1. Introduction
Imagine a world where aging is not a challenge but an opportunity for empowerment and independence. As our global population ages [
1], the need for innovative solutions to enhance the lives of seniors and those with mobility limitations becomes increasingly urgent. Traditionally, individuals facing mobility issues have had to rely heavily on caregivers, be it family members or hired professionals, creating strains on both sides. However, technological advancements, particularly intelligent control, are witnessing a transformative shift toward a more inclusive and accessible future. Safety and accuracy improvements are paramount to fully realizing these systems’ potential. Furthermore, for individuals grappling with severe paralysis and potential cognitive impairments, the complexity of current interface systems presents a significant barrier. Although numerous methods exist to control machines, each approach has challenges. For instance, brain–computer interface (BCI) systems [
2] promise to enhance communication and control for individuals with diverse abilities. However, their widespread adoption faces obstacles such as extensive training and calibration, which are particularly challenging for users with cognitive impairments or limited motor function. Moreover, the reliability of brain signals can fluctuate due to factors like fatigue or external distractions, leading to inconsistent performance. Similarly, eyeball control systems [
3] rely on eye movements for navigation but encounter limitations in range and precision, especially for individuals with restricted mobility. Environmental factors like lighting conditions or visual distractions can also impact the accuracy of eye-tracking systems, potentially causing control errors and user discomfort. Arm muscle electrical signal control systems face challenges such as muscle fatigue with prolonged use [
4], difficulty in generating reliable muscle signals for individuals with severe paralysis, and the need for frequent recalibration due to variability in muscle signal strength. Nevertheless, muscle fatigue in EMG signals has been extensively studied, and established approaches, such as zero-crossing rate (ZCR) and median frequency (MDF) analysis, are routinely used for reliable fatigue assessments [
5].
Therefore, we propose a simpler method for wheelchair control: the use of facial muscles, particularly the occlusal (masseter and temporalis) muscles. These muscles were deliberately selected not only because they are generally preserved even in patients with severe motor impairments but also because their activation is intuitive, requires minimal training, and produces high-amplitude sEMG signals compared to the baseline. While other facial muscle groups (e.g., around the eyes or lips) may also provide EMG signals, they are more easily influenced by involuntary actions such as blinking, speech, or facial expressions, which reduces their robustness for control purposes. In contrast, occlusal muscles allow for voluntary, deliberate activation with lower susceptibility to unintentional interference.
In addition, although surface electrodes are known to be more noise-sensitive compared to invasive electrodes, they were deliberately chosen in this study to maintain a non-invasive, low-cost, and user-friendly setup. Importantly, in our experiments, the amplitude of occlusion-induced sEMG signals was approximately 7–9 times higher than the baseline (28–29 μV at rest vs. 196–255 μV during occlusion), ensuring that reliable recognition could be achieved with simple filtering and thresholding (Tables 1–3). This quantitative difference justifies the feasibility of using surface electrodes in this proof-of-concept design.
Therefore, we turn to a simpler method for wheelchair control: facial muscles. Harnessing the power of occlusal myoelectric signal control brings numerous advantages to users with mobility impairments. Facial muscles offer natural and intuitive control, allowing users to navigate their environment using familiar facial movements like smiling or biting. This intuitive control mechanism promotes a sense of independence, liberating users from reliance on external assistance or complex interfaces. Moreover, facial muscle control preserves upper limb functionality, which is particularly beneficial for individuals with upper limb impairments. Facial muscle control typically incurs minimal fatigue compared to other methods, ensuring that users can operate their wheelchairs comfortably for extended periods.
More importantly, unlike prior works that only introduced occlusion-based control as a concept or in limited pilot tests, our study is the first to implement closed-loop control with an actual powered wheelchair, integrate cloud-based communication and safety mechanisms, and quantitatively benchmark system performance against existing EMG-based approaches. These distinctions highlight both the novelty and necessity of our design.
Therefore, further research is required to develop a simple system with a user-friendly interface and less training. In biomedical engineering, EMG signals [
6] for control have been widely used. Myoelectric signals [
7] can record human movements, such as athlete training and analysis [
8], athlete stability training, and a systematic and grouped scientific training method for athletes’ muscle group linkage [
9] and power muscle groups by recording myoelectric signals [
10,
11].
In the design of electric wheelchairs, there has been research proposed in the prior literature on the control of electric wheelchairs using myoelectric signals [
9,
10,
11,
12,
13,
14], such as the control of electric wheelchairs using signals that detect eye movements and the control of wheelchairs by arm myoelectric signals. These are the forefront of pioneering techniques to empower users with greater autonomy. By reviewing the related works, it can be observed that almost all studies on the control of electric wheelchairs by myoelectric signals set the electrodes as surface electrodes, and it can also be observed that for the surface electromyographic signal (sEMG) analysis, the signal recognition is relatively high. The movement status of the subject can be judged intuitively. Most importantly, since patients with severe paralysis can only utilize their facial muscles to control electric wheelchair systems, systems based on occlusal movements may be more appropriate in this patient population. Chang et al. developed a human brain–computer interface (hBCI) system that could be controlled based on masseter sEMG signals. Li et al. further demonstrated that different occlusal movements could be used to move and stop a BCI-based wheelchair. Chai et al. used the EMG patterns to build the hBCI-controlled smart home system. Moreover, products to assist users with limb impairment include arm muscle signal-controlled prostheses, eye movement signal-controlled wheelchairs, and arm muscle signal-controlled wheelchairs [
12,
13,
14,
15,
16,
17,
18,
19,
20].
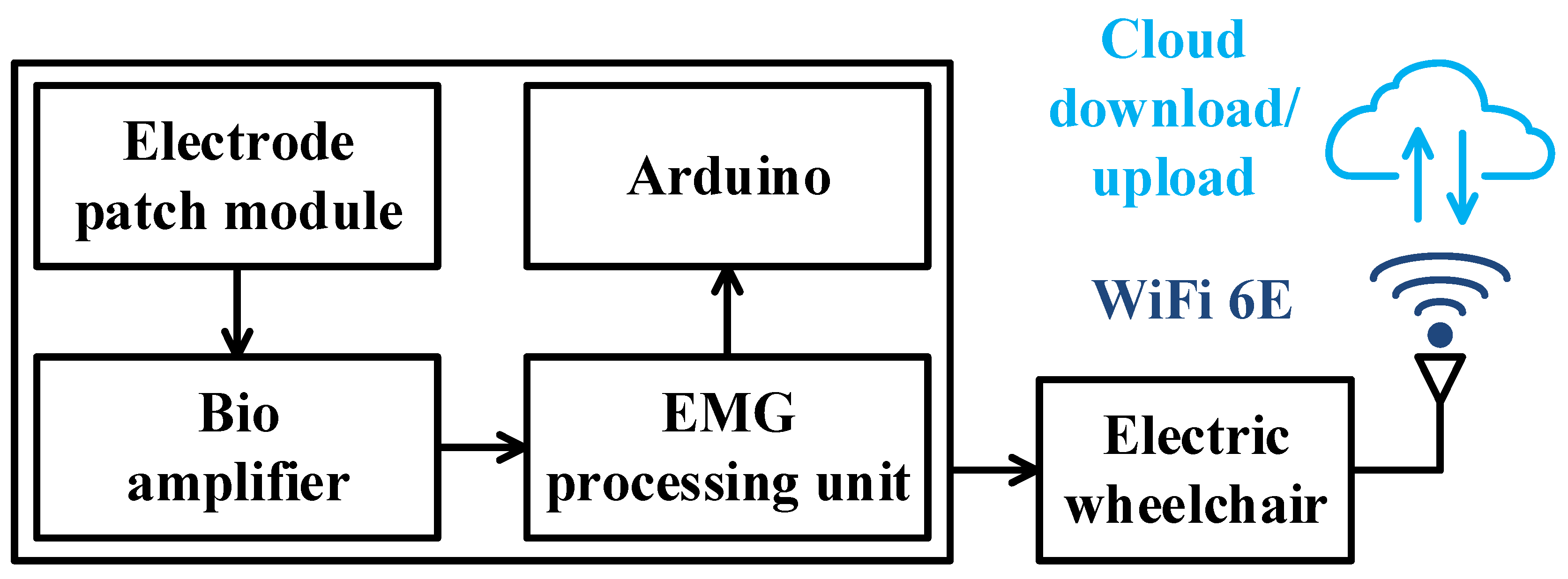
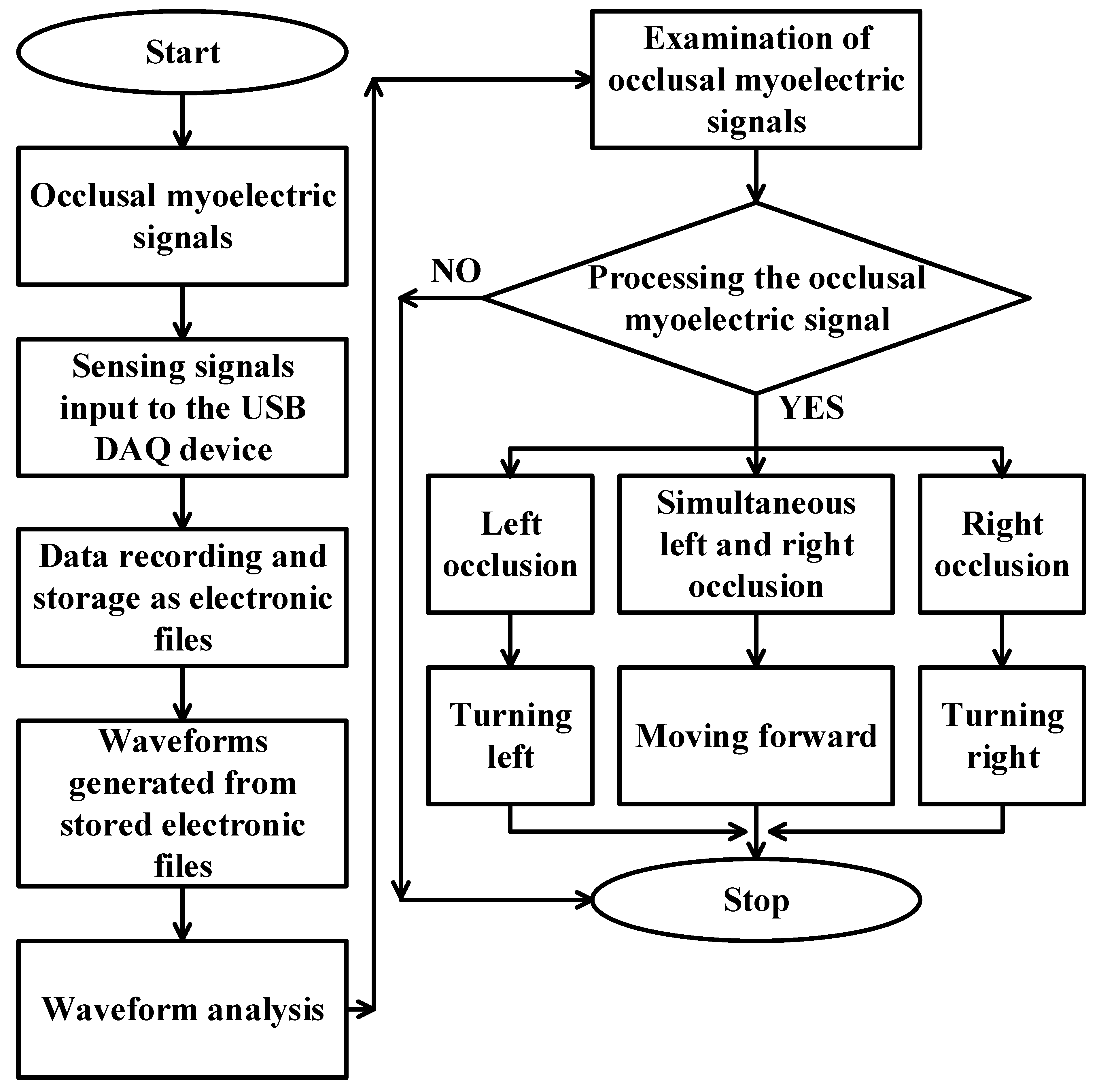
This work aims to help users with limited mobility or lower body paralysis but who still have an average bite ability to operate the wheelchair by reading the bite signal and converting it into a usable control signal. In this way, users with limited mobility can control the movement of the electric wheelchair by themselves through the bite action, reducing the burden of bothering others. These innovations offer newfound freedom and empower users to navigate their world with confidence and dignity. This work chose to set up surface electrodes to receive myoelectric signals. After receiving the bite signal, the amplitude of the sEMG signal generated during the bite was identifiable. Hence, the amplitude difference of the original signal waveform was analyzed to find the signal generated during the bite as the basis for controlling the electric wheelchair. A schematic diagram of the proposed electric wheelchair control system is depicted in
Figure 1. The functional block diagram of Arduino control is depicted in
Figure 2. The principle of controlling wheels to move forward, backward, left, and right with Arduino board (Arduino, Monza, Lombardia, Italy) is mainly achieved by controlling DC motors or servo motors. To drive the motors, an H-Bridge motor driver using the L298N chip (STMicroelectronics Geneva, Switzerland) is usually used to control the direction and speed of the motors. Below is the detailed explanation of how it works. The direction of rotation of a DC motor is determined by the direction of the current flowing through it. The motion of the wheels (forward, backward, or turning) is controlled by changing the direction of the current through the motors. A half-bridge circuit is allowed to control the direction of a DC motor. It consists of four switches (such as MOSFETs), which are controlled by input signals to change the direction of the current flow through the motor. The half-bridge circuit allows control in two directions: clockwise (forward) and counterclockwise (backward). The half-bridge has four pins: IN1, IN2, IN3, and IN4. To make the wheel move forward, you can make IN1 and IN3 have high levels and IN2 and IN4 have low levels. If you want to make the wheel go backward, you can make IN1 and IN3 have low levels and IN2 and IN4 have high levels. To make the wheel stationary, make IN1 and IN2, and IN3 and IN4 low at the same time so that the motor will not rotate. The schematic of the hardware architecture is shown in
Figure 3 (
https://en.hwlibre.com/l298n/, accessed on 21 January 2022).
The background and purpose of this study and operation of the electric wheelchair using the occlusion signal are explained in detail in
Section 2 and
Section 3, respectively. The discussion is described in
Section 4. Finally, the conclusions are presented in
Section 5.
Although previous studies have explored myoelectric- or brain–computer interface (BCI)-based wheelchair control, existing systems often suffer from high training requirements, susceptibility to noise, muscle fatigue, or poor reliability under real-world conditions. To date, no study has systematically validated occlusal sEMG-driven wheelchair control in a proof-of-concept setting with quantitative benchmarking.
More importantly, to the best of our knowledge, no prior study has systematically validated an occlusal sEMG-driven wheelchair with quantitative performance benchmarking. Unlike prior works that only introduced occlusion-based control as a concept or in limited pilot tests, our study is the first to implement closed-loop control with an actual powered wheelchair, integrate cloud-based communication and safety mechanisms, and quantitatively benchmark system performance against existing EMG-based approaches.
Our proposed system fills this gap by (1) utilizing facial occlusal muscles, which remain functional even in users with severe limb paralysis; (2) demonstrating surface electrode-based, non-invasive acquisition with minimal user training; and (3) providing quantitative validation of control accuracy (100% success rate), surpassing prior reports that achieved 82–99% recognition accuracy using other muscle groups. These distinctions highlight both the novelty and necessity of our design. This work not only demonstrates technical feasibility but also aims to address the clinical reality that masticatory muscles are often preserved even in individuals with severe paralysis, making them an ideal input source for assistive mobility systems.
The hypothesis of this study is as follows: “Using a control method based on the sEMG of the articulatory muscles, we verified its feasibility as an electric wheelchair control system and further explored the effectiveness of this method in improving the mobility of people with limited mobility or paralysis of the lower limbs, which has potential for patient autonomy.”
2. Materials and Methods
There were five males aged between 20 and 45 years included in this experiment, all with normal occlusal function and no occlusal impairment, and each subject was informed of the procedure and the purpose of the experiment and asked for permission before beginning the experiment. In this study, electrode patches were applied to the occlusal muscles on both sides of the cheeks, and the test results were investigated using the same experimental procedure and analysis method. Moreover, this study extends the research presented in [
21,
22]; however, the textual content and experimental figures have been updated.
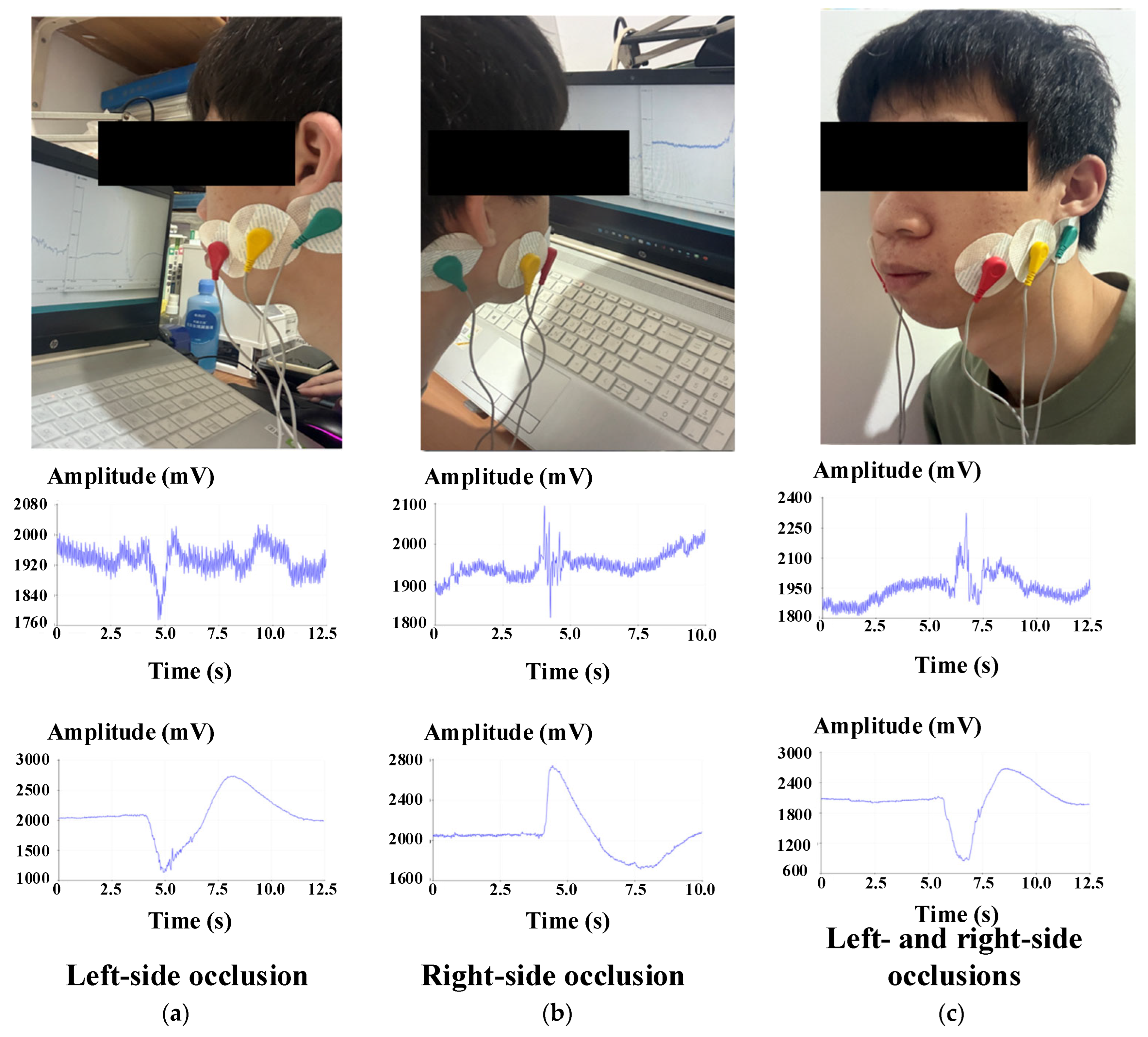
Figure 4 shows a schematic diagram of the experimental setup [
23]. By scheduling the time, the subjects were told to perform a specific occlusion behavior (left occlusion, right occlusion, left and right simultaneous occlusion every 5 s) during the fixed period of the test to observe the occlusion status of different subjects. The data from the same waveform were analyzed to determine whether the electrode patch was successfully set.
The operation diagram of occlusion signal reception is shown in
Figure 5. First, the occlusal signals generated by the occluding muscles were observed in a non-interference environment to find the variation and obtain usable feature points. A subject was placed in a fixed position and sEMG signals [
24] were received through a data acquisition (DAQ) device with a sampling rate of 1 kHz. Six testing sessions were repeated for each subject, each with 30 s of sEMG data. As mentioned, the differences in the obtained occlusion signals were analyzed and compared to determine the appropriate drive signal for controlling the electric wheelchair. On the other hand, to reduce the human interference caused by swallowing and talking, confirming the status of the subject before the test and reducing the swallowing and talking movements within 30 s were necessary to reduce the fatigue of the subject due to repeated testing; so, the subject was allowed to rest for 30 s to 1 min after the collection of each set of signals.
As shown in
Figure 6, the sEMG signal electrodes were set on the left and right occlusal muscles [
19], and the reference potentials were put in the mastoid area behind the ear to receive the signals generated by the subject’s occlusal movements [
12] and to obtain the energy transfer generated during occlusion. The received signal indicates the change in energy generated during occlusion [
18,
19,
20,
21,
22].
This study uses notch filters to suppress noise signals at specific frequencies effectively. Since power frequency noise (60 Hz) can interfere with the performance and accuracy of the equipment, notch filters are usually designed to establish a deep filtering band near the power frequency to effectively attenuate noise signals at this frequency to ensure stable operation and high precision. In this way, the signal-to-noise ratio of the muscle signal driven by the cheek muscles will be greater than 99, and there is no need to use complicated methods for signal processing.
There are two physiological signal electrode setups: the inline and surface electrodes. Firstly, the advantages of the inlay electrode are less error, less interference from noise, and the ability to measure deep muscle. However, the disadvantage is that the electrode setup is more complicated and costly. On the other hand, the advantage of surface electrodes is that they are simple, can be set up without surgery, and the setup cost is low, but the disadvantage is that they have a lot of noise and cannot receive signals from deeper muscles. After analysis and comparison, this work received myoelectric signals through surface electrodes [
27,
28,
29,
30,
31]. The comparison between the inline electrode and surface electrode is listed in
Table 1.
The rationale for selecting surface electrodes in this prototype was to prioritize non-invasiveness, low cost, and ease of deployment. Although surface electrodes are more susceptible to noise compared to invasive methods, the occlusion task in our study produced signal amplitudes that were substantially higher than the baseline. Specifically, the resting baseline averaged 28–29 μV, whereas occlusion conditions produced average amplitudes of 196–255 μV (approximately 7–9 times higher, see
Table 2 and
Table 3). Occlusion-induced EMG amplitudes were significantly higher than the baseline (mean ± SD, 95% CI). This large amplitude difference allowed reliable detection with simple filtering and thresholding, making surface electrodes suitable for early-stage assistive device development.
By analyzing sEMG signals [
12], the wheelchair can be controlled using myoelectric signals and adjusted to correspond to the occlusal signal when the subject makes different bite movements [
32,
33,
34,
35,
36]. The Wi-Fi 6E [
37], an extension of the Wi-Fi 6 standard, represents a significant leap forward in wireless communication technology by operating in the 6 GHz frequency range. This extension brings many improvements and innovations, setting it apart from its predecessor, Wi-Fi 6 (802.11ax). One of the key enhancements introduced by Wi-Fi 6E is the availability of an additional spectrum. By utilizing the 6 GHz frequency band, Wi-Fi 6E alleviates the congestion in the 2.4 GHz and 5 GHz bands, which are shared by many devices. This increased spectrum not only provides more channels for data transmission but also allows for wider bandwidths, leading to faster and more efficient wireless communication. This is particularly crucial in today’s densely populated urban environments where multiple devices vie for limited airspace. The wider channels of the 6 GHz frequency band possibly contribute to improved data rates. Wi-Fi 6E supports higher throughput, enabling faster and more reliable connections. This is especially beneficial for bandwidth-intensive applications such as video streaming, online gaming, and virtual reality experiences, where seamless and high-quality connectivity is paramount. Moreover, Wi-Fi 6E brings a lower latency, significantly reducing the time it takes for devices to communicate with each other. This is essential for real-time responsiveness of applications like online gaming or video conferencing. The lower latency enhances the overall user experience, making interactions smoother and more instantaneous. In this study, recognition accuracy was defined as the proportion of correctly executed commands relative to the total number of intended commands. Each subject performed ten repetitions of three commands (forward, left, and right), resulting in 150 total trials (5 subjects × 3 commands × 10 repetitions). Accuracy was defined as the proportion of correctly recognized and executed commands relative to the intended inputs, consistent with prior sEMG-based control studies. A trial was counted as correct when the system’s recognized output matched the subject’s intended command. This definition of accuracy is consistent with prior sEMG-based wheelchair control studies and was applied throughout the evaluation. To statistically validate the observed differences, we applied descriptive and inferential analyses. Specifically, we report means ± standard deviations (SDs) and 95% confidence intervals (CI) for occlusion versus non-occlusion EMG amplitudes (
Table 2 and
Table 3). Given the small sample size (n = 5) and potential non-normality, we employed the Wilcoxon signed-rank test to assess whether occlusion amplitudes were significantly higher than the baseline. In addition, effect sizes were calculated to quantify the magnitude of the observed differences. These analyses ensure that the performance evaluation is supported by statistical evidence beyond raw accuracy percentages.
3. Results
To investigate how the human body generates, operates, and receives myoelectric signals, the surface electrodes were placed on the occlusal muscles on both sides of the cheeks to receive sEMG signals in this experiment. This experiment shows that the human body can have many physiological and cardiac signals, and the surface electrode method was chosen to receive myoelectric signals. This method may cause an increase in noise. Still, after the signal analysis, it can be found that there is a significant variation in the sEMG signal, which can be utilized with a simple analysis. The noise and electrode setting errors are within tolerable ranges. The experimental environment of an electric wheelchair with occlusion myoelectric signal control is shown in
Figure 7.
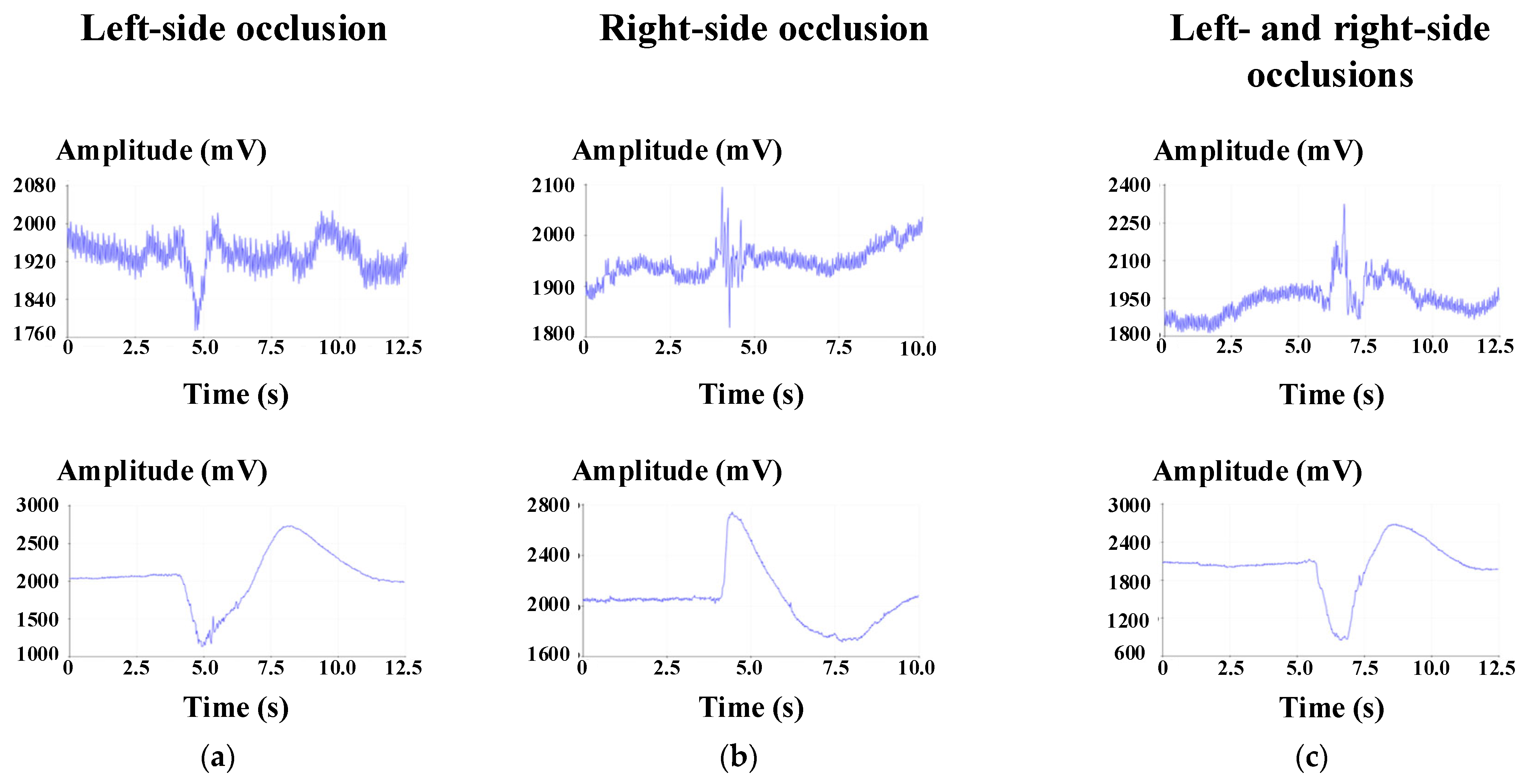
The measured waveforms of the occlusion signal are shown in
Figure 8. After analyzing the occlusion signals, it was found that there was a significant difference in the signal intensity measured during occlusion and non-occlusion.
Table 2 and
Table 3 list the measured signal intensity without and with occlusion, respectively. Statistical analysis confirmed that EMG amplitudes during occlusion were significantly greater than the baseline (
p < 0.05, Wilcoxon signed-rank test). In addition, descriptive statistics (means ± SDs, 95% CIs) are reported in
Table 2 and
Table 3 to highlight the substantial amplitude differences across subjects. Effect size calculations further indicated a strong magnitude of difference, underscoring the robustness of the observed effect. These results confirm that the amplitude differences are not only descriptive but also statistically meaningful. The experimental values indicated that the signal intensity measured during occlusion was not the same for each subject; so, it can be inferred that the occlusion intensity was different for each subject. If the signal is more prominent on the right side, it can be assumed that the subject is used to chewing mainly on the right side. The analyzed occlusion signal was filtered through a high-pass filter to convert the occlusion signal into an on/off signal. When the computer received a high voltage level, the electric wheelchair would activate; conversely, the electric wheelchair would turn off when the computer did not receive a control request within a fixed period, and then stop at a safe speed, ensuring user safety. Based on this definition, accuracy was calculated for each subject and each command. As summarized in
Table 4,
Table 5 and
Table 6, all 150 trials (5 subjects × 3 commands × 10 repetitions) were correctly recognized, yielding an overall accuracy of 100%.
Table 4,
Table 5 and
Table 6 summarize the experimental outcomes for the three motor commands: right turn, left turn, and forward movement. Five subjects (denoted as I to V) each performed ten trials per task, and in all instances, the intended control commands were successfully recognized and executed by the system. As shown in
Table 4, all subjects achieved a 100% success rate in executing the right turn command across all trials. This result indicates that the proposed system demonstrates excellent reliability and precision in detecting and processing the corresponding sEMG signals. Moreover, the system exhibited consistent performance across different individuals, underscoring its robustness and generalizability.
Table 5 presents the results for the left turn task. Similarly, every subject completed all trials successfully, yielding a total success rate of 100%. These findings further confirm the system’s ability to accurately and consistently recognize sEMG signals associated with the left turn operation and convert them into reliable control outputs.
Table 6 details the results of the forward movement task. All participants were able to trigger the forward command with complete accuracy in every trial, once again achieving a perfect success rate. This demonstrates the system’s capability to handle directional commands with high fidelity and responsiveness in real time. Collectively, these results validate the effectiveness of the proposed sEMG-based control system in supporting multiple directional commands. The consistent 100% success rates across all subjects and tasks highlight the system’s high accuracy, operational stability, and user independence, indicating strong potential for practical application in assistive mobility technologies.
A concise and precise description of the experimental results, their interpretation, and the experimental conclusions that can be drawn is provided. One of the remarkable aspects of this technology is its ability to dismantle physical barriers that may have previously restricted individuals with mobility issues. Whether it be due to age, injury, or disability, these barriers can significantly impede one’s ability to move freely and engage in daily activities. With cutting-edge mobility solutions, individuals can navigate their surroundings with newfound freedom.
Moreover, the technology goes beyond just providing physical mobility; it extends to ensuring the safety and security of users. This is achieved through the seamless transmission of user data and GPS paths to the cloud, facilitated by the state-of-the-art Wi-Fi 6E communication technology. By leveraging cloud connectivity, the system can instantly relay critical information, such as the user’s location and movement patterns, ensuring a prompt emergency response.
The significance of Wi-Fi 6E in this context cannot be overstated. This advanced communication technology operates in the 6 GHz frequency band, offering faster data transfer speeds and lower latency. This enhances the mobility devices’ real-time tracking capabilities and contributes to a more responsive and reliable overall user experience.
In practical terms, individuals using these advanced mobility solutions can confidently explore their surroundings, knowing that their location and movements are monitored in real time. This newfound autonomy can be life-changing for those who may have felt confined or dependent on others for assistance.
4. Discussion
These findings also address a key research gap in the existing literature. While prior approaches based on myoelectric signals or BCIs have shown promise, most suffer from high training demands, noise interference, muscle fatigue, or limited reliability in practical scenarios. In contrast, our study represents the first systematic and quantitative validation of an occlusal sEMG-driven wheelchair, achieving a 100% command success rate. Compared with the recognition accuracy reported for other muscle groups (82–99%), our results highlight the advantages of using occlusal muscles as a control source and further emphasize the novelty and necessity of this approach. Importantly, unlike the original submission, the revised analysis incorporated statistical validation, including descriptive statistics, non-parametric testing, and effect size reporting. The Wilcoxon signed-rank test confirmed the significance of increases in the EMG amplitude during occlusion (p < 0.05), and effect size metrics provided further evidence of robustness. These additions enhance the scientific rigor of our findings and strengthen confidence in the proposed approach. From an application perspective, our prototype demonstrated real-time responsiveness: once occlusion was performed, EMG signals were filtered and detected, and motor commands were executed with millisecond-level latency. In terms of comfort, the use of non-invasive surface electrodes ensured that participants experienced no discomfort during short trial sessions (~30 s each). Nevertheless, we recognize that long-term comfort and usability have not yet been evaluated. Future studies will therefore incorporate extended-duration trials, user feedback surveys, and additional comfort-oriented features such as pressure ulcer alert systems and ergonomic adjustments, ensuring that the system remains reliable and user-friendly in daily use scenarios.
The small sample size (n = 5, healthy young males), the controlled laboratory setting, and the absence of long-term and real-world testing limit the generalizability of the findings. We also acknowledge that target populations (e.g., paralyzed or elderly users) may present different muscle activation patterns, fatigue resistance, and comorbidities that influence control performance. In addition, the perfect success rate observed here may be partly attributable to the simplicity of the experimental task design and the large amplitude gap between occlusion and rest (7–9 times), factors that reduce the error likelihood in short-term controlled conditions. Moreover, this study only recruited five healthy young males, which limits its clinical relevance. Target user populations such as elderly individuals or patients with paralysis may exhibit different muscle activation patterns, fatigue characteristics, or comorbidities that could influence control performance. Future studies will therefore expand to include these clinical cohorts to better establish real-world applicability.
These conditions may not fully represent real-world scenarios involving fatigue, swallowing, or other involuntary facial actions. Future studies will expand the validation to larger and more diverse populations; incorporate error metrics such as false activation/rejection rates, confusion matrices, and response times; and evaluate robustness under real-world conditions such as obstacle navigation, prolonged continuous use (≥30 min), and multi-day repeatability testing.
Beyond recognition accuracy, this work distinguishes itself from prior occlusion-based control studies through three major contributions. First, we implemented closed-loop control with an actual powered wheelchair across three directional commands (forward, left, and right), enabling systematic validation under realistic operation rather than conceptual demonstrations alone. Second, we integrated cloud-based communication (Wi-Fi 6E) and a built-in safety mechanism that automatically stops the wheelchair in the absence of valid control input, thus enhancing user safety and system robustness. Third, we strengthened the evaluation’s rigor by reporting descriptive statistics, applying non-parametric significance testing, and conducting quantitative comparisons with prior EMG-based approaches. In future work, we also plan to implement advanced signal-processing methods such as machine learning (e.g., SVM and CNN) to replace simple thresholding, which—although sufficient for the large amplitude differences in our current controlled setup—may not be robust enough in noisy or more complex clinical environments.
The investigation into myoelectric signals and their application in machine control has yielded significant insights into the potential of utilizing facial articulatory muscles for wheelchair navigation. In this experiment, surface electrodes were strategically placed on the occlusal muscles on both sides of the cheeks to receive sEMG signals. Despite the potential increase in noise associated with this method, the analysis revealed a significant variation in the sEMG signal, which could be effectively utilized with simple analytical techniques. Notably, noise and electrode setting errors remained within tolerable ranges, validating the feasibility of this approach [
36]. The experimental setup of an electric wheelchair controlled by occlusal myoelectric signals provided a conducive environment for testing. The analysis of the measured occlusion signals demonstrated a marked difference in signal intensity between occlusion and non-occlusion states. This variation in intensity was further observed to differ across subjects, indicating individual-specific occlusion intensities. Additionally, the occlusion signal was filtered through a high-pass filter to convert it into an on/off signal for wheelchair control, ensuring user safety by activating or deactivating the wheelchair based on signal presence. The experimental results showcased the efficacy of the proposed control method, with a 100% success rate across all subjects in executing maneuvers such as turning to the right, turning to the left, and moving forward. We cite relevant research [
37] to compare the success rate of the control method in this study with other myoelectric control methods, highlighting the effectiveness and innovation of the system. However, this comparison should be interpreted cautiously, as the laboratory setup in our study likely reduced variability compared to studies conducted in more complex or uncontrolled environments.
Even under conditions of random occlusion strength, flexibility, and primary occlusion side, the electric wheelchair functioned seamlessly, affirming the robustness and adaptability of the occlusion myoelectric signal control system, and the experimental findings underscore this approach’s naturalness, effectiveness, and safety, providing valuable insights into advancing assistive technologies for individuals with diverse abilities. At the same time, we also consider that the muscle signal of occlusion can be used to control the direction of the wheelchair very well. Specifically, the average signal intensity during occlusion reached 255.2 μV under left-only occlusion (left channel) and 228.2 μV under bilateral occlusion (right channel), compared with a baseline of 28–29 μV without occlusion (
Table 2 and
Table 3), representing nearly a nine-fold increase in amplitude. Across all 150 trials (five subjects × three commands × ten repetitions), the system achieved a 100% command recognition success rate (
Table 4,
Table 5 and
Table 6), quantitatively confirming its reliability and robustness.
This study compares the control results of other similar muscle electrical signals, as shown in
Table 7. The study by Khushaba et al. [
38] showed a success rate of 93% using surface electrodes. In comparison, the method of Gijsberts et al. [
39] had a slightly lower success rate of 82% using surface electrodes. The method of Witman et al. [
40] achieved a very high success rate of 99.1%. The signal identification success rate of Fang et al. is 90.85% [
41]. This may be because the parts that collect muscle electrical signals are fingers with smaller muscles, which can be controlled more accurately and achieve extremely high accuracy.
Beyond recognition accuracy, our proposed occlusal sEMG control approach offers several practical advantages over other control modalities. Occlusal-based control induces low fatigue due to the natural strength and endurance of the masticatory muscles, provides intuitive operation requiring minimal training, and does not rely on upper- or lower-limb mobility. In addition, the system requires only minimal calibration, which makes it more accessible for long-term daily use. Nonetheless, further evaluation is necessary to examine how the system performs with electrode reattachment, long-term fatigue accumulation, and interference from involuntary facial activities such as swallowing, speech, or coughing. Future enhancements will also focus on integrating ultrasonic obstacle detection, ensuring compliance with ISO 7176 and IEC 60601 standards, and developing long-term comfort features such as pressure ulcer alert systems. Together, these improvements will strengthen both the clinical applicability and the safety profile of the proposed wheelchair control method.
Regarding the electrode type, most studies have chosen surface electrodes, probably due to their ease of application and lower cost, non-invasiveness, and convenience. However, there are a few studies that used single-fiber needles as electrodes. Notably, our proposed method achieved a 100% success rate while selecting surface electrodes, which aligns with most other studies. Our method shows advantages in research, signal identification, control success rates, and electrode types. In addition,
Table 7 has been updated to reflect the most recent research findings. For example, Witman et al. [
40] reported a recognition accuracy of 99.1%, and Fang et al. [
41] reported 90.85%. We have double-checked the references and confirmed their accuracy to ensure that
Table 7 reflects the latest progress in this field.
To further advance this field, future studies should elucidate the potential effects of muscle activity on the temporomandibular joint (TMJ) and develop corresponding risk control strategies [
44].
It may be difficult for patients with limited mobility to detect obstacles in the wheelchair’s path when moving the wheelchair. Therefore, in the future, we will add an ultrasonic sensing system to this design, which can be used as a warning to detect obstacles when the electric wheelchair is moving. The user’s safety is greatly improved when using this electric wheelchair to move. In addition, we will also optimize this system in the future and add a long-term alarm reminder system to prevent pressure ulcers and improve ride comfort. In the future, safety will be further considered, and relevant tests of medical equipment ISO 7176-part 1 to part 10 will be carried out to verify the design, such as static stability tests, braking efficiency tests, energy consumption tests, maximum speed, acceleration and deceleration, static force, impact and fatigue strength, weather resistance, obstacle surmounting ability, power and control system tests, posture support device fire tests, etc., to ensure the safety of users. For safety considerations, electrical safety verification by IEC 60601-1-1 and EMC verification by IEC 60601-1-2 will also be included to ensure user safety and compliance with standards. In conclusion, by providing critical interpretations, acknowledging limitations, considering alternative explanations, and outlining a roadmap for future research, this revised Discussion now moves beyond a superficial summary and aligns with the standards of a peer-reviewed scientific analysis.
This newfound autonomy is life-changing for those who may have once felt confined or dependent on others for assistance. This newfound autonomy is life-changing for those who have ever felt restricted or dependent on others for help. In conclusion, using occlusal myoelectric signals for wheelchair control provides a promising method for enhancing the mobility of people with mobility impairments.
In conclusion, this revised Discussion moves beyond a superficial summary by providing critical interpretations, acknowledging limitations, considering alternative explanations, and outlining a roadmap for future work. By situating our findings within the broader literature and highlighting both strengths and limitations, we believe this section now aligns with the expectations of a peer-reviewed scientific analysis.
5. Conclusions
In conclusion, our innovative electric wheelchair control system, driven by occlusal sEMG signals, has demonstrated exceptional potential to enhance user independence and mobility, achieving a success rate exceeding 99%. In detail, the system achieved a 100% recognition success rate across all three directional commands, with five subjects performing ten repetitions of three directional commands (150/150 correct trials). The average sEMG amplitude increased from a baseline of 28–29 μV (no occlusion) to 255.2 μV (left-only occlusion, left channel) and 228.2 μV (bilateral occlusion, right channel), indicating a nearly nine-fold signal enhancement. These quantitative results clearly demonstrate the sensitivity, stability, and robustness of the proposed occlusal control system. However, this study should be interpreted as a proof-of-concept feasibility demonstration rather than a comprehensive clinical validation. The small sample size (n = 5), the recruitment of only healthy young males, and the controlled laboratory setting limit the generalizability of these findings. Clinical populations such as elderly individuals or patients with paralysis may exhibit different muscle activation patterns, fatigue resistance, or comorbidities that could influence performance.
To address these limitations, future developments will integrate ultrasonic sensing for obstacle detection and a long-term alarm system to prevent pressure ulcers and improve comfort. Larger-scale evaluations including diverse and clinical cohorts, real-world navigation scenarios, and long-term usability studies will be essential to establish clinical relevance. The current prototype already incorporates a fail-safe stop mechanism that automatically halts the wheelchair in the absence of valid control input. Future iterations will integrate ultrasonic obstacle detection and undergo systematic safety validation to ensure a robust emergency response and user protection. Additionally, comprehensive safety and compliance tests, including ISO 7176 and IEC 60601-1/60601-1-2 standards, will be conducted to ensure reliability and user safety.
This work demonstrates the feasibility of an occlusal sEMG-driven wheelchair control system as an early-stage prototype, laying the groundwork for future translational research and clinical validation. These enhancements aim to further optimize the system’s functionality and expand its practical application for individuals with mobility impairments.