A Practical Guide to ECG Device Performance Testing According to International Standards
Abstract
1. Introduction
2. Materials and Methods
2.1. International ECG Standards: 60601-2-25, 60601-2-27, and 60601-2-47
2.2. Experimental Setup
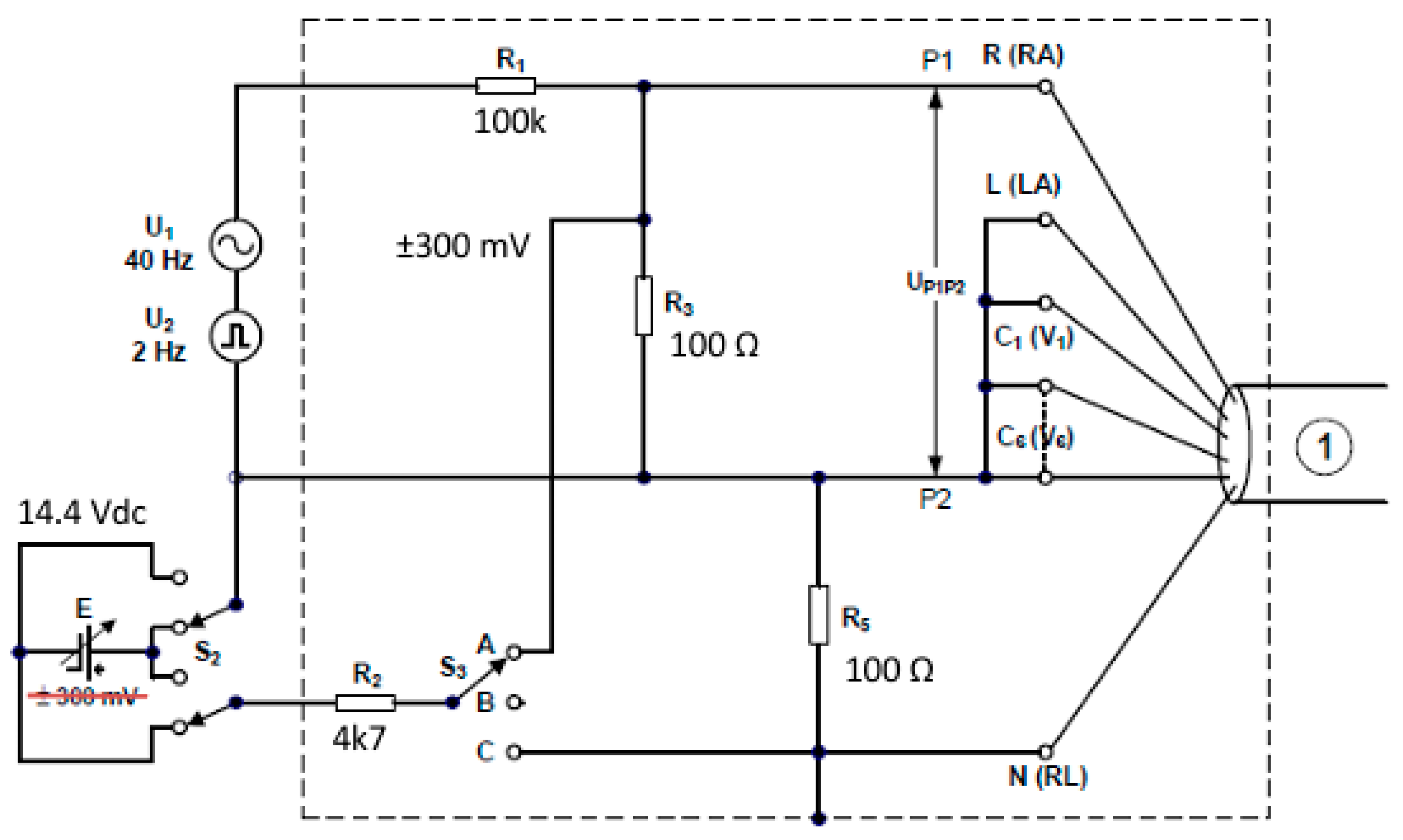
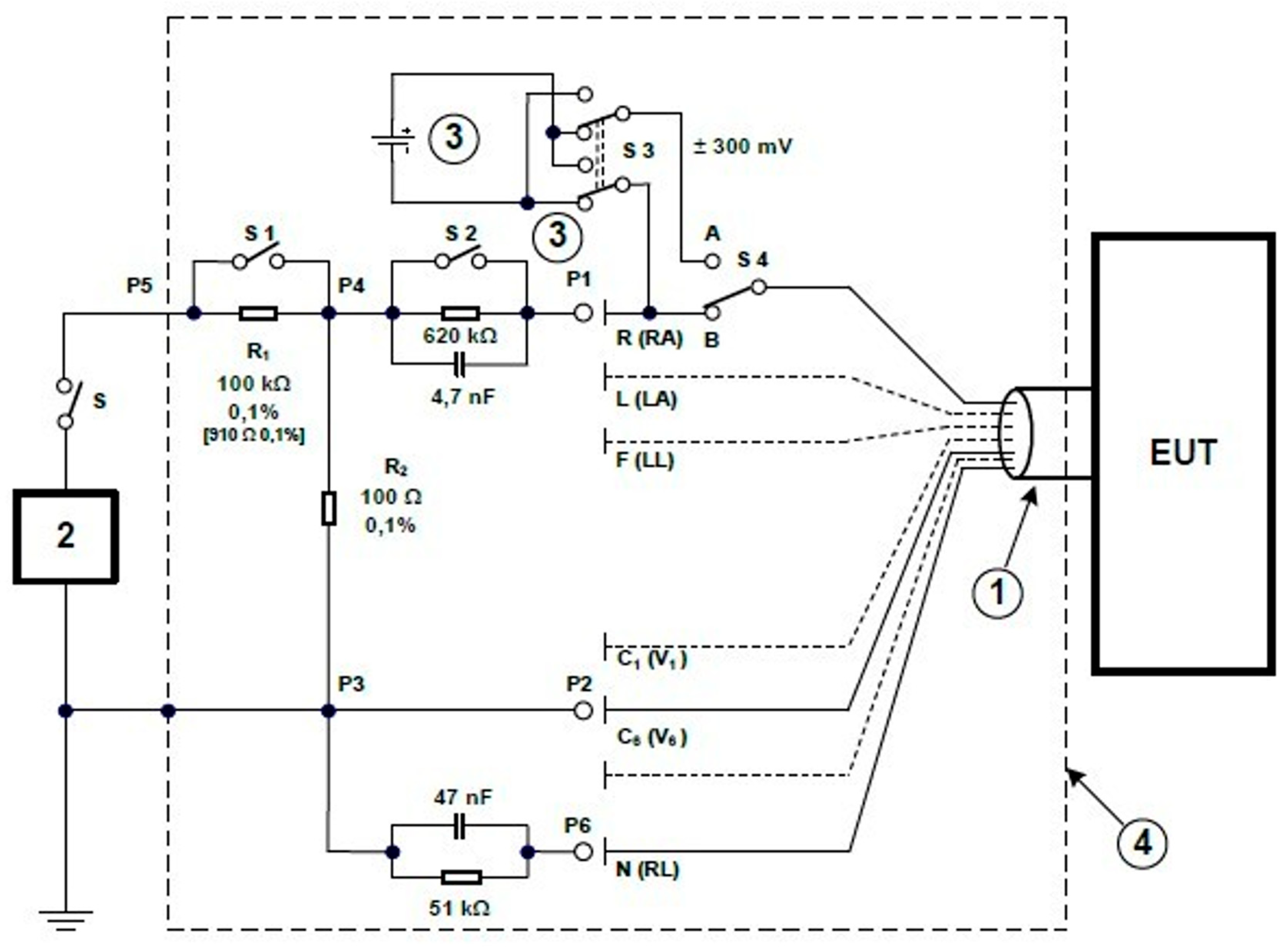
2.2.1. ECG Tester TEST
- 201.12.1.101.1—Signal reproduction accuracy;
- 201.12.1.101.2—Dynamic range and differential offset voltage;
- 201.12.1.101.3—Input impedance;
- 201.12.1.101.5—Inter-channel crosstalk;
- 201.12.1.101.6—Gain adjustment and stability;
- 201.12.1.101.7—Slew rate;
- 201.12.1.101.8—Frequency and impulse response;
- 201.12.1.101.9—Gain indicator—characteristic;
- 201.12.1.101.11—Isoline reset;
- 201.12.1.101.14—Cardioversion pulse synchronization;
- 201.12.1.101.15—HR range and accuracy, QRS detection range.
- P1 Group of parallel connected sockets (yellow) for connecting selected ECG track inputs;
- P2 Group of parallel connected sockets (red) for connecting the remaining ECG track inputs;
- P3 Group of parallel connected sockets (black) for the reference signal, e.g., ECG track N input;
- G1 Socket for connecting a pulse signal generator (signal fed to P1);
- G2 Socket for connecting a pulse signal generator connected directly to P2;
- G+ Socket for connecting a sinusoidal signal generator (signal fed to P1);
- G− Socket for connecting a sinusoidal signal generator connected directly to P2.
- SW1 RC two-terminal bypass switch for measuring the input impedance of the ECG input;
- SW2 Offset voltage polarity change switch;
- SW3 Offset switch differentially between P1 and P2 or jointly with respect to P3;
- SW4 Switch shorting the upper resistor of the signal divider fed from the generator between G+ and GSW5.
- SW6 Switch shunting the RC two-terminal in the P3 reference electrode path;
- SW7 Switch changing the method of supplying the offset signal;
- SW8 Switch enabling and disabling the offset supplied in series with the input signal;
- SW9 Switch shorting groups P2 and P3;
- SW10 Switch selecting the offset voltage source (it requires the presence of two batteries to obtain 14.4 V);
- SW11 Offset generation system power switch;
- RV1 Potentiometer for offset voltage correction for SW10 in position 1;
- RV2 Potentiometer for offset voltage correction for SW10 in position 3.
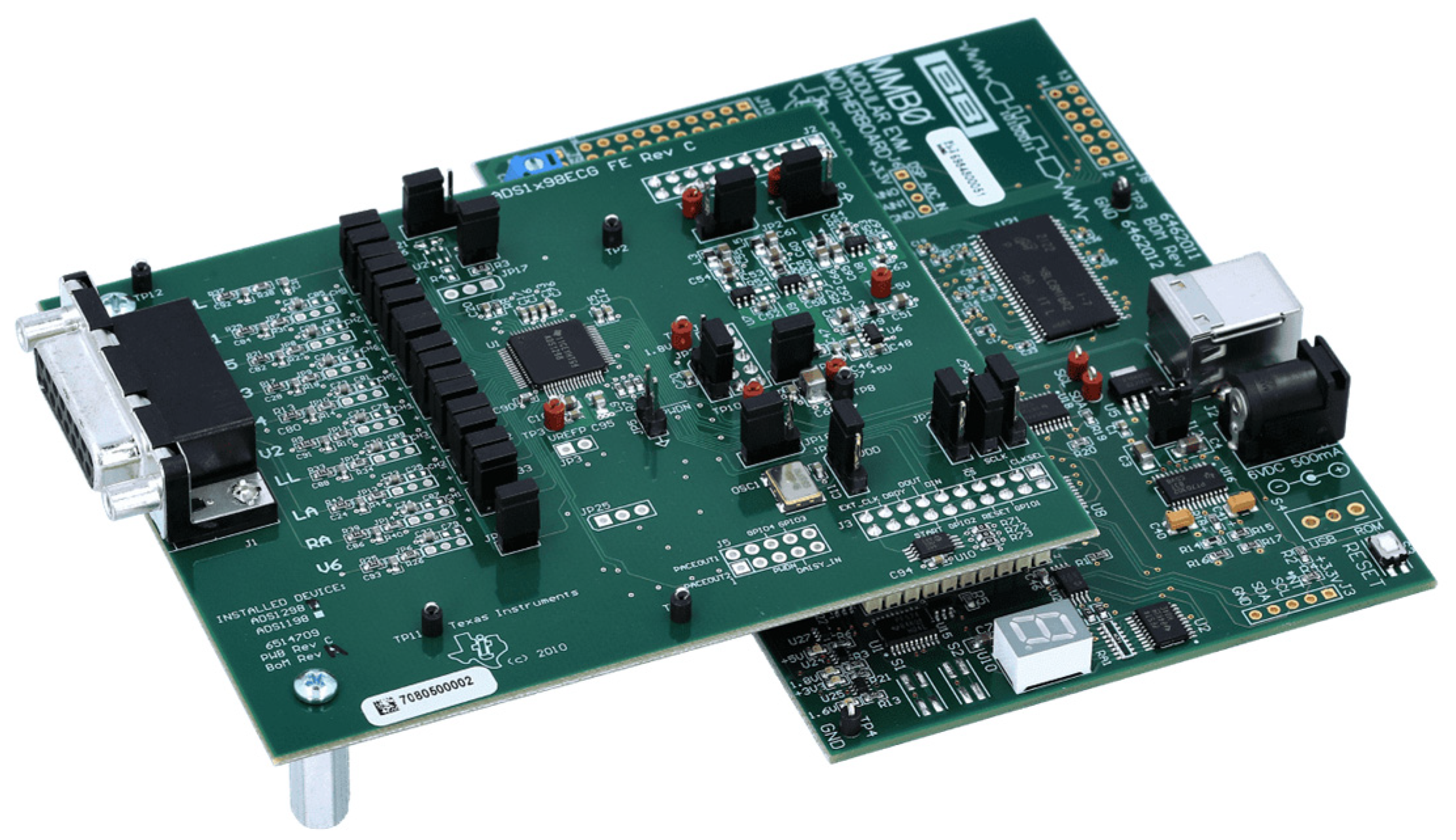
2.2.2. ADS1298 Analog Front-End
2.2.3. Cables
2.2.4. BNC Connectors
2.3. Performance Metrics
3. Results
- Input impedance (12.4.103) (should be greater than 2.5 MΩ);
- ECG path sensitivity (12.4.104) (should be changed stepwise with an accuracy of 5% in the presence of differential and common voltages of ±300 mV);
- Resistance to overdrive (12.4.105.2) (differential voltage of 1 V for 10 s);
- Noise level (12.4.106.1) (with only the mains filter switched on, should not exceed 30 µV);
- Channel crosstalk (12.4.106.2) (should not exceed an amplitude of 0.5 mm at normal sensitivity, i.e., 2% of the amplitude of the given signal multiplied by the set sensitivity);
- Frequency response (12.4.107.1) (in the frequency range up to 150 Hz);
- Linearity and range of transfer (12.4.107.2) (should be ensured in each lead for 20 mV with an accuracy of 5% over the entire width of the recording field, also in the presence of a common or differential voltage of 300 mV of any polarity).
3.1. Input Impedance
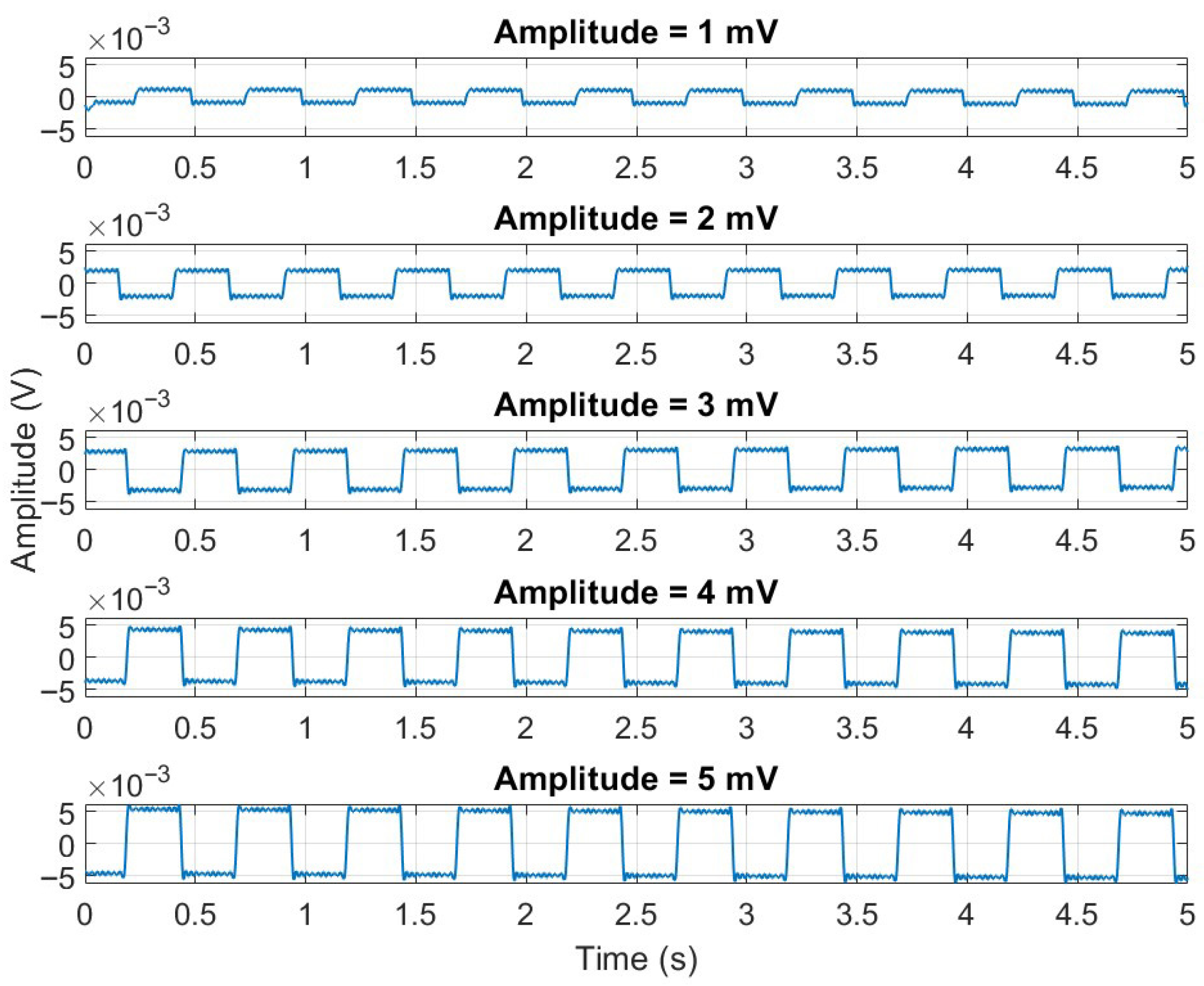
3.2. ECG Gain Accuracy
3.3. Overload Tolerance
3.4. Noise Level
- The peak-to-peak noise amplitude measured over the 10 s window must not exceed 30 microvolts (µV).
- The root mean square (RMS) noise over the same interval must be no greater than 15 µV.
3.5. Channel Crosstalk
3.6. Frequency Response
3.6.1. High-Frequency Response
3.6.2. Low-Frequency (Impulse) Response
- The maximum allowable baseline offset must not exceed ±100 µV when measured 350 ms after the end of the pulse. This limit ensures that the system does not introduce spurious baseline shifts which could distort the clinical interpretation—especially in the evaluation of ST-segment deviations that are typically in the range of hundreds of microvolts.
- The maximum slope of the baseline recovery must not exceed 300 µV per second. This criterion controls the rate at which the baseline returns to its steady state following the impulse. A slope steeper than this limit may produce artefacts that resemble dynamic cardiac events, while an overly gradual return may obscure real low-frequency components such as ischemic ST shifts or T-wave abnormalities.
3.7. Linearity and Dynamic Range
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFE | Analog front-end |
| ADC | Analog-to-digital converter |
| CMRR | Common-mode rejection ratio |
| CT | Central terminal |
| DSP | Digital signal processor |
| ECG | Electrocardiography |
| FIR | Finite impulse response |
| IEC | International Electrotechnical Commission |
| IIR | Infinite impulse response |
| ISO | International Organization for Standardization |
| LA | Left arm |
| LPF | Low-pass filter |
| OIML | International Organization of Legal Metrology (in French: Organisation Internationale de Métrologie Légale) |
| PGA | Programmable gain amplifier |
| PLI | Production-linked incentive |
| pp | Peak-to-peak |
| QMS | Quality management system |
| RA | Right arm |
| RC | Resistor capacitor |
| RLD | Right-leg drive |
| RMS | Root mean square |
| SNR | Signal-to-noise ratio |
| SPI | Serial peripheral interface |
Appendix A
High-Frequency Filtering
References
- IEC 60601-2-25:2016-01; Medical Electrical Equipment—Part 2-25: Particular Requirements for the Basic Safety and Essential Performance of Electrocardiographs. IEC: Geneva, Switzerland, 2016.
- IEC 60601-2-47:2015; Medical Electrical Equipment—Part 2-47: Particular Requirements for the Basic Safety and Essential Performance of Ambulatory Electrocardiographic Systems. IEC: Geneva, Switzerland, 2015.
- IEC 60601-2-27:2014; Medical Electrical Equipment—Part 2-27: Particular Requirements for the Basic Safety and Essential Performance of Electrocardiographic Monitoring Equipment. Comitato Elettrotecnico Italiano: Milan, Italy, 2014.
- Silva, M.C.; Gusmao, L.A.P.; Hall Barbosa, C.R.; Costa Monteiro, E. System for Conformity Assessment of Electrocardiographs. In Proceedings of the 13th International Conference on Biomedical Engineering, Singapore, 3–6 December 2008; Lim, C.T., Goh, J.C.H., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; Volume 23, pp. 1124–1127. [Google Scholar]
- IEC 60601-2-51:2005; Medical Electrical Equipment—Part 2-51: Particular Requirements for the Basic Safety and Essential Performance of Medical Electrocardiographic (ECG) Equipment. IEC: Geneva, Switzerland, 2005.
- OIML R90:2013; Metrological Regulation for Load Cells (1st ed.). OIML: Paris, France, 2013.
- Gordillo-Roblero, L.A.; Soto-Cajiga, J.A.; Romo-Fuentes, C.; Martinez-Soto, L.F.; Rodriguez-Olivares, N.A. A Methodology for the Design of a Compliant Electrocardiograph: A Case Study. Electronics 2024, 13, 4238. [Google Scholar] [CrossRef]
- Campillo, D.; Guardarrama, R.; Gonzalez, R.; Rodriguez, J.; Jimenez, D. A real time ECG preprocessing system based on ADS1298. Comput. Cardiol. 2013, 2013, 947–950. [Google Scholar]
- Badnjevi, A.; Deumic, A.; Softic, A.; Pokvic, L.G. A novel method for conformity assessment testing of patient monitors for post-market surveillance purposes. Technol. Health Care 2023, 31, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Badnjevi, A.; Magjarevic, R.; Mrdjanovic, E.; Pokvic, L.G. A novel method for conformity assessment testing of electrocardiographs for post-market surveillance purposes. Technol. Health Care 2023, 31, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Young, B. New standards for ECG equipment. J. Electrocardiol. 2019, 57S, S1–S4. [Google Scholar] [CrossRef] [PubMed]
- Zepeda-Echavarria, A.; van de Leur, R.R.; van Sleuwen, M.; Hassink, R.J.; Wildbergh, T.X.; Doevendans, P.A.; Jaspers, J.; van Es, R. Electrocardiogram Devices for Home Use: Technological and Clinical Scoping Review. JMIR Cardio 2023, 7, e44003. [Google Scholar] [CrossRef] [PubMed]
- Texas Instruments. ADS129x Low-Power, 8-channel, 24-bit Analog Front-End for Biopotential Measurements Datasheet (Rev. K); Texas Instruments: Dallas, TX, USA, 2020; Available online: https://www.ti.com/lit/ds/symlink/ads1299.pdf (accessed on 1 September 2025).
- Cosoli, G.; Spinsante, S.; Scardulla, F.; D’Acquisto, L.; Scalise, L. Wireless ECG and cardiac monitoring systems: State of the art, available commercial devices and useful electronic components. Measurement 2021, 177, 109243. [Google Scholar] [CrossRef]
- Rahman, M.; Morshed, B.I. Resource-Constrained On-Chip AI Classifier for Beat-by-Beat Real-Time Arrhythmia Detection with an ECG Wearable System. Electronics 2025, 14, 2654. [Google Scholar] [CrossRef]
- Wang, Y. Emerging epidermal electrodes towards digital health and on-skin digitalization. Soft Sci. 2024, 4, 5. [Google Scholar] [CrossRef]
- Zhu, P.; Niu, M.; Liang, S.; Yang, W.; Zhang, Y.; Chen, K.; Pan, Z.; Mao, Y. Non-hand-worn, load-free VR hand rehabilitation system assisted by deep learning based on ionic hydrogel. Nano Res. 2025, 18, 94907301. [Google Scholar] [CrossRef]
- Webster, J.G. Medical Instrumentation: Application and Design, 4th ed.; Wiley: New York, NY, USA, 2009; ISBN 978-0471676003. [Google Scholar]
- Clifford, G.D.; Azuaje, F.; McSharry, P. Advanced Methods and Tools for ECG Data Analysis; Artech House: Norwood, MA, USA, 2006; ISBN 978-1580539661. [Google Scholar]
- Sornmo, L.; Laguna, P. Bioelectrical Signal Processing in Cardiac and Neurological Applications; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2005. [Google Scholar]
- Emon, M.H.; Ashikuzzaman, M.; Babu, M.U.; Ahmed, S.; Islam, M.T. Design and Analysis of a High-Gain, Low-Noise, and Low-Power Analog Front End for Electrocardiogram Acquisition in 45 nm Technology Using gm_mm/ID_DD Method. Electronics 2024, 13, 2190. [Google Scholar] [CrossRef]
- Faruk, N.; Abdulkarim, A.; Emmanuel, I.; Folawiyo, Y.Y.; Adewole, K.S.; Mojeed, H.A.; Oloyede, A.A.; Olawoyin, L.A.; Sikiru, I.A.; Nehemiah, M.; et al. A comprehensive survey on low-cost ECG acquisition systems: Advances on design specifications, challenges and future direction. Biocybern. Biomed. Eng. 2021, 41, 474–502. [Google Scholar] [CrossRef]







| Switch Marking on the Tester Housing | Switch Position |
|---|---|
| SW3 | 4 (top) |
| SW4 | Open (top) |
| SW5 | Closed (bottom) |
| SW6 | Closed (bottom) |
| SW7 | 1A-B (bottom) |
| SW8 | No offs (bottom) |
| SW9 | Open (top) |
| SW10 | 3 (top) |
| SW11 | OFF (top) ON (bottom) in the case of using offset |
| Switch Marking on the Tester Housing | Switch Position |
|---|---|
| SW3 | 2 (middle) |
| SW4 | Open (bottom) |
| SW5 | Closed (bottom) |
| SW6 | Open (top) |
| SW7 | 2A-B (top) |
| SW8 | Offs (2B) (bottom) |
| SW9 | Closed (bottom) |
| SW10 | 1 (bottom) |
| SW11 | OFF (top) ON (bottom) in the case of using offset |
| Switch Marking on the Tester Housing | Switch Position |
|---|---|
| SW3 | 2 (middle) |
| SW4 | Open (bottom) |
| SW5 | Closed (bottom) |
| SW6 | Open (top) |
| SW7 | 2A-B (top) |
| SW8 | Offs (2B) (bottom) |
| SW9 | Closed (bottom) |
| SW10 | 1 (bottom) |
| SW11 | OFF (top) ON (bottom) in the case of using offset |
| Switch Marking on the Tester Housing | Switch Position |
|---|---|
| S2 | SW2 |
| S3 | SW3 |
| Switch Marking on the Tester Housing | Switch Position |
|---|---|
| S | SW1 |
| S2 | SW5 |
| S3 | SW2 |
| S4 | SW8 |
| S5 | SW4 |
| Switch Marking on the Tester Housing | Switch Position |
|---|---|
| S | SW5 |
| S2 | SW1 |
| S3 | SW2 |
| S4 | SW8 |
| S5 | SW4 |
| Switch | Switch Position |
|---|---|
| SW1 | TOP |
| SW2 | TOP |
| SW3 | -> |
| SW4 | TOP |
| SW5 | BOTTOM |
| SW6 | TOP |
| SW7 | TOP |
| SW8 | BOTTOM |
| SW9 | TOP |
| SW10 | BOTTOM |
| SW11 | TOP |
| ECG Lead | Amplitude (mV pp)
Without Offset | Relative
Error [%] |
|---|---|---|
| F | 4.985 | 0.30 |
| L | 4.982 | 0.36 |
| R | 5.019 | 0.38 |
| V1 | 4.983 | 0.34 |
| V2 | 4.981 | 0.38 |
| V3 | 4.986 | 0.28 |
| V4 | 4.986 | 0.28 |
| V5 | 5.142 | 2.84 |
| V6 | 5.307 | 6.14 |
| ECG Channel | Amplitude (mV pp) | Relative Error [%] |
|---|---|---|
| F | 1.989 | 0.55 |
| L | 1.989 | 0.55 |
| R | 1.988 | 0.60 |
| V1 | 2.000 | 0.00 |
| V2 | 1.999 | 0.05 |
| V3 | 2.004 | 0.20 |
| V4 | 2.001 | 0.05 |
| V5 | 2.003 | 0.15 |
| V6 | 2.001 | 0.05 |
| ECG Channel | Amplitude [mV pp] (Overload) | Amplitude [mV pp] (Post-Overload) | Relative Error [%] |
|---|---|---|---|
| F | 602.19 | 4.98 | 0.36 |
| L | 597.22 | 4.75 | 4.94 |
| V1 | 604.22 | 4.88 | 2.40 |
| V2 | 604.22 | 4.88 | 2.40 |
| V3 | 576.16 | 4.86 | 2.75 |
| V4 | 596.00 | 5.25 | 4.91 |
| V5 | 601.82 | 5.48 | 9.63 |
| V6 | 595.65 | 4.97 | 0.63 |
| ECG Channel | pp Amplitude(μV) | RMS (μV) |
|---|---|---|
| F | 17.96 | 2.69 |
| L | 25.81 | 3.34 |
| V1 | 23.97 | 2.43 |
| V2 | 17.60 | 2.67 |
| V3 | 17.89 | 2.77 |
| V4 | 22.61 | 2.92 |
| V5 | 16.90 | 2.77 |
| V6 | 14.44 | 2.22 |
| Source ECG Lead | Observed ECG Lead | |||||||
|---|---|---|---|---|---|---|---|---|
| Lead I | Lead II | V1 | V2 | V3 | V4 | V5 | V6 | |
| I | 100% | 0.8% | 0.3% | 0.2% | 0.2% | 0.2% | 0.2% | 0.3% |
| II | 0.8% | 100% | 0.2% | 0.3% | 0.2% | 0.2% | 0.1% | 0.1% |
| V1 | 0.1% | 0.1% | 100% | 0.4% | 0.2% | 0.1% | 0.1% | 0.1% |
| V2 | 0.1% | 0.1% | 0.4% | 100% | 0.4% | 0.2% | 0.1% | 0.1% |
| V3 | 0.1% | 0.1% | 0.2% | 0.4% | 100% | 0.3% | 0.2% | 0.1% |
| V4 | 0.1% | 0.1% | 0.1% | 0.2% | 0.3% | 100% | 0.3% | 0.2% |
| V5 | 0.1% | 0.1% | 0.1% | 0.1% | 0.2% | 0.3% | 100% | 0.4% |
| V6 | 0.1% | 0.1% | 0.1% | 0.1% | 0.1% | 0.2% | 0.4% | 100% |
| ECG Lead | Sine Wave of 1 mV at 0.67 Hz | Sine Wave of 1 mV at 10 Hz | Sine Wave of 1 mV at 40 Hz | Relative Error [%] (0.67 Hz) | Relative Error [%] (0.10 Hz) | Relative Error [%] (40 Hz) |
|---|---|---|---|---|---|---|
| F | 0.9993 | 0.9991 | 0.9903 | 0.07 | 0.09 | 0.97 |
| L | 0.9992 | 0.9987 | 0.9898 | 0.08 | 0.13 | 1.02 |
| R | 0.9990 | 0.9986 | 0.9896 | 0.10 | 0.14 | 1.04 |
| V1 | 1 | 0.9990 | 0.9900 | 0.00 | 0.10 | 1.00 |
| V2 | 0.9990 | 0.9980 | 0.9894 | 0.11 | 0.20 | 1.06 |
| V3 | 0.9992 | 0.9991 | 0.9900 | 0.08 | 0.09 | 1.00 |
| V4 | 0.9994 | 0.9995 | 0.9913 | 0.06 | 0.05 | 0.87 |
| V5 | 0.9998 | 0.9988 | 0.9904 | 0.02 | 0.12 | 0.96 |
| V6 | 0.9988 | 0.9990 | 0.9899 | 0.12 | 0.10 | 1.01 |
|
ECG
Lead | Triangle Wave of 1.5 mV at 0.67 Hz for 20 ms Base Width | Triangle Wave of 1.5 mV at 0.67 Hz for 200 ms Base Width—Reference Signal | Relative Error [%] |
|---|---|---|---|
| F | 1.487 | 1.539 | 3.43 |
| L | 1.470 | 1.569 | 6.32 |
| R | 1.479 | 1.589 | 6.92 |
| V1 | 1.605 | 1.730 | 7.23 |
| V2 | 1.620 | 1.734 | 6.62 |
| V3 | 1.598 | 1.708 | 6.46 |
| V4 | 1.581 | 1.683 | 6.07 |
| V5 | 1.603 | 1.728 | 7.26 |
| V6 | 1.606 | 1.699 | 5.51 |
| ECG Channels | pp Amplitude [mV] | Mean Max Displacement [µV/s] | Mean Max Slope [µV] |
|---|---|---|---|
| F | 3.110 | 21.085 | 265.13 |
| L | 3.109 | 21.336 | 223.40 |
| R | 3.109 | 19.795 | 245.79 |
| V1 | 3.110 | 20.116 | 241.56 |
| V2 | 3.109 | 19.840 | 251.51 |
| V3 | 3.110 | 20.865 | 257.15 |
| V4 | 3.112 | 20.141 | 225.68 |
| V5 | 3.109 | 20.338 | 251.36 |
| V6 | 3.108 | 20.202 | 267.82 |
| Performance Parameter | IEC 60601-2-25 Requirement | The Current Study (ADS1298) | Gordillo-Roblero et al. [7] (ADAS1000) | Campillo et al. [8] (ADS1298) |
|---|---|---|---|---|
| System Noise | ≤30 μV | 14.44 µV on V6 | 5.17 µV | ≈9 μV |
| Amplitude Quantization | ≤5 μV/LSB | ≤5 μV/LSB | 0.1009 µV/LSB | ≈3.1 μV/LSB |
| Input Impedance | ≥2.5 MΩ | >2.5 MΩ | - | - |
| Inter-Channel Crosstalk | ≤2% | 0.1–0.8% | - | <2% |
| Common-Mode Rejection | ≥89 dB | - | - | ≈93.7 dB |
| Sampling Frequency | ≥500 Hz | 1 kHz | 2 kHz (up to 16 kHz) | 500 Hz |
| Inter-Channel Skew | <100 μs | - | 0.0 µs | - |
| Frequency Response (Passband) | 0.05–150 Hz (diagnostic) | 0.05–150 Hz | 0.05–150 Hz | 0.05–150 Hz |
| Frequency Response (Amplitude Error at 40 Hz) | ±10% of 10 Hz | 0.88% (with external filter) | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raus-Jarzabek, E.; Czerw, M.; Skowronek, A.; Dąbrowska-Boruch, A.; Jamro, E.; Olejarczyk, E. A Practical Guide to ECG Device Performance Testing According to International Standards. Electronics 2025, 14, 3878. https://doi.org/10.3390/electronics14193878
Raus-Jarzabek E, Czerw M, Skowronek A, Dąbrowska-Boruch A, Jamro E, Olejarczyk E. A Practical Guide to ECG Device Performance Testing According to International Standards. Electronics. 2025; 14(19):3878. https://doi.org/10.3390/electronics14193878
Chicago/Turabian StyleRaus-Jarzabek, Elzbieta, Marek Czerw, Andrzej Skowronek, Agnieszka Dąbrowska-Boruch, Ernest Jamro, and Elzbieta Olejarczyk. 2025. "A Practical Guide to ECG Device Performance Testing According to International Standards" Electronics 14, no. 19: 3878. https://doi.org/10.3390/electronics14193878
APA StyleRaus-Jarzabek, E., Czerw, M., Skowronek, A., Dąbrowska-Boruch, A., Jamro, E., & Olejarczyk, E. (2025). A Practical Guide to ECG Device Performance Testing According to International Standards. Electronics, 14(19), 3878. https://doi.org/10.3390/electronics14193878






