Cu2O Nanowire Chemiresistors for Detection of Organophosphorus CWA Simulants
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and Sensor Fabrication
2.2. Characterization
2.3. Gas Exposure Setup
2.4. Simulants’ Similarity to CWAs
2.5. Data Analysis
3. Results and Discussion
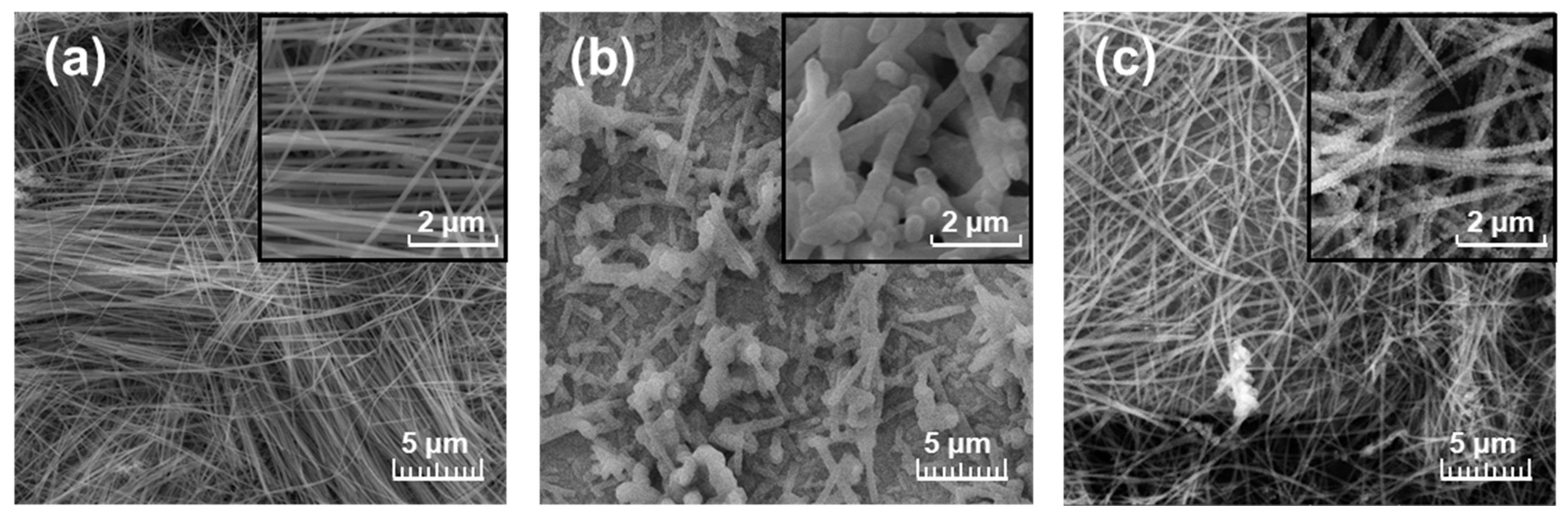
3.1. Scanning Electron Microscopy
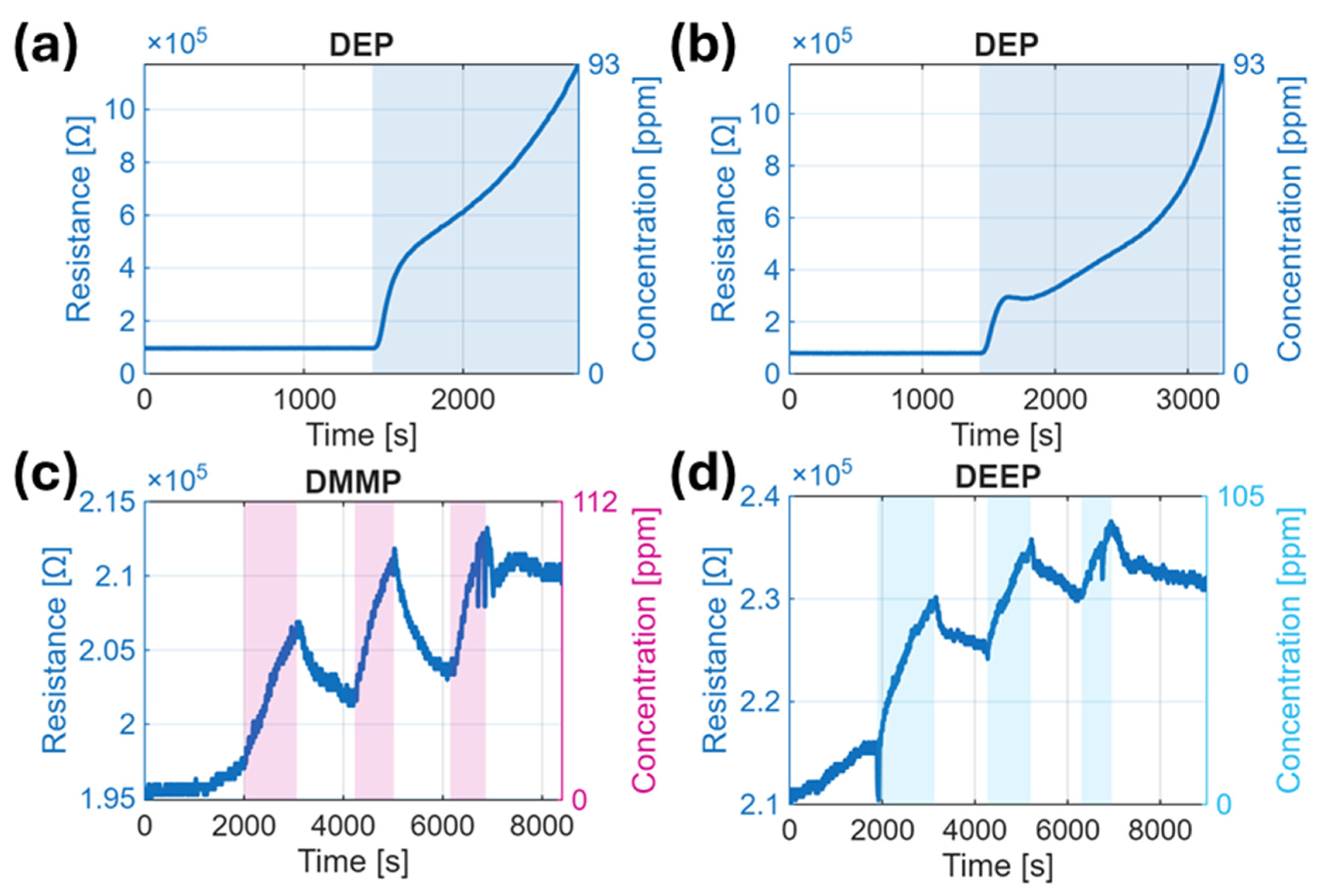
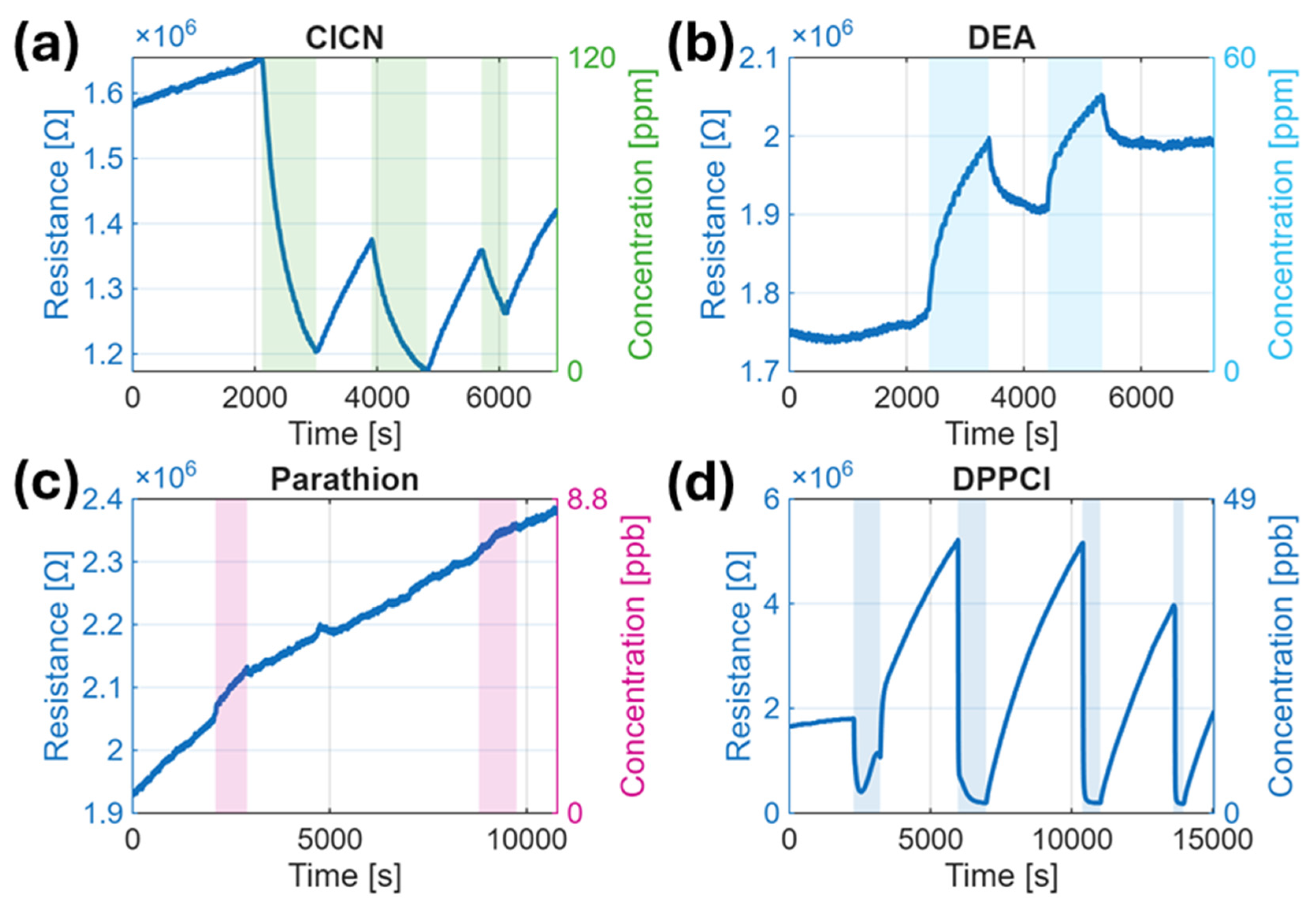
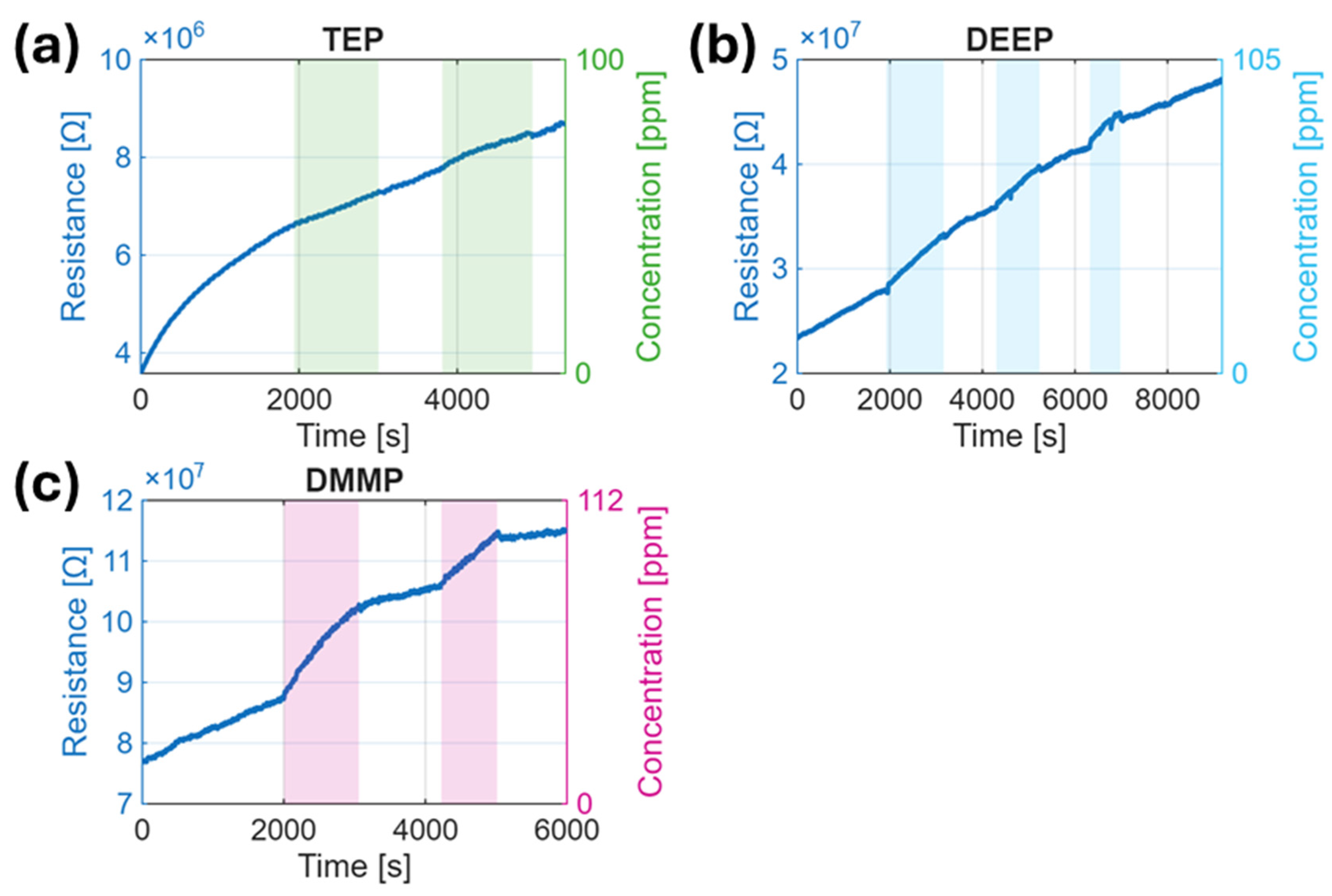
3.2. Sensor Response Overview
3.3. Response Kinetics and Reproducibility
3.4. Surface Analysis and Sensor Stoichiometry Effects
3.5. Selectivity Considerations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CWA | Chemical Warfare Agent |
| Cu2O | Copper(I) oxide |
| CuO | Copper(II) oxide |
| DMMP | Dimethyl Methylphosphonate |
| DEP | Diethyl Phosphite |
| DPPCl | Diphenyl Phosphoryl Chloride |
| DEEP | Diethyl Ethylphosphonate |
| TEP | Triethyl Phosphate |
| DEA | Diethyl Adipate |
| ED | Euclidean Distance |
| GC-MS | Gas Chromatography–Mass Spectrometry |
| HD | Distilled Mustard |
| ClCN | Cyanogen Chloride |
| MOS | Metal Oxide Semiconductor |
| SEM | Scanning Electron Microscopy |
| EDS | Energy-Dispersive X-ray Spectroscopy |
| XPS | X-ray Photoelectron Spectroscopy |
| NAP-XPS | Near-Ambient Pressure XPS |
| DFT | Density Functional Theory |
| RR | Relative Response |
| Pt | Platinum |
| TC | Tanimoto Coefficient |
References
- Witkiewicz, Z.; Jasek, K.; Grabka, M. Semiconductor gas sensors for detecting chemical warfare agents and their simulants. Sensors 2023, 23, 3272. [Google Scholar] [CrossRef]
- Diauudin, F.N.; Rashid, J.I.A.; Knight, V.F.; Yunus, W.M.Z.W.; Ong, K.K.; Kasim, N.A.M.; Halim, N.A.; Noor, S.A.M. A review of current advances in the detection of organophosphorus chemical warfare agents based biosensor approaches. Sens. BioSens. Res. 2019, 26, 100305. [Google Scholar] [CrossRef]
- Sferopoulos, R. A Review of Chemical Warfare Agent (CWA) Detector Technologies and Commercial-Off-The-Shelf Items. 2009. Available online: https://apps.dtic.mil/sti/tr/pdf/ADA502856.pdf (accessed on 29 July 2025).
- White, S.; Miller, P.; Sorge, M.; Curtis, C.; Hare, A.; Whiting, J.; Sammon, J.; Corbin, W.C. Colorimetric/Electrical Sensing of Chemical Warfare Agent Surrogates with Polydiacetylenes. In Proceedings of the 2024 IEEE SENSORS, Kobe, Japan, 20–23 October 2024. [Google Scholar] [CrossRef]
- Kanu, A.B.; Haigh, P.E.; Hill, H.H. Surface detection of chemical warfare agent simulants and degradation products. Anal. Chim. Acta 2005, 553, 148–159. [Google Scholar] [CrossRef]
- Barreca, D.; Maccato, C.; Gasparotto, A. Metal Oxide Nanosystems As Chemoresistive Gas Sensors for Chemical Warfare Agents: A Focused Review. Adv. Mater. Interfaces 2022, 9, 2102525. [Google Scholar] [CrossRef]
- Head, A.R.; Tsyshevsky, R.; Trotochaud, L.; Yu, Y.; Karslıoǧlu, O.; Eichhorn, B.; Kuklja, M.M.; Bluhm, H. Dimethyl methylphosphonate adsorption and decomposition on MoO2 as studied by ambient pressure x-ray photoelectron spectroscopy and DFT calculations. J. Phys. Condens. Matter 2018, 30, 134005. [Google Scholar] [CrossRef]
- Head, A.R.; Tsyshevsky, R.V.; Trotochaud, L.; Yu, Y.; Kyhl, L.; Karslıoǧlu, O.; Kuklja, M.M.; Bluhm, H. Adsorption of Dimethyl Methylphosphonate on MoO3: The Role of Oxygen Vacancies. J. Phys. Chem. C 2016, 120, 29077–29088. [Google Scholar] [CrossRef]
- Trotochaud, L.; Head, A.R.; Büchner, C.; Yu, Y.; Karslıoğlu, O.; Tsyshevsky, R.; Holdren, S.; Eichhorn, B.; Kuklja, M.M.; Bluhm, H. Room temperature decomposition of dimethyl methylphosphonate on cuprous oxide yields atomic phosphorus. Surf. Sci. 2019, 680, 75–87. [Google Scholar] [CrossRef]
- Ponzoni, A.; Baratto, C.; Bianchi, S.; Comini, E.; Ferroni, M.; Pardo, M.; Vezzoli, M.; Vomiero, A.; Faglia, G.; Sberveglieri, G. Metal Oxide Nanowire and Thin-Film-Based Gas Sensors for Chemical Warfare Simulants Detection. IEEE Sens. J. 2008, 8, 735–742. [Google Scholar] [CrossRef]
- Fumian, F.; Chierici, A.; Bianchelli, M.; Martellucci, L.; Rossi, R.; Malizia, A.; Gaudio, P.; D’eRrico, F.; Di Giovanni, D. Development and performance testing of a miniaturized multi-sensor system combining MOX and PID for potential UAV application in TIC, VOC and CWA dispersion scenarios. Eur. Phys. J. Plus 2021, 136, 913. [Google Scholar] [CrossRef]
- Panigrahi, P.; Pal, Y.; Kaewmaraya, T.; Bae, H.; Nasiri, N.; Hussain, T. Molybdenum Carbide MXenes as Efficient Nanosensors toward Selected Chemical Warfare Agents. ACS Appl. Nano Mater. 2023, 6, 8404–8415. [Google Scholar] [CrossRef]
- Devi, K.S.S.; Anantharamakrishnan, A.; Krishnan, U.M. Expanding Horizons of Metal Oxide-based Chemical and Electrochemical Sensors. Electroanalysis 2021, 33, 1979–1996. [Google Scholar] [CrossRef]
- Xu, M.; Hu, X.; Zhang, H.; Miao, T.; Ma, L.; Liang, J.; Zhu, Y.; Zhu, H.; Cheng, Z.; Sun, X. Detection and Pattern Recognition of Chemical Warfare Agents by MOS-Based MEMS Gas Sensor Array. Sensors 2025, 25, 2633. [Google Scholar] [CrossRef]
- Xu, M.; Zhong, Y.; Hu, X.; Tao, Y.; Shen, Q.; Zhang, S.; Zhang, P.; Zhang, H.; Cheng, Z.; Sun, X. Detection of CWA Simulants by Electronic Nose Based on Low-Powered MEMS Gas Sensor Array. IEEE Sens. J. 2025, 25, 30361–30369. [Google Scholar] [CrossRef]
- Dhull, V. Fabrication of AChE/SnO2-cMWCNTs/Cu Nanocomposite-Based Sensor Electrode for Detection of Methyl Parathion in Water. Int. J. Anal. Chem. 2018, 2018, 2874059. [Google Scholar] [CrossRef] [PubMed]
- Sakdarat, P.; Chongsuebsirikul, J.; Thanachayanont, C.; Prichanont, S.; Pungetmongkol, P.; Azzazy, H. Development of a Nonenzymatic Electrochemical Sensor for Organophosphate Pesticide Detection Using Copper (II) Oxide Nanorod Electrodes. J. Nanomater. 2021, 2021, 1–11. [Google Scholar] [CrossRef]
- Sen, S.; Roy, A.; Sanyal, A.; Devi, P.S. A Nonenzymatic Reduced Graphene Oxide-Based Nanosensor for Parathion. Beilstein J. Nanotechnol. 2022, 13, 730–744. [Google Scholar] [CrossRef]
- Bao, J.; Hou, C.; Chen, M.; Li, J.; Huo, D.; Yang, M.; Luo, X.; Lei, Y. Plant Esterase–Chitosan/Gold Nanoparticles–Graphene Nanosheet Composite-Based Biosensor for the Ultrasensitive Detection of Organophosphate Pesticides. J. Agric. Food Chem. 2015, 63, 10319–10326. [Google Scholar] [CrossRef]
- Bhusari, R.; Thomann, J.-S.; Guillot, J.; Leturcq, R. Oxygen Adsorption and Desorption Kinetics in CuO Nanowire Bundle Networks: Implications for MOx-Based Gas Sensors. ACS Appl. Nano Mater. 2022, 5, 10248–10257. [Google Scholar] [CrossRef]
- Qin, Y.; Zhang, F.; Chen, Y.; Zhou, Y.; Li, J.; Zhu, A.; Luo, Y.; Tian, Y.; Yang, J. Hierarchically Porous CuO Hollow Spheres Fabricated via a One-Pot Template-Free Method for High-Performance Gas Sensors. J. Phys. Chem. C 2012, 116, 11994–12000. [Google Scholar] [CrossRef]
- Yang, B.; Myung, N.V.; Tran, T. 1D Metal Oxide Semiconductor Materials for Chemiresistive Gas Sensors: A Review. Adv. Electron. Mater. 2021, 7, 2100271. [Google Scholar] [CrossRef]
- Barsan, N.; Weimar, U. Conduction Model of Metal Oxide Gas Sensors. J. ElectroCeram. 2001, 7, 143–167. [Google Scholar] [CrossRef]
- Comini, E.; Faglia, G.; Ferroni, M.; Ponzoni, A.; Vomiero, A.; Sberveglieri, G. Metal oxide nanowires: Preparation and application in gas sensing. J. Mol. Catal. A Chem. 2009, 305, 170–177. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Cho, B. Instability of metal oxide-based conductometric gas sensors and approaches to stability improvement (short survey). Sens. Actuators B Chem. 2011, 156, 527–538. [Google Scholar] [CrossRef]
- Filipič, G.; Cvelbar, U. Copper oxide nanowires: A review of growth. Nanotechnology 2012, 23, 194001. [Google Scholar] [CrossRef]
- Zhou, K.; Wang, R.; Xu, B.; Li, Y. Synthesis, characterization and catalytic properties of CuO nanocrystals with various shapes. Nanotechnology 2006, 17, 3939–3943. [Google Scholar] [CrossRef]
- Izaki, M.; Abe, S.; Nakakita, K.; Khoo, P.L. Photoelctrochemically Fabricated and Heated Cu2O/CuO Bilayers with Enhanced Photovoltaic Characteristics. ACS Omega 2021, 6, 27587–27597. [Google Scholar] [CrossRef]
- Hozák, P.; Vorokhta, M.; Khalakhan, I.; Jarkovská, K.; Cibulková, J.; Fitl, P.; Vlček, J.; Fara, J.; Tomeček, D.; Novotný, M.; et al. New Insight into the Gas-Sensing Properties of CuOx Nanowires by Near-Ambient Pressure XPS. J. Phys. Chem. C 2019, 123, 29739–29749. [Google Scholar] [CrossRef]
- Trotochaud, L.; Tsyshevsky, R.; Holdren, S.; Fears, K.; Head, A.R.; Yu, Y.; Karslıoğlu, O.; Pletincx, S.; Eichhorn, B.; Owrutsky, J.; et al. Spectroscopic and Computational Investigation of Room-Temperature Decomposition of a Chemical Warfare Agent Simulant on Polycrystalline Cupric Oxide. Chem. Mater. 2017, 29, 7483–7496. [Google Scholar] [CrossRef]
- Schlur, L.; Agostini, P.; Thomas, G.; Gerer, G.; Grau, J.; Spitzer, D. Detection of Organophosphorous Chemical Agents with CuO-Nanorod-Modified Microcantilevers. Sensors 2020, 20, 1061. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, J.; Srinivasan, S.; Nagarajan, R. Using cheminformatics to find simulants for chemical warfare agents. J. Hazard. Mater. 2011, 194, 85–91. [Google Scholar] [CrossRef]
- Lupan, O.; Ababii, N.; Mishra, A.K.; Gronenberg, O.; Vahl, A.; Schürmann, U.; Duppel, V.; Krüger, H.; Chow, L.; Kienle, L.; et al. Single CuO/Cu2O/Cu Microwire Covered by a Nanowire Network as a Gas Sensor for the Detection of Battery Hazards. ACS Appl. Mater. Interfaces 2020, 12, 42248–42263. [Google Scholar] [CrossRef] [PubMed]
- Maier, C.; Egger, L.; Köck, A.; Becker, S.; Niehaus, J.S.; Reichmann, K. Investigation of Gas Sensing Performance of CuO/Cu2O Thin Films as a Function of Au-NP Size for CO, CO2, and Hydrocarbons Mixtures. Nanomaterials 2025, 15, 705. [Google Scholar] [CrossRef] [PubMed]
- Hruška, M.; Kejzlar, J.; Otta, J.; Fitl, P.; Novotný, M.; Čížek, J.; Melikhova, O.; Mičušík, M.; Machata, P.; Vrňata, M. Hydrogen Sensing Capabilities of Highly Nanoporous Black Gold Films. Appl. Surf. Sci. 2023, 647, 158618. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, A.; Das, M.; Kim, S.S.; Kim, H.W.; Kumar, M. Ag catalysts boosted NO2 gas sensing performance of RF sputtered α-Fe2O3 films. Sens. Actuators B Chem. 2023, 393, 134307. [Google Scholar] [CrossRef]
- Kumar, S.; Betal, A.; Kumar, A.; Chakkar, A.G.; Kumar, P.; Kwoka, M.; Sahu, S.; Kumar, M. Enhancing NO2 Gas Sensing: The Dual Impact of UV and Thermal Activation on Vertically Aligned Nb-MoS2 for Superior Response and Selectivity. ACS Sens. 2025, 10, 2191–2202. [Google Scholar] [CrossRef]
- Sharma, A.K.; Mahajan, A. Chapter 8-Potential applications of chemiresistive gas sensors. In Carbon Nanomaterials and Their Nanocomposite-Based Chemiresistive Gas Sensors; Dhall, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 223–245. [Google Scholar] [CrossRef]
- Torrisi, A.; Vacík, J.; Ceccio, G.; Cannavò, A.; Lavrentiev, V.; Horák, P.; Yatskiv, R.; Vaniš, J.; Grym, J.; Fišer, L.; et al. Chemiresistors based on Li-doped CuO–TiO2 films. Chemosensors 2021, 9, 246. [Google Scholar] [CrossRef]
- Yoo, R.; Cho, S.; Song, M.-J.; Lee, W. Highly sensitive gas sensor based on Al-doped ZnO nanoparticles for detection of dimethyl methylphosphonate as a chemical warfare agent simulant. Sens. Actuators B Chem. 2015, 221, 217–223. [Google Scholar] [CrossRef]
- Holdren, S.; Tsyshevsky, R.; Fears, K.; Owrutsky, J.; Wu, T.; Wang, X.; Eichhorn, B.W.; Kuklja, M.M.; Zachariah, M.R. Adsorption and Destruction of the G-Series Nerve Agent Simulant Dimethyl Methylphosphonate on Zinc Oxide. ACS Catal. 2018, 9, 902–911. [Google Scholar] [CrossRef]
- Yoo, R.; Yoo, S.; Lee, D.; Kim, J.; Cho, S.; Lee, W. Highly selective detection of dimethyl methylphosphonate (DMMP) using CuO nanoparticles/ZnO flowers heterojunction. Sens. Actuators B Chem. 2017, 240, 1099–1105. [Google Scholar] [CrossRef]
- Wang, X.; Li, B.; Xia, Z.; Zhou, W.; Wu, Y.; Zhu, Z.; Zhu, G. Effects of copper(II) oxide on the co-pyrolysis of waste polyester enameled wires and poly(vinyl chloride). Polymers 2023, 16, 27. [Google Scholar] [CrossRef] [PubMed]
- Bassett, H.; Taylor, H.S. CLVIII.—The interaction of metallic oxides and phosphoryl chloride, alone and in the presence of certain organic compounds. J. Chem. Soc. Trans. 1911, 99, 1402–1414. [Google Scholar] [CrossRef]
- Claros, M.; Gràcia, I.; Figueras, E.; Vallejos, S. Hydrothermal synthesis and annealing effect on the properties of gas-sensitive copper oxide nanowires. Chemosensors 2022, 10, 353. [Google Scholar] [CrossRef]
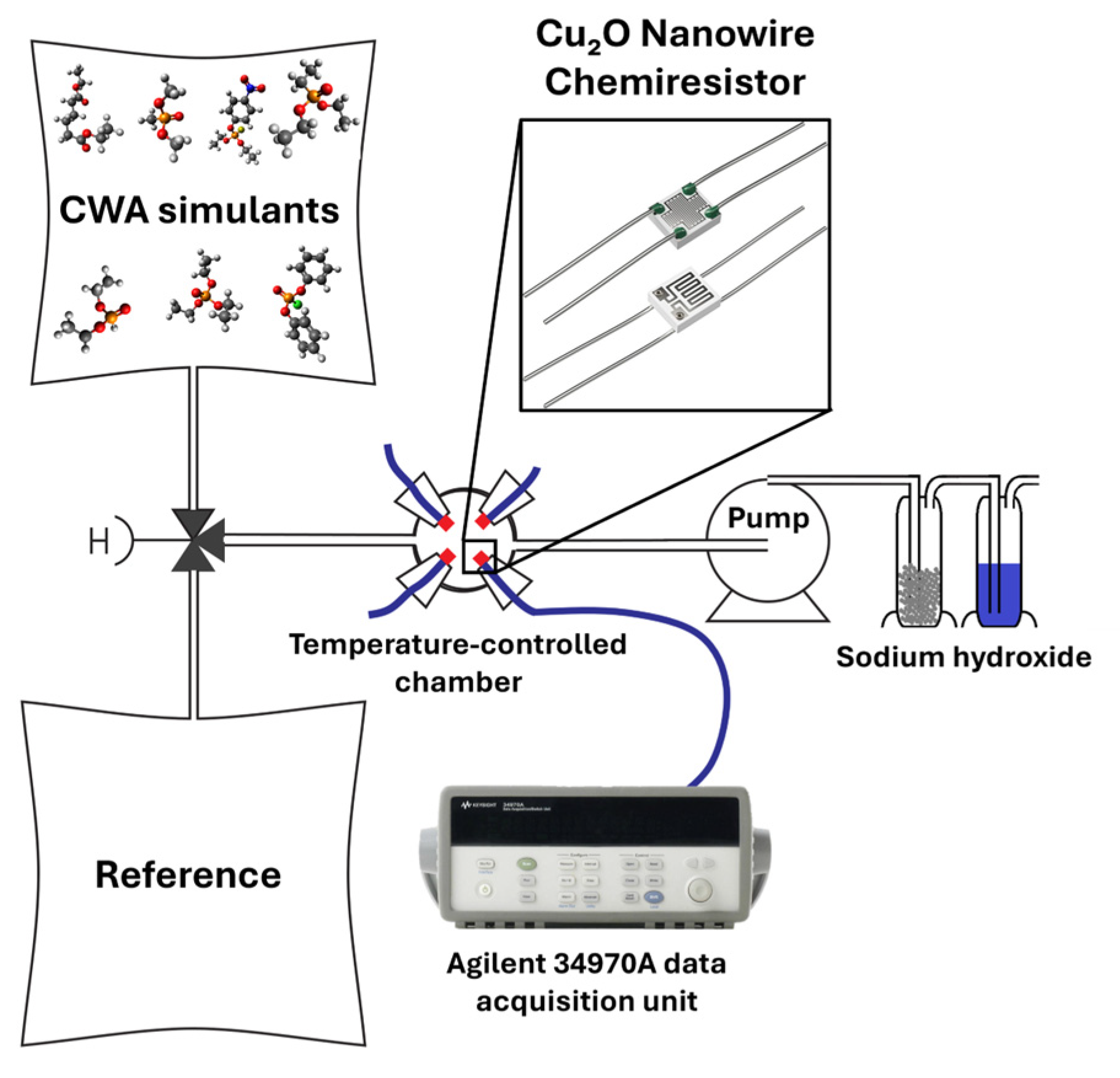
| Simulant | Saturated Vapor Pressure [Pa] | Prepared Concentration [ppm] | Distributor | Purity [%] |
|---|---|---|---|---|
| ClCN | 1,987,000 | 120 | Draslovka a.s., Kolín, Czech Republic | ≤100.0 |
| DEA | 7.70 | 60 | Sigma-Aldrich®, St. Louis, MI, USA | ≥98.0 |
| DEEP | 41.2 | 105 | Sigma-Aldrich®, St. Louis, MI, USA | ≥98.0 |
| DEP | 1490 | 93 | Sigma-Aldrich®, St. Louis, MI, USA | ≥98.0 |
| DMMP | 128 | 112 | Sigma-Aldrich®, St. Louis, MI, USA | ≥98.0 |
| DPPCl | 0.0049 | 0.049 | Sigma-Aldrich®, St. Louis, MI, USA | ≥99.0 |
| Parathion | 0.00089 | 0.0089 | Supelco® Bellefonte, PA, USA | ≥98.0 |
| TEP | 51.8 | 100 | Sigma-Aldrich®, St. Louis, MI, USA | ≥99.8 |
| Simulant | CAS No. | ED (Sarin) | ED (Soman) | ED (Tabun) | ED (VX) | ED (HD) | TC (Sarin) | TC (Soman) | TC (Tabun) | TC (VX) | TC (HD) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| DMMP | 756-79-6 | 0.193 | 0.303 | 0.265 | – | – | 0.667 | 0.640 | 0.432 | – | – |
| DEEP | 78-38-6 | 0.242 | 0.239 | 0.245 | – | – | 0.429 | 0.417 | 0.537 | – | – |
| TEP | 78-40-0 | 0.308 | 0.302 | 0.286 | – | – | 0.455 | 0.441 | 0.525 | – | – |
| DEP | 762-04-9 | 0.230 | 0.275 | 0.238 | – | – | 0.382 | 0.371 | 0.463 | – | – |
| DPPCl | 2524-64-3 | 0.541 | 0.541 | 0.511 | – | – | 0.500 | 0.485 | 0.245 | – | – |
| Parathion | 56-38-2 | – | – | – | 0.395 | – | – | – | – | 0.509 | – |
| DEA | 141-28-6 | – | – | – | – | 0.560 | – | – | – | – | 0.310 |
| ClCN | 506-77-4 | – | – | – | – | – | – | – | – | – | – |
| Analyte | C (at. %) | O (at. %) | P (at. %) | Cl (at. %) | Cu (at. %) |
|---|---|---|---|---|---|
| Before | 33.48 ± 1.28 | 20.48 ± 0.97 | – | – | 46.04 ± 2.18 |
| DPPCl | 18.65 ± 0.80 | 2.40 ± 0.17 | – | 40.79 ± 1.07 | 37.32 ± 1.64 |
| DEP | 7.49 ± 0.80 | 53.86 ± 3.26 | 12.31 ± 0.42 | – | 26.34 ± 1.46 |
| Simulant | ClCN | DEA | DEP | DEEP | DMMP | TEP * | DEEP * | DMMP * | DPPCl | Parathion | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| RR (%) | −9.46 | 9.94 | 203.75 | 4.58 | 3.39 | 0.20 | 4.23 | 5.76 | −94.67 | 1.82 | |
| Response | τ(s) | 484 | 510 | - | 1063 | 1120 | - | 1095 | 1139 | 94 | 196 |
| τ10%(s) | 51 | 54 | - | 112 | 118 | - | 115 | 120 | 10 | 196 | |
| τ90%(s) | 1115 | 1175 | - | 2447 | 118 | - | 2521 | 2623 | 10 | 21 | |
| Recovery | τ(s) | 1944 | 157 | - | 780 | 606 | - | 1838 | 1510 | 3372 | 1984 |
| τ10%(s) | 205 | 17 | - | 82 | 64 | - | 194 | 159 | 355 | 209 | |
| τ90%(s) | 4477 | 362 | - | 1795 | 1396 | - | 4233 | 3476 | 7763 | 4569 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otta, J.; Mišek, J.; Fišer, L.; Kejzlar, J.; Hruška, M.; Kukal, J.; Vrňata, M. Cu2O Nanowire Chemiresistors for Detection of Organophosphorus CWA Simulants. Electronics 2025, 14, 3478. https://doi.org/10.3390/electronics14173478
Otta J, Mišek J, Fišer L, Kejzlar J, Hruška M, Kukal J, Vrňata M. Cu2O Nanowire Chemiresistors for Detection of Organophosphorus CWA Simulants. Electronics. 2025; 14(17):3478. https://doi.org/10.3390/electronics14173478
Chicago/Turabian StyleOtta, Jaroslav, Jan Mišek, Ladislav Fišer, Jan Kejzlar, Martin Hruška, Jaromír Kukal, and Martin Vrňata. 2025. "Cu2O Nanowire Chemiresistors for Detection of Organophosphorus CWA Simulants" Electronics 14, no. 17: 3478. https://doi.org/10.3390/electronics14173478
APA StyleOtta, J., Mišek, J., Fišer, L., Kejzlar, J., Hruška, M., Kukal, J., & Vrňata, M. (2025). Cu2O Nanowire Chemiresistors for Detection of Organophosphorus CWA Simulants. Electronics, 14(17), 3478. https://doi.org/10.3390/electronics14173478







