Studies on the Control of Dermanyssus gallinae via High-Voltage Impulse
Abstract
1. Introduction
2. Materials and Methods
2.1. Voltage Applied Target
2.2. Experimental Procedure
2.3. Experimental Apparatus
2.4. Variation in Discharge Voltage Due to Different Electrode Spacing and Insulation Materials
2.5. Variation of Mortality by Electrical Parameters
2.6. Variation of Mortality Due to Ultraviolet Light and Ozone
2.7. Observation of D. gallinae Killed by High-Voltage Impulse Discharges using EDS
2.8. Statistical Analysis
3. Results
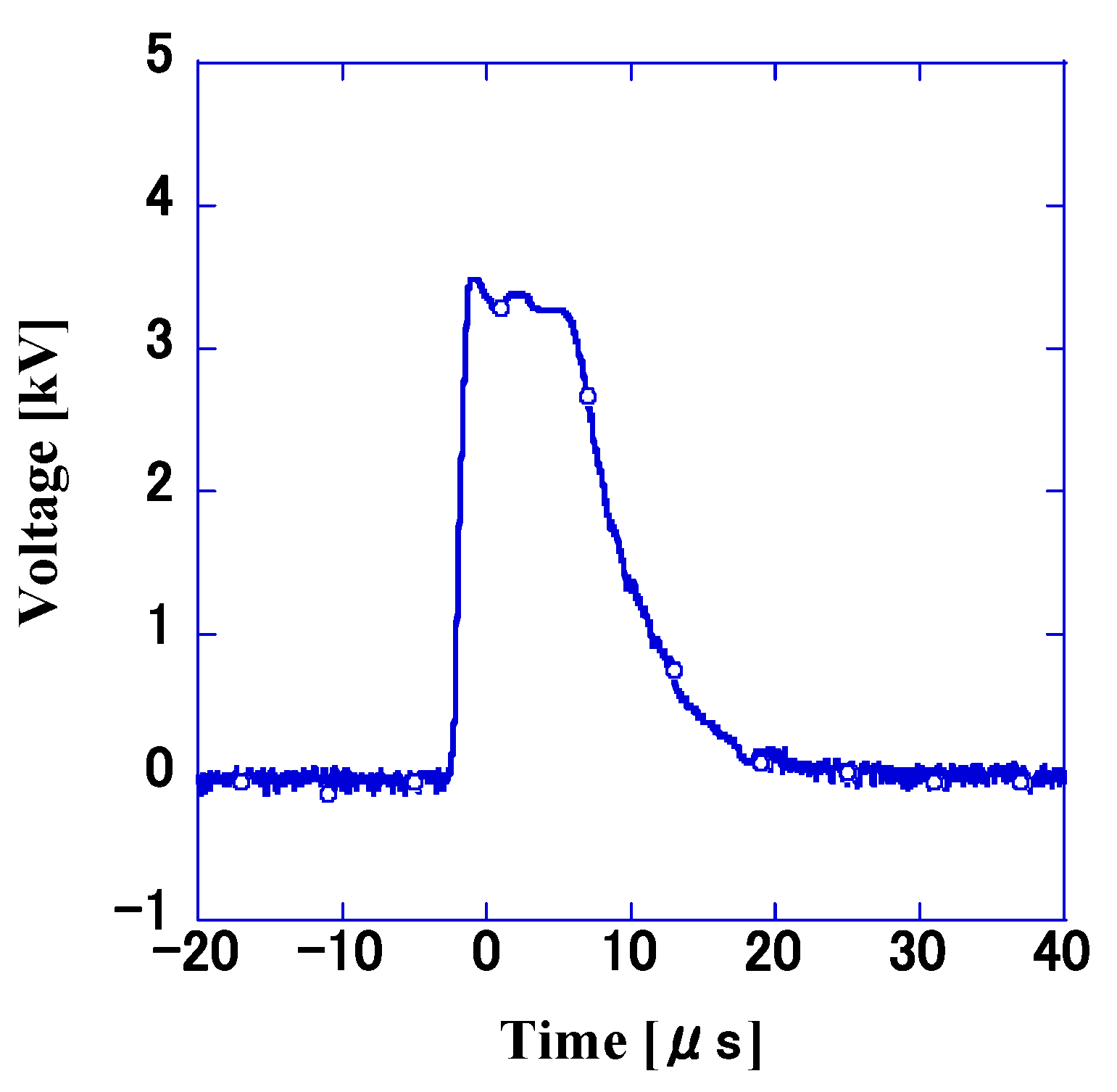
3.1. Measurement of Discharge Voltage via Electrode Gap and Polarity
3.2. Variation in Mortality of D. gallinae Using Electrical Parameters
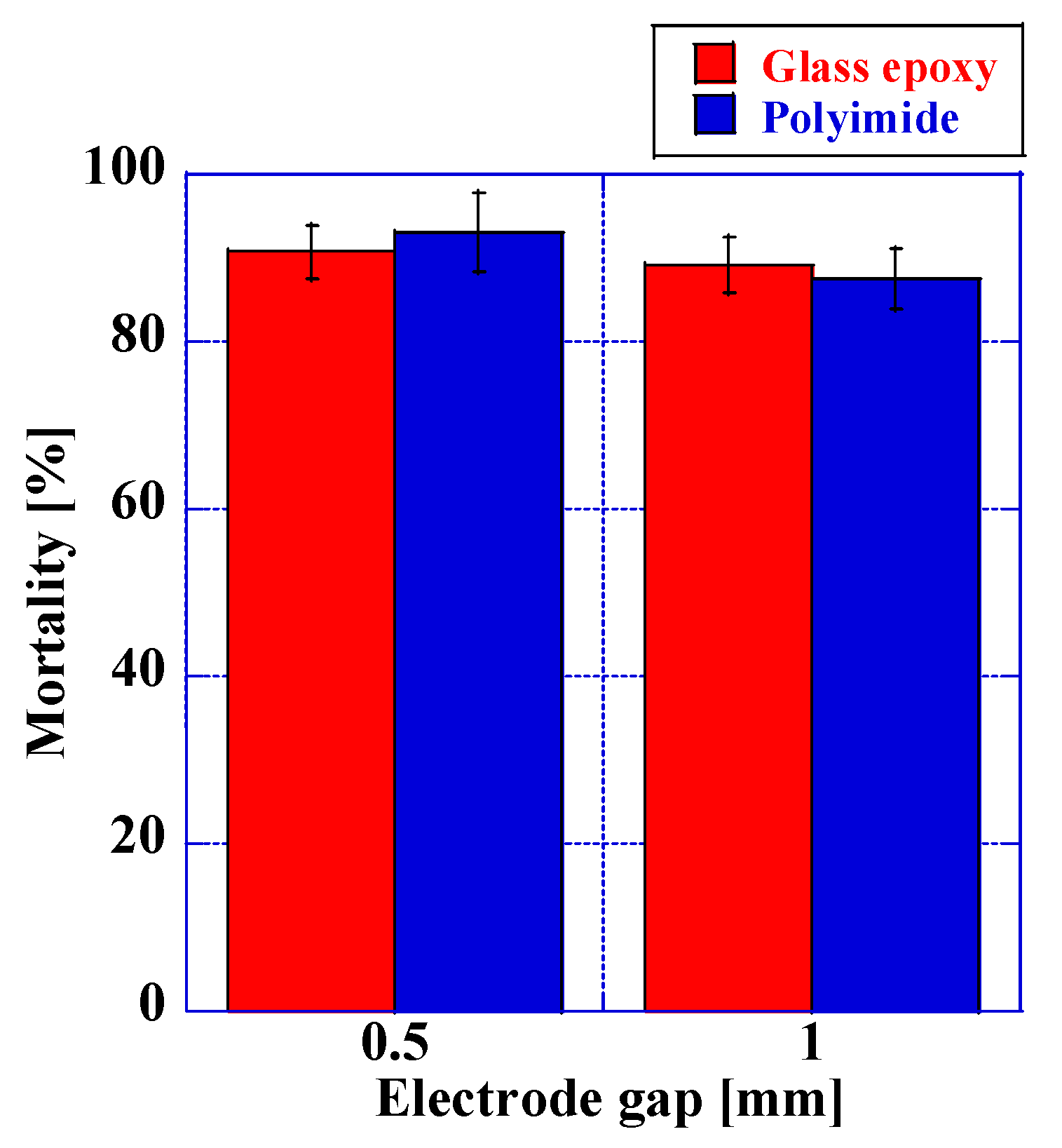
3.2.1. Variation in Mortality Depending on the Type of Insulator
3.2.2. Variation of Mortality as a Function of Discharge Voltage
3.2.3. Mortality as a Function of Frequency
3.2.4. Variation of Mortality with Current
3.2.5. Variation of Mortality with Pulse Width
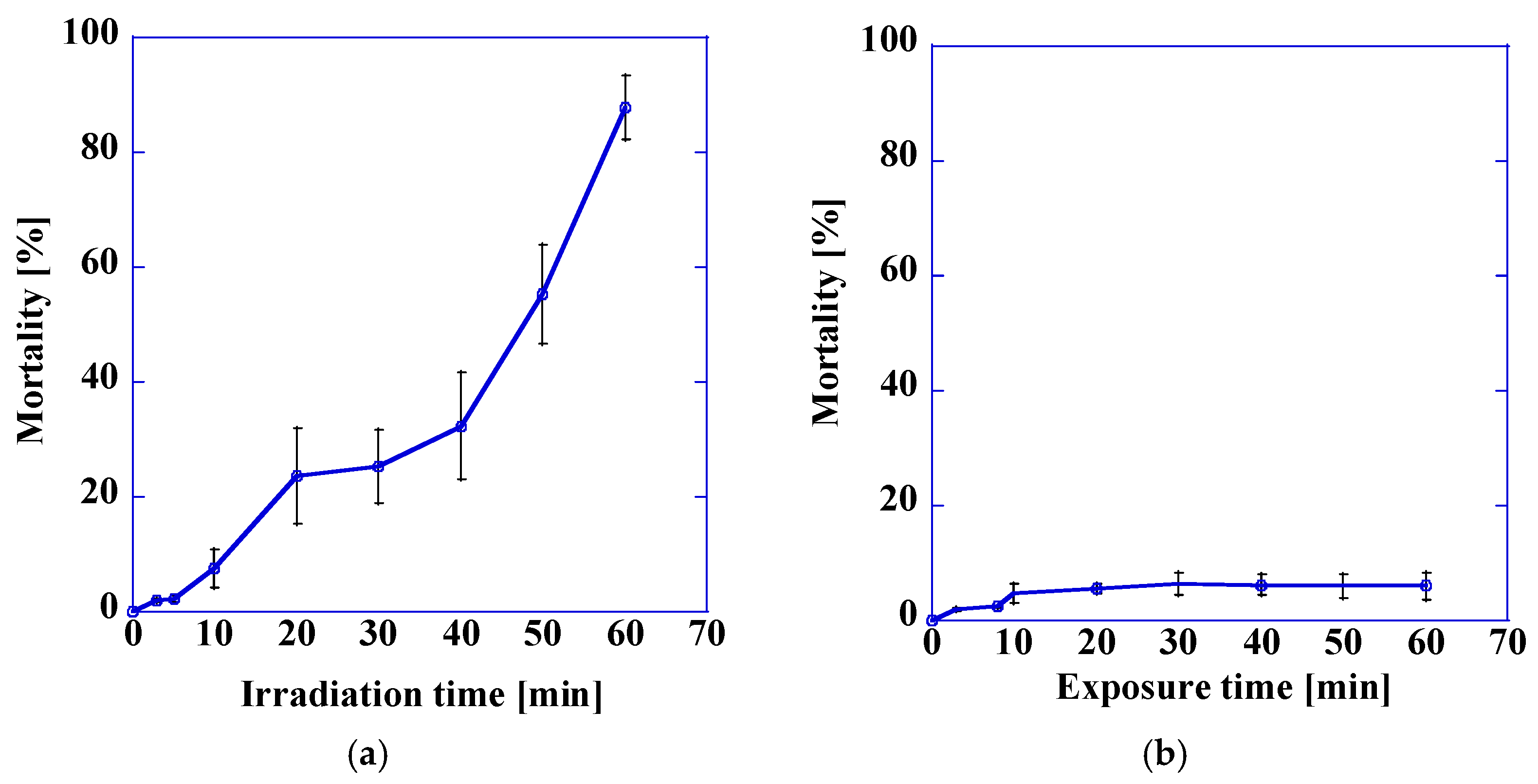
3.3. Variation in Mortality with Ultraviolet Light
3.4. Variation in Mite Control Efficacy with Ozone
3.5. Comparison of D. gallinae Body Surfaces after Voltage Application
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galvani, L. Aloysii Galvani De Viribus Electricitatis in Motu Musculari Commentarius; Ex Typographia Instituti Scientiarium: Bononiae, Italy, 1791. [Google Scholar] [CrossRef]
- Houston, E.J. Electricity in Every-Day Life; P. F. Collier & Son: New York, NY, USA, 1905. [Google Scholar]
- Finkelstein, G. Emil du Bois-Reymond vs Ludimar Hermann. Comptes Rendus Biol. 2006, 329, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Clarke, E.; Jacyna, L.S. Nineteenth-Century Origins of Neuroscientific Concepts; University of California Press: Berkeley, CA, USA, 1987. [Google Scholar]
- Wells, D.A. The Science of Common Things: A Familiar Explanation of the First Principles of Physical Science. For Schools, Families, and Young Students. Illustrated with Numerous Engravings; Ivison, Phinney & Company: New York, NY, USA, 1860. [Google Scholar]
- Theruvath, T.P.; Ikonomidis, J.S. Historical perspectives of The American Association for Thoracic Surgery: Claude S. Beck (1894–1971). J. Thorac. Cardiovasc. Surg. 2015, 149, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Beloucif, S. Informed consent for special procedures: Electroconvulsive therapy and psychosurgery. Curr. Opin. Anaesthesiol. 2013, 26, 182–185. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, J.H.; Vasu, D. The use of electroconvulsive therapy in atypical psychotic presentations: A case review. Psychiatry 2007, 4, 30–39. [Google Scholar]
- Sale, A.J.H.; Hamilton, W.A. Effects of high electric fields on micro-organisms: III. Lysis of erythrocytes and protoplasts. Biochim. Et Biophys. Acta (BBA) Biomembr. 1968, 163, 37–43. [Google Scholar] [CrossRef]
- Neumann, E.; Rosenheck, K. Permeability changes induced by electric impulses in vesicular membranes. J. Membr. Biol. 1972, 10, 279–290. [Google Scholar] [CrossRef]
- Nuccitelli, R.; Pliquett, U.; Chen, X.; Ford, W.; James Swanson, R.; Beebe, S.J.; Kolb, J.F.; Schoenbach, K.H. Nanosecond pulsed electric fields cause melanomas to self-destruct. Biochem. Biophys. Res. Commun. 2006, 343, 351–360. [Google Scholar] [CrossRef]
- Dev, S.B.; Rabussay, D.P.; Widera, G.; Hofmann, G.A. Medical applications of electroporation. IEEE Trans. Plasma Sci. 2000, 28, 206–223. [Google Scholar] [CrossRef]
- Beebe, S.J.; Blackmore, P.F.; White, J.; Joshi, R.P.; Schoenbach, K.H. Nanosecond pulsed electric fields modulate cell function through intracellular signal transduction mechanisms. Physiol. Meas. 2004, 25, 1077–1093. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Maeda, T.; Ikeda, M.; Akiyama, H. Application of pulsed power to mushroom culturing. In Proceedings of the Digest of Technical Papers, PPC-2003, 14th IEEE International Pulsed Power Conference (IEEE Cat. No.03CH37472), Dallas, TX, USA, 15–18 June 2003; Volume 1112, pp. 1116–1119. [Google Scholar]
- Shimizu, H.; Hiraguri, T.; Kimoto, M.; Ota, K.; Shindo, T.; Hoshino, Y.; Takaki, K. Stimulatory growth effect of lightning strikes applied in the vicinity of shiitake mushroom bed logs. J. Phys. D Appl. Phys. 2020, 53, 204002. [Google Scholar] [CrossRef]
- Eing, C.J.; Bonnet, S.; Pacher, M.; Puchta, H.; Frey, W. Effects of nanosecond pulsed electric field exposure on Arabidopsis thaliana. IEEE Trans. Dielectr. Electr. Insul. 2009, 16, 1322–1328. [Google Scholar] [CrossRef]
- Wang, D.; Lin, X.; Hirayama, K.; Li, Z.; Ohno, T.; Zhang, W.; Namihira, T.; Katsuki, S.; Takano, H.; Takio, S.; et al. A New Application of Underwater Pulsed Streamerlike Discharge to Transcriptional Activation of Retrotransposon of Porphyra yezoensis. IEEE Trans. Plasma Sci. 2010, 38, 39–46. [Google Scholar] [CrossRef]
- Wood, H.P. The Chicken Mite: Its Life History and Habits; U.S. Dept. of Agriculture: Washington, DC, USA, 1917; Volume 553. [Google Scholar]
- Collins, D. Mites in the poultry house. J. Agric. North. Irel. 1976, 51, 24–26. [Google Scholar]
- Kilpinen, O.; Roepstorff, A.; Permin, A.; Nørgaard-Nielsen, G.; Lawson, L.G.; Simonsen, H.B. Influence of Dermanyssus gallinae and Ascaridia galli infections on behaviour and health of laying hens (Gallus gallus domesticus). Br. Poult. Sci. 2005, 46, 26–34. [Google Scholar] [CrossRef]
- Sparagano, O.; Pavlićević, A.; Murano, T.; Camarda, A.; Sahibi, H.; Kilpinen, O.; Mul, M.; van Emous, R.; le Bouquin, S.; Hoel, K.; et al. Prevalence and key figures for the poultry red mite Dermanyssus gallinae infections in poultry farm systems. Exp. Appl. Acarol. 2009, 48, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Cencek, T. Prevalence of dermanyssus gallinae in poultry farms in silesia region in poland. J. Bull. Vet. Inst. Pulawy 2003, 47, 465–470. [Google Scholar]
- Guy, J.H.; Khajavi, M.; Hlalel, M.M.; Sparagano, O. Red mite (Dermanyssus gallinae) prevalence in laying units in Northern England. Br. Poult. Sci. 2004, 45 (Suppl. S1), S15–S16. [Google Scholar] [CrossRef]
- Thind, B.B.; Ford, H.L. Assessment of susceptibility of the poultry red mite Dermanyssus gallinae (Acari: Dermanyssidae) to some acaricides using an adapted filter paper based bioassay. Vet. Parasitol. 2007, 144, 344–348. [Google Scholar] [CrossRef]
- Zeman, P.; Železný, J. The susceptibility of the poultry red mite, Dermanyssus gallinae (De Geer, 1778), to some acaricides under laboratory conditions. Exp. Appl. Acarol. 1985, 1, 17–22. [Google Scholar] [CrossRef]
- Frings, H. Factors determining the effects of radio-frequency electromagnetic fields on insects and materials they infest. J. Econ. Entomol. 1952, 45, 396–408. [Google Scholar] [CrossRef]
- Wang, S.; Tang, J.; Johnson, J.A.; Mitcham, E.; Hansen, J.D.; Hallman, G.; Drake, S.R.; Wang, Y. Dielectric Properties of Fruits and Insect Pests as related to Radio Frequency and Microwave Treatments. Biosyst. Eng. 2003, 85, 201–212. [Google Scholar] [CrossRef]
- Wang, S.; Tiwari, G.; Jiao, S.; Johnson, J.A.; Tang, J. Developing postharvest disinfestation treatments for legumes using radio frequency energy. Biosyst. Eng. 2010, 105, 341–349. [Google Scholar] [CrossRef]
- Pickens, L.G.; Mills, G.D., Jr. Solar-powered electrocuting trap for controlling house flies and stable flies (Diptera: Muscidae). J. Med. Entomol. 1993, 30, 872–877. [Google Scholar] [CrossRef]
- Urban, J.E.; Broce, A. Killing of flies in electrocuting insect traps releases bacteria and viruses. Curr. Microbiol. 2000, 41, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Pickens, L.G. Battery-powered, electrocuting trap for stable flies (Diptera: Muscidae). J. Med. Entomol. 1991, 28, 822–830. [Google Scholar] [CrossRef]
- O’Dwyer, J.J. Theory of Dielectric Breakdown in Solids. J. Electrochem. Soc. 1969, 116, 239. [Google Scholar] [CrossRef]
- Verweij, J.F.; Klootwijk, J.H. Dielectric breakdown I: A review of oxide breakdown. Microelectron. J. 1996, 27, 611–622. [Google Scholar] [CrossRef]
- Beroual, A.; Kebbabi, L. Analysis of cumulative number and polarity of creeping discharges initiated at solid/liquid interfaces subjected to AC voltage. In Proceedings of the 2009 IEEE Conference on Electrical Insulation and Dielectric Phenomena, Virginia Beach, VA, USA, 18–21 October 2009; pp. 380–383. [Google Scholar]
- Li, C.; Cao, B.; Li, X.; Liu, X.; Cheng, J.; Liu, Z.; Zhao, Z.; Yang, Z. Surface discharge characteristics of silicone gel and DBC under positive repetitive square voltage. Power Electron. Devices Compon. 2022, 3, 100021. [Google Scholar] [CrossRef]
- Donkó, Z.; Zajičková, L.; Sugimoto, S.; Harumningtyas, A.A.; Hamaguchi, S. Modeling characterisation of a bipolar pulsed discharge. Plasma Sources Sci. Technol. 2020, 29, 104001. [Google Scholar] [CrossRef]
- Fukuma, M.; Nagao, M.; Kosaki, M. Numerical Analysis of Electrical Breakdown Characteristics in Polypropylene Film based on Thermal and Electronic Composite Breakdown Model. IEEJ Trans. Fundam. Mater. 1994, 114, 230–235. [Google Scholar] [CrossRef]
- Nagao, M.; Yamaguchi, F.; Tokumaru, K.; Sugide, I.; Kozaki, M.; Ieda, M. DC dielectric breakdown of polypropylene films in the high temperature range. IEEJ Trans. Electr. Electron. Eng. 1985, 105, 177–182. [Google Scholar] [CrossRef]
- Yamadera, T. Environment-Friendry Printed Wiring Boards (PWB) and the Technologies for their Inflammabilities. J. Jpn. Inst. Electron. Packag. 2014, 17, 96–99. [Google Scholar] [CrossRef]
- Matsuo, M. Glass Epoxy (FR-4). Circuit Technol. 1993, 8, 402–411. [Google Scholar] [CrossRef]
- Crowe, J.H. Studies on acarine cuticles. III. Cuticular ridges in the citrus red mite. Trans. Am. Microsc. Soc. 1975, 94, 98–108. [Google Scholar] [CrossRef]
- Alberti, G.; Storch, V.; Renner, H. Über den feinstrukturellen Aufbau der Milbencuticula (Acari, Arachnida). Zoologische Jahrbücher fair Anatomie; G. Fischer: Jena, Germany, 1981; Volume 105, pp. 183–236. [Google Scholar]
- Lah, E.F.C.; Musa, R.N.A.R.; Ming, H.T. Effect of germicidal UV-C light (254 nm) on eggs and adult of house dustmites, Dermatophagoides pteronyssinus and Dermatophagoides farinae (Astigmata: Pyroglyhidae). Asian Pac. J. Trop. Biomed. 2012, 2, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Watanabe, M.; Takeda, M. UV tolerance in the two-spotted spider mite, Tetranychus urticae. J. Insect Physiol. 2009, 55, 649–654. [Google Scholar] [CrossRef]
- Beggs, C.B. A quantitative method for evaluating the photoreactivation of ultraviolet damaged microorganisms. Photochem. Photobiol. Sci. 2002, 1, 431–437. [Google Scholar] [CrossRef]
- Chamberlain, J.; Moss, S.H. Lipid peroxidation and other membrane damage produced in Escherichia coli K1060 by near-UV radiation and deuterium oxide. Photochem. Photobiol. 1987, 45, 625–630. [Google Scholar] [CrossRef]
- Meng, J.-Y.; Zhang, C.-Y.; Zhu, F.; Wang, X.-P.; Lei, C.-L. Ultraviolet light-induced oxidative stress: Effects on antioxidant response of Helicoverpa armigera adults. J. Insect Physiol. 2009, 55, 588–592. [Google Scholar] [CrossRef]
- Sang, W.; Ma, W.-H.; Qiu, L.; Zhu, Z.-H.; Lei, C.-L. The involvement of heat shock protein and cytochrome P450 genes in response to UV-A exposure in the beetle Tribolium castaneum. J. Insect Physiol. 2012, 58, 830–836. [Google Scholar] [CrossRef]
- Zhou, L.-J.; Zhu, Z.-H.; Liu, Z.-X.; Ma, W.-H.; Desneux, N.; Lei, C.-L. Identification and transcriptional profiling of differentially expressed genes associated with response to UVA radiation in Drosophila melanogaster (Diptera: Drosophilidae). Environ. Entomol. 2013, 42, 1110–1117. [Google Scholar] [CrossRef]
- Grasso, C.; Eramo, V.; Lembo, M.; Forniti, R.; Carboni, C.; Botondi, R. Effects of gaseous ozone treatment on the mite pest control and qualitative properties during ripening storage of pecorino cheese. J. Sci. Food Agric. 2023, 103, 2124–2133. [Google Scholar] [CrossRef]
- Mahmoud, R.; Abdel-Khalik, A.R. Comparison between Two Physical Methods to Control the Stored Dates Fruit Mites, Tyrophagus putrescentiae (Schrank) and Rhizoglyphus robini Claparede (Astigmata: Acaridae). Egypt. Acad. J. Biol. Sci. B. Zool. 2022, 14, 149–158. [Google Scholar] [CrossRef]
- Pritchard, J.; Kuster, T.; Sparagano, O.; Tomley, F. Understanding the biology and control of the poultry red mite Dermanyssus gallinae: A review. Avian Pathol. 2015, 44, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Marangi, M.; Cafiero, M.A.; Capelli, G.; Camarda, A.; Sparagano, O.A.; Giangaspero, A. Evaluation of the poultry red mite, Dermanyssus gallinae (Acari: Dermanyssidae) susceptibility to some acaricides in field populations from Italy. Exp. Appl. Acarol. 2009, 48, 11–18. [Google Scholar] [CrossRef]
- Beugnet, F.; Chauve, C.; Gauthey, M.; Beert, L. Resistance of the red poultry mite to pyrethroids in France. Vet. Rec. 1997, 140, 577–579. [Google Scholar] [CrossRef] [PubMed]
- Nordenfors, H.; Höglund, J.; Tauson, R.; Chirico, J. Effect of permethrin impregnated plastic strips on Dermanyssus gallinae in loose-housing systems for laying hens. Vet. Parasitol. 2001, 102, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Murano, T. Red mite, Dermanyssus gallinae; Current problem and trials for control in Japan. J. Jpn. Soc. Poult. Dis. 2007, 43, 23–30. [Google Scholar]
- IEC 60479-1:2018; Effects of Current on Human Beings and Livestock—Part 1: General Aspects. IEC: London, UK, 2018.
- Coleman, D.C.; Callaham, M.A.; Crossley, D.A. Fundamentals of Soil Ecology, 3rd ed.; Coleman, D.C., Callaham, M.A., Crossley, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Mohammadi, K.; Fathi, S.A.A.; Razmjou, J.; Naseri, B. Evaluation of the effect of strip intercropping green bean/garlic on the control of Tetranychus urticae in the field. Exp. Appl. Acarol. 2021, 83, 183–195. [Google Scholar] [CrossRef]
- Castilho, R.C.; Venancio, R.; Narita, J.P.Z. Mesostigmata as biological control agents, with emphasis on Rhodacaroidea and Parasitoidea. Prospect. Biol. Control Plant Feed. Mites Other Harmful Org. 2015, 19, 1–31. [Google Scholar]
- Rueda-Ramírez, D.; Palevsky, E.; Ruess, L. Soil Nematodes as a Means of Conservation of Soil Predatory Mites for Biocontrol. Agronomy 2023, 13, 32. [Google Scholar] [CrossRef]
- MAFF. Statistics on the Agricultural Labor Force: Population of Agricultural Workers and Number of Core Agricultural Workers. 2020. Available online: https://www.maff.go.jp/j/tokei/sihyo/data/08.html (accessed on 14 February 2023).
- MAFF. Food Price Trends Survey (Meat & Eggs). Available online: https://www.maff.go.jp/j/zyukyu/anpo/kouri/k_gyuniku/index.html (accessed on 14 February 2023).
- Mul, M.F.; Koenraadt, C.J. Preventing introduction and spread of Dermanyssus gallinae in poultry facilities using the HACCP method. Exp. Appl. Acarol. 2009, 48, 167–181. [Google Scholar] [CrossRef] [PubMed]
- George, D.R.; Olatunji, G.; Guy, J.H.; Sparagano, O.A.E. Effect of plant essential oils as acaricides against the poultry red mite, Dermanyssus gallinae, with special focus on exposure time. Vet. Parasitol. 2010, 169, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Ghaffar, F.; Sobhy, H.M.; Al-Quraishy, S.; Semmler, M. Field study on the efficacy of an extract of neem seed (Mite-Stop) against the red mite Dermanyssus gallinae naturally infecting poultry in Egypt. Parasitol. Res. 2008, 103, 481–485. [Google Scholar] [CrossRef] [PubMed]





| (a) Control | (b) High-Voltage Impulse | (c) Heat | |
|---|---|---|---|
| Carbon (%) | 57.8 ± 0.15 | 57.9 ± 0.13 | 58.4 ± 0.13 |
| Oxygen (%) | 42.2 ± 0.15 | 42.1 ± 0.12 | 41.6 ± 0.13 |
| Coefficients | T Stat | p-Value | |
|---|---|---|---|
| Intercept | −2.27 × 10−1 | −1.82 × 100 | 6.98 × 10−2 |
| Voltage | 2.04 × 10−1 | 5.23 × 100 | 4.44 × 10−7 |
| Frequency | −1.42 × 10−6 | −1.01 × 100 | 3.14 × 10−1 |
| Current | 4.48 × 10−4 | 3.87 × 100 | 1.49 × 10−4 |
| Pulse width | −4.43 × 10−8 | −2.85 × 100 | 4.89 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ueno, T.; Mizobe, Y.; Ninomiya, J.; Inoue, T.; Furukawa, T.; Hatta, T. Studies on the Control of Dermanyssus gallinae via High-Voltage Impulse. Electronics 2023, 12, 1038. https://doi.org/10.3390/electronics12041038
Ueno T, Mizobe Y, Ninomiya J, Inoue T, Furukawa T, Hatta T. Studies on the Control of Dermanyssus gallinae via High-Voltage Impulse. Electronics. 2023; 12(4):1038. https://doi.org/10.3390/electronics12041038
Chicago/Turabian StyleUeno, Takahisa, Yuma Mizobe, Junko Ninomiya, Takahiro Inoue, Takashi Furukawa, and Takeshi Hatta. 2023. "Studies on the Control of Dermanyssus gallinae via High-Voltage Impulse" Electronics 12, no. 4: 1038. https://doi.org/10.3390/electronics12041038
APA StyleUeno, T., Mizobe, Y., Ninomiya, J., Inoue, T., Furukawa, T., & Hatta, T. (2023). Studies on the Control of Dermanyssus gallinae via High-Voltage Impulse. Electronics, 12(4), 1038. https://doi.org/10.3390/electronics12041038





