Exploring AI in Healthcare Systems: A Study of Medical Applications and a Proposal for a Smart Clinical Assistant
Abstract
1. Introduction
- RQ1: How do we envision the next generation of intelligent clinical assistants integrating clinical decision-making workflows to autonomously analyze the adverse effects of combination medications while providing decision support to medical doctors?
- RQ2: What are the essential architectural elements and methodologies needed to create and execute a successful intelligent clinical support system?
- RQ3: What are the basic components of a framework that ensures the scalability and reliability of AI-based medical assistants, how has this concept evolved over time, and what is the potential of these assistants in modern healthcare systems?
2. Materials and Methods
2.1. MoodGYM
2.2. Woebot
2.3. Wysa
2.4. COVID-19 Canada Pediatric Chatbot
- Strengths:
- 60–70% reduction in triage time
- Reduced risk of exposure for medical staff
- Very high user satisfaction (parents, doctors)
- Easy to scale to other medical contexts
- Frees up medical personnel
- Limits COVID-19 exposure in waiting rooms
- Free, anonymous access
- High acceptability in hospital settings
- Weaknesses:
- Limited to a specific domain (COVID-19 screening)
- Requires strong integration with local IT infrastructure
- Does not perform complex diagnoses
- Depends on regular updates to medical guidelines
- Needs to be embedded within hospital systems
2.5. COVID-19 Symptom Evaluation Chatbot for India
- Strengths:
- Millions of users within a few months;
- Extremely fast and accurate automated triage (specificity >90%);
- Relieves pressure on medical personnel;
- Reduces hospital overcrowding;
- Provides verified official information;
- Linguistic adaptations for regional languages.
- Weaknesses:
- Focused on a single pathology (COVID-19);
- Requires continuous updates (medical guidelines);
- Initial challenges with regionalization and digital literacy;
- Controversies around privacy and personal data.
2.6. Platforms Selection Criteria and Metrics
- Geographic Diversity: Platforms were chosen to represent a range of regions and health system contexts, including high, middle, and low income settings, and both Western and non-Western digital health innovation;
- Technology Type Representation: The selection includes both chatbot based (Woebot, Wysa, COVID-19 chatbots) and non-chatbot (MoodGYM) digital health solutions, enabling comparison between conversational artificial intelligence and traditional digital interventions;
- Clinical Domain Coverage: Platforms address both chronic (mental health: Cognitive Behavioral Therapy, anxiety, depression) and acute (COVID-19 triage) care domains;
- Validation Level: Inclusion required peer reviewed evidence (randomized controlled trials, real-world studies, systematic reviews) or, for COVID-19 chatbots, documented operational impact and integration with healthcare systems.
2.7. Comparison and Analysis
- Platform name
- Type of digital intervention
- Objective/domain
- Target audience
- AI technology (yes/no and what type)
- Key benefits
- Major limitations
- Reported clinical outcomes
3. System Design and Workflow
3.1. Proposed System Architecture and Workflow
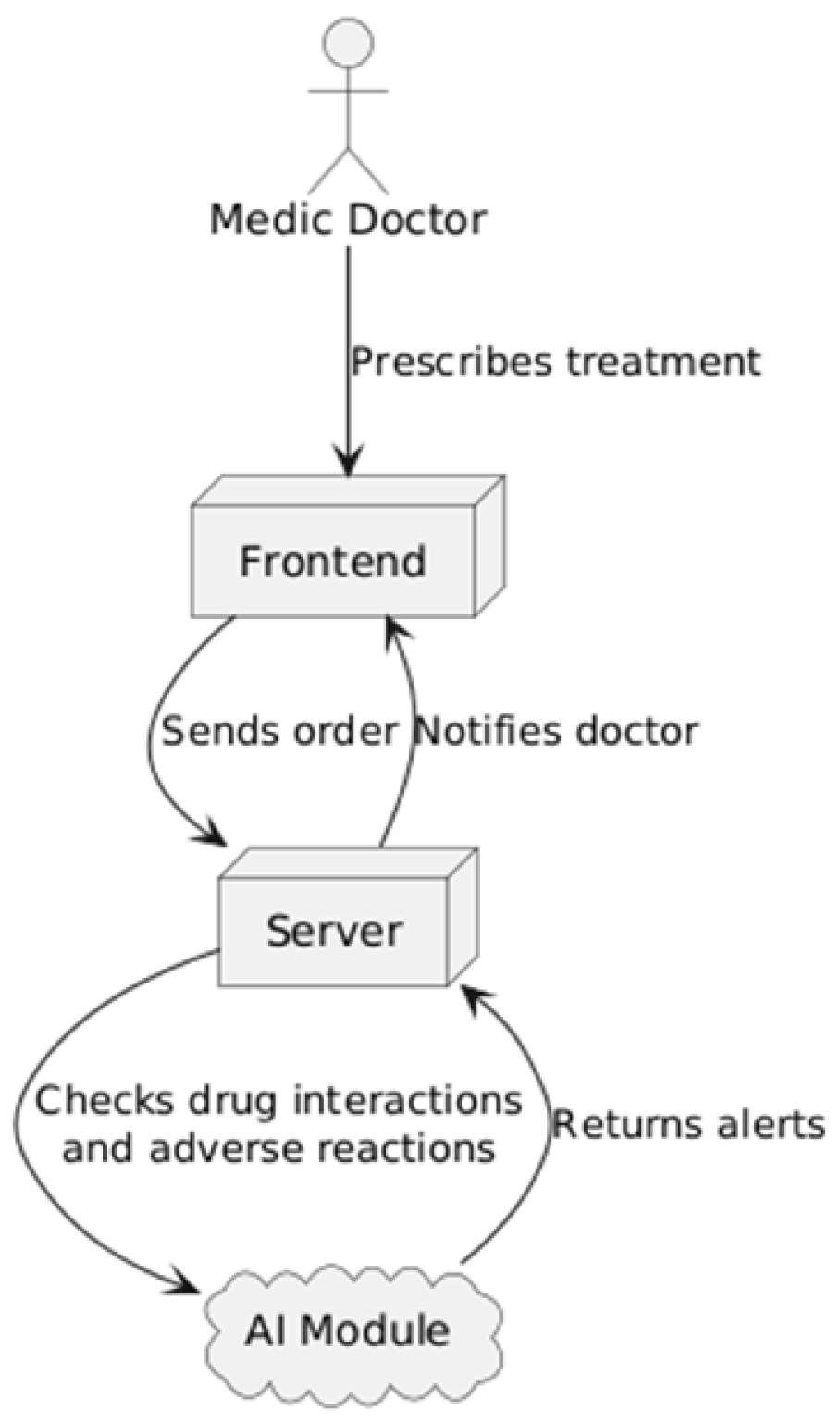
3.2. Proposed System Overview
- The frontend, representing the user interface, developed using the React library (based on the JavaScript language), which provides an interactive environment through which the doctor can access patient information and enter medical orders.
- The backend, built with NestJS (a Node.js framework based on the JavaScript language), forming a robust core that manages the application logic, data processing, and integration with external services.
- A centralized relational SQL database that stores all medical data, including patient records, hospitalized patients in each department, prescribed treatments, laboratory results, and appointments, ensuring data consistency and accessibility at the national level.
- An AI module (implemented in Python 3.13.0) integrated into the back end to provide decision support. This AI engine is responsible for analyzing prescribed treatments and laboratory results. A more detailed presentation of the AI module architecture and its evaluation follows in Section 3.4 and Section 3.5.
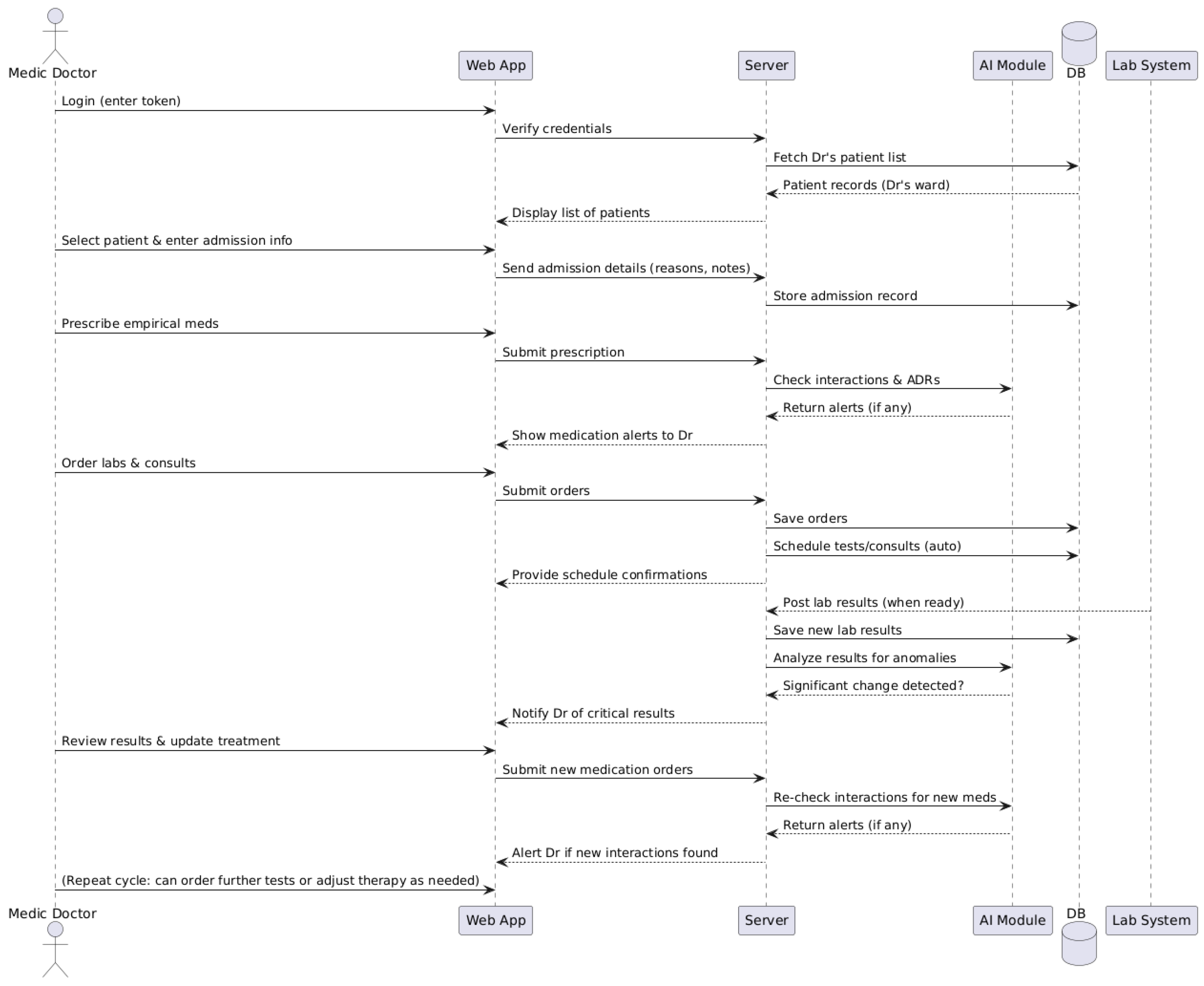
3.3. Operational Workflow for the Doctor
- Authentication in the system and display of patients;
- Selecting the patient and recording the initial data;
- Prescribing the initial treatment and verifying it with the help of the AI module;
- Requesting investigations and automatic scheduling;
- Receiving the results and interpreting them with the assistance of the AI module;
- Re-evaluating and adapting the treatment.
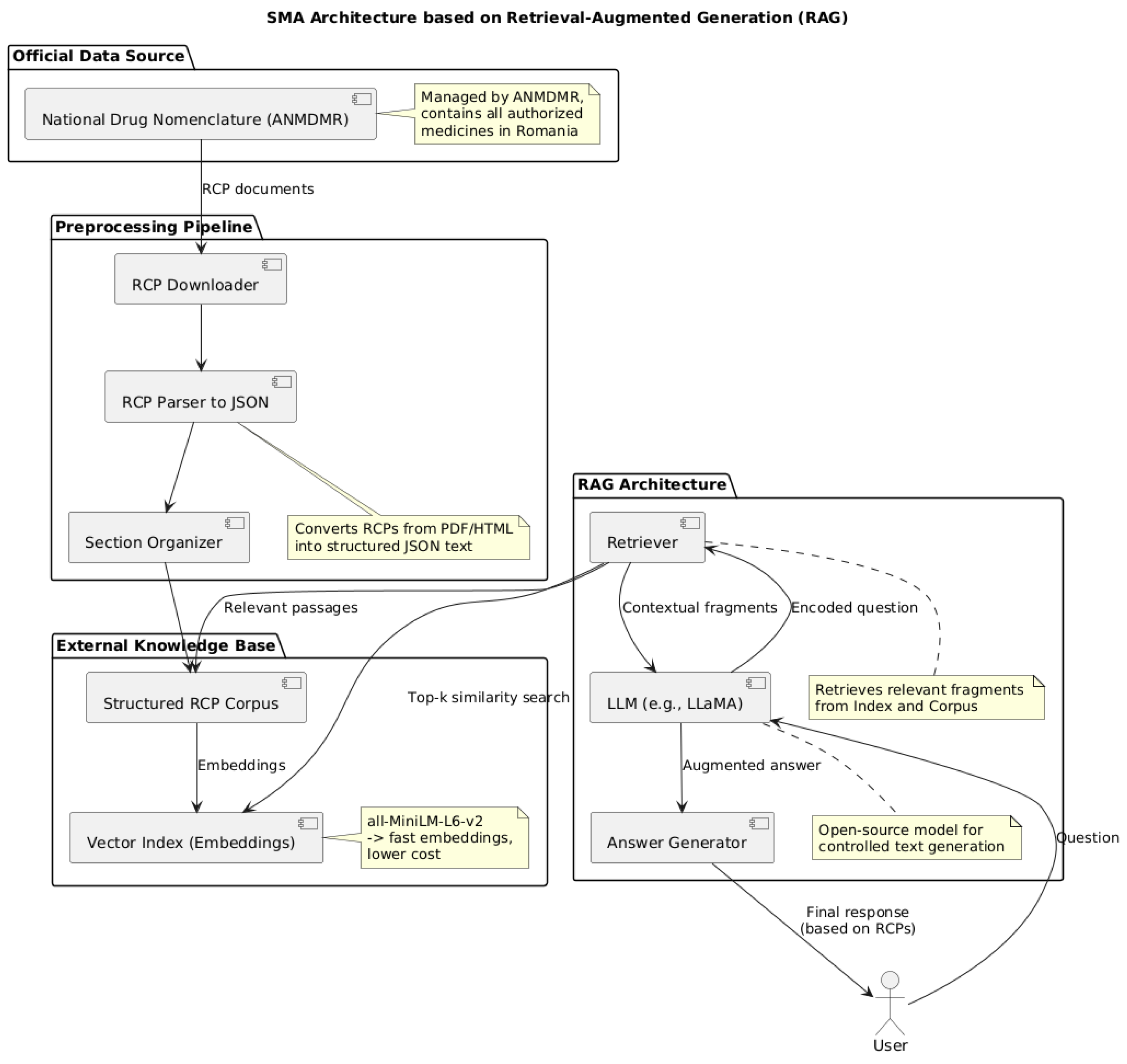
3.4. AI Module Architecture: Retrieval-Augmented Generation (RAG) Approach
3.5. AI Module Limitations, Ethical Considerations and Future Directions
3.6. Considerations on Research Questions
- The Data Perception Module is designed to aggregate and interpret clinical data from heterogeneous sources, including laboratory test results, EHRs, prescription data, and structured medical databases. This module employs advanced data integration techniques to harmonize disparate inputs into a unified, structured format. By transforming raw clinical information into standardized datasets, the system facilitates downstream analytical processes, enabling robust clinical decision support, predictive modeling, and research-driven insights.
- Knowledge-based reasoning engines, which examine data to find patterns in adverse events or possible drug interactions. These engines can use AI models for clinical reasoning, including large language models, machine learning (ML) classifiers, and knowledge graphs.
- Memory and Learning Components. These components interact with clinicians over time to improve prediction accuracy by learning from new cases and storing pertinent medical knowledge (such as medication databases and clinical guidelines).
- The Interaction and Integration Module functions as a communication interface between the clinical decision support system and medical personnel, ensuring alignment with established clinical practices. This module incorporates both a conversational agent and an interactive dashboard designed to elucidate system-generated alerts and recommendations. Furthermore, it provides integration capabilities with hospital IT infrastructures, enabling the suggestion of prescription adjustments and the formulation of patient monitoring plans. By facilitating transparent and actionable communication, the module enhances clinical workflow efficiency and supports informed decision-making. The SMA operates through interconnected modules that collect data before using AI reasoning to ensure medication safety and interact with users while following integration principles and security standards and maintainability protocols. The modular design with strict implementation will enable us to create an analysis system which performs effectively while being deployable in clinical environments and scalable between departments and hospitals and adaptable to upcoming requirements.
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADEs | Adverse Drug Events |
| AI | Artificial Intelligence |
| ANMDMR | National Agency for Medicines and Medical Devices of Romania |
| API | Application Programming Interface |
| BLEU | Bilingual Evaluation Understudy |
| CBT | Cognitive Behavioral Therapy |
| CDSS | Clinical Decision Support System |
| EHR | Electronic Health Record |
| EMR | Electronic Medical Record |
| LIS | Laboratory Information System |
| LLM | Large Language Model |
| ML | Machine Learning |
| NLP | Natural Language Processing |
| RAG | Retrieval-Augmented Generation |
| RBAC | Role-Based Access Control |
| ROUGE | Recall-Oriented Understudy for Gisting Evaluation |
| SMA | Smart Clinical Assistant |
| SPC | Summaries of Product Characteristics |
| SQL | Structured Query Language |
| VPN | Virtual Private Network |
| XAI | Explainable Artificial Intelligence |
References
- Li, W.; Chai, Y.; Khan, F.; Jan, S.R.U.; Verma, S.; Menon, V.G.; Kavita; Li, X. A Comprehensive Survey on Machine Learning-Based Big Data Analytics for IoT-Enabled Smart Healthcare System. Mob. Netw. Appl. 2021, 26, 234–252. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Navimipour, N.J. A Comprehensive and Systematic Review of the IoT-Based Medical Management Systems: Applications, Techniques, Trends and Open Issues. Sustain. Cities Soc. 2022, 82, 103914. [Google Scholar] [CrossRef]
- Ullah, H.; Manickam, S.; Obaidat, M.; Laghari, S.U.A.; Uddin, M. Exploring the Potential of Metaverse Technology in Healthcare: Applications, Challenges, and Future Directions. IEEE Access 2023, 11, 69686–69707. [Google Scholar] [CrossRef]
- Anamaria, N.; Monica Mihaela, M.M.; Cristina, M. Artificial Intelligence: Friend or Foe? Experts’ Concerns on European AI Act. Econ. Comput. Econ. Cybern. Stud. Res. 2023, 57, 5–22. [Google Scholar] [CrossRef]
- Alowais, S.A.; Alghamdi, S.S.; Alsuhebany, N.; Alqahtani, T.; Alshaya, A.I.; Almohareb, S.N.; Aldairem, A.; Alrashed, M.; Bin Saleh, K.; Badreldin, H.A.; et al. Revolutionizing Healthcare: The Role of Artificial Intelligence in Clinical Practice. BMC Med. Educ. 2023, 23. [Google Scholar] [CrossRef]
- Al Kuwaiti, A.; Nazer, K.; Al-Reedy, A.; Al-Shehri, S.; Al-Muhanna, A.; Subbarayalu, A.V.; Al Muhanna, D.; Al-Muhanna, F.A. A Review of the Role of Artificial Intelligence in Healthcare. J. Pers. Med. 2023, 13, 951. [Google Scholar] [CrossRef]
- Younis, H.A.; Eisa, T.A.E.; Nasser, M.; Sahib, T.M.; Noor, A.A.; Alyasiri, O.M.; Salisu, S.; Hayder, I.M.; Younis, H.A. A Systematic Review and Meta-Analysis of Artificial Intelligence Tools in Medicine and Healthcare: Applications, Considerations, Limitations, Motivation and Challenges. Diagnostics 2024, 14, 109. [Google Scholar] [CrossRef]
- Khang, A. (Ed.) Driving Smart Medical Diagnosis Through AI-Powered Technologies and Applications; Advances in Medical Diagnosis, Treatment, and Care; IGI Global: Hershey, PA, USA, 2024; ISBN 979-8-3693-3679-3. [Google Scholar]
- Gao, X.; He, P.; Zhou, Y.; Qin, X. Artificial Intelligence Applications in Smart Healthcare: A Survey. Future Internet 2024, 16, 308. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Le, L.K.-D.; Lal, A.; Engel, L.; Mihalopoulos, C. The Cost-Effectiveness of Delivering an e-Health Intervention, MoodGYM, to Prevent Anxiety Disorders among Australian Adolescents: A Model-Based Economic Evaluation. Ment. Health Prev. 2021, 24, 200210. [Google Scholar] [CrossRef]
- Twomey, C.; O’Reilly, G. Effectiveness of a Freely Available Computerised Cognitive Behavioural Therapy Programme (MoodGYM) for Depression: Meta-Analysis. Aust. N. Z. J. Psychiatry 2017, 51, 260–269. [Google Scholar] [CrossRef]
- Mohamed Jasim, K.; Malathi, A.; Bhardwaj, S.; Aw, E.C.-X. A Systematic Review of AI-Based Chatbot Usages in Healthcare Services. J. Health Organ. Manag. 2025. [Google Scholar] [CrossRef]
- Haque, M.D.R.; Rubya, S. An Overview of Chatbot-Based Mobile Mental Health Apps: Insights From App Description and User Reviews. JMIR MHealth UHealth 2023, 11, e44838. [Google Scholar] [CrossRef]
- Prochaska, J.J.; Vogel, E.A.; Chieng, A.; Kendra, M.; Baiocchi, M.; Pajarito, S.; Robinson, A. A Therapeutic Relational Agent for Reducing Problematic Substance Use (Woebot): Development and Usability Study. J. Med. Internet Res. 2021, 23, e24850. [Google Scholar] [CrossRef]
- Hoffman, V.; Flom, M.; Mariano, T.Y.; Chiauzzi, E.; Williams, A.; Kirvin-Quamme, A.; Pajarito, S.; Durden, E.; Perski, O. User Engagement Clusters of an 8-Week Digital Mental Health Intervention Guided by a Relational Agent (Woebot): Exploratory Study. J. Med. Internet Res. 2023, 25, e47198. [Google Scholar] [CrossRef]
- Sinha, P.; Matthay, M.A.; Calfee, C.S. Is a “Cytokine Storm” Relevant to COVID-19? JAMA Intern. Med. 2020, 180, 1152. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Malik, T.; Sinha, C. Delivery of a Mental Health Intervention for Chronic Pain Through an Artificial Intelligence–Enabled App (Wysa): Protocol for a Prospective Pilot Study. JMIR Res. Protoc. 2022, 11, e36910. [Google Scholar] [CrossRef]
- Inkster, B.; Kadaba, M.; Subramanian, V. Understanding the Impact of an AI-Enabled Conversational Agent Mobile App on Users’ Mental Health and Wellbeing with a Self-Reported Maternal Event: A Mixed Method Real-World Data mHealth Study. Front. Glob. Womens Health 2023, 4, 1084302. [Google Scholar] [CrossRef] [PubMed]
- Sinha, C.; Meheli, S.; Kadaba, M. Understanding Digital Mental Health Needs and Usage With an Artificial Intelligence–Led Mental Health App (Wysa) During the COVID-19 Pandemic: Retrospective Analysis. JMIR Form. Res. 2023, 7, e41913. [Google Scholar] [CrossRef]
- Chang, C.L.; Sinha, C.; Roy, M.; Wong, J.C.M. AI-Led Mental Health Support (Wysa) for Health Care Workers During COVID-19: Service Evaluation. JMIR Form. Res. 2024, 8, e51858. [Google Scholar] [CrossRef] [PubMed]
- Meheli, S.; Sinha, C.; Kadaba, M. Understanding People With Chronic Pain Who Use a Cognitive Behavioral Therapy–Based Artificial Intelligence Mental Health App (Wysa): Mixed Methods Retrospective Observational Study. JMIR Hum. Factors 2022, 9, e35671. [Google Scholar] [CrossRef]
- Iglesias, M.; Sinha, C.; Vempati, R.; Grace, S.E.; Roy, M.; Chapman, W.C.; Rinaldi, M.L. Evaluating a Digital Mental Health Intervention (Wysa) for Workers’ Compensation Claimants: Pilot Feasibility Study. J. Occup. Environ. Med. 2023, 65, e93–e99. [Google Scholar] [CrossRef] [PubMed]
- Palanica, A.; Fossat, Y. COVID-19 Has Inspired Global Healthcare Innovation. Can. J. Public Health 2020, 111, 645–648. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Shukla, V.; Islam, N.; Upadhyay, A.; Duong, L. Applying Artificial Intelligence in Healthcare: Lessons from the COVID-19 Pandemic. Int. J. Prod. Res. 2025, 63, 594–627. [Google Scholar] [CrossRef]
- Celuppi, I.C.; Lima, G.D.S.; Rossi, E.; Wazlawick, R.S.; Dalmarco, E.M. Uma Análise Sobre o Desenvolvimento de Tecnologias Digitais Em Saúde Para o Enfrentamento Da COVID-19 No Brasil e No Mundo. Cad. Saúde Pública 2021, 37, e00243220. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, A.; Amanzadeh, M.; Hamedan, M.; Naemi, R. Artificial Intelligence Based Chatbots to Combat COVID-19 Pandemic: A Scoping Review. Shiraz E-Med. J. 2023, 24, e139627. [Google Scholar] [CrossRef]
- Thwala, E.; Adegun, A.; Adigun, M. Self-Assessment Chatbot for COVID-19 Prognosis Using Deep Learning-Based Natural Language Processing (NLP). In Proceedings of the 2023 International Conference on Science, Engineering and Business for Sustainable Development Goals (SEB-SDG), Omu-Aran, Nigeria, 5 April 2023; IEEE: New York, NY, USA, 2023; pp. 1–8. [Google Scholar]
- Tzelios, C.; Contreras, C.; Istenes, B.; Astupillo, A.; Lecca, L.; Ramos, K.; Ramos, L.; Roca, K.; Galea, J.T.; Tovar, M.; et al. Using Digital Chatbots to Close Gaps in Healthcare Access during the COVID-19 Pandemic. Public Health Action 2022, 12, 180–185. [Google Scholar] [CrossRef]
- Yuan, S.; Yang, Z.; Li, J.; Wu, C.; Liu, S. AI-Powered Early Warning Systems for Clinical Deterioration Significantly Improve Patient Outcomes: A Meta-Analysis. BMC Med. Inform. Decis. Mak. 2025, 25, 203. [Google Scholar] [CrossRef]
- Khare, A.; Reddy Penubaka, K.K.; Chithrakumar, T.; Geetha, M.; Kamalavalli, K.; Bhagirath Jadhav, A. AI-Driven Patient Flow Management in Hospitals: Reducing Wait Times and Enhancing Care. J. Neonatal Surg. 2025, 14, 696–708. [Google Scholar] [CrossRef]
- Atlam, H.F.; Yang, Y. Enhancing Healthcare Security: A Unified RBAC and ABAC Risk-Aware Access Control Approach. Future Internet 2025, 17, 262. [Google Scholar] [CrossRef]
- Ferreira, T.R.; Lopes, L.C.; Bergamaschi, C.D.C. Frequency and Severity of Adverse Drug Reactions to Medications Prescribed for Alzheimer’s Disease in a Brazilian City: Cross-Sectional Study. Front. Pharmacol. 2020, 11, 538095. [Google Scholar] [CrossRef] [PubMed]
- Graafsma, J.; Murphy, R.M.; Van De Garde, E.M.W.; Karapinar-Çarkit, F.; Derijks, H.J.; Hoge, R.H.L.; Klopotowska, J.E.; Van Den Bemt, P.M.L.A. The Use of Artificial Intelligence to Optimize Medication Alerts Generated by Clinical Decision Support Systems: A Scoping Review. J. Am. Med. Inform. Assoc. 2024, 31, 1411–1422. [Google Scholar] [CrossRef] [PubMed]
- Maleki Varnosfaderani, S.; Forouzanfar, M. The Role of AI in Hospitals and Clinics: Transforming Healthcare in the 21st Century. Bioengineering 2024, 11, 337. [Google Scholar] [CrossRef]
- Spies, N.C.; Farnsworth, C.W.; Jackups, R. Data-Driven Anomaly Detection in Laboratory Medicine: Past, Present, and Future. J. Appl. Lab. Med. 2023, 8, 162–179. [Google Scholar] [CrossRef]
- Dodig, S.; Čepelak, I.; Dodig, M. Are We Ready to Integrate Advanced Artificial Intelligence Models in Clinical Laboratory? Biochem. Medica 2025, 35, 010501. [Google Scholar] [CrossRef]
- Sabin, O. Information Sources in Romanian on Medicines. Medic.ro 2019, 4, 28. [Google Scholar] [CrossRef]
- Shen, Z.; Spruit, M. Automatic Extraction of Adverse Drug Reactions from Summary of Product Characteristics. Appl. Sci. 2021, 11, 2663. [Google Scholar] [CrossRef]
- Sharma, C. Retrieval-Augmented Generation: A Comprehensive Survey of Architectures, Enhancements, and Robustness Frontiers. arXiv 2025, arXiv:2506.00054. [Google Scholar]
- Gupta, S. Retrieval-Augmented Generation and Hallucination in Large Language Models: A Scholarly Overview. Sch. J. Eng. Technol. 2025, 13, 328–330. [Google Scholar] [CrossRef]
- Wada, A.; Tanaka, Y.; Nishizawa, M.; Yamamoto, A.; Akashi, T.; Hagiwara, A.; Hayakawa, Y.; Kikuta, J.; Shimoji, K.; Sano, K.; et al. Retrieval-Augmented Generation Elevates Local LLM Quality in Radiology Contrast Media Consultation. npj Digit. Med. 2025, 8, 395. [Google Scholar] [CrossRef]
- UKPLab (Ubiquitous Knowledge Processing Lab) at Technische Universität Darmstadt, H.F. (Hugging Face) Pretrained Models—Sentence Transformers Documentation. Available online: https://www.sbert.net/docs/sentence_transformer/pretrained_models.html (accessed on 29 August 2025).
- Charter Global Open-Source vs. Closed-Source LLM Software (Pros and Cons). Available online: https://www.charterglobal.com/open-source-vs-closed-source-llm-software-pros-and-cons (accessed on 29 August 2025).
- e-Cancer AI Tech Should Augment Physician Decision-Making, Not Replace It. Available online: https://ecancer.org/en/news/24837-ai-tech-should-augment-physician-decision-making-not-replace-it (accessed on 29 August 2025).
- Saarela, M.; Podgorelec, V. Recent Applications of Explainable AI (XAI): A Systematic Literature Review. Appl. Sci. 2024, 14, 8884. [Google Scholar] [CrossRef]
- Rosenbacke, R.; Melhus, Å.; McKee, M.; Stuckler, D. How Explainable Artificial Intelligence Can Increase or Decrease Clinicians’ Trust in AI Applications in Health Care: Systematic Review. JMIR AI 2024, 3, e53207. [Google Scholar] [CrossRef]
- Chinta, S.V.; Wang, Z.; Palikhe, A.; Zhang, X.; Kashif, A.; Smith, M.A.; Liu, J.; Zhang, W. AI-Driven Healthcare: A Review on Ensuring Fairness and Mitigating Bias. PLoS Digit. Health 2025, 4, e0000864. [Google Scholar] [CrossRef]
- Bouderhem, R. Shaping the Future of AI in Healthcare through Ethics and Governance. Humanit. Soc. Sci. Commun. 2024, 11, 416. [Google Scholar] [CrossRef]
- Hassan, M.; Kushniruk, A.; Borycki, E. Barriers to and Facilitators of Artificial Intelligence Adoption in Health Care: Scoping Review. JMIR Hum. Factors 2024, 11, e48633. [Google Scholar] [CrossRef]
- Wong, A.; Berenbrok, L.A.; Snader, L.; Soh, Y.H.; Kumar, V.K.; Javed, M.A.; Bates, D.W.; Sorce, L.R.; Kane-Gill, S.L. Facilitators and Barriers to Interacting With Clinical Decision Support in the ICU: A Mixed-Methods Approach. Crit. Care Explor. 2023, 5, e0967. [Google Scholar] [CrossRef]
- Rotaru, N.; Edelhauser, E. Digital Transformation: A Challenge for the Romanian Health System. Systems 2024, 12, 366. [Google Scholar] [CrossRef]
- Marino, C.A.; Diaz Paz, C. Enhancing Interoperability for a Sustainable, Patient-Centric Health Care Value Chain: Systematic Review for Taxonomy Development. J. Med. Internet Res. 2025, 27, e69465. [Google Scholar] [CrossRef] [PubMed]
- Nastasa, I.V.; Furtunescu, F.-L.; Mincă, D.G. Challenges and Progress in General Data Protection Regulation Implementation in Romanian Public Healthcare. Cureus 2025, 17, e78008. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.C.L.; Chen, M.H.; Ng, N.; Elangovan, K.; Tan, N.Y.T.; Jin, L.; Xie, Q.; Ting, D.S.W.; Rodriguez-Monguio, R.; Bates, D.W.; et al. A Scoping Review on Generative AI and Large Language Models in Mitigating Medication Related Harm. npj Digit. Med. 2025, 8, 182. [Google Scholar] [CrossRef]
- Patel, D.; Raut, G.; Cheetirala, S.N.; Glicksberg, B.; Levin, M.A.; Nadkarni, G.; Freeman, R.; Klang, E.; Timsina, P. AI Agents in Modern Healthcare: From Foundation to Pioneer—A Comprehensive Review and Implementation Roadmap for Impact and Integration in Clinical Settings. Med. Pharmacol. 2025. [Google Scholar] [CrossRef]
- Agafonov, O.; Babic, A.; Sousa, S.; Alagaratnam, S. Editorial: Trustworthy AI for Healthcare. Front. Digit. Health 2024, 6, 1427233. [Google Scholar] [CrossRef] [PubMed]
- Steerling, E.; Siira, E.; Nilsen, P.; Svedberg, P.; Nygren, J. Implementing AI in Healthcare—The Relevance of Trust: A Scoping Review. Front. Health Serv. 2023, 3, 1211150. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A.; Hakim Azizul, Z.; Zakariah, M.; Brahim Belhaouari, S.; Altameem, A.; Ramli, R.; Almazyad, A.S.; Mat Kiah, M.L.; Azzuhri, S.R. Implementing Federated Learning over VPN-Based Wireless Backhaul Networks for Healthcare Systems. PeerJ Comput. Sci. 2024, 10, e2422. [Google Scholar] [CrossRef] [PubMed]
| Platform | Geographic Origin | User Engagement Metrics | Clinical Efficacy Measures |
|---|---|---|---|
| MoodGYM | Australia/Europe | Over 1 million users, high dropout | Modest reduction in depression/anxiety (randomized controlled trials) |
| Woebot | USA | 12.14 average sessions (randomized controlled trial), high engagement | Small-moderate reduction in depression/anxiety (Patient Health Questionnaire-9, Generalized Anxiety Disorder-7) |
| Wysa | India/global | 10.9 average sessions (healthcare workers), 67.7% positive feedback | Modest reduction in depression/anxiety (Patient Health Questionnaire-9, Patient Health Questionnaire-4 |
| COVID-19 pediatric chatbot | Canada | High parental satisfaction, rapid adoption | 60–70% reduction in triage time |
| COVID-19 chatbot | India | Millions of users, rapid scale-up | Over 90% specificity in triage |
| Characteristic | MoodGYM | Woebot | Wysa | Chabot COVID-19 Pediatrician | Chatbot COVID-19 India |
|---|---|---|---|---|---|
| Has chatbot | No | Yes | Yes | Yes | Yes |
| Type of Digital Intervention | Self-guided modular app | Text-based CBT chatbot | Chatbot + journaling + exercises | Hospital-integrated chatbot | National self-assessment chatbot |
| Objective/Domain | Mental health, depression prevention | Anxiety, depression | Psychological support for employees | COVID-19 screening | COVID-19 screening and triage |
| Key Benefits | Accessible, scalable, low cost | Empathetic, 24/7, low cost | Anonymous, CBT technique combination | 60–70% triage time reduction, lower risk | Millions of users, >90% specificity |
| Major Limitations | No personalization, low adherence | Small/moderate effects, limited adaptation | Pilot, modest effects, limited validation | Narrow domain, requires IT integration | Continuous updates, early localization issues |
| AI/Chatbot | No | Basic conversational AI | Moderate conversational AI | Advanced conversational AI | Advanced conversational AI |
| Reported Clinical Outcomes | Variable, sometimes small-moderate effects | Significant but small symptom reductions | Reduced anxiety/depression in pilot groups | Reduced triage time, high satisfaction | Reduced overcrowding, efficient triage |
| Target Audience | Adolescents, adults (LMIC) | Adults | Corporate employees | Parents, children | General population (India) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zota, R.D.; Cîmpeanu, I.A.; Lungu, M.A. Exploring AI in Healthcare Systems: A Study of Medical Applications and a Proposal for a Smart Clinical Assistant. Electronics 2025, 14, 3727. https://doi.org/10.3390/electronics14183727
Zota RD, Cîmpeanu IA, Lungu MA. Exploring AI in Healthcare Systems: A Study of Medical Applications and a Proposal for a Smart Clinical Assistant. Electronics. 2025; 14(18):3727. https://doi.org/10.3390/electronics14183727
Chicago/Turabian StyleZota, Răzvan Daniel, Ionuț Alexandru Cîmpeanu, and Mihai Adrian Lungu. 2025. "Exploring AI in Healthcare Systems: A Study of Medical Applications and a Proposal for a Smart Clinical Assistant" Electronics 14, no. 18: 3727. https://doi.org/10.3390/electronics14183727
APA StyleZota, R. D., Cîmpeanu, I. A., & Lungu, M. A. (2025). Exploring AI in Healthcare Systems: A Study of Medical Applications and a Proposal for a Smart Clinical Assistant. Electronics, 14(18), 3727. https://doi.org/10.3390/electronics14183727






