang, H.; Kim, D.; Kim, J.; Moon, D.; Song, H.S.; Lee, M.; Hong, S.; Park, T.H. Nanodisc-Based Bioelectronic Nose Using Olfactory Receptor Produced in Escherichia coli for the Assessment of the Death-Associated Odor Cadaverine. ACS Nano 2017, 11, 11847–11855. [Google Scholar] [CrossRef]
- Choi, D.; Lee, S.J.H.; Baek, D.; Kim, S.O.; Shin, J.; Choi, Y.; Cho, Y.; Bang, S.; Park, J.Y.; Lee, S.J.H.; et al. Bioelectrical Nose Platform Using Odorant-Binding Protein as a Molecular Transporter Mimicking Human Mucosa for Direct Gas Sensing. ACS Sens. 2022, 7, 3399–3408. [Google Scholar] [CrossRef]
- Kim, B.; Lee, J.; Namgung, S.; Kim, J.; Park, J.Y.; Lee, M.S.; Hong, S. DNA sensors based on CNT-FET with floating electrodes. Sens. Actuators B Chem. 2012, 169, 182–187. [Google Scholar] [CrossRef]
- Lin, Z.; Wu, G.; Zhao, L.; Lai, K.W.C. Detection of bacterial metabolic volatile indole using a graphene-based field-effect transistor biosensor. Nanomaterials 2021, 11, 1155. [Google Scholar] [CrossRef]
- Park, S.J.; Seo, S.E.; Kim, K.H.; Lee, S.H.; Kim, J.; Ha, S.; Song, H.S.; Lee, S.H.; Kwon, O.S. Real-time monitoring of geosmin based on an aptamer-conjugated graphene field-effect transistor. Biosens. Bioelectron. 2021, 174, 112804. [Google Scholar] [CrossRef]
- Kwon, O.S.; Song, H.S.; Park, S.J.; Lee, S.H.; An, J.H.; Park, J.W.; Yang, H.; Yoon, H.; Bae, J.; Park, T.H. An Ultrasensitive, Selective, Multiplexed Superbioelectronic Nose That Mimics the Human Sense of Smell. Nano Lett. 2015, 15, 6559–6567. [Google Scholar] [CrossRef]
- Oh, J.; Yang, H.; Jeong, G.E.; Moon, D.; Jang, J. Ultrasensitive, Selective, and Highly Stable Bioelectronic Nose That Detects the Liquid and Gaseous Cadaverine. Anal. Chem. 2019, 91, 12181–12190. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, A.E.; Komarova, N.V.; Kuznetsov, E.V.; Andrianova, M.S.; Grudtsov, V.P.; Rybachek, E.N.; Puchnin, K.V.; Ryazantsev, D.V.; Saurov, A.N. Integration of a field effect transistor-based aptasensor under a hydrophobic membrane for bioelectronic nose applications. Biosens. Bioelectron. 2019, 129, 29–35. [Google Scholar] [CrossRef]
- Terutsuki, D.; Mitsuno, H.; Sakurai, T.; Okamoto, Y.; Kanzaki, R. Increasing cell–device adherence using cultured insect cells for receptor-based biosensors. R. Soc. Open Sci. 2018, 5, 172366. [Google Scholar] [CrossRef]
- Obořilová, R.; Kučerová, E.; Botka, T.; Vaisocherová-Lísalová, H.; Skládal, P.; Farka, Z. Piezoelectric biosensor with dissipation monitoring enables the analysis of bacterial lytic agent activity. Sci. Rep. 2025, 15, 3419. [Google Scholar] [CrossRef]
- Siddiq, M.A. Novel plant extract-chitosan nanocomposite (SCE/ChNPs) biosensor for trace level arsenic sensing in water samples using quartz crystal microbalance with dissipation monitoring. Nano Express 2025, 6, 015004. [Google Scholar] [CrossRef]
- Park, S.; Gerber, A.; Santa, C.; Aktug, G.; Hengerer, B.; Clark, H.A.; Jonas, U.; Dostalek, J.; Sergelen, K. Molecularly Responsive Aptamer-Functionalized Hydrogel for Continuous Plasmonic Biomonitoring. J. Am. Chem. Soc. 2025, 147, 11485–11500. [Google Scholar] [CrossRef] [PubMed]
- Ogi, H. Ultrasensitive wireless quartz crystal microbalance bio/gas sensors. Jpn. J. Appl. Phys. 2024, 63, 040802. [Google Scholar] [CrossRef]
- Su, X.; Chen, D.; Li, N.; Stevenson, A.C.; Li, G.; Hu, R. A wireless electrode-free QCM-D in a multi-resonance mode for volatile organic compounds discrimination. Sens. Actuators A Phys. 2020, 305, 111938. [Google Scholar] [CrossRef]
- Chen, D.; Li, H.; Su, X.; Li, N.; Wang, Y.; Stevenson, A.C.; Hu, R.; Li, G. A wireless-electrodeless quartz crystal microbalance method for non-enzymatic glucose monitoring. Sens. Actuators B Chem. 2019, 287, 35–41. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, K.; Zhou, H.; Fan, G.; Wang, Y.; Li, G.; Hu, R. A wireless-electrodeless quartz crystal microbalance with dissipation DMMP sensor. Sens. Actuators B Chem. 2018, 261, 408–417. [Google Scholar] [CrossRef]
- Chen, T.; Sha, P.; Xu, Y.; Su, X.; Chen, D.; Li, N.; Li, G.; Hu, R. Online pH monitoring based on a wireless electrodeless quartz crystal microbalance with dissipation. Sens. Actuators A Phys. 2021, 331, 112952. [Google Scholar] [CrossRef]
- Du, L.; Wu, C.; Peng, H.; Zou, L.; Zhao, L.; Huang, L.; Ping, W. Piezoelectric olfactory receptor biosensor prepared by aptamer-assisted immobilization. Sens. Actuators B Chem. 2013, 187, 481–487. [Google Scholar] [CrossRef]
- Sankaran, S.; Panigrahi, S.; Mallik, S. Olfactory receptor based piezoelectric biosensors for detection of alcohols related to food safety applications. Sens. Actuators B Chem. 2011, 155, 8–18. [Google Scholar] [CrossRef]
- Nardiello, M.; Scieuzo, C.; Salvia, R.; Farina, D.; Franco, A.; Cammack, J.A.; Tomberlin, J.K.; Falabella, P.; Persaud, K.C. Odorant binding proteins from Hermetia illucens: Potential sensing elements for detecting volatile aldehydes involved in early stages of organic decomposition. Nanotechnology 2022, 33, 205501. [Google Scholar] [CrossRef] [PubMed]
- Wasilewski, T.; Szulczyński, B.; Wojciechowski, M.; Kamysz, W.; Gębicki, J. Determination of long-chain aldehydes using a novel quartz crystal microbalance sensor based on a biomimetic peptide. Microchem. J. 2020, 154, 104509. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, C.; Liang, C.; Liu, Y.; Li, H.; Zhang, C.; Duan, X.; Pan, Y. Sensitive Materials Used in Surface Acoustic Wave Gas Sensors for Detecting Sulfur-Containing Compounds. Polymers 2024, 16, 457. [Google Scholar] [CrossRef]
- Liang, C.; Yan, C.; Zhai, S.; Wang, Y.; Hu, A.; Wang, W.; Pan, Y. Recent Progress in Flexible Surface Acoustic Wave Sensing Technologies. Micromachines 2024, 15, 357. [Google Scholar] [CrossRef]
- Zhao, H.; Jiao, C.; Wang, Q.; Gao, C.; Sun, J. Surface Acoustic Wave Sensor for Selective Multi-Parameter Measurements in Cardiac Magnetic Field Detection. Appl. Sci. 2025, 15, 3583. [Google Scholar] [CrossRef]
- Zeng, Y.; Yuan, R.; Fu, H.; Xu, Z.; Wei, S. Foodborne pathogen detection using surface acoustic wave biosensors: A review. RSC Adv. 2024, 14, 37087–37103. [Google Scholar] [CrossRef]
- Aleixandre, M.; Horrillo, M.C. Recent Advances in SAW Sensors for Detection of Cancer Biomarkers. Biosensors 2025, 15, 88. [Google Scholar] [CrossRef]
- Kukaev, A.; Shalymov, E.; Shevchenko, S.; Sorvina, M.; Venediktov, V. Advancements in Surface Acoustic Wave Gyroscope Technology in 2015–2024. Sensors 2025, 25, 877. [Google Scholar] [CrossRef] [PubMed]
- Mandal, D.; Banerjee, S. Surface Acoustic Wave (SAW) Sensors: Physics, Materials, and Applications. Sensors 2022, 22, 820. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.; Wang, W.; Cheng, L.; Jin, J.; Hu, A.; Ren, Z.; Xue, X.; Liang, Y. Acoustic impedance-based surface acoustic wave chip for gas leak detection and respiratory monitoring. Commun. Eng. 2025, 4, 15. [Google Scholar] [CrossRef] [PubMed]
- Caliendo, C.; Benetti, M.; Cannatà, D.; Laidoudi, F.; Petrone, G. Interface Acoustic Waves in 128° YX-LiNbO3/SU-8/Overcoat Structures. Micromachines 2025, 16, 99. [Google Scholar] [CrossRef]
- Stubbs, D.D.; Hunt, W.D.; Lee, S.H.; Doyle, D.F. Gas phase activity of anti-FITC antibodies immobilized on a surface acoustic wave resonator device. Biosens. Bioelectron. 2002, 17, 471–477. [Google Scholar] [CrossRef]
- Wu, C.; Du, L.; Wang, D.; Wang, L.; Zhao, L.; Wang, P. A novel surface acoustic wave-based biosensor for highly sensitive functional assays of olfactory receptors. Biochem. Biophys. Res. Commun. 2011, 407, 18–22. [Google Scholar] [CrossRef]
- Cannatà, D.; Di Pietrantonio, F.; Varriale, A.; D’Auria, S.; Staiano, M.; Verona, E.; Benetti, M. Detection of odorant molecules via surface acoustic wave biosensor array based on odorant-binding proteins. Biosens. Bioelectron. 2013, 41, 328–334. [Google Scholar] [CrossRef]
- Palla-Papavlu, A.; Patrascioiu, A.; Di Pietrantonio, F.; Fernández-Pradas, J.M.; Cannatà, D.; Benetti, M.; D’Auria, S.; Verona, E.; Serra, P. Preparation of surface acoustic wave odor sensors by laser-induced forward transfer. Sens. Actuators B Chem. 2014, 192, 369–377. [Google Scholar] [CrossRef]
- Wang, J.; Du, L.; Krause, S.; Wu, C.; Wang, P. Surface modification and construction of LAPS towards biosensing applications. Sens. Actuators B Chem. 2018, 265, 161–173. [Google Scholar] [CrossRef]
- Hou, C.; Dong, J.; Qin, H.; Huo, D.; Shen, C. Development of Cell-Based Olfactory Biosensors. Anal. Lett. 2012, 45, 202–218. [Google Scholar] [CrossRef]
- Li, X.; Liu, S.; Tan, J.; Wu, C. Light-Addressable Potentiometric Sensors in Microfluidics. Front. Bioeng. Biotechnol. 2022, 10, 833481. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Ye, W.; Hu, N.; Cai, H.; Yu, H.; Wang, P. Olfactory receptor cells respond to odors in a tissue and semiconductor hybrid neuron chip. Biosens. Bioelectron. 2010, 26, 1672–1678. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Ye, W.; Yu, H.; Hu, N.; Du, L.; Wang, P.; Yang, M. Olfactory mucosa tissue-based biosensor: A bioelectronic nose with receptor cells in intact olfactory epithelium. Sens. Actuators B Chem. 2009, 146, 527–533. [Google Scholar] [CrossRef]
- Wu, C.; Chen, P.; Yu, H.; Liu, Q.; Zong, X.; Hua, C.; Ping, W. A novel biomimetic olfactory-based biosensor for single olfactory sensory neuron monitoring. Biosens. Bioelectron. 2008, 24, 1498–1502. [Google Scholar] [CrossRef]
- Wu, C.S.; Chen, P.H.; Yuan, Q.; Wang, P. Response enhancement of olfactory sensory neurons-based biosensors for odorant detection. J. Zhejiang Univ. B 2009, 10, 285–290. [Google Scholar] [CrossRef]
- Du, L.; Wu, C.; Peng, H.; Zhao, L.; Huang, L.; Wang, P. Bioengineered olfactory sensory neuron-based biosensor for specific odorant detection. Biosens. Bioelectron. 2013, 40, 401–406. [Google Scholar] [CrossRef]
- Du, L.; Chen, W.; Tian, Y.; Zhu, P.; Wang, J.; Cai, W.; Wu, C. A light-addressable microfluidic device for label-free functional assays of bioengineered taste receptor cells via extracellular recording. Biophys. Rep. 2019, 5, 73–79. [Google Scholar] [CrossRef]
- Du, L.; Zou, L.; Zhao, L.; Huang, L.; Wang, P.; Wu, C. Label-free functional assays of chemical receptors using a bioengineered cell-based biosensor with localized extracellular acidification measurement. Biosens. Bioelectron. 2014, 54, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Misawa, N.; Fujii, S.; Kamiya, K.; Osaki, T.; Takaku, T.; Takahashi, Y.; Takeuchi, S. Construction of a Biohybrid Odorant Sensor Using Biological Olfactory Receptors Embedded into Bilayer Lipid Membrane on a Chip. ACS Sens. 2019, 4, 711–716. [Google Scholar] [CrossRef]
- Yamada, T.; Sugiura, H.; Mimura, H.; Kamiya, K.; Osaki, T.; Takeuchi, S. Highly sensitive VOC detectors using insect olfactory receptors reconstituted into lipid bilayers. Sci. Adv. 2021, 7, eabd2013. [Google Scholar] [CrossRef]
- Fujii, S.; Nobukawa, A.; Osaki, T.; Morimoto, Y.; Takeuchi, S. Pesticide vapor sensing using an aptamer, nanopore, and agarose gel on a chip. Lab a Chip 2017, 17, 2421–2425. [Google Scholar] [CrossRef] [PubMed]
- Pérez, R.L.; Ayala, C.E.; Park, J.Y.; Choi, J.W.; Warner, I.M. Coating-based quartz crystal microbalance detection methods of environmentally relevant volatile organic compounds. Chemosensors 2021, 9, 153. [Google Scholar] [CrossRef]
- Stensmyr, M.C.; Dweck, H.K.M.; Farhan, A.; Ibba, I.; Strutz, A.; Mukunda, L.; Linz, J.; Grabe, V.; Steck, K.; Lavista-Llanos, S.; et al. A conserved dedicated olfactory circuit for detecting harmful microbes in drosophila. Cell 2012, 151, 1345–1357. [Google Scholar] [CrossRef] [PubMed]
- Son, M.; Cho, D.G.; Lim, J.H.; Park, J.; Hong, S.; Ko, H.J.; Park, T.H. Real-time monitoring of geosmin and 2-methylisoborneol, representative odor compounds in water pollution using bioelectronic nose with human-like performance. Biosens. Bioelectron. 2015, 74, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Termtanasombat, M.; Mitsuno, H.; Misawa, N.; Yamahira, S.; Sakurai, T.; Yamaguchi, S.; Nagamune, T.; Kanzaki, R. Cell-Based Odorant Sensor Array for Odor Discrimination Based on Insect Odorant Receptors. Chem. Ecol. 2016, 42, 716–724. [Google Scholar] [CrossRef]
- Deng, H.; Mitsuno, H.; Kanzaki, R.; Nomoto, S.; Nakamoto, T. Gas-phase Odorant Fast Quantification by Odor Biosensor Based on Reference Response Model. IEEE Sens. J. 2023, 23, 24169–24178. [Google Scholar] [CrossRef]
- Anderson, M.J.; Sullivan, J.G.; Horiuchi, T.K.; Fuller, S.B.; Daniel, T.L. A bio-hybrid odor-guided autonomous palm-sized air vehicle. Bioinspiration Biomim. 2020, 16, 026002. [Google Scholar] [CrossRef]
- Lochmatter, T.; Raemy, X.; Matthey, L.; Indra, S.; Martinoli, A. A comparison of casting and spiraling algorithms for odor source localization in laminar flow. In Proceedings of the 2008 IEEE International Conference on Robotics and Automation, Pasadena, CA, USA, 19–23 May 2008. [Google Scholar] [CrossRef]
- Terutsuki, D.; Uchida, T.; Fukui, C.; Sukekawa, Y.; Okamoto, Y.; Kanzaki, R. Real-time odor concentration and direction recognition for efficient odor source localization using a small bio-hybrid drone. Sens. Actuators B Chem. 2021, 339, 129770. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Q.; Li, X.; Li, K.; Huang, D.; Zou, K.; Zhang, Z.; Qian, Y.; Zhu, C.; Kong, X.Y. Olfactory-Inspired Separation-Sensing Nanochannel-Based Electronics for Wireless Sweat Monitoring. ACS Nano 2025, 3, 3781–3790. [Google Scholar] [CrossRef]
- Pelosi, P.; Calvello, M.; Ban, L. Diversity of odorant-binding proteins and chemosensory proteins in insects. Chem. Senses 2005, 30, i291–i292. [Google Scholar] [CrossRef]
- Oncu, S.; Yeniterzi, D.; Karakurt, O.; Cakmaktepe, S.; Cirpan, A.; Soylemez, S.; Toppare, L. Synthesis and nanoparticle formation of pyrazine, benzodithiophene (BDT) and fluorene containing conjugated polymer (P-PBF) and its biosensing performance toward catechol. Microchem. J. 2024, 207, 112158. [Google Scholar] [CrossRef]
- Bordbar, M.M.; Tashkhourian, J.; Tavassoli, A.; Bahramali, E.; Hemmateenejad, B. Ultrafast detection of infectious bacteria using optoelectronic nose based on metallic nanoparticles. Sens. Actuators B Chem. 2020, 319, 128262. [Google Scholar] [CrossRef]
- Dent, A.G.; Sutedja, T.G.; Zimmerman, P.V. Exhaled breath analysis for lung cancer. J. Thorac. Dis. 2013, 5 (Suppl. S5), S540–S550. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Chien, P.J.; Toma, K.; Arakawa, T.; Mitsubayashi, K. An acetone bio-sniffer (gas phase biosensor) enabling assessment of lipid metabolism from exhaled breath. Biosens. Bioelectron. 2015, 73, 208–213. [Google Scholar] [CrossRef]
- Toda, K.; Koga, T.; Kosuge, J.; Kashiwagi, M.; Oguchi, H.; Arimoto, T. Micro gas analyzer measurement of nitric oxide in breath by direct wet scrubbing and fluorescence detection. Anal. Chem. 2009, 81, 7031–7037. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Pan, F.; Liu, J.; Wang, K.; Zhang, C.; Cheng, S.; Lu, L.; Zhang, W.; Zhang, Z.; et al. Breath Analysis Based on Surface-Enhanced Raman Scattering Sensors Distinguishes Early and Advanced Gastric Cancer Patients from Healthy Persons. ACS Nano 2016, 10, 8169–8179. [Google Scholar] [CrossRef]
- Nakano-Baker, O.; Fong, H.; Shukla, S.; Lee, R.V.; Cai, L.; Godin, D.; Hennig, T.; Rath, S.; Novosselov, I.; Dogan, S.D.; et al. Data-driven design of a multiplexed, peptide-sensitized transistor to detect breath VOC markers of COVID-19. Biosens. Bioelectron. 2023, 229, 115237. [Google Scholar] [CrossRef]
- Hu, J.; Hu, N.; Pan, D.; Zhu, Y.; Jin, X.; Wu, S.; Lu, Y. Smell cancer by machine learning-assisted peptide/MXene bioelectronic array. Biosens. Bioelectron. 2024, 262, 116562. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Selyanchyn, R.; Wakamatsu, S.; Hayashi, K. Nanoassembled thin-film-coated quartz crystal microbalance odor sensors for environmental and human breath ammonia assessments. Sens. Mater. 2018, 30, 1133–1144. [Google Scholar] [CrossRef]
- Sankaran, S.; Panigrahi, S.; Mallik, S. Odorant binding protein based biomimetic sensors for detection of alcohols associated with Salmonella contamination in packaged beef. Biosens. Bioelectron. 2011, 26, 3103–3109. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, W.; Yan, S.; Chen, R.; Qin, G. High-Performance Odorant Binding Protein-Derived Peptide Biosensor for Salmonella Contamination in Food Detection Based on Mace Structure. ACS Sens. 2025, 10, 897–906. [Google Scholar] [CrossRef]
- Son, M.; Kim, D.; Kang, J.; Lim, J.H.; Lee, S.H.; Ko, H.J.; Hong, S.; Park, T.H. Bioelectronic nose using odorant binding protein-derived peptide and carbon nanotube field-effect transistor for the assessment of salmonella contamination in food. Anal. Chem. 2016, 88, 11283–11287. [Google Scholar] [CrossRef]
- Cui, W.; Hu, G.; Lv, E.; Li, C.; Wang, Z.; Li, Q.; Qian, Z.; Wang, J.; Xu, S.; Wang, R. A label-free and enzyme-free fluorescent aptasensor for amplified detection of kanamycin in milk sample based on target-triggered catalytic hairpin assembly. Food Control 2021, 133, 108654. [Google Scholar] [CrossRef]
- Wasilewski, T.; Gębicki, J.; Kamysz, W. Bio-inspired approaches for explosives detection. TrAC Trends Anal. Chem. 2021, 142, 116330. [Google Scholar] [CrossRef]
- Otto, J.; Brown, M.F.; Long, W. Training rats to search and alert on contraband odors. Appl. Anim. Behav. Sci. 2002, 77, 217–232. [Google Scholar] [CrossRef]
- Persaud, K.C. Biomimetic olfactory sensors. IEEE Sens. J. 2012, 12, 3108–3112. [Google Scholar] [CrossRef]
- Scorsone, E.; Manai, R.; Cali, K.; Ricatti, M.J.; Mucignat, C. Biosensor array based on ligand binding proteins for narcotics and explosives detection. Sens. Actuators B Chem. 2021, 334, 129587. [Google Scholar] [CrossRef]
- Radhika, V.; Proikas-Cezanne, T.; Jayaraman, M.; Onesime, D.; Ha, J.H.; Dhanasekaran, D.N. Chemical sensing of DNT by engineered olfactory yeast strain. Nat. Chem. Biol. 2007, 3, 325–330. [Google Scholar] [CrossRef]
- Yokoshiki, Y.; Nagayoshi, K.; Nakamoto, T. Improving Performance of Odor Biosensor System Using Olfactory Receptor. Sens. Mater. 2022, 34, 1617–1626. [Google Scholar] [CrossRef]
- Mujiono, T.; Sukekawa, Y.; Nakamoto, T.; Mitsuno, H.; Termtanasombat, M.; Kanzaki, R.; Misawa, N. Sensitivity improvement by applying lock-in technique to fluorescent instrumentation for cell-based odor sensor. Sens. Mater. 2017, 29, 65–76. [Google Scholar] [CrossRef]
- Deng, H.; Mitsuno, H.; Kanzaki, R.; Nakamoto, T. Extending lifetime of gas-phase odor biosensor using liquid thickness control and liquid exchange. Biosens. Bioelectron. 2022, 199, 113887. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Mitsuno, H.; Kanzaki, R.; Nakamoto, T. Discrete and continuous odor quantification in gas-phase odor biosensor. Meas. Sci. Technol. 2024, 35, 075105. [Google Scholar] [CrossRef]
- Deng, H.; Sukekawa, Y.; Mitsuno, H.; Kanzaki, R.; Nakamoto, T. Active Tracking of Temporally Changing Gas-Phase Odor Mixture Using an Array of Cells Expressing Olfactory Receptors. Anal. Chem. 2023, 95, 11558–11565. [Google Scholar] [CrossRef] [PubMed]


| Transducer Type | Detection Principle | Sensing Material | Response Time | Feature | References |
|---|---|---|---|---|---|
| Microelectrode | Current, potential, and impedance change | OSN, OB, and olfactory epithelium | Around ten seconds | Multiple cells or sites; invasive condition with high sensitivity but short lifetime; non-invasive condition with easy operation but low sensitivity | [39,73] |
| Fluorescence | Fluorescent intensity change | OSN and cell expressing OR | Around half minute | Multiple cells; non-invasive with long lifetime; easily affected by ambient light | [82,87,88] |
| SPR | Resonance wavelength change | Cell, OR protein, and OBP | Several seconds | Rapid and label-free detection; good stability and reproducibility; complex operation | [103] |
| FET | Conductivity change | OR protein, OBP, and aptamer | Several seconds | Very high sensitivity and selectivity; complex fabrication | [72,116,119] |
| QCM | Resonant frequency or dissipation change | OR protein and OBP | Several seconds | Low cost; high sensitivity; complex experimental setup | [127,134] |
| SAW | Resonant frequency change | OR protein and OBP | Several seconds | High sensitivity; moderate reproducibility | [140,141,143,144] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, H.; Chen, Z.; Feng, P.; Tian, L.; Zong, H.; Nakamoto, T. Recent Advances and Applications of Odor Biosensors. Electronics 2025, 14, 1852. https://doi.org/10.3390/electronics14091852
Deng H, Chen Z, Feng P, Tian L, Zong H, Nakamoto T. Recent Advances and Applications of Odor Biosensors. Electronics. 2025; 14(9):1852. https://doi.org/10.3390/electronics14091852
Chicago/Turabian StyleDeng, Hongchao, Zhangyu Chen, Pengfei Feng, Lifeng Tian, Huijuan Zong, and Takamichi Nakamoto. 2025. "Recent Advances and Applications of Odor Biosensors" Electronics 14, no. 9: 1852. https://doi.org/10.3390/electronics14091852
APA StyleDeng, H., Chen, Z., Feng, P., Tian, L., Zong, H., & Nakamoto, T. (2025). Recent Advances and Applications of Odor Biosensors. Electronics, 14(9), 1852. https://doi.org/10.3390/electronics14091852





