Age-Associated Changes on Gait Smoothness in the Third and the Fourth Age
Abstract
1. Introduction
2. Materials and Methods
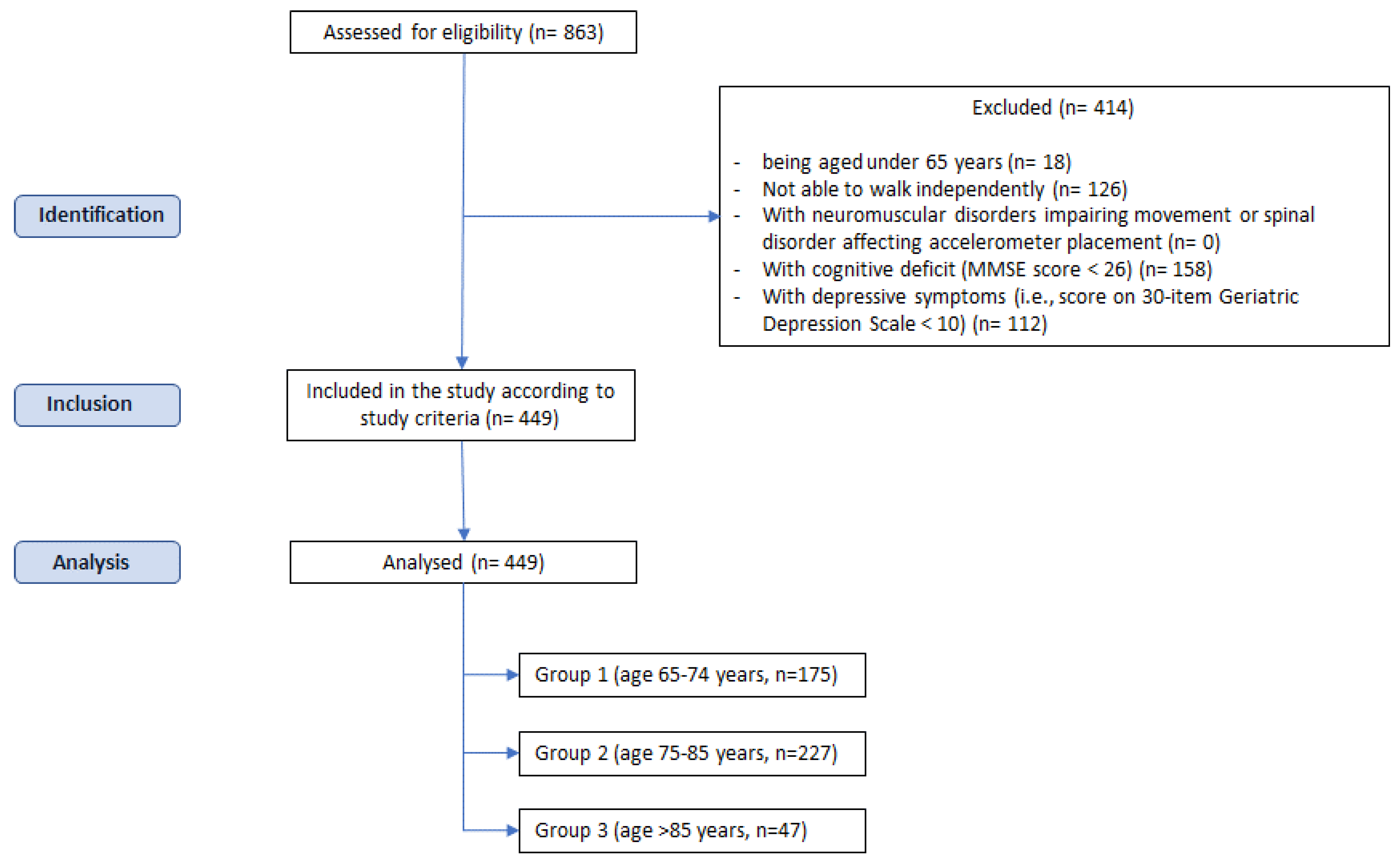
2.1. Participants
- Group 1 (age 65–74 years, n = 175);
- Group 2 (age 75–85 years, n = 227);
- Group 3 (age >85 years, n = 47).
2.2. Data Acquisition
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- DeVita, P.; Hortobagyi, T. Age Causes a Redistribution of Joint Torques and Powers during Gait. J. Appl. Physiol. 2000, 88, 1804–1811. [Google Scholar] [CrossRef] [PubMed]
- McGibbon, C.A.; Krebs, D.E. Age-Related Changes in Lower Trunk Coordination and Energy Transfer during Gait. J. Neurophysiol. 2001, 85, 1923–1931. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.; Stenholm, S.; Metter, E.J.; Ferrucci, L. Age-Associated Gait Patterns and the Role of Lower Extremity Strength - Results from the Baltimore Longitudinal Study of Aging. Arch. Gerontol. Geriatr. 2012, 55, 474–479. [Google Scholar] [CrossRef]
- Harada, C.N.; Natelson Love, M.C.; Triebel, K.L. Normal Cognitive Aging. Clin. Geriatr. Med. 2013, 29, 737–752. [Google Scholar] [CrossRef]
- Lajoie, Y.; Teasdale, N.; Bard, C.; Fleury, M. Upright Standing and Gait: Are There Changes in Attentional Requirements Related to Normal Aging? Exp. Aging Res. 1996, 22, 185–198. [Google Scholar] [CrossRef]
- Woollacott, M.; Shumway-Cook, A. Attention and the Control of Posture and Gait: A Review of an Emerging Area of Research. Gait Posture 2002, 16, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sunderaraman, P.; Maidan, I.; Kozlovski, T.; Apa, Z.; Mirelman, A.; Hausdorff, J.M.; Stern, Y. Differential Associations between Distinct Components of Cognitive Function and Mobility: Implications for Understanding Aging, Turning and Dual-Task Walking. Front. Aging Neurosci. 2019, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Boyé, N.D.A.; Mattace-Raso, F.U.S.; Van der Velde, N.; Van Lieshout, E.M.M.; De Vries, O.J.; Hartholt, K.A.; Kerver, A.J.H.; Bruijninckx, M.M.M.; Van der Cammen, T.J.M.; Patka, P.; et al. Circumstances Leading to Injurious Falls in Older Men and Women in the Netherlands. Injury 2014, 45, 1224–1230. [Google Scholar] [CrossRef]
- Zijlstra, W.; Hof, A.L. Assessment of Spatio-Temporal Gait Parameters from Trunk Accelerations during Human Walking. Gait Posture 2003, 18, 1–10. [Google Scholar] [CrossRef]
- Lord, S.; Galna, B.; Verghese, J.; Coleman, S.; Burn, D.; Rochester, L. Independent Domains of Gait in Older Adults and Associated Motor and Nonmotor Attributes: Validation of a Factor Analysis Approach. J. Gerontol Ser. A Biol. Sci. Med. Sci. 2013, 68, 820–827. [Google Scholar] [CrossRef]
- Roetenberg, D.; Luinge, H.; Slycke, P. Xsens MVN: Full 6DOF Human Motion Tracking Using Miniature Inertial Sensors. Xsens Motion Technol. BV 2009, 1–7. [Google Scholar]
- Seel, T.; Raisch, J.; Schauer, T. IMU-Based Joint Angle Measurement for Gait Analysis. Sensors 2014, 14, 6891–6909. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, S.; Trung, T.Q.; Lee, N.E. Recent Progress, Challenges, and Prospects of Fully Integrated Mobile and Wearable Point-of-Care Testing Systems for Self-Testing. Chem. Soc. Rev. 2020, 49, 1812–1866. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Rao, Z.; Zhang, W.; Liu, C.; Wang, Z.; Zhang, S.; Zhang, B.; Hu, M.; Servati, P.; Xiao, X. Airline Point-of-Care System on Seat Belt for Hybrid Physiological Signal Monitoring. Micromachines 2022, 13, 1880. [Google Scholar] [CrossRef]
- Cho, Y.-S.; Jang, S.-H.; Cho, J.-S.; Kim, M.-J.; Lee, H.D.; Lee, S.Y.; Moon, S.-B. Evaluation of Validity and Reliability of Inertial Measurement Unit-Based Gait Analysis Systems. Ann. Rehabil. Med. 2018, 42, 872–883. [Google Scholar] [CrossRef] [PubMed]
- Horak, F.; King, L.; Mancini, M. Role of Body-Worn Movement Monitor Technology for Balance and Gait Rehabilitation. Phys. Ther. 2015, 95, 461–470. [Google Scholar] [CrossRef]
- Maetzler, W.; Rochester, L. Body-Worn Sensors--the Brave New World of Clinical Measurement? Mov. Disord. Off. J. Mov. Disord. Soc. 2015, 30, 1203–1205. [Google Scholar] [CrossRef]
- Del Din, S.; Godfrey, A.; Galna, B.; Lord, S.; Rochester, L. Free-Living Gait Characteristics in Ageing and Parkinson’s Disease: Impact of Environment and Ambulatory Bout Length. J. Neuroeng. Rehabil. 2016, 13, 1–12. [Google Scholar] [CrossRef]
- Labini, F.S.; Meli, A.; Ivanenko, Y.P.; Tufarelli, D. Recurrence Quantification Analysis of Gait in Normal and Hypovestibular Subjects. Gait Posture 2012, 35, 48–55. [Google Scholar] [CrossRef]
- Bisi, M.C.; Riva, F.; Stagni, R. Measures of Gait Stability: Performance on Adults and Toddlers at the Beginning of Independent Walking. J. Neuroeng. Rehabil. 2014, 11, 131. [Google Scholar] [CrossRef]
- Menz, H.B.; Lord, S.R.; Fitzpatrick, R.C. Acceleration Patterns of the Head and Pelvis When Walking Are Associated with Risk of Falling in Community-Dwelling Older People. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2003, 58, M446–M452. [Google Scholar] [CrossRef]
- Bellanca, J.L.; Lowry, K.A.; VanSwearingen, J.M.; Brach, J.S.; Redfern, M.S. Harmonic Ratios: A Quantification of Step to Step Symmetry. J. Biomech. 2013, 46, 828–831. [Google Scholar] [CrossRef] [PubMed]
- Lowry, K.A.; Lokenvitz, N.; Smiley-Oyen, A.L. Age- and Speed-Related Differences in Harmonic Ratios during Walking. Gait Posture 2012, 35, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Menz, H.B.; Lord, S.R.; St George, R.; Fitzpatrick, R.C. Walking Stability and Sensorimotor Function in Older People with Diabetic Peripheral Neuropathy. Arch. Phys. Med. Rehabil. 2004, 85, 245–252. [Google Scholar] [CrossRef]
- Lowry, K.A.; Smiley-Oyen, A.L.; Carrel, A.J.; Kerr, J.P. Walking Stability Using Harmonic Ratios in Parkinson’s Disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2009, 24, 261–267. [Google Scholar] [CrossRef]
- Pau, M.; Mandaresu, S.; Pilloni, G.; Porta, M.; Coghe, G.; Marrosu, M.G.; Cocco, E. Smoothness of Gait Detects Early Alterations of Walking in Persons with Multiple Sclerosis without Disability. Gait Posture 2017, 58, 307–309. [Google Scholar] [CrossRef] [PubMed]
- Iosa, M.; Paradisi, F.; Brunelli, S.; Delussu, A.S.; Pellegrini, R.; Zenardi, D.; Paolucci, S.; Traballesi, M. Assessment of Gait Stability, Harmony, and Symmetry in Subjects with Lower-Limb Amputation Evaluated by Trunk Accelerations. J. Rehabil. Res. Dev. 2014, 51, 623–634. [Google Scholar] [CrossRef]
- Cimolin, V.; Cau, N.; Sartorio, A.; Capodaglio, P.; Galli, M.; Tringali, G.; Leban, B.; Porta, M.; Pau, M. Symmetry of Gait in Underweight, Normal and Overweight Children and Adolescents. Sensors 2019, 19, 2054. [Google Scholar] [CrossRef]
- Brach, J.S.; McGurl, D.; Wert, D.; Vanswearingen, J.M.; Perera, S.; Cham, R.; Studenski, S. Validation of a Measure of Smoothness of Walking. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2011, 66, 136–141. [Google Scholar] [CrossRef]
- Leban, B.; Cimolin, V.; Porta, M.; Arippa, F.; Pilloni, G.; Galli, M.; Pau, M. Age-Related Changes in Smoothness of Gait of Healthy Children and Early Adolescents. J. Mot. Behav. 2020, 52, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Lowry, K.A.; Sebastian, K.; Perera, S.; Van Swearingen, J.; Smiley-Oyen, A.L. Age-Related Differences in Locomotor Strategies During Adaptive Walking. J. Mot. Behav. 2017, 49, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Misu, S.; Asai, T.; Doi, T.; Sawa, R.; Ueda, Y.; Saito, T.; Nakamura, R.; Murata, S.; Sugimoto, T.; Yamada, M.; et al. Association between Gait Abnormality and Malnutrition in a Community-Dwelling Elderly Population. Geriatr. Gerontol. Int. 2017, 17, 1155–1160. [Google Scholar] [CrossRef]
- Asai, T.; Misu, S.; Sawa, R.; Doi, T.; Yamada, M. The Association between Fear of Falling and Smoothness of Lower Trunk Oscillation in Gait Varies According to Gait Speed in Community-Dwelling Older Adults. J. Neuroeng. Rehabil. 2017, 14, 1–9. [Google Scholar] [CrossRef]
- Row Lazzarini, B.S.; Kataras, T.J. Treadmill Walking Is Not Equivalent to Overground Walking for the Study of Walking Smoothness and Rhythmicity in Older Adults. Gait Posture 2016, 46, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Pau, M.; Mulas, I.; Putzu, V.; Asoni, G.; Viale, D.; Mameli, I.; Leban, B.; Allali, G. Smoothness of Gait in Healthy and Cognitively Impaired Individuals: A Study on Italian Elderly Using Wearable Inertial Sensor. Sensors 2020, 20, 3577. [Google Scholar] [CrossRef]
- Hollman, J.H.; McDade, E.M.; Petersen, R.C. Normative Spatiotemporal Gait Parameters in Older Adults. Gait Posture 2011, 34, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Brach, J.S.; Perera, S.; Studenski, S.; Katz, M.; Hall, C.; Verghese, J. Meaningful Change in Measures of Gait Variability in Older Adults. Gait Posture 2010, 31, 175–179. [Google Scholar] [CrossRef]
- Bugané, F.; Benedetti, M.G.; Casadio, G.; Attala, S.; Biagi, F.; Manca, M.; Leardini, A. Estimation of Spatial-Temporal Gait Parameters in Level Walking Based on a Single Accelerometer: Validation on Normal Subjects by Standard Gait Analysis. Comput. Methods Programs Biomed. 2012, 108, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Ilaria Viscione; Francesca D’Elia; Rodolfo Vastola; Maurizio Sibilio the Correlation Between Technologies and Rating Scales in Gait Analysis. J. Sport. Sci. 2016, 4, 119–123. [CrossRef]
- Pau, M.; Porta, M.; Pilloni, G.; Corona, F.; Fastame, M.C.; Hitchcott, P.K.; Penna, M.P. Texting While Walking Induces Gait Pattern Alterations in Healthy Older Adults. Proc. Hum. Factors Ergon. Soc. Annu. Meet. 2018, 62, 1908–1912. [Google Scholar] [CrossRef]
- Zijlstra, W. Assessment of Spatio-Temporal Parameters during Unconstrained Walking. Eur. J. Appl. Physiol. 2004, 92, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Mazzà, C.; Iosa, M.; Pecoraro, F.; Cappozzo, A. Control of the Upper Body Accelerations in Young and Elderly Women during Level Walking. J. Neuroeng. Rehabil. 2008, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Abellan van Kan, G.; Rolland, Y.; Andrieu, S.; Bauer, J.; Beauchet, O.; Bonnefoy, M.; Cesari, M.; Donini, L.M.; Gillette Guyonnet, S.; Inzitari, M.; et al. Gait Speed at Usual Pace as a Predictor of Adverse Outcomes in Community-Dwelling Older People an International Academy on Nutrition and Aging (IANA) Task Force. J. Nutr. Health Aging 2009, 13, 881–889. [Google Scholar] [CrossRef]
- Studenski, S.; Perera, S.; Patel, K.; Rosano, C.; Faulkner, K.; Inzitari, M.; Brach, J.; Chandler, J.; Cawthon, P.; Connor, E.B.; et al. Gait Speed and Survival in Older Adults. JAMA 2011, 305, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Dommershuijsen, L.J.; Ragunathan, J.; Ruiter, T.R.; Groothof, D.; Mattace-Raso, F.U.S.; Ikram, M.A.; Polinder-Bos, H.A. Gait Speed Reference Values in Community-Dwelling Older Adults – Cross-Sectional Analysis from the Rotterdam Study. Exp. Gerontol. 2022, 158, 111646. [Google Scholar] [CrossRef] [PubMed]
- Aboutorabi, A.; Arazpour, M.; Bahramizadeh, M.; Hutchins, S.W.; Fadayevatan, R. The Effect of Aging on Gait Parameters in Able-Bodied Older Subjects: A Literature Review. Aging Clin. Exp. Res. 2016, 28, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Brach, J.S.; Studenski, S.A.; Perera, S.; VanSwearingen, J.M.; Newman, A.B. Gait Variability and the Risk of Incident Mobility Disability in Community-Dwelling Older Adults. J. Gerontol. Ser. A 2007, 62, 983–988. [Google Scholar] [CrossRef]
- Brach, J.S.; Studenski, S.; Perera, S.; VanSwearingen, J.M.; Newman, A.B. Stance Time and Step Width Variability Have Unique Contributing Impairments in Older Persons. Gait Posture 2008, 27, 431–439. [Google Scholar] [CrossRef]
- Jones, L.M.; Waters, D.L.; Legge, M. Walking Speed at Self-Selected Exercise Pace Is Lower but Energy Cost Higher in Older versus Younger Women. J. Phys. Act. Health 2009, 6, 327–332. [Google Scholar] [CrossRef]
- Kavanagh, J.J.; Barrett, R.S.; Morrison, S. Age-Related Differences in Head and Trunk Coordination during Walking. Hum. Mov. Sci. 2005, 24, 574–587. [Google Scholar] [CrossRef]
- Mesure, S.; Azulay, J.P.; Pouget, J.; Amblard, B. Strategies of Segmental Stabilization during Gait in Parkinson’s Disease. Exp. Brain Res. 1999, 129, 573–581. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, S.M.; Kuo, A.D. Direction-Dependent Control of Balance during Walking and Standing. J. Neurophysiol. 2009, 102, 1411–1419. [Google Scholar] [CrossRef]
- Brodie, M.A.D.; Menz, H.B.; Lord, S.R. Age-Associated Changes in Head Jerk While Walking Reveal Altered Dynamic Stability in Older People. Exp. Brain Res. 2014, 232, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Chou, L.-S.; Kaufman, K.R.; Hahn, M.E.; Brey, R.H. Medio-Lateral Motion of the Center of Mass during Obstacle Crossing Distinguishes Elderly Individuals with Imbalance. Gait Posture 2003, 18, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Cofré Lizama, L.E.; Pijnappels, M.; Reeves, N.P.; Verschueren, S.M.; van Dieën, J.H. Centre of Pressure or Centre of Mass Feedback in Mediolateral Balance Assessment. J. Biomech. 2015, 48, 539–543. [Google Scholar] [CrossRef]
- Singer, J.C.; McIlroy, W.E.; Prentice, S.D. Kinetic Measures of Restabilisation during Volitional Stepping Reveal Age-Related Alterations in the Control of Mediolateral Dynamic Stability. J. Biomech. 2014, 47, 3539–3545. [Google Scholar] [CrossRef]
- Maki, B.E. Gait Changes in Older Adults: Predictors of Falls or Indicators of Fear. J. Am. Geriatr. Soc. 1997, 45, 313–320. [Google Scholar] [CrossRef]
- Casas-Herrero, A.; Anton-Rodrigo, I.; Zambom-Ferraresi, F.; Sáez de Asteasu, M.L.; Martinez-Velilla, N.; Elexpuru-Estomba, J.; Marin-Epelde, I.; Ramon-Espinoza, F.; Petidier-Torregrosa, R.; Sanchez-Sanchez, J.L.; et al. Effect of a Multicomponent Exercise Programme (VIVIFRAIL) on Functional Capacity in Frail Community Elders with Cognitive Decline: Study Protocol for a Randomized Multicentre Control Trial. Trials 2019, 20, 362. [Google Scholar] [CrossRef]
- Amorese, A.J.; Ryan, A.S. Home-Based Tele-Exercise in Musculoskeletal Conditions and Chronic Disease: A Literature Review. Front. Rehabil. Sci. 2022, 3. [Google Scholar] [CrossRef]
- Baez, M.; Khaghani Far, I.; Ibarra, F.; Ferron, M.; Didino, D.; Casati, F. Effects of Online Group Exercises for Older Adults on Physical, Psychological and Social Wellbeing: A Randomized Pilot Trial. PeerJ 2017, 5, e3150. [Google Scholar] [CrossRef]
- Sherrington, C.; Fairhall, N.J.; Wallbank, G.K.; Tiedemann, A.; Michaleff, Z.A.; Howard, K.; Clemson, L.; Hopewell, S.; Lamb, S.E. Exercise for Preventing Falls in Older People Living in the Community. Cochrane Database Syst. Rev. 2019, 1, CD012424. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Low, L.-F.; Gwynn, J.D.; Clemson, L. Interventions to Improve Gait in Older Adults with Cognitive Impairment: A Systematic Review. J. Am. Geriatr. Soc. 2019, 67, 381–391. [Google Scholar] [CrossRef] [PubMed]

| Group 1 (65–74 Years) | Group 2 (75–85 Years) | Group 3 (>85 Years) | |
|---|---|---|---|
| Participants # (F, M) | 175 (103 F, 72 M) | 228 (128 F, 100 M) | 47 (28 F, 19 M) |
| Participants percentage (F, M) | F 59%, M 41% | F 56%, M 44% | F 60%, M 40% |
| Age (years) | 70.4 ± 2.5 | 79.1 ± 2.8 a | 86.5 ± 1.7 a,b |
| Body Mass (kg) | 66.8 ± 12.4 | 65.6 ± 11.4 | 61.3 ± 13.6 a,b |
| Height (cm) | 162.0 ± 8.4 | 160.0 ± 8.7 a | 158.6 ± 8.5 a |
| Group 1 (65–74 Years) | Group 2 (75–85 Years) | Group 3 (>85 Years) | ||
|---|---|---|---|---|
| Spatial-temporal parameters of gait | Gait speed (m s−1) | 1.08 ± 0.24 | 0.98 ± 0.24 a | 0.91 ± 0.26 a |
| Stride length (m) | 1.16 ± 0.21 | 1.05 ± 0.22 a | 1.04 ± 0.27 a | |
| Cadence (steps min−1) | 111.20 ± 9.46 | 111.61 ± 10.75 | 105.20 ± 13.00 a,b | |
| Stance phase (% of the GC) | 60.46 ± 2.55 | 60.82 ± 1.97 | 61.28 ± 2.74 | |
| Swing phase (% of the GC) | 39.42 ± 3.00 | 39.06 ± 2.73 | 39.04 ± 3.32 | |
| Double support phase (% of the GC) | 10.56 ± 2.08 | 10.80 ± 1.98 | 11.21 ± 2.71 | |
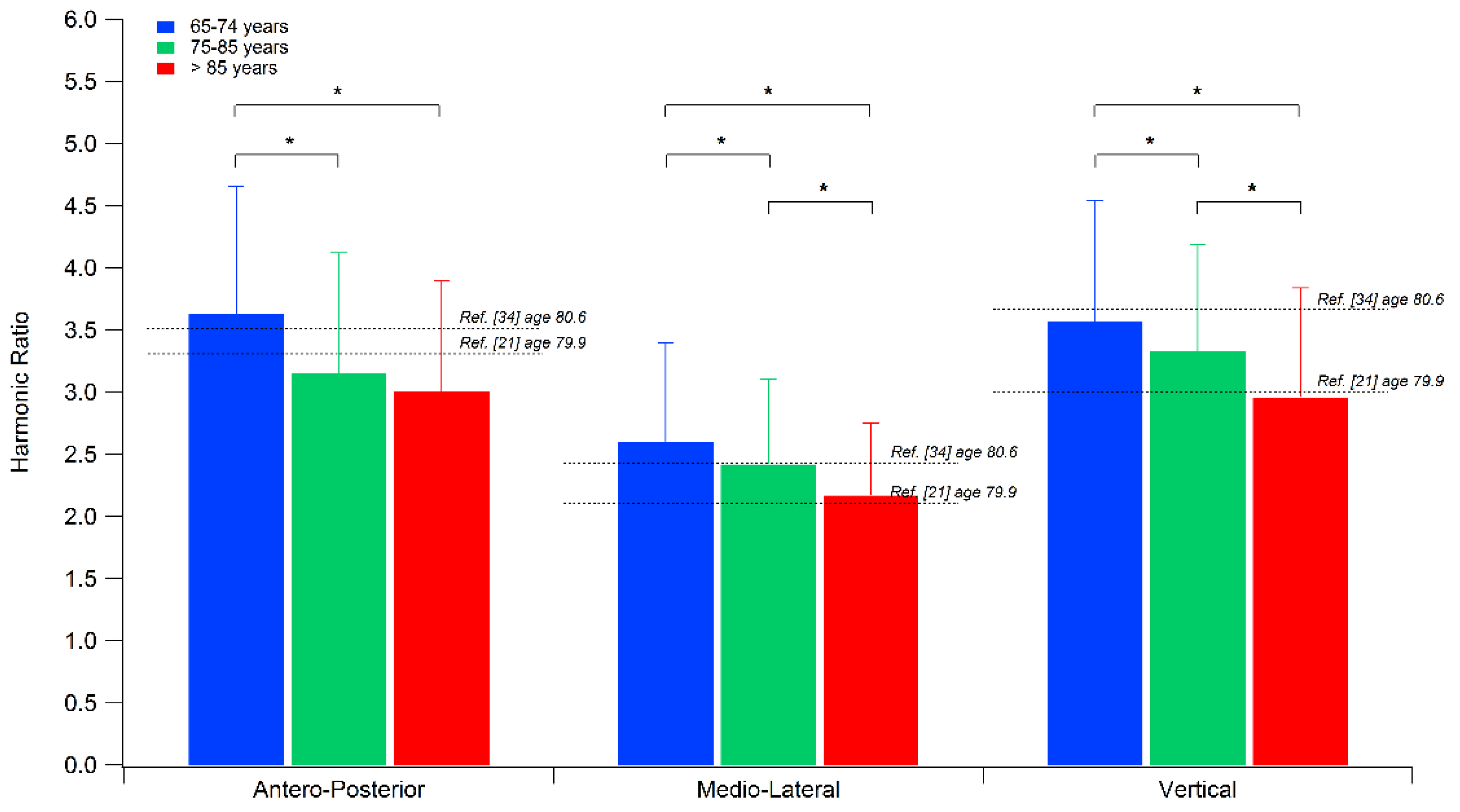
| Harmonic Ratio * | AP direction * | 3.63 ± 1.03 | 3.15 ± 0.97 a | 3.01 ± 0.89 a |
| ML direction * | 2.60 ± 0.80 | 2.42 ± 0.69 a | 2.17 ± 0.58 a,b | |
| V direction * | 3.57 ± 0.97 | 3.33 ±0.86 a | 2.96 ± 0.88 a,b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pau, M.; Bernardelli, G.; Leban, B.; Porta, M.; Putzu, V.; Viale, D.; Asoni, G.; Riccio, D.; Cerfoglio, S.; Galli, M.; et al. Age-Associated Changes on Gait Smoothness in the Third and the Fourth Age. Electronics 2023, 12, 637. https://doi.org/10.3390/electronics12030637
Pau M, Bernardelli G, Leban B, Porta M, Putzu V, Viale D, Asoni G, Riccio D, Cerfoglio S, Galli M, et al. Age-Associated Changes on Gait Smoothness in the Third and the Fourth Age. Electronics. 2023; 12(3):637. https://doi.org/10.3390/electronics12030637
Chicago/Turabian StylePau, Massimiliano, Giuseppina Bernardelli, Bruno Leban, Micaela Porta, Valeria Putzu, Daniela Viale, Gesuina Asoni, Daniela Riccio, Serena Cerfoglio, Manuela Galli, and et al. 2023. "Age-Associated Changes on Gait Smoothness in the Third and the Fourth Age" Electronics 12, no. 3: 637. https://doi.org/10.3390/electronics12030637
APA StylePau, M., Bernardelli, G., Leban, B., Porta, M., Putzu, V., Viale, D., Asoni, G., Riccio, D., Cerfoglio, S., Galli, M., & Cimolin, V. (2023). Age-Associated Changes on Gait Smoothness in the Third and the Fourth Age. Electronics, 12(3), 637. https://doi.org/10.3390/electronics12030637










