Abstract
Patients with sleep apnea syndrome (SAS) have a risk of stroke that is more than three times higher than that of healthy individuals. Early detection and appropriate treatment are crucial for preventing serious complications, and detecting cyclic variation in heart rate (CVHR) plays a key role in early diagnosis. This study investigated the feasibility of detecting CVHR during sleep using a wearable, comfortable device and evaluated the ability to assess weekly fluctuations. Heart rate, blood oxygen saturation, and bio-acceleration were measured for seven consecutive nights in eight healthy subjects (45.7 ± 10.1 years old). The CVHR values obtained using a ring-type sensor were compared to those derived from the apnea–hypopnea index (AHI) measured with a Holter ECG. The results revealed that CVHR values measured with the ring-type sensor were higher than those measured with the Holter monitor. Although correction is required, the ring-type sensor successfully detected intra-weekly fluctuations. These findings suggest that a ring-type sensor could be a practical tool for monitoring CVHR and identifying weekly trends in a comfortable, non-invasive manner.
1. Introduction
Sleep apnea syndrome (SAS) is a prevalent and serious sleep disorder characterized by repeated interruptions to breathing during sleep. These interruptions, or apneas, lead to a decrease in blood oxygen levels and place a considerable strain on the cardiovascular system [1,2,3,4,5,6]. SAS, through recurrent apneic events, leads to hypoxemia and blood pressure fluctuations. In response to hypoxia, the heart works harder to deliver sufficient oxygen, resulting in elevated blood pressure and increased stress on blood vessels, which subsequently raise the risk of cardiovascular events like stroke and myocardial infarction.
In recent years, SAS has drawn increasing attention from researchers and clinicians due to its association with cardiovascular diseases, metabolic disorders, and notably, stroke. Studies show that the risk of stroke is 2.8 times higher in patients with SAS compared to healthy individuals, and among those with severe SAS, the risk rises to approximately 3.6 times that of healthy individuals [7,8,9,10,11,12,13]. Moreover, stroke itself can disrupt normal breathing patterns, sometimes resulting in central sleep apnea syndrome. During the recovery period from stroke, neuroplasticity (the brain’s capacity to grow or reorganize neural networks) is essential. And, sleep disturbances such as central sleep apnea and insomnia are commonly observed in stroke patients and are concerning for their potential impact on neuroplasticity, potentially affecting recovery outcomes [14,15]. This bidirectional relationship, where SAS increases the risk of stroke and stroke can exacerbate sleep-related breathing disorders, underscores the importance of early detection and timely intervention. Recognizing the link between stroke and SAS can motivate individuals who may be at risk to reevaluate their sleep habits as part of a self-care regimen, as good sleep can contribute significantly to overall health and recovery from neurological events [16,17,18,19]. Consequently, early detection methods that are accessible, accurate, and comfortable for patients are essential for both prevention and management.
One promising biomarker for SAS detection is the cyclic variation in heart rate (CVHR), a distinctive pattern of heart rate fluctuation in response to apneic episodes and the subsequent recovery of blood oxygen levels [20,21,22,23,24,25]. Recent advancements in wearable sensor technology have enabled researchers to explore non-invasive, comfortable methods for CVHR monitoring that can enhance patient compliance and facilitate long-term observation in everyday settings. In particular, silicon-based ring sensors offer significant potential for monitoring CVHR, as they are comfortable to wear, unlikely to cause discomfort, and provide a visible record of physiological data, thus minimizing recording errors. Additionally, these sensors are cost effective, which is advantageous for large-scale screenings and for populations who may otherwise lack access to standard sleep monitoring tools. For dementia patients, such technology can be especially beneficial. Individuals with cognitive impairment may have difficulty expressing discomfort or distress, making it challenging to assess sleep quality through self-reporting or behavioral cues. Wearable sleep sensors provide an objective means of evaluating sleep patterns, which can inform caregivers and healthcare providers in developing strategies for enhancing sleep and, by extension, overall well-being. Despite the appeal of wearable technology for sleep monitoring, certain challenges remain. The accuracy and reliability of CVHR detection using wearable sensors, especially compared to established methods like a Holter ECG, are central to ongoing research. Studies have shown that CVHR values obtained from ring-type sensors may differ from those measured by a traditional ECG, necessitating calibration to ensure reliability for clinical and self-monitoring applications. Moreover, understanding the variability of CVHR over time, including intra-weekly fluctuations, could provide further insights into how SAS and related disorders impact cardiovascular and autonomic functions in varying contexts, such as different sleep stages or stress levels [26,27,28,29,30].
In this study, we aim to investigate the feasibility of using a ring-type silicon sensor to detect CVHR during sleep, focusing on both the device’s accuracy relative to a Holter ECG and its capability to capture intra-weekly variability. Our study involves seven consecutive nights of monitoring in healthy subjects, measuring heart rate, blood oxygen saturation, and bio-acceleration, to provide a comprehensive picture of physiological status across sleep stages. By evaluating intra-weekly CVHR variability, it has potential offering a practical tool for SAS detection and more personalized, preventative care approaches in naturalistic settings, so our aim is to assess the sensor’s sensitivity to subtle changes over time.
2. Materials and Methods
2.1. Materials
In this study, we used a ring-shaped silicon sensor (Check-me Ring, SAN-EI MEDISYS, Kyoto, Japan) for data measurement. This device is specially designed for comfortable, long-term physiological monitoring during sleep. The sensor is compact and lightweight, and fits snugly on the finger, minimizing discomfort even when worn overnight. This sensor detects heart rate, blood oxygen saturation (SpO2), and acceleration.
The sensor is attached, the power automatically turns on, and measurement begins a few seconds later. The display alternately shows the measured values of blood oxygen saturation (SpO2) and heart rate (PR) (Figure 1). Four sets of measurement data can be stored, with a maximum of 10 h per set. The data can be exported to smartphone or tablet apps via Bluetooth. The device is typing BF (in medical electrical equipment, this is a connection part with protection against electric shock that is higher than that of a Type B connection part, and the patient connection part is separated from the other parts of the ME equipment), and uses electromagnetic compatibility. It weighs 15 g, has external dimensions of 38 × 30 × 38 mm, and uses a 3.7 Vdc (rechargeable lithium polymer) battery. It can be used for 12 to 16 h under normal conditions. The wireless system uses Bluetooth 4.0 BLE, and the measurement range is 70–99 for SpO2 and 30–250 bpm for heart rate. Measurement accuracy is 80–99 ± 2% (in the 70–79 range, ±3%) for SpO2 and 30–250 ± 2 (bpm) for heart rate.
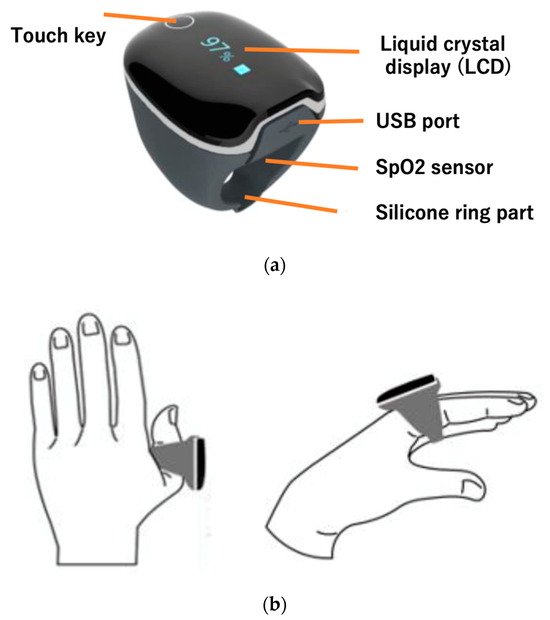
Figure 1.
Attaching the ring-type silicon sensor. (a) The ring is equipped with a liquid crystal display, which allows you to check human heart rate. The sensor embedded in the silicone measures acceleration and heart rate (medical device certification number is 304AABZX00029000, a specified maintenance management medical device). (b) The position where the ring is attached. It is attached to the index finger or thumb and used.
The sensor conforms to the requirements of EN IEC 60601-1-2 for EMC of medical electrical equipment and medical electrical systems. Electromagnetic compatibility (EMC) refers to both the ability to prevent the emission of noise that could cause unacceptable interference to other electronic devices in the surrounding environment (electromagnetic emissions) and the ability to withstand the electromagnetic environment of the location where the device is used, including noise emitted from other electronic devices in the surrounding environment, and to function normally (electromagnetic immunity). Even if other devices conform to the requirements of the International Special Committee on Radio Interference (CISPR), they may still interfere with the sensor. In such cases, if the input signal falls below the minimum amplitude, there is a risk of incorrect measurement, so each subjects took care to avoid using any electronic devices other than the smartphone for data transfer during measurement.
2.2. Methods
2.2.1. Continuous Measurement for One Week
We enrolled 8 healthy subjects (45.7 ± 10.1 years old, 2 females) and monitored heart rate, blood oxygen saturation (SpO2), and bio-acceleration during sleep over seven consecutive nights using a ring-type silicon sensor. The sampling frequency was set at 5 Hz, and bio-acceleration data were used to calculate composite acceleration.
Data were recorded on each subject’s smartphone via the app (Check-me Ring Application, SAN-EI MEDISYS, Kyoto, Japan) through a wireless connection and collected in CSV format via cloud transfer. To protect privacy, subjects were identified using sensor serial numbers linked with participant ID numbers.
2.2.2. Consistency with a Holter Electrocardiogram (ECG)
In order to check the consistency of a Holter electrocardiogram (ECG) data and ring-type silicon sensors, we compared Holter electrocardiogram (Poco303, Suzuken, Nagoya, Japan) data. The subject was one healthy female (38 years old) who wore both the Holter monitor and ring sensor to bed while sleeping. The data obtained from the Holter electrocardiogram was converted to CSV format using dedicated software (Cardy Analyzer 03, Kenz, Japan). The Holter ECG device can record electrocardiogram RR intervals and bio-acceleration. The RR interval sampling frequency was 125 Hz, and bio-acceleration was measured at 31.25 Hz.
2.2.3. Calculation of CVHR Frequency
The frequency of CVHR was calculated by comparing the aFcv values obtained from the silicon ring sensor at 1 Hz and 2 Hz, and the oxygen desaturation index (ODI) was calculated from SpO2 values, and the two were compared. The ODI is a value that appears in the results of a simple monitoring test for sleep apnea syndrome, and it refers to the index of oxygen desaturation. The ODI is a value obtained by counting the number of times the SpO2 of arterial blood drops by 3% or more during sleep, while a pulse oximeter is attached to the wrist, and it is considered normal if it is less than 15 times per hour [31]. Here, we will define sleep apnea as a 3% ODI, in accordance with previous research.
3. Results
3.1. Correlation Between Holter ECG and CVHR Values for a Ring-Type Senser
The results of comparing CVHR values between the Holter ECG and the ring-type sensor showed a correlation between the measurements of the two, and a certain degree of consistency with the Holter ECG data was confirmed. However, the ring sensor showed higher CVHR values, and it was found that correction was necessary to improve accuracy (Table 1, Figure 2). A comparison of bioacceleration data collected using both the silicon ring sensor and the Holter monitor revealed that the measured acceleration during sleep was minimal for both devices.

Table 1.
Comparison of sleep-related indices calculated from a Holter ECG and ring sensor.
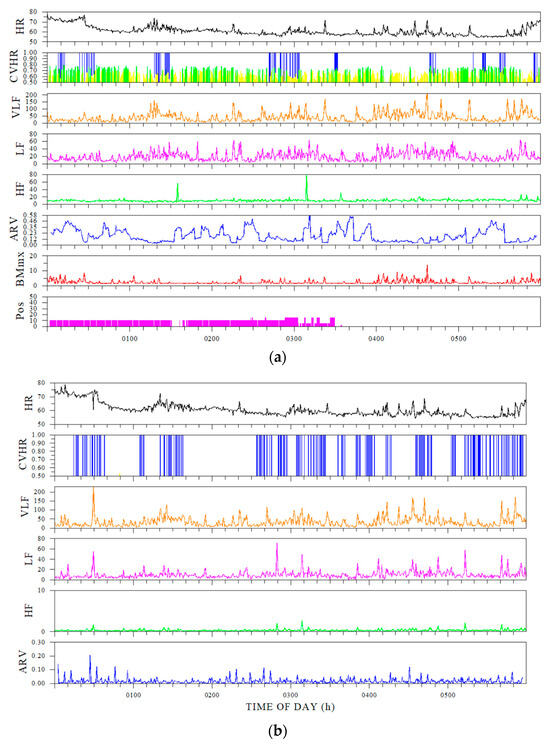
Figure 2.
Comparison of time-series indices for Holter ECG and ring sensors. (a) Sleep indices calculated from the Holter electrocardiogram, and (b) ring-sensor indices. (a) From the top down, HR (heart rate), CVHR, VLF (very-low-frequency component), LF (low-frequency component), HF (high-frequency component), ARV (heart rate variability using an autoregressive model), body movement, and posture. (b) HR, CVHR, VLF, LF, HF, and ARV.
Comparison of Holter electrocardiogram and ring sensor. The sampling frequency used to calculate the FCV from the ring sensor was set at 1 Hz. The explanation of each indicator is shown in Table 2.

Table 2.
Explanation of sleep-related indicators.
3.2. Detecting CVHR Using a Ring-Type Sensor
The ring-type sensor was suitable for capturing the pattern of CVHR during sleep, but as with the results in Section 3.1, the overall CVHR was high (Figure 3, Table 3).
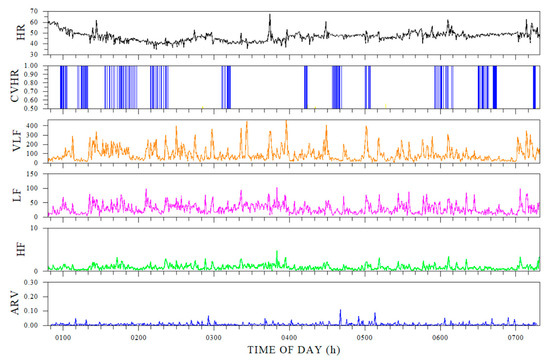
Figure 3.
Example of CVHR extracted from a ring-type sensor.

Table 3.
Comparison of sleep indicators: 1 Hz and 2 Hz sampling.
CVHR values extracted from the ring sensor tended to be calculated as high for all subjects. The average number of times per hour (CVHRI) is used as an evaluation index for CVHR. If the CVHRI is 15 or more, moderate or severe sleep apnea is suspected, and if the CVHRI exceeds 30, a sleep test is recommended [21].
3.3. Intra-Weekly Rhythm of Measured Signals
Analysis of the measured data over the course of a week showed that the CVHR values of the subjects showed a certain degree of intra-weekly fluctuation, and in particular, patterns related to the rhythm of sleep were suggested. This indicates that CVHR monitoring using a ring-type sensor can also be applied to the evaluation of intra-weekly physiological fluctuations.
The CVHR values tended to be higher at the weekend for subjects 5 and 8, and higher during the week for subject 6. The 3% ODI values tended to be higher at the weekend for subjects 1, 2, and 8, and higher during the week for subjects 3, 4, 5, 6, and 7. As is shown in Figure 4, although there were individual differences, it was confirmed that there was a circadian rhythm within the week.
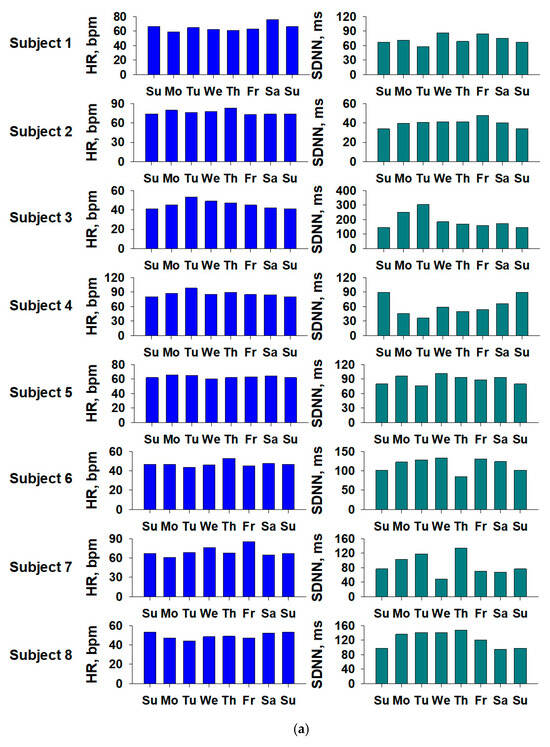
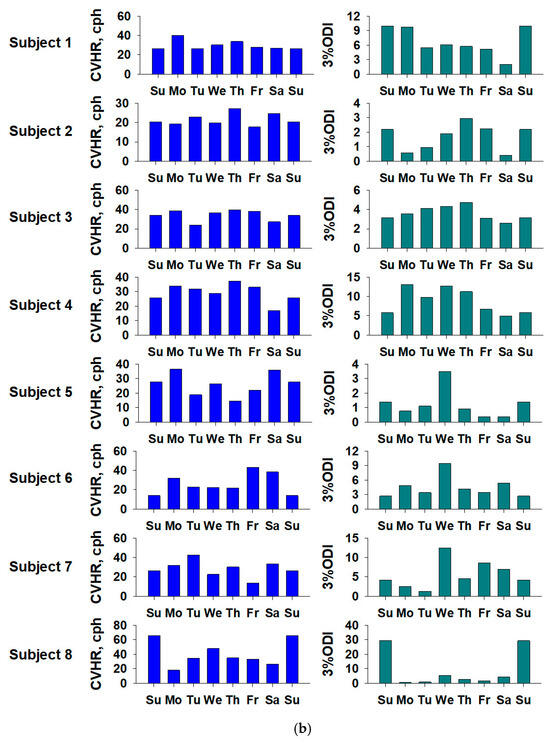
Figure 4.
Weekly fluctuations in sleep-related indices for each subject. (a) Heart rate (HR) and SDNN (standard deviation of the NN [normal-to-normal] intervals), and (b) CVHR (cyclic variation in heart rate) and 3 percent ODI (oxygen desaturation index), respectively. The graph shows the values for the eight subjects. The horizontal axis shows the day of the week, and to make it easier to see the fluctuations by day of the week, the same data are plotted twice for the first and last Sundays.
4. Discussion
The heart rate variability values obtained using a ring-type sensor were always higher than those obtained using a Holter electrocardiogram. This difference is probably due to the differences in the measurement principles and data processing algorithms of the two devices. The ring-type sensor, which relies on photoplethysmography (PPG) to detect the heart rate, is susceptible to motion artifacts and fluctuations in peripheral blood flow. These factors can amplify even the slightest fluctuations, resulting in a higher CVHR value. On the other hand, Holter monitors measure the electrical signals from the heart, so the detection of R-R intervals is more accurate and stable (differences in the measurement principles of the devices). Also, if the algorithm of the ring sensor does not sufficiently smooth the data, instantaneous fluctuations will be reflected in the CVHR value as they are, resulting in a higher value (differences in data smoothing and filtering). Furthermore, there are also significant differences depending on the measurement location. As the ring sensor measures at the fingertip, it is affected by peripheral blood vessels. This effect can make the heart rate fluctuation appear larger than it actually is. On the other hand, the Holter monitor directly detects signals close to the heart, so it is not affected by peripheral factors. In this study, we compared the two under the same conditions. One possible solution is to use the Holter monitor as the gold standard and apply corrections to the ring-sensor data. The results of this study showed that the CVHR detected was about twice as high as that of a Holter monitor, so it is believed that correcting the output value of the ring sensor with a constant would be effective. It is believed that correction can improve the accuracy of screening. Also, since it cannot be denied that the tendency for the CVHR output to be large may be due to this being a characteristic of silicon types, it will be necessary to compare it with ring sensors made of other materials in the future. Ring sensors are made of materials such as metals, ceramics, and organic polymers, and the sensor material is responsible for converting information on the object of sensing, such as light, temperature, magnetism, and pressure, into an electrical signal. In recent years, wearable sensors have emerged that are made of various materials, including self-powered and composite fabrics made of nylon coated with reduced graphene oxide (rGOx) [32]. However, devices with similar structures and measurement principles are expected to be highly versatile.
In addition to the CVHR method, which utilizes heart rate and pulse intervals, several other approaches are available for screening SAS, including direct breath detection, ECG analysis, polysomnography (PSG), and radar-based techniques (Table 4). Direct breath detection involves the use of a nasal cannula or mask to measure airflow and oxygen saturation directly. This method offers the advantage of accurately detecting apnea and hypopnea. However, its drawbacks include patient discomfort due to the equipment, the need for dedicated and costly devices, and the associated financial burden [33,34,35]. ECG analysis detects apnea-related changes, such as ST-segment and QRS-complex variations, from electrocardiogram waveforms. While it provides insight into the impact of apnea on heart rate, ECG measurement requires a high sampling frequency, leading to a substantial data volume and increased processing demands [36,37,38]. PSG is considered to be the gold standard for SAS diagnosis. It provides the most detailed and accurate assessments, allowing comprehensive evaluation of sleep quality, electroencephalogram, electromyogram, electrocardiogram, oxygen saturation, and apnea/hypopnea events. However, PSG is expensive, necessitates hospitalization, involves bulky equipment, and can cause discomfort to patients during the testing process [39,40]. Radar-based methods use radio waves to measure breathing and heart rate in a non-contact manner. This approach places minimal physical burden on patients due to its non-invasive nature. Nevertheless, it is expensive, requires technical expertise, and is susceptible to environmental noise, which can affect measurement accuracy [41,42]. Given this context, the CVHR method exhibits exceptional potential as a screening tool for SAS. Its advantages include its suitability for early diagnosis, use in community healthcare settings, and home care applications. This study introduces a novel approach by utilizing a ring-type sensor to detect CVHR. However, for definitive diagnoses or severe cases, conventional methods—especially PSG and direct breath detection—remain indispensable due to their high accuracy. To optimize SAS diagnosis, it is essential to analyze clinical data and integrate CVHR analysis with these established methods, creating an efficient and comprehensive diagnostic system. This study analyzed the CVHR method, which analyzes heart rate intervals, using a commercially available silicon ring sensor, and addressing this challenge represents a critical future direction for advancing SAS diagnosis and care.

Table 4.
Comparison of SAS detection using the CVHR method and others.
The findings of this study suggest that CVHR values extracted from ring-type sensors, particularly when combined with the oxygen desaturation index (ODI) derived from SpO2, can approximate ECG-based measurements such as those from Holter electrocardiography. Recent advances in wearable devices with high-resolution PPG sensors and improved artifact correction algorithms have shown potential for enhancing measurement accuracy. In this study, we demonstrated that even a compact, ring-shaped sensor can detect CVHR fluctuations and monitor weekly trends, although calibration remains necessary. The ability to capture weekly fluctuations is a unique contribution, shedding light on the dynamic nature of CVHR over time, a topic not well explored in prior research. However, several challenges and areas for improvement remain. First, robust calibration methods must be developed to align CVHR values from ring-type sensors with Holter ECG measurements, enhancing their clinical applicability for SAS diagnosis. Second, validation in larger and more diverse populations is needed, as this study focused on a small sample of healthy subjects. Future research should include cohorts with varying SAS severity to assess generalizability. It is also necessary to analyze demographic data such as age and gender to increase the detection power for groups with a high prevalence of SAS. Finally, improvements in artifact reduction and signal processing for PPG-based devices are essential to ensure accurate CVHR detection, particularly in the presence of body motion. These advancements will also support reliable long-term time-series monitoring, further increasing the utility of wearable sensors in clinical and home settings.
5. Conclusions
This study demonstrated the feasibility of using a ring-type silicone sensor to detect cyclic variation in heart rate (CVHR) during sleep and evaluate intra-weekly variability. The findings indicate that while CVHR values obtained with the ring-type sensor were higher than those measured with a Holter ECG, the device successfully detected trends and fluctuations over a week. These results suggest that ring-type sensors, with proper calibration, could serve as practical and non-invasive tools for monitoring CVHR and potentially aiding in the diagnosis of sleep apnea syndrome (SAS).
Author Contributions
Conceptualization, E.Y. and J.H.; methodology, E.Y.; software, J.H.; validation, E.Y., J.H. and H.E.; formal analysis, E.Y.; investigation, E.Y.; resources, J.H. and K.H.; data curation, E.Y.; writing—original draft preparation, E.Y.; writing—review and editing, J.H.; visualization, E.Y.; supervision, E.Y.; project administration, E.Y.; funding acquisition, E.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Nagoya City University Hospital (protocol code 60-00-0709, 17 August 2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets analyzed during the current study are not publicly available; however, they can be distributed by email to those who request them. For additional information, please contact the corresponding author.
Acknowledgments
The authors would like to express their sincere gratitude to all participants who contributed to this study.
Conflicts of Interest
Two of the authors of this study (Hiroyuki Edamatsu and Kenji Hosomi) are affiliated with a medical device manufacturer, which manufactures and sells medical devices. These authors provided technical support for the data measurement and experiments in this study, but were not involved in the study design, data analysis, or interpretation of the results. This study was conducted based on the independent scientific judgment of all authors, and we here by state that no misconduct or manipulation to lead to results favorable to the manufacturer was ethical guidelines to ensure the fairness and reliability of the research results conducted. In addition, the study was carried out in accordance with appropriate procedures and ethical guidelines to ensure the fairness and reliability of the research results.
References
- Javaheri, S.; Barbe, F.; Campos-Rodriguez, F.; Dempsey, J.A.; Khayat, R.; Javaheri, S.; Malhotra, A.; Martinez-Garcia, M.A.; Mehra, R.; Pack, A.I.; et al. Sleep Apnea: Types, Mechanisms, and Clinical Cardiovascular Consequences. J. Am. Coll. Cardiol. 2017, 69, 841–858. [Google Scholar] [CrossRef]
- Peker, Y.; Akdeniz, B.; Altay, S.; Balcan, B.; Başaran, Ö.; Baysal, E.; Çelik, A.; Dursunoğlu, D.; Dursunoğlu, N.; Fırat, S.; et al. Obstructive Sleep Apnea and Cardiovascular Disease: Where Do We Stand? Anatol. J. Cardiol. 2023, 27, 375–389. [Google Scholar] [CrossRef] [PubMed]
- Floras, J.S. Sleep Apnea and Cardiovascular Disease: An Enigmatic Risk Factor. Circ. Res. 2018, 122, 1741–1764. [Google Scholar] [CrossRef]
- Seravalle, G.; Grassi, G. Sleep Apnea and Hypertension. High. Blood Press. Cardiovasc. Prev. 2022, 29, 23–31. [Google Scholar] [CrossRef]
- Ludka, O. Sleep Apnea and Cardiovascular Disease. Cas. Lek. Cesk. 2019, 158, 178–184. [Google Scholar]
- Drager, L.F.; McEvoy, R.D.; Barbe, F.; Lorenzi-Filho, G.; Redline, S.; INCOSACT Initiative (International Collaboration of Sleep Apnea Cardiovascular Trialists). Sleep Apnea and Cardiovascular Disease: Lessons From Recent Trials and Need for Team Science. Circulation 2017, 136, 1840–1850. [Google Scholar] [CrossRef]
- Klar Yaggi, H.; Concato, J.; Kernan, W.N.; Lichtman, J.H.; Brass, L.M.; Mohsenin, V. Obstructive Sleep Apnea as a Risk Factor for Stroke and Death. N. Engl. J. Med. 2005, 353, 2034–2041. [Google Scholar] [CrossRef]
- Valham, F.; Mooe, T.; Rabben, T.; Stenlund, H.; Wiklund, U.; Franklin, K.A. Increased Risk of Stroke in Patients with Coronary Artery Disease and Sleep Apnea: A 10-Year Follow-Up. Circulation 2008, 118, 955–960. [Google Scholar] [CrossRef] [PubMed]
- Baillieul, S.; Dekkers, M.; Brill, A.K.; Schmidt, M.H.; Detante, O.; Pépin, J.L.; Tamisier, R.; Bassetti, C.L.A. Sleep Apnoea and Ischaemic Stroke: Current Knowledge and Future Directions. Lancet Neurol. 2022, 21, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, R.F.; Lutsey, P.L.; Benveniste, H.; Brown, D.L.; Full, K.M.; Lee, J.M.; Osorio, R.S.; Pase, M.P.; Redeker, N.S.; Redline, S.; et al. Impact of Sleep Disorders and Disturbed Sleep on Brain Health: A Scientific Statement From the American Heart Association. Stroke 2024, 55, e61–e76. [Google Scholar] [CrossRef]
- Khot, S.P.; Morgenstern, L.B. Sleep and Stroke. Stroke 2019, 50, 1612–1617. [Google Scholar] [CrossRef] [PubMed]
- Culebras, A. Central Sleep Apnea May Be Central to Acute Stroke. Sleep Med. 2021, 77, 302–303. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, C.L. Sleep and Stroke. Semin. Neurol. 2005, 25, 19–32. [Google Scholar] [CrossRef]
- Abad, V.C.; Guilleminault, C. Pharmacological Treatment of Sleep Disorders and Its Relationship With Neuroplasticity. Curr. Top. Behav. Neurosci. 2015, 25, 503–553. [Google Scholar] [CrossRef] [PubMed]
- Lanza, G.; DelRosso, L.M.; Ferri, R. Sleep and Homeostatic Control of Plasticity. Handb. Clin. Neurol. 2022, 184, 53–72. [Google Scholar] [CrossRef]
- Berryhill, S.; Morton, C.J.; Dean, A.; Berryhill, A.; Provencio-Dean, N.; Patel, S.I.; Estep, L.; Combs, D.; Mashaqi, S.; Gerald, L.B.; et al. Effect of Wearables on Sleep in Healthy Individuals: A Randomized Crossover Trial and Validation Study. J. Clin. Sleep. Med. 2020, 16, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Huysmans, D.; Borzée, P.; Buyse, B.; Testelmans, D.; Van Huffel, S.; Varon, C. Sleep Diagnostics for Home Monitoring of Sleep Apnea Patients. Front. Digit. Health 2021, 3, 685766. [Google Scholar] [CrossRef]
- Silva, F.B.; Uribe, L.F.S.; Cepeda, F.X.; Alquati, V.F.S.; Guimarães, J.P.S.; Silva, Y.G.A.; Santos, O.L.D.; Oliveira, A.A.; Aguiar, G.H.M.; Andersen, M.L.; et al. Sleep Staging Algorithm Based on Smartwatch Sensors for Healthy and Sleep Apnea Populations. Sleep Med. 2024, 119, 535–548. [Google Scholar] [CrossRef]
- Hsiou, D.A.; Gao, C.; Matlock, R.C.; Scullin, M.K. Validation of a Nonwearable Device in Healthy Adults With Normal and Short Sleep Durations. J. Clin. Sleep Med. 2022, 18, 751–757. [Google Scholar] [CrossRef]
- Hayano, J.; Watanabe, E.; Saito, Y.; Sasaki, F.; Kawai, K.; Kodama, I.; Sakakibara, H. Diagnosis of Sleep Apnea by the Analysis of Heart Rate Variation: A Mini Review. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2011, 2011, 7731–7734. [Google Scholar] [CrossRef]
- Hayano, J.; Watanabe, E.; Saito, Y.; Sasaki, F.; Fujimoto, K.; Nomiyama, T.; Kawai, K.; Kodama, I.; Sakakibara, H. Screening for obstructive sleep apnea by cyclic variation of heart rate. Circ. Arrhythm. Electrophysiol. 2011, 4, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Hayano, J.; Ueda, N.; Kisohara, M.; Yuda, E.; Watanabe, E.; Carney, R.M.; Blumenthal, J.A. Risk stratification after acute myocardial infarction by amplitude-frequency mapping of cyclic variation of heart rate. Ann. Noninvasive Electrocardiol. 2021, 26, e12825. [Google Scholar] [CrossRef]
- Hayano, J.; Yasuma, F.; Watanabe, E.; Carney, R.M.; Stein, P.K.; Blumenthal, J.A.; Arsenos, P.; Gatzoulis, K.A.; Takahashi, H.; Ishii, H.; et al. Blunted cyclic variation of heart rate predicts mortality risk in post-myocardial infarction, end-stage renal disease, and chronic heart failure patients. Europace 2017, 19, 1392–1400. [Google Scholar] [CrossRef]
- Hayano, J.; Yamamoto, H.; Nonaka, I.; Komazawa, M.; Itao, K.; Ueda, N.; Tanaka, H.; Yuda, E. Quantitative detection of sleep apnea with wearable watch device. PLoS ONE 2020, 15, e0237279. [Google Scholar] [CrossRef]
- Hayano, J.; Yuda, E. Night-to-night variability of sleep apnea detected by cyclic variation of heart rate during long-term continuous ECG monitoring. Ann. Noninvasive Electrocardiol. 2022, 27, e12901. [Google Scholar] [CrossRef]
- Tobaldini, E.; Nobili, L.; Strada, S.; Casali, K.R.; Braghiroli, A.; Montano, N. Heart rate variability in normal and pathological sleep. Front. Physiol. 2013, 4, 294. [Google Scholar] [CrossRef] [PubMed]
- Arnaud, C.; Bochaton, T.; Pépin, J.L.; Belaidi, E. Obstructive sleep apnoea and cardiovascular consequences: Pathophysiological mechanisms. Arch. Cardiovasc. Dis. 2020, 113, 350–358. [Google Scholar] [CrossRef]
- De Nys, L.; Anderson, K.; Ofosu, E.F.; Ryde, G.C.; Connelly, J.; Whittaker, A.C. The effects of physical activity on cortisol and sleep: A systematic review and meta-analysis. Psychoneuroendocrinology 2022, 143, 105843. [Google Scholar] [CrossRef]
- Tobaldini, E.; Costantino, G.; Solbiati, M.; Cogliati, C.; Kara, T.; Nobili, L.; Montano, N. Sleep, sleep deprivation, autonomic nervous system and cardiovascular diseases. Neurosci. Biobehav. Rev. 2017, 74, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Koskenvuo, M. Cardiovascular stress and sleep. Ann. Clin. Res. 1987, 19, 110–113. [Google Scholar] [PubMed]
- Williams, A.J.; Yu, G.; Santiago, S.; Stein, M. Screening for Sleep Apnea Using Pulse Oximetry and a Clinical Score. Chest 1991, 100, 631–635. [Google Scholar] [CrossRef]
- Hallfors, N.G.; Abi Jaoude, M.; Liao, K.; Ismail, M.; Isakovic, A.F. Graphene oxide—Nylon ECG sensors for wearable IoT healthcare. In Proceedings of the 2017 Sensors Networks Smart and Emerging Technologies (SENSET), Beiriut, Lebanon, 12–14 September 2017. [Google Scholar] [CrossRef]
- Schäfer, T. Method for Measuring Respiration in Sleep: Capnography for Determining Ventilation. Biomed. Tech. 2003, 48, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.H.; Kern, N.B.; Costantino, J.P.; Stiller, R.A.; Strollo, P.J., Jr.; Studnicki, K.A.; Coates, J.A.; Richards, T.J. Accuracy of End-Tidal and Transcutaneous PCO2 Monitoring During Sleep. Chest 1994, 106, 472–483. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, B.D.; Shapiro, B.A. Capnography. Respir. Care Clin. N. Am. 1995, 1, 107–117. [Google Scholar] [PubMed]
- Yeo, M.; Byun, H.; Lee, J.; Byun, J.; Rhee, H.Y.; Shin, W.; Yoon, H. Robust Method for Screening Sleep Apnea with Single-Lead ECG Using Deep Residual Network: Evaluation with Open Database and Patch-Type Wearable Device Data. IEEE J. Biomed. Health Inform. 2022, 26, 5428–5438. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Nakayama, C.; Fujiwara, K.; Sumi, Y.; Matsuo, M.; Kano, M.; Kadotani, H. Screening of Sleep Apnea Based on Heart Rate Variability and Long Short-Term Memory. Sleep Breath. 2021, 25, 1821–1829. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Tu, Y.; Huang, C.; Ye, S.; Chen, H. An Algorithm Based on ECG Signal for Sleep Apnea Syndrome Detection. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi 2013, 30, 999–1002. [Google Scholar] [PubMed]
- Wang, S.; Xuan, W.; Chen, D.; Gu, Y.; Liu, F.; Chen, J.; Xia, S.; Dong, S.; Luo, J. Machine Learning Assisted Wearable Wireless Device for Sleep Apnea Syndrome Diagnosis. Biosensors 2023, 13, 483. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Chen, L.; Xu, Q.; Xu, J.; Zhang, L.; Wang, J. Association Between Obstructive Sleep Apnea and Risk for Post-Stroke Anxiety: A Chinese Hospital-Based Study in Noncardiogenic Ischemic Stroke Patients. Sleep Med. 2023, 107, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Koda, T.; Okumura, S.; Taki, H.; Hamada, S.; Sunadome, H.; Sato, S. Noncontact Detection of Sleep Apnea Using Radar and Expectation–Maximization Algorithm. IEEE Sens. J. 2024, 24, 32748–32756. [Google Scholar] [CrossRef]
- Koda, T.; Sakamoto, T.; Wu, S.; Okumura, S.; Taki, H.; Hamada, S.; Sato, S.; Chin, K. Comparison of Machine Learning and Non-Machine Learning Methods for the Sleep Apnea Detection Using Millimeter-Wave Radar. IEICE Commun. Express 2022, 11, 355–360. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).