Trial of Brain–Computer Interface for Continuous Motion Using Electroencephalography and Electromyography
Abstract
1. Introduction
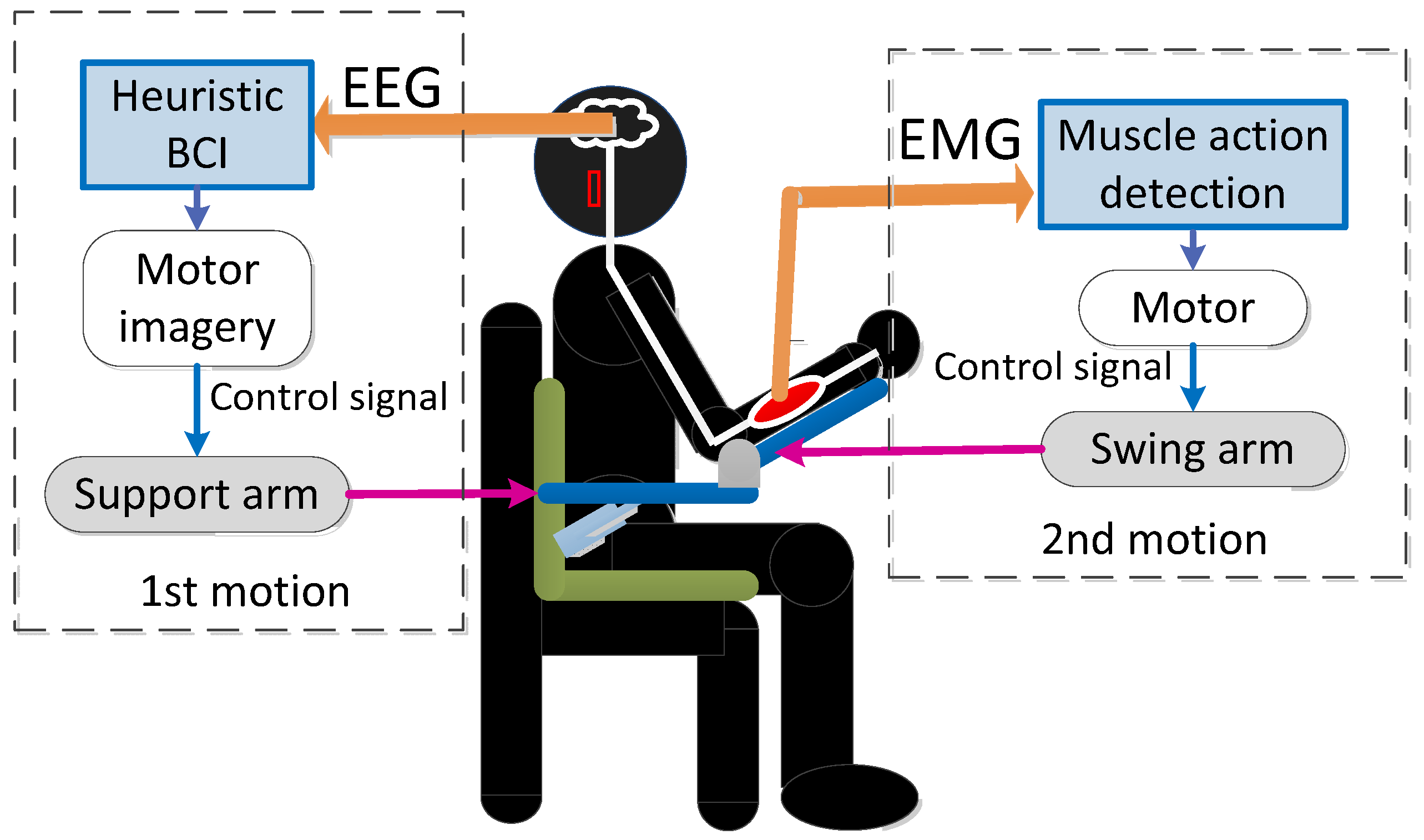
- Heuristic BCIs can generate a motor intention detection algorithm in a short time by binarizing the measured CH EEG power to LOW or HIGH and generating patterns without seeking the optimal conditions for detection, such as machine learning.
- The algorithm is based on the assumption that the target movement is skill training and is realized by using EEG and EMG potentials to handle continuous movements rather than monotonous movements of flexion and extension.
- The proposed system has the potential to be widely used because it simplifies the settings necessary for the system to work as much as possible, enabling users to perform advanced medical treatment at home by themselves, while reducing costs.
2. Materials and Methods
2.1. Shoulder-Joint Motion Intention Detection Using a Heuristic BCI
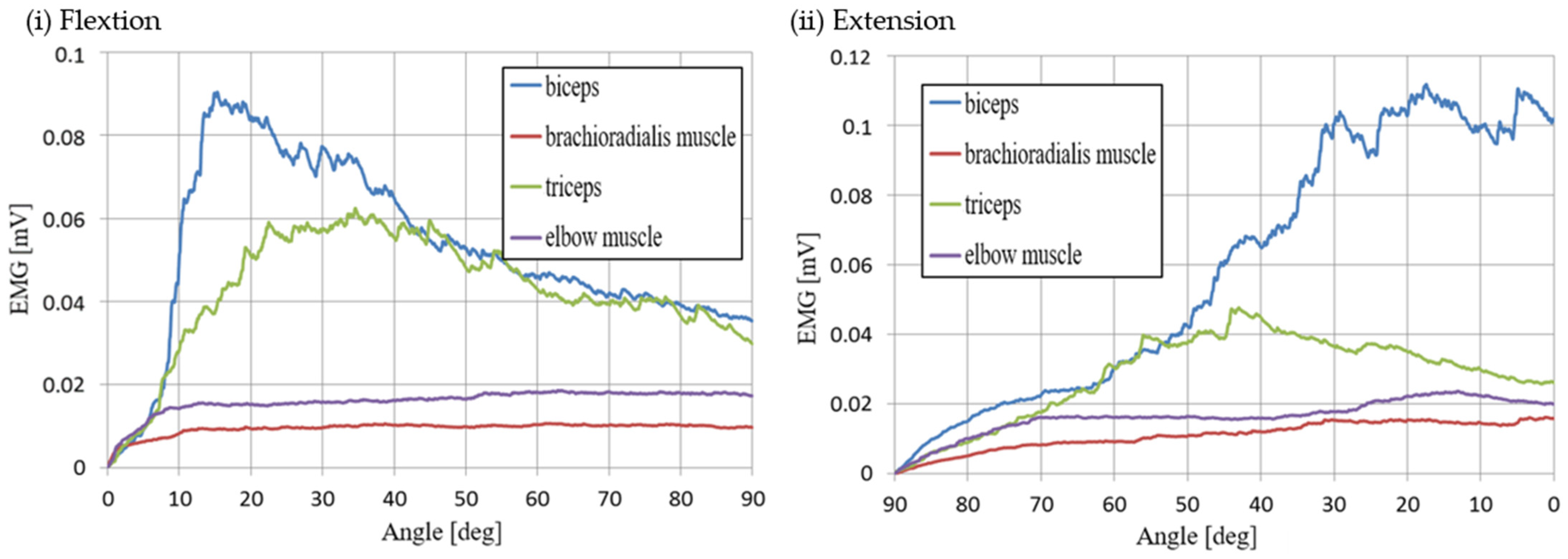
2.2. Elbow-Joint Motion Intention Detection by Muscle Activity Detection Section
3. Experiments
3.1. Participants
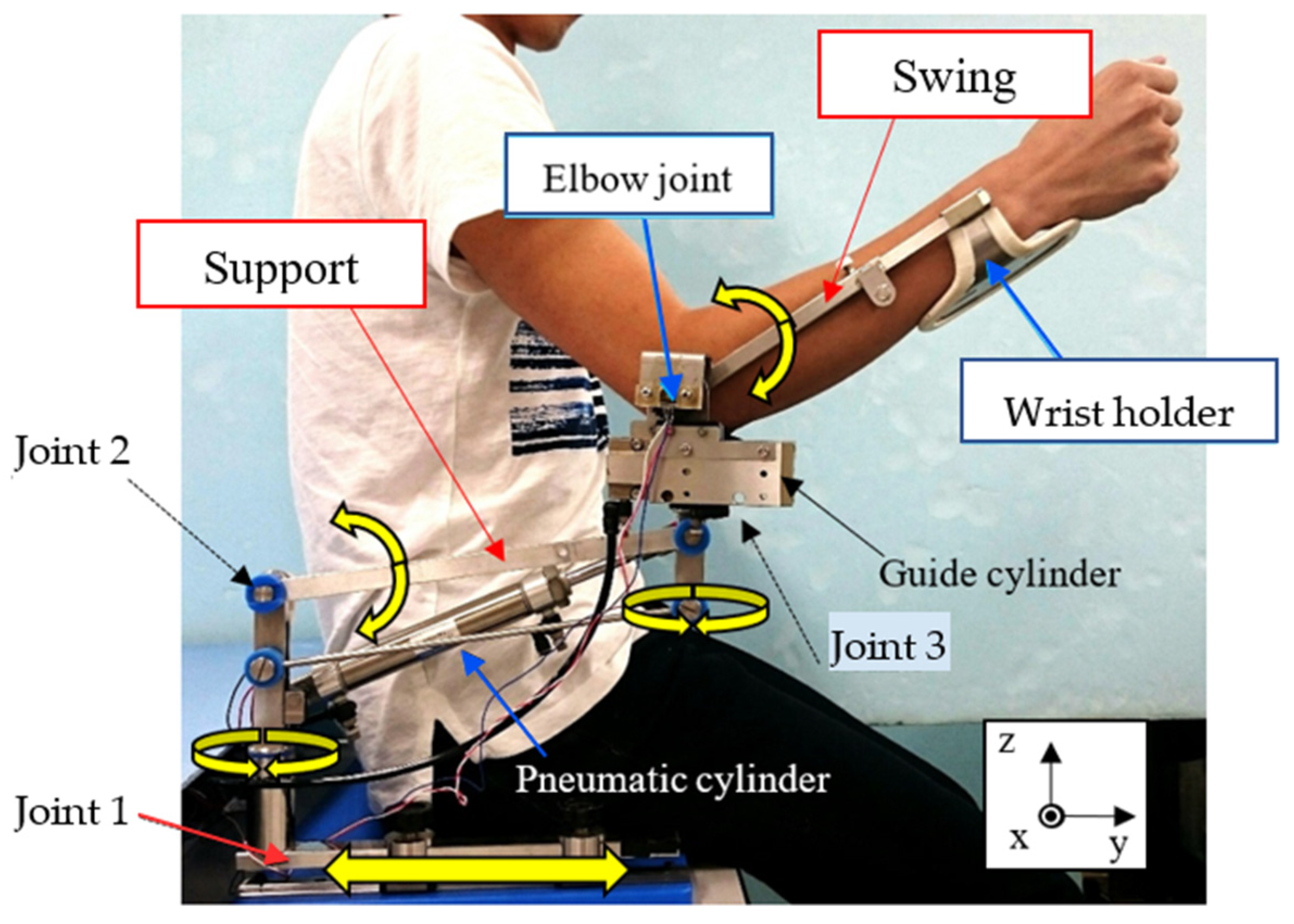
3.2. Overview of the Upper Limb Support Devices
3.3. Setup for Motor Intent Detection by the EEG
3.4. Setup for Motor Intent Detection by the EMG
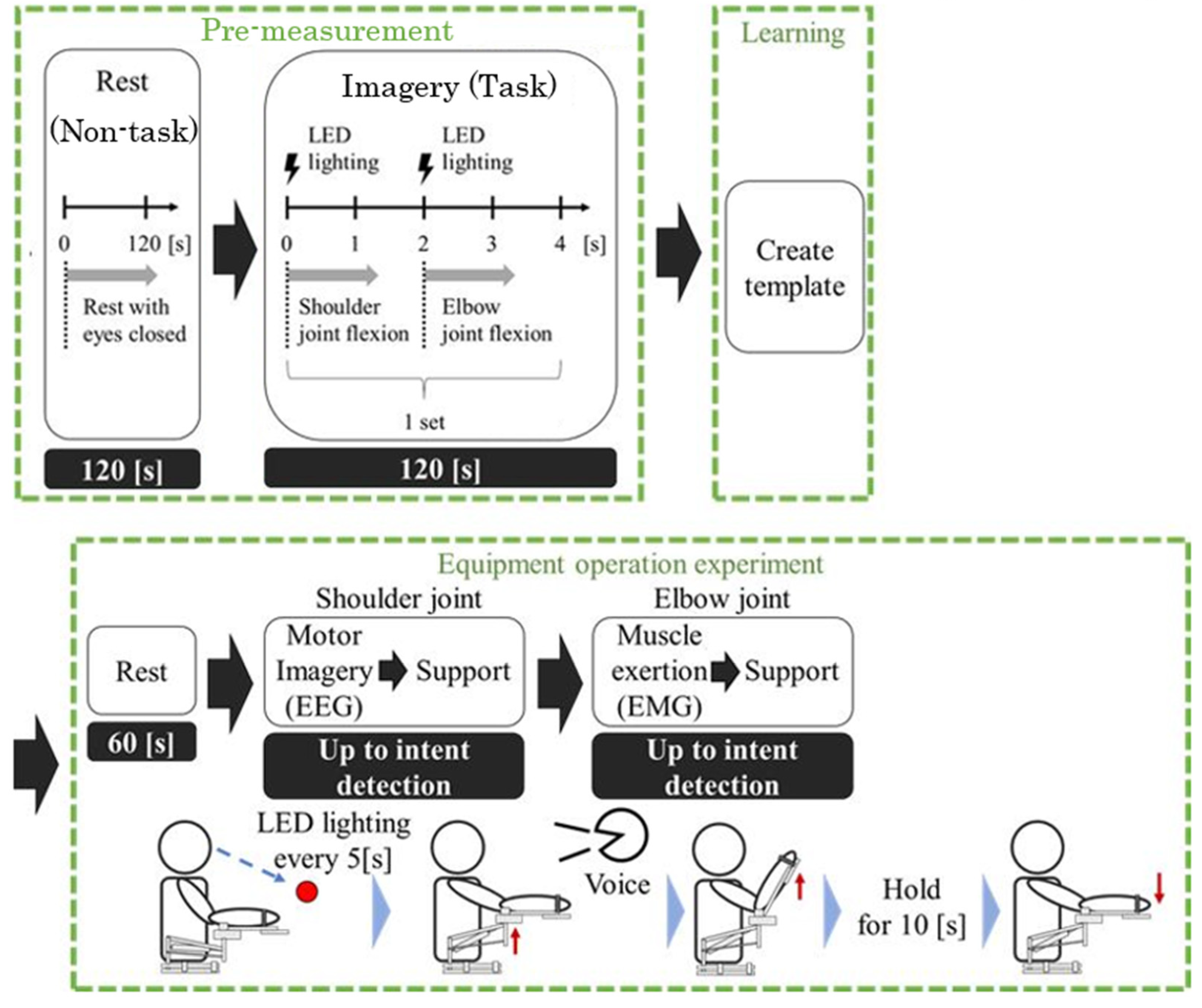
3.5. Experimental Overview of the Hybrid BCI System
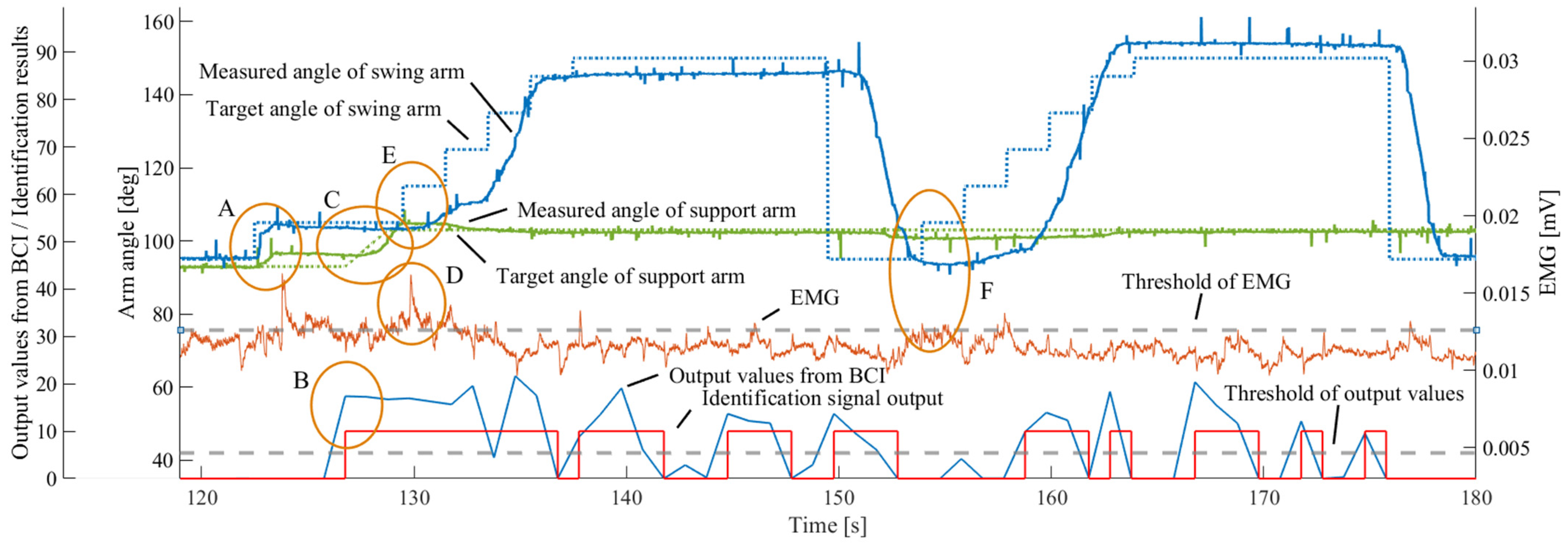
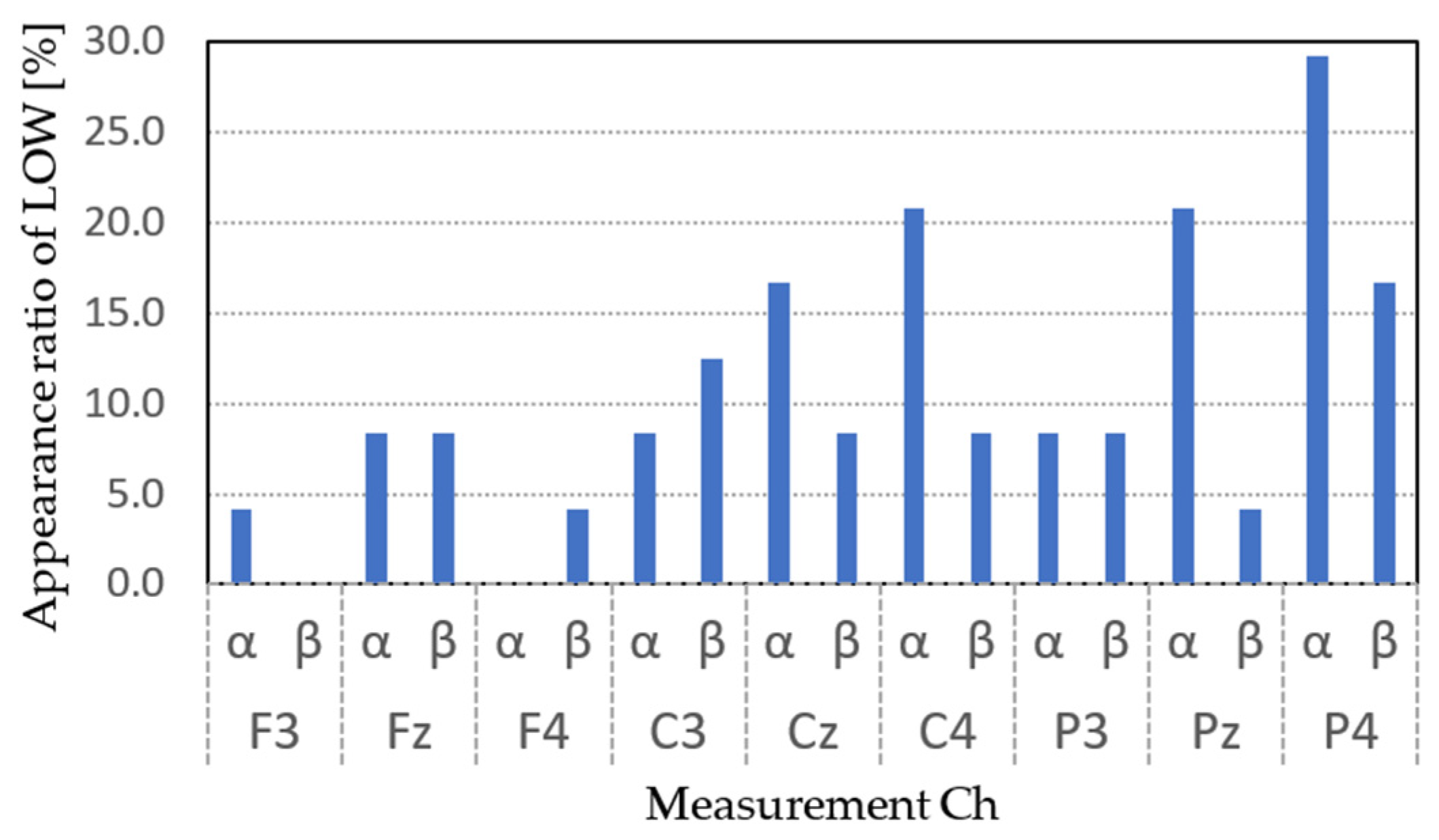
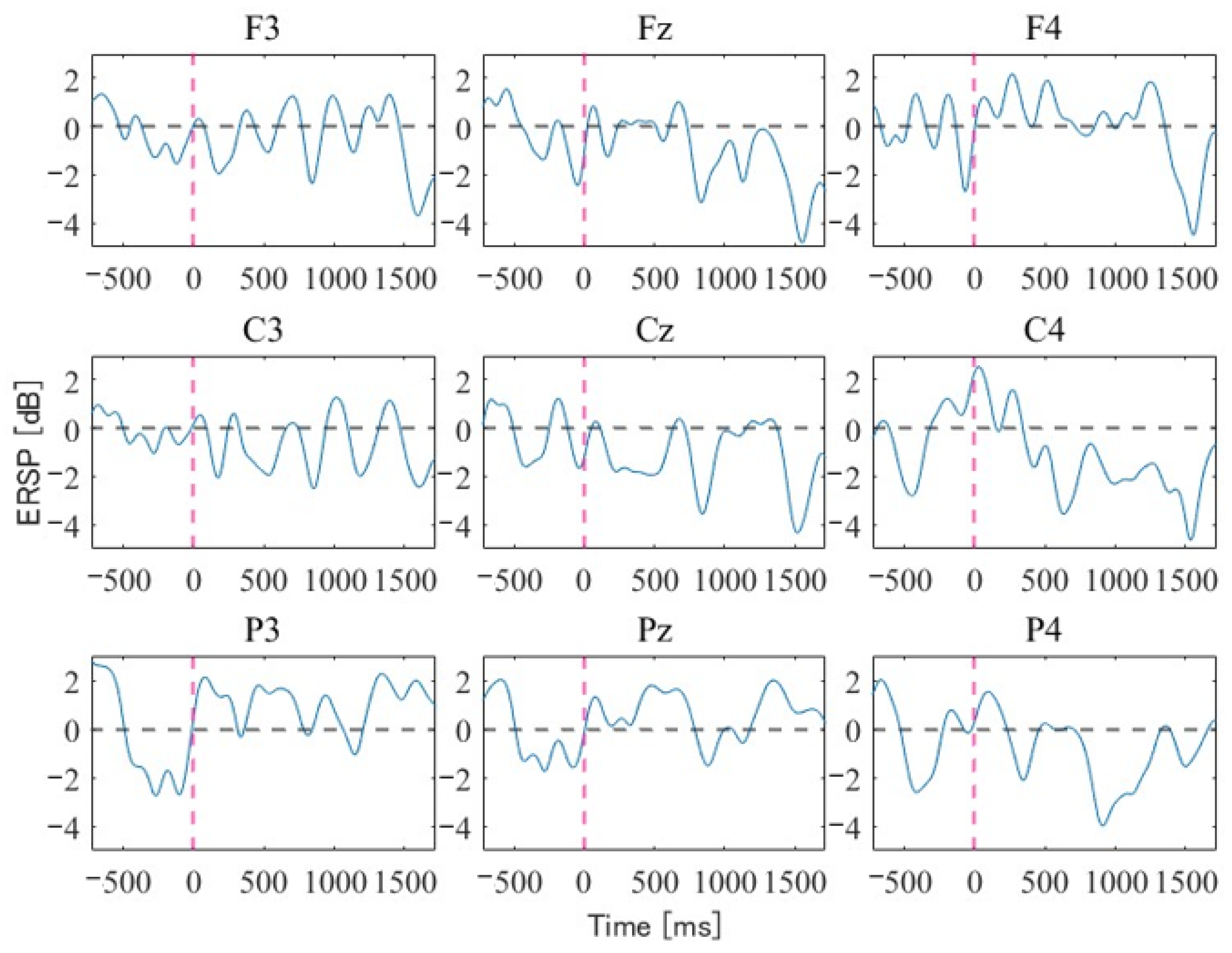
4. Experimental Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pfurtscheller, G.; Neuper, C. Future prospects of ERD/ERS in the context of brain–computer interface (BCI) developments. Prog. Brain Res. 2006, 159, 433–437. [Google Scholar] [CrossRef]
- Jackson, A.; Zimmermann, J.B. Neural interfaces for the brain and spinal cord—Restoring motor function. Nat. Rev. Neurosci. 2012, 8, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Ethier, C.; Gallego, J.A.; Miller, L.E. Brain-controlled neuromuscular stimulation to drive neural plasticity and functional recovery. Curr. Opin. Neurobiol. 2015, 33, 95–102. [Google Scholar] [CrossRef]
- Chaudhary, U.; Birbaumer, N.; Murguialday, A.R. Brain–computer interfaces for communication and rehabilitation. Nat. Rev. Neurosci. 2016, 12, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Caria, A.; Weber, C.; Brötz, D.; Ramos, A.; Ticini, L.F.; Gharabaghi, A.; Braun, C.; Birbaumer, N. Chronic stroke recovery after combined BCI training and physiotherapy: A case report. Psychophysiology 2011, 48, 578–582. [Google Scholar] [CrossRef]
- Sitaram, R.; Ros, T.; Stoeckel, L.; Haller, S.; Scharnowski, F.; Peacock, J.L.; Weiskopf, N.; Blefari, M.L.; Rana, M.; Oblak, E.; et al. Closed-loop brain training: The science of neurofeedback. Nat. Rev. Neurosci. 2017, 18, 86–100. [Google Scholar] [CrossRef]
- Halme, H.L.; Parkkonen, L. Comparing Features for Classification of MEG Responses to Motor Imagery. PLoS ONE 2016, 11, e0168766. [Google Scholar] [CrossRef]
- Kim, Y.J.; Park, S.W.; Yeom, H.G.; Bang, M.S.; Kim, J.S.; Chung, C.K.; Kim, S. A study on a robot arm driven by three-dimensional trajectories predicted from non-invasive neural signals. Biomed. Eng. Online 2015, 14, 81. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Mukae, N.; Arata, J.; Iwata, H.; Iramina, K.; Iihara, K.; Hashizume, M. A multichannel-near-infrared-spectroscopy-triggered robotic hand rehabilitation system for stroke patients. IEEE Int. Conf. Rehabil. Robot. ICORR 2017, 2017, 158–163. [Google Scholar] [CrossRef]
- Logothetis, N.K. What we can do and what we cannot do with fMRI. Nature 2008, 453, 869–878. [Google Scholar] [CrossRef]
- Boas, D.A.; Elwell, C.E.; Ferrari, M.; Taga, G. Twenty years of functional near-infrared spectroscopy: Introduction for the special issue. Neuroimage 2014, 85, 1–5. [Google Scholar] [CrossRef]
- Ferrari, M.; Quaresima, V. A brief review on the history of human functional near-infrared spectroscopy (fNIRS) development and fields of application. Neuroimage 2012, 63, 921–935. [Google Scholar] [CrossRef] [PubMed]
- Nunez, P.L.; Srinivasan, R. Electric Fields of the Brain: The Neurophysics of EEG, 2nd ed.; Oxford University Press: New York, NY, USA, 2006. [Google Scholar] [CrossRef]
- Wang, Y.; Nakanishi, M.; Zhang, D. EEG-Based Brain-Computer Interfaces. Adv. Exp. Med. Biol. 2019, 1101, 41–65. [Google Scholar] [CrossRef] [PubMed]
- Pfurtscheller, G. Event-related synchronization (ERS): An electrophysiological correlate of cortical areas at rest. Electroencephalogr. Clin. Neurophysiol. 1992, 83, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Pfurtscheller, G.; Aranibar, A. Event-related cortical desynchronization detected by power measurements of scalp EEG. Electroencephalogr. Clin. Neurophysiol. 1977, 42, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Farwell, L.A.; Donchin, E. Talking off the top of your head: Toward a mental prosthesis utilizing event-related brain potentials. Electroencephalogr. Clin. Neurophysiol. 1988, 70, 510–523. [Google Scholar] [CrossRef]
- Kaufmann, T.; Herweg, A.; Kübler, A. Toward brain-computer interface based wheelchair control utilizing tactually-evoked event-related potentials. J. Neuroeng. Rehabil. 2014, 11, 7. [Google Scholar] [CrossRef]
- Herweg, A.; Gutzeit, J.; Kleih, S.; Kübler, A. Wheelchair control by elderly participants in a virtual environment with a brain-computer interface (BCI) and tactile stimulation. Biol. Psychol. 2016, 121, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Eidel, M.; Kübler, A. Wheelchair Control in a Virtual Environment by Healthy Participants Using a P300-BCI Based on Tactile Stimulation: Training Effects and Usability. Front. Hum. Neurosci. 2020, 14, 265. [Google Scholar] [CrossRef]
- Pritchard, W.S. Psychophysiology of P300. Psychol. Bull. 1981, 89, 506–540. [Google Scholar] [CrossRef]
- Hillyard, S.A.; Kutas, M. Electrophysiology of cognitive processing. Annu. Rev. Psychol. 1983, 34, 33–61. [Google Scholar] [CrossRef] [PubMed]
- Rebsamen, B.; Burdet, E.; Guan, C.; Zhang, H.; Teo, C.L.; Zeng, Q.; Laugier, C.; Ang, M.H. Controlling a Wheelchair Indoors Using Thought. IEEE Intell. Syst. 2007, 22, 18–24. [Google Scholar] [CrossRef]
- Cheng, M.; Gao, X.; Gao, S.; Xu, D. Design and implementation of a brain-computer interface with high transfer rates. IEEE. Trans. Biomed. Eng. 2002, 49, 1181–1186. [Google Scholar] [CrossRef] [PubMed]
- Chi, X.; Wan, C.; Wang, C.; Zhang, Y.; Chen, X.; Cui, X. A Novel Hybrid Brain-Computer Interface Combining Motor Imagery and Intermodulation Steady-State Visual Evoked Potential. IEEE Trans. Neural Syst. Rehabil. Eng. 2022, 30, 1525–1535. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, B.; Wang, Y.; Cui, H.; Dong, J.; Ma, R.; Li, N.; Gao, X. Optimizing Stimulus Frequency Ranges for Building a High-Rate High Frequency SSVEP-BCI. IEEE Trans. Neural Syst. Rehabil. Eng. 2023, 31, 1277–1286. [Google Scholar] [CrossRef] [PubMed]
- Lotte, F.; Bougrain, L.; Cichocki, A.; Clerc, M.; Congedo, M.; Rakotomamonjy, A.; Yger, F. A review of classification algorithms for EEG-based brain-computer interfaces: A 10 year update. J. Neural Eng. 2018, 15, 31005. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Xu, J.; Li, H.; Liu, Z.; Zhou, H.; You, Y.; Song, T.; Zuo, G. A Multi-Scale Temporal Convolutional Network with Attention Mechanism for Force Level Classification during Motor Imagery of Unilateral Upper-Limb Movements. Entropy 2023, 25, 464. [Google Scholar] [CrossRef] [PubMed]
- Pfurtscheller, G. EEG event-related desynchronization (ERD) and synchronization (ERS). Electroencephalogr. Clin. Neurophysiol. 1997, 103, 26. [Google Scholar] [CrossRef]
- Benzy, V.K.; Vinod, A.P.; Subasree, R.; Alladi, S.; Raghavendra, K. Motor Imagery Hand Movement Direction Decoding Using Brain Computer Interface to Aid Stroke Recovery and Rehabilitation. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 3051–3062. [Google Scholar] [CrossRef]
- Prasad, G.; Herman, P.; Coyle, D.; McDonough, S.; Crosbie, J. Applying a brain-computer interface to support motor imagery practice in people with stroke for upper limb recovery: A feasibility study. J. Neuroeng. Rehabil. 2010, 7, 60. [Google Scholar] [CrossRef]
- Blankertz, B.; Dornhege, G.; Krauledat, M.; Müller, K.R.; Curio, G. The non-invasive Berlin Brain-Computer Interface: Fast acquisition of effective performance in untrained subjects. NeuroImage 2007, 37, 539–550. [Google Scholar] [CrossRef]
- Krauledat, M.; Schröder, M.; Blankertz, B.; Müller, K.R. Reducing Calibration Time for Brain-Computer Interfaces: A Clustering Approach. In Advances in Neural Information Processing Systems 19: Proceedings of the 2006 Conference, Vancouver, Canada, 4–7 December 2006; MIT Press: Cambridge, MA, USA, 2007; pp. 753–760. [Google Scholar]
- Van Erp, J.B.F.; Lotte, F.; Tangermann, M. Brain-Computer Interfaces: Beyond Medical Applications. Comput. J. 2012, 45, 26–34. [Google Scholar] [CrossRef]
- Saga, N.; Tanaka, Y.; Doi, A.; Oda, T.; Kudoh, S.N.; Fujie, H. Prototype of an Ankle Neurorehabilitation System with Heuristic BCI Using Simplified Fuzzy Reasoning. Appl. Sci. 2019, 9, 2429. [Google Scholar] [CrossRef]
- Saga, N.; Doi, A.; Oda, T.; Kudoh, S.N. Elucidation of EEG Characteristics of Fuzzy Reasoning-Based Heuristic BCI and Its Application to Patient with Brain Infarction. Front. Neurorobot. 2021, 14, 607706. [Google Scholar] [CrossRef]
- Bansal, A.K.; Truccolo, W.; Irwin, C.E.V.; Donoghue, J.P. Decoding 3D reach and grasp from hybrid signals in motor and premotor cortices: Spikes, multiunit activity, and local field potentials. J. Neurophysiol. 2012, 107, 1337–1355. [Google Scholar] [CrossRef]
- Jeong, J.H.; Shim, K.H.; Kim, D.J.; Lee, S.W. Trajectory Decoding of Arm Reaching Movement Imageries for Brain-Controlled Robot Arm System. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 5544–5547. [Google Scholar] [CrossRef]
- Kim, J.H.; Bießmann, F.; Lee, S.W. Decoding Three-Dimensional Trajectory of Executed and Imagined Arm Movements from Electroencephalogram Signals. IEEE Trans. Neural Syst. Rehabil. Eng. 2015, 23, 867–876. [Google Scholar] [CrossRef]
- Malesevic, N.; Markovic, D.; Kanitz, G.; Controzzi, M.; Cipriani, C.; Antfolk, C. Decoding of individual finger movements from surface EMG signals using vector autoregressive hierarchical hidden Markov models (VARHHMM). IEEE Int. Conf. Rehabil. Robot. (ICORR) 2017, 2017, 1518–1523. [Google Scholar]
- Zhang, Q.; Liu, R.; Chen, W.; Xiong, C. Simultaneous and Continuous Estimation of Shoulder and Elbow Kinematics from Surface EMG Signals. Front. Neurosci. 2017, 11, 280. [Google Scholar] [CrossRef]
- Pan, L.; Crouch, D.L.; Huang, H. Comparing EMG-Based Human-Machine Interfaces for Estimating Continuous, Coordinated Movements. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 2145–2154. [Google Scholar] [CrossRef]
- Tsukahara, A.; Hasegawa, Y.; Eguchi, K.; Sankai, Y. Restoration of gait for spinal cord injury patients using HAL with intention estimator for preferable swing speed. IEEE Trans. Neural Syst. Rehabil. Eng. 2015, 23, 308–318. [Google Scholar] [CrossRef]
- Bernshteĭn, N.A. The Coordination and Regulation of Movements; Pergamon Press: Oxford, UK, 1967. [Google Scholar]
- d’Avella, A.; Portone, A.; Fernandez, L.; Lacquaniti, F. Control of fast-reaching movements by muscle synergy combinations. J. Neurosci. 2006, 26, 7791–7810. [Google Scholar] [CrossRef] [PubMed]
- Namikawa, Y.; Kawamoto, H.; Sankai, Y. Gait Evaluation with Bioelectrical Signal Patterns during Cybernic Treatment. In Proceedings of the 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Virtual, 1–5 November 2021; pp. 6728–6733. [Google Scholar] [CrossRef]
- Saga, N.; Umeki, R.; Saito, N.; Nagase, J.; Satoh, T. Development of a Meal Support Device for Functional Recovery Using EMG Signals. IEEE Access 2020, 8, 79586–79593. [Google Scholar] [CrossRef]
- Towle, V.L.; Bolaños, J.; Suarez, D.; Tan, K.; Grzeszczuk, R.; Levin, D.N.; Cakmur, R.; Frank, S.A.; Spire, J.P. The spatial location of EEG electrodes: Locating the bestfitting sphere relative to cortical anatomy. Electroencephalogr. Clin. Neurophysiol. 1993, 86, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Cael, C. Functional Anatomy: Musculoskeletal Anatomy, Kinesiology, and Palpation for Manual Therapists, 2nd ed.; Jones & Bartlett Learning: Burlington, MA, USA, 2022. [Google Scholar]
- Koehler, P.J. The Barrés test and Mingazzini test. In Neurological Eponyms; Oxford Academic-Oxford University Press: Oxford, UK, 2000; pp. 119–126. [Google Scholar] [CrossRef]
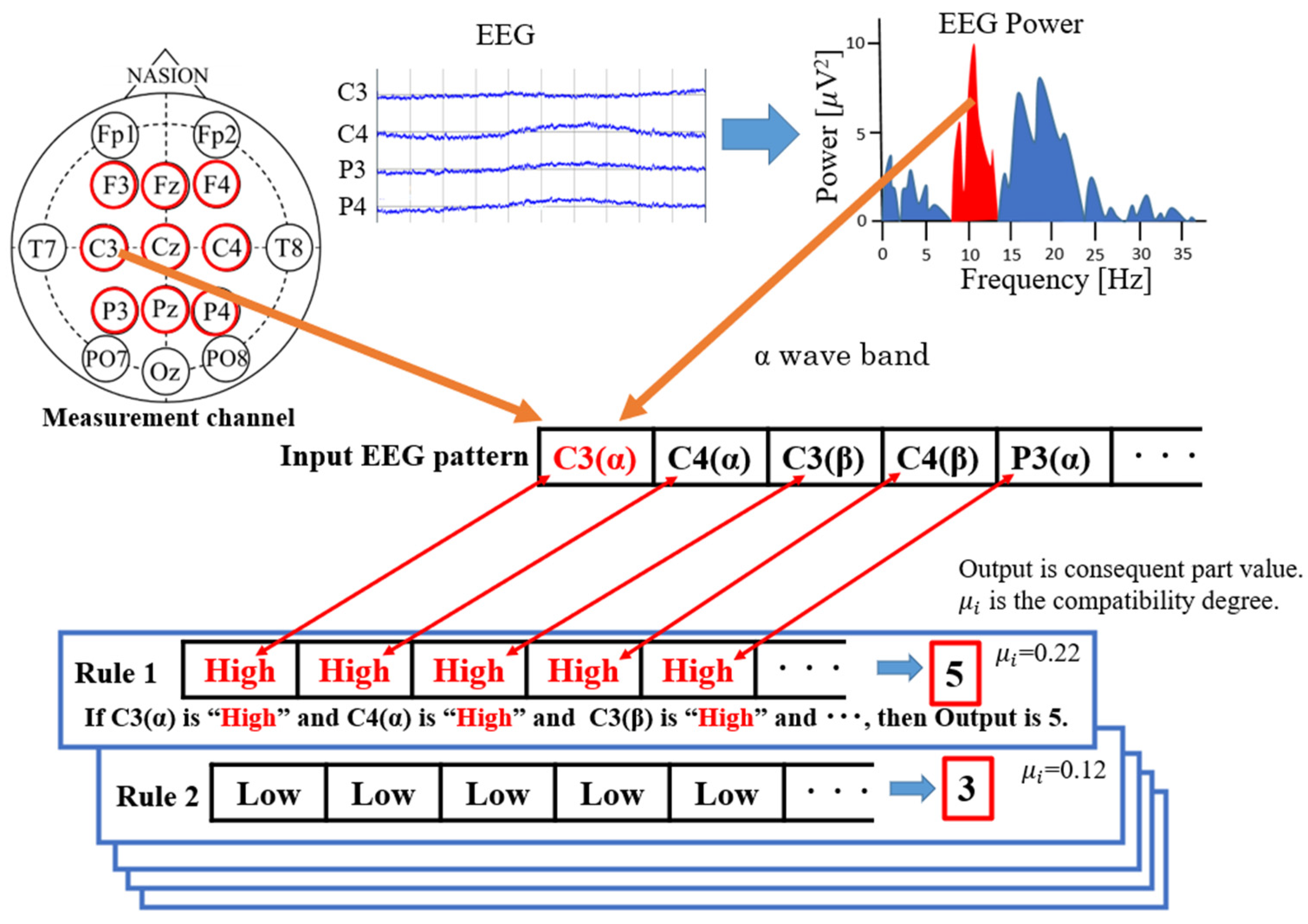


| Top | F3 | Fz | F4 | C3 | Cz | C4 | P3 | Pz | P4 | Consequent Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| α | β | α | β | α | β | α | β | α | β | α | β | α | β | α | β | α | β | ||
| 1 | High | High | High | High | High | High | High | High | High | High | Low | High | High | High | High | High | High | High | 3.289 |
| 2 | High | High | High | High | High | High | Low | High | High | High | High | High | High | High | High | High | High | High | 3.099 |
| 3 | High | High | High | High | High | High | High | High | High | Low | High | Low | High | High | High | High | Low | High | 2.080 |
| 4 | High | High | High | High | High | High | Low | High | Low | High | Low | High | Low | Low | Low | High | High | High | 1.794 |
| 5 | High | High | High | High | High | High | High | Low | High | High | High | Low | High | High | High | High | High | High | 1.619 |
| 6 | High | High | High | High | High | Low | High | High | High | High | High | High | High | High | High | High | High | High | 1.465 |
| 7 | High | High | High | High | High | High | High | High | High | High | High | High | High | High | High | High | Low | Low | 1.388 |
| 8 | High | High | High | High | High | High | High | Low | High | Low | High | High | High | High | High | High | High | High | 1.384 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saga, N.; Okawa, Y.; Saga, T.; Satoh, T.; Saito, N. Trial of Brain–Computer Interface for Continuous Motion Using Electroencephalography and Electromyography. Electronics 2024, 13, 2770. https://doi.org/10.3390/electronics13142770
Saga N, Okawa Y, Saga T, Satoh T, Saito N. Trial of Brain–Computer Interface for Continuous Motion Using Electroencephalography and Electromyography. Electronics. 2024; 13(14):2770. https://doi.org/10.3390/electronics13142770
Chicago/Turabian StyleSaga, Norihiko, Yukina Okawa, Takuma Saga, Toshiyuki Satoh, and Naoki Saito. 2024. "Trial of Brain–Computer Interface for Continuous Motion Using Electroencephalography and Electromyography" Electronics 13, no. 14: 2770. https://doi.org/10.3390/electronics13142770
APA StyleSaga, N., Okawa, Y., Saga, T., Satoh, T., & Saito, N. (2024). Trial of Brain–Computer Interface for Continuous Motion Using Electroencephalography and Electromyography. Electronics, 13(14), 2770. https://doi.org/10.3390/electronics13142770






