Characterization of a Novel Packaged Hydrogel Wound Dressing by 2.35 T Magnetic Resonance Imaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Novel Dressing Production Process
2.3. Hydrogel Samples Design
2.4. Hydrogel Samples Preparation
2.5. Gamma Irradiation
2.6. MRI Hardware
2.7. MRI Acquisitions
2.8. MRI Data Statistical Analysis
2.9. Swelling Measurements
3. Results
3.1. Quantitative MRI
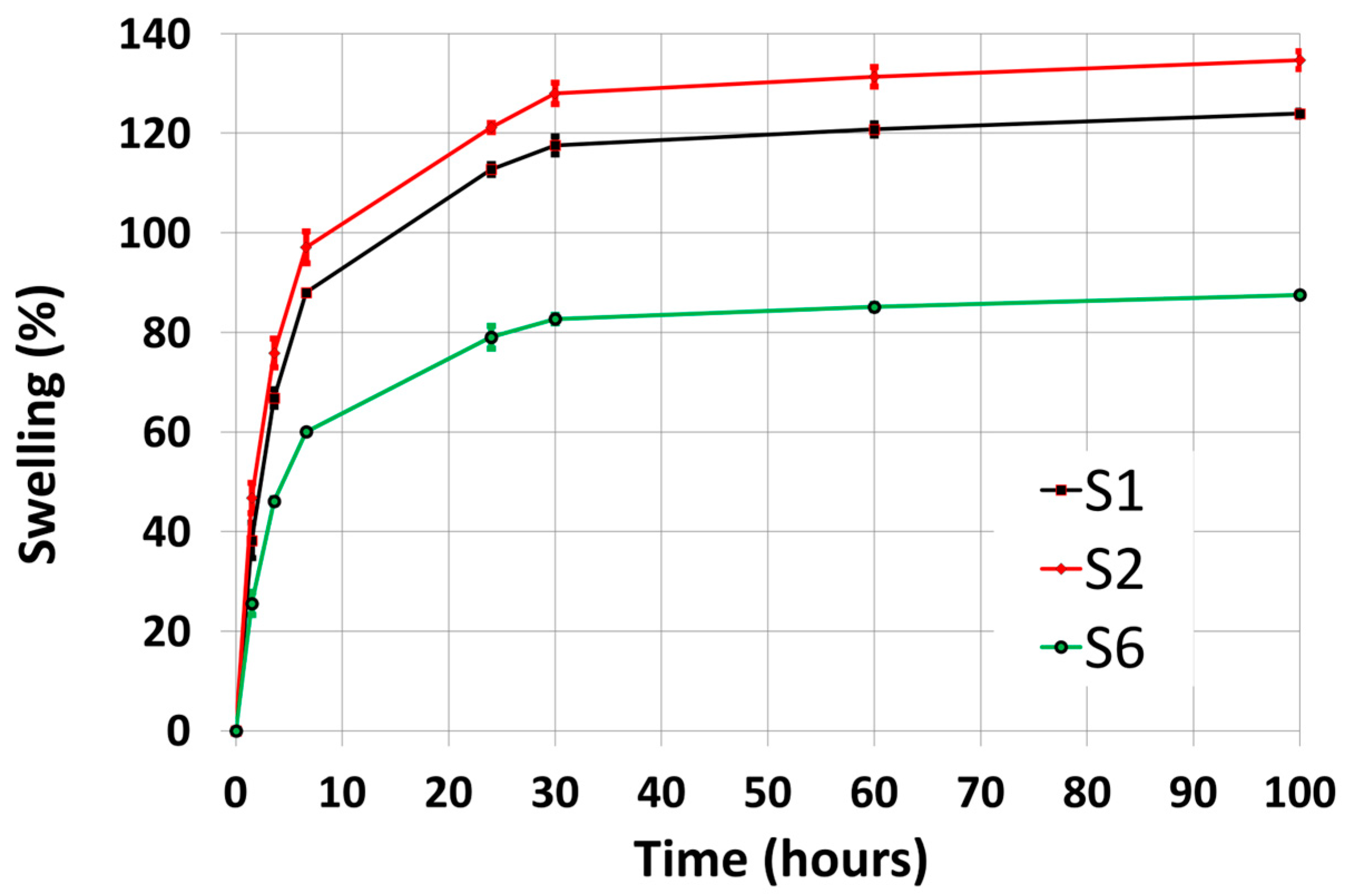
3.2. Swelling
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PVP | Polyvinylpyrrolidone |
| MRI | Magnetic Resonance Imaging |
| PD | Proton Density |
| T1 | Longitudinal relaxation times |
| T2 | Transverse relaxation times |
| T2* | Phase-coherence relaxation times |
| ADC | Apparent Diffusion Coefficient |
| PEG | Polyethylene Glycol |
| PET | Polyethylene terephthalate |
| DSV | Diameter Spherical Volume |
| RF | Radio Frequency |
| NSPEC | Single Pulse Sequence |
| NMR | Nuclear Magnetic Resonance |
| TR | Repetition Time |
| tp | Pulse Length |
| BW | Bandwidth |
| NEX | Number of Excitations |
| TACQ | Total Acquisition Time |
| GE | Gradient Echo |
| FLASH | Fast Low Angle Shot |
| TE | Echo Time |
| FOV | Field Of View |
| AP | Anterior-posterior |
| RAREVTR | Rapid Images with Refocused Echoes and Variable Repetition Time |
| MSME | Multi-Slice Multi-Echo |
| MGE | Multiple Gradient Echo |
| SE | Spin Echo |
| b | Diffusion gradients |
| ROI | Region-of-Interest |
| SF | Swelling Degree |
| CAL | Crosslinked calcium alginate |
References
- Ladet, S.; David, L.; Domard, A. Multi-membrane hydrogels. Nature 2008, 452, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Vermonden, T.; Klumperman, B. The past, present and future of hydrogels. Europ. Polym. J. 2015, 72, 341–343. [Google Scholar] [CrossRef]
- Naahidi, S.; Jafari, M.; Logan, M.; Wang, Y.; Yuan, Y.; Bae, H.; Dixon, B.; Chen, P. Biocompatibility of hydrogel-based scaffolds for tissue engineering applications. Biotechnol. Adv. 2017, 35, 530–544. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Shim, K.Y.; Kim, B.; Sung, J.H. Hydrogel-based three-dimensional cell culture for organ-on-a-chip applications. Biotechnol. Prog. 2017, 33, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Ivanovska, J.; Zehnder, T.; Lennert, P.; Sarker, B.; Boccaccini, A.R.; Hartmann, A.; Schneider-Stock, R.; Detsch, R. Biofabrication of 3D Alginate-Based Hydrogel for Cancer Research: Comparison of Cell Spreading, Viability, and Adhesion Characteristics of Colorectal HCT116 Tumor Cells. Tissue Eng. Part C Methods 2016, 22, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Foyt, D.A.; Norman, M.D.A.; Yu, T.T.L.; Gentleman, E. Exploiting Advanced Hydrogel Technologies to Address Key Challenges in Regenerative Medicine. Adv. Healthcare Mater. 2018, 7, 1700939. [Google Scholar] [CrossRef] [PubMed]
- Bakker, M.H.; Tseng, C.C.S.; Keizer, H.M.; Seevinck, P.R.; Janssen, H.M.; Van Slochteren, F.J.; Chamuleau, S.A.J.; Dankers, P.Y.W. MRI Visualization of Injectable Ureidopyrimidinone Hydrogelators by Supramolecular Contrast Agent Labeling. Adv. Healthcare Mater. 2018, 7, 1701139. [Google Scholar] [CrossRef]
- Pourshahrestani, S.; Zeimaran, E.; Kadri, N.A.; Mutlu, N.; Boccaccini, A.R. Polymeric Hydrogel Systems as Emerging Biomaterial Platforms to Enable Hemostasis and Wound Healing. Adv. Healthcare Mater. 2020, 9, 2000905. [Google Scholar] [CrossRef]
- Tang, N.; Zheng, Y.; Cui, D.; Haick, H. Multifunctional Dressing for Wound Diagnosis and Rehabilitation. Adv. Healthcare Mater. 2021, 10, 2101292. [Google Scholar] [CrossRef]
- Subchapter, H. (Ed.) US Food and Drug Administration Department of Health and Human Services; Title 21, Chapter I; Medical Devices: London, UK.
- Commissione Regionale Dispositivi Medici (Delibera Giunta Regionale n. 1523/2008), Le Medicazioni Avanzate per il Trattamento Delle Ferite Acute e Croniche. Available online: https://salute.regione.emilia-romagna.it/normativa-e-documentazione/rapporti/dispositivi-medici/le-medicazioni-avanzate-per-il-trattamento-delle-ferite-acute-e-croniche-2016 (accessed on 20 October 2022).
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Alam, P.; Shakeel, F.; Anwer, M.K.; Foudah, A.I.; Alqarni, M.H. Wound Healing Study of Eucalyptus Essential Oil Containing Nanoemulsion in Rat Model. J. Oleo Sci. 2018, 67, 957–968. [Google Scholar] [CrossRef]
- Murakami, Y.; Fujino, T.; Kurachi, R.; Hasegawa, T.; Usui, T.; Hayase, F.; Watanabe, H. Identification of pyridinoline, a collagen crosslink, as a novel intrinsic ligand for the receptor for advanced glycation end-products (RAGE). Biosci. Biotechnol. Biochem. 2018, 82, 1508–1514. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.M.S. ARTIFICIAL CELL evolves into nanomedicine, biotherapeutics, blood substitutes, drug delivery, enzyme/gene therapy, cancer therapy, cell/stem cell therapy, nanoparticles, liposomes, bioencapsulation, replicating synthetic cells, cell encapsulation/scaffold, biosorbent/immunosorbent haemoperfusion/plasmapheresis, regenerative medicine, encapsulated microbe, nanobiotechnology, nanotechnology. Artif. Cells Nanomed. Biotechnol. 2019, 47, 997–1013. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Tang, M.; Dai, J.; Wang, T.; Bai, R. Effect of multiwalled carbon nanotube-grafted polymer brushes on the mechanical and swelling properties of polyacrylamide composite hydrogels. Polymer 2016, 85, 67–76. [Google Scholar] [CrossRef]
- Li, Z.; Tang, M.; Bai, W.; Bai, R. Preparation of Hydrophilic Encapsulated Carbon Nanotubes with Polymer Brushes and Its Application in Composite Hydrogels. Langmuir 2017, 33, 6092–6101. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lin, Z. Recent advances in polysaccharide-based hydrogels for synthesis and applications. Aggregate 2021, 2, e21. [Google Scholar] [CrossRef]
- Rosiak, J.; Rucinska-Rybus, A.; Pekala, W. Method of Manufacturing Hydrogel Dressings. U.S. Patent No. 4,871,490, 30 December 1987. [Google Scholar]
- Olejnik, A.K. Hydrogel Wound Coverings. EP1789102 B1, 9 September 2004. [Google Scholar]
- Ajji, Z.; Othman, I.; Rosiak, J.M. Production of hydrogel wound dressings using gamma radiation. Nucl. Instr. Meth. Phys. Res. B 2005, 229, 375–380. [Google Scholar] [CrossRef]
- Singh, R.; Singh, D. Radiation synthesis of PVP/alginate hydrogel containing nanosilver as wound dressing. J. Mater. Sci. Mater. Med. 2012, 23, 2649–2658. [Google Scholar] [CrossRef]
- Pajewsky, L.A. Biocompatible Alginate Hydrogels. Italian Patent IT1278419, 20 November 1997. [Google Scholar]
- Yanli, X.; Xueying, Y.; Jun, D.; Lianzhu, D.; Changlin, L.; LinJing, H.; Chunming, S. Anti-Microbial Healing-Promoting Hydrogel Dressing and Preparation Method Therefor. Chinese Patent CN103520767, 22 January 2014. [Google Scholar]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Progr. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Kenawy, E.S.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Potter, K.; Carpenter, T.A.; Hall, L.D. Mapping of spatial variation in alginate with calcium ions studied by magnetic resonance imaging (MRI). Carbohydr. Res. 1993, 246, 43–49. [Google Scholar] [CrossRef]
- Degrassi, A.; Toffanin, R.; Paoletti, S.; Hall, L.D. A better understanding of the properties of alginate solutions and gels by quantitative MRI. Carbohydr. Res. 1998, 306, 19–26. [Google Scholar] [CrossRef]
- Simpson, N.E.; Grant, S.C.; Blackband, S.J.; Constantinidis, I. NMR properties of alginate microbeads. Biomaterials 2003, 24, 4941–4948. [Google Scholar] [CrossRef]
- Grant, S.C.; Celper, S.; Gauffin-Holmberg, I.; Simpson, N.E.; Blackband, S.J.; Constantinidis, I. Alginate assessment by NMR microscopy. J. Mater. Sci. Mater. Med. 2005, 16, 511–514. [Google Scholar] [CrossRef]
- Bascelli, M.; Pajewski, L.A.; Fracassi, A.; Sotgiu, A.; Alecci, M. NMR Spectroscopy and Imaging of Calcium Alginate Beads Reveals Structural Heterogeneity. Magn. Reson. Mater. Phy. 2008, 21, 307–308. [Google Scholar]
- Austin, D.T.R.; Hills, B.P. Two-dimensional NMR relaxation study of the pore structure in silicone hydrogel contact lenses. Appl. Magn. Reson. 2009, 35, 581–591. [Google Scholar] [CrossRef]
- Iuliano, C.; Piggott, R.B.; Venturi, L.; Hills, B.P. A two-dimensional relaxation study of the evolving microstructure in a mixed biopolymer gel. Appl. Magn. Reson. 2010, 38, 307–320. [Google Scholar] [CrossRef]
- Chan, K.W.; Liu, G.; Song, X.; Kim, H.; Yu, T.; Arifin, D.R.; Gilad, A.A.; Hanes, J.; Walczak, P.; van Zijl, P.C.; et al. MRI-detectable pH nanosensors incorporated into hydrogels for in vivo sensing of transplanted-cell viability. Nat. Mater. 2013, 12, 268–275. [Google Scholar] [CrossRef]
- Zhao, Y.; Terai, W.; Hoshijima, Y.; Gotoh, K.; Matsuura, K.; Matsumura, K. Development and Characterization of a Poly (Vinyl Alcohol)/Graphene Oxide Composite Hydrogel as An Artificial Cartilage Material. Appl. Sci. 2018, 8, 2772. [Google Scholar] [CrossRef]
- El-banna, F.S.; Mahfouz, M.E.; Leporatti, S.; El-Kemary, M.; Hanafy, N.A.N. Chitosan as a Natural Copolymer with Unique Properties for the Development of Hydrogels. Appl. Sci. 2019, 9, 2193. [Google Scholar] [CrossRef]
- Patel, M.; Nakaji-Hirabayashi, T.; Matsumura, K. Effect of dual- drug- releasing micelle-hydrogel composite on wound healing in vivo in full-thickness excision wound rat model. J. Biomed. Mat. Res. Part A 2019, 107, 1094–1106. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, K. Special Issue: Nanocomposite Hydrogels for Biomedical Applications. App. Sci. 2020, 10, 389. [Google Scholar] [CrossRef]
- Mathur, A.M.; AB Scranton, A.B. Characterization of hydrogels using nuclear magnetic resonance spectroscopy. Biomaterials 1996, 17, 547–557. [Google Scholar] [CrossRef]
- Potter, K.; Balcom, B.J.; Carpenter, T.A.; Hall, L.D. The gelation of sodium alginate with calcium ions studied by magnetic resonance imaging (MRI). Carbohydr. Res. 1994, 257, 117–126. [Google Scholar] [CrossRef]
- Manetti, C.; Casciani, L.; Pescosolido, N. Diffusive contribution to permeation of hydrogel contact lenses: Theoretical model and experimental evaluation by nuclear magnetic resonance techniques. Polymer 2002, 43, 87–92. [Google Scholar] [CrossRef]
- Chaudhry, Z.; Sammet, S.; Coffey, R.; Crockett, A.; Yuh, W.T.C.; Miller, S. Assessing the safety and compatibility of silver based wound dressings in a magnetic resonance environment. Burns 2009, 35, 1080–1085. [Google Scholar] [CrossRef]
- Bailey, J.K.; Sammet, S.; Overocker, J.; Craft-Coffman, B.; Acevedo, C.M.; Cowan, M.E.; Powell, H.M. MRI compatibility of silver based wound dressings. Burns 2018, 44, 1940–1946. [Google Scholar] [CrossRef]
- Pajewsky, L.A.; Corradini, V.; Alecci, M. Procedimento di Produzione di un Bendaggio Idrogel Confezionato. Italian Patent N. 102016000105584, 10 April 2019. [Google Scholar]
- Pajewsky, L.A.; Corradini, V.; Alecci, M. Process for Producing a Packaged Hydrogel Dressing. European Patent EP3311853B1, 15 July 2020. [Google Scholar]
- Brandolini, L.; Cristiano, L.; Fidoamore, A.; De Pizzol, M.; Di Giacomo, E.; Florio, T.M.; Confalone, G.; Galante, A.; Cinque, B.; Benedetti, E.; et al. Targeting CXCR1 on breast cancer stem cells: Signaling pathways and clinical application modelling. Oncotarget 2015, 6, 43375–43394. [Google Scholar] [CrossRef]
- Dellarosa, N.; Laghi, L.; Ragni, L.; Dalla Rosa, M.; Galante, A.; Ranieri, B.; Florio, T.M.; Alecci, M. Pulsed electric fields processing of apple tissue: Spatial distribution of electroporation by means of magnetic resonance imaging and computer vision system. Innov. Food Sci. Emerg. Technol. 2018, 47, 120–126. [Google Scholar] [CrossRef]
- Brown, R.W.; Cheng, Y.-C.N.; Haacke, E.M.; Thompson, M.R.; Venkatesan, R. Magnetic Resonance Imaging: Physical Principles and Sequence Design; John Wiley & Sons: New York, NY, USA, 2014. [Google Scholar]
- Rasband, W.S.; ImageJ. U.S. National Institutes of Health. Bethesda, Maryland, USA, 1997–2011. Available online: http://imagej.nih.gov/ij (accessed on 20 October 2022).
- Sievers, J.; Sperlich, K.; Stahnke, T.; Kreiner, C.; Eickner, T.; Martin, H.; Guthoff, R.F.; Schünemann, M.; Bohn, S.; Stachs, O. Determination of hydrogel swelling factors by two established and a novel non-contact continuous method. J. Appl. Polym. Sci. 2021, 138, e50326. [Google Scholar] [CrossRef]
- Wagner, F.; Laun, F.B.; Kuder, T.A.; Mlynarska, A.; Maier, F.; Faust, J.; Demberg, K.; Lindemann, L.; Rivkin, B.; Nagel, A.M.; et al. Temperature and concentration calibration of aqueous polyvinylpyrrolidone (PVP) solutions for isotropic diffusion MRI phantoms. PLoS ONE 2017, 19, e0179276. [Google Scholar] [CrossRef]
- Baker, J.B.; Stephens, D.R.; Blanch, H.W.; Prausnitz, J.M. Swelling equilibria for acrylamide-based polyampholyte hydrogels. Macromolecules 1992, 25, 1955–1958. [Google Scholar] [CrossRef]
- Cohen, Y.; Ramon, O.; Kopelman, I.J.; Mizrahi, S. Characterization of inhomogeneous polyacrylamide hydrogels. J. Polym. Sci. Part B 1992, 30, 1055–1067. [Google Scholar] [CrossRef]
- Feil, H.; Bae, Y.H.; Feijen, J.; Kim, S.W. Mutual influence of pH and temperature on the swelling of ionizable and thermosensitive hydrogels. Macromolecules 1992, 25, 5528–5530. [Google Scholar] [CrossRef]
- McConville, P.; Whittaker, M.K.; Pope, J.M. Water and Polymer Mobility in Hydrogel Biomaterials Quantified by 1H NMR: A Simple Model Describing Both T1 and T2 Relaxation. Macromolecules 2002, 35, 6961–6969. [Google Scholar] [CrossRef]
- Lai, S.; Locci, E.; Saba, G.; Husu, I.; Masci, G.; Crescenzi, V.; Lai, A. Solid-state 13C and 129Xe NMR study of poly(vinyl alcohol) and poly(vinyl alcohol)/lactosilated chitosan gels. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 3123–3131. [Google Scholar] [CrossRef]
- Simpson, N.E.; Grant, S.C.; Gustavsson, L.; Peltronen, V.; Blackband, S.J.; Constantinidis, I. Biochemical consequencies of alginate encapsulation: A NMR study of insulin secreting cells. Biomaterials 2006, 27, 2577–2586. [Google Scholar] [CrossRef]
- Trefná, H.D.; Ström, A. Hydrogels as a water bolus during hyperthermia treatment. Phys. Med. Biol. 2019, 64, 115025. [Google Scholar] [CrossRef]
- Goh, C.H.; Heng, P.W.; Huang, E.P.; Li, B.K.; Chan, L.W. Interactions of antimicrobial compounds with cross-linking agents of alginate dressings. J. Antimicrob. Chemother. 2008, 62, 105–108. [Google Scholar] [CrossRef]
- Paul, W.; Sharma, C.P. Chitosan and alginate wound dressings: A short review. Trends Biomater. Artif. Organs. 2004, 18, 18–23. [Google Scholar]

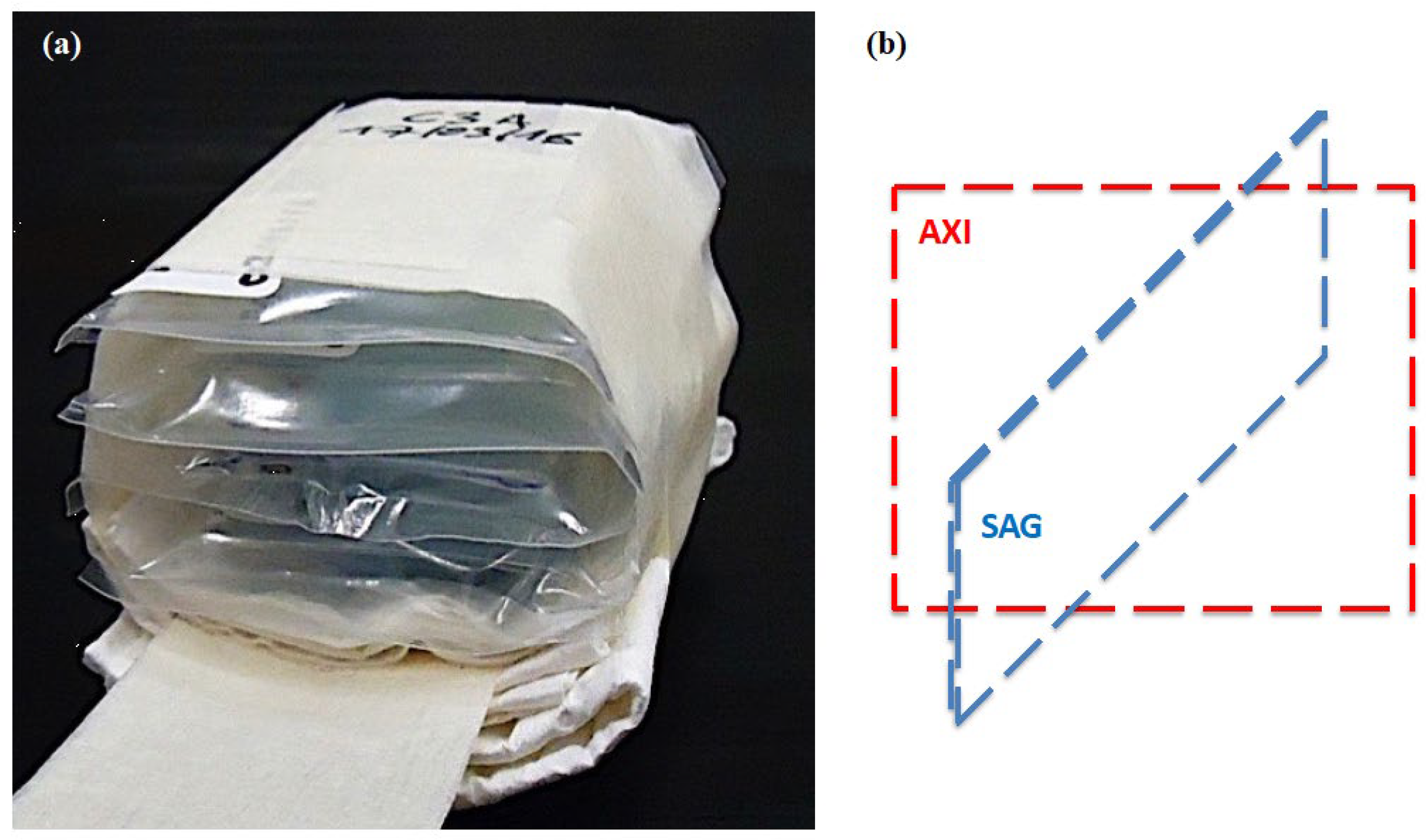
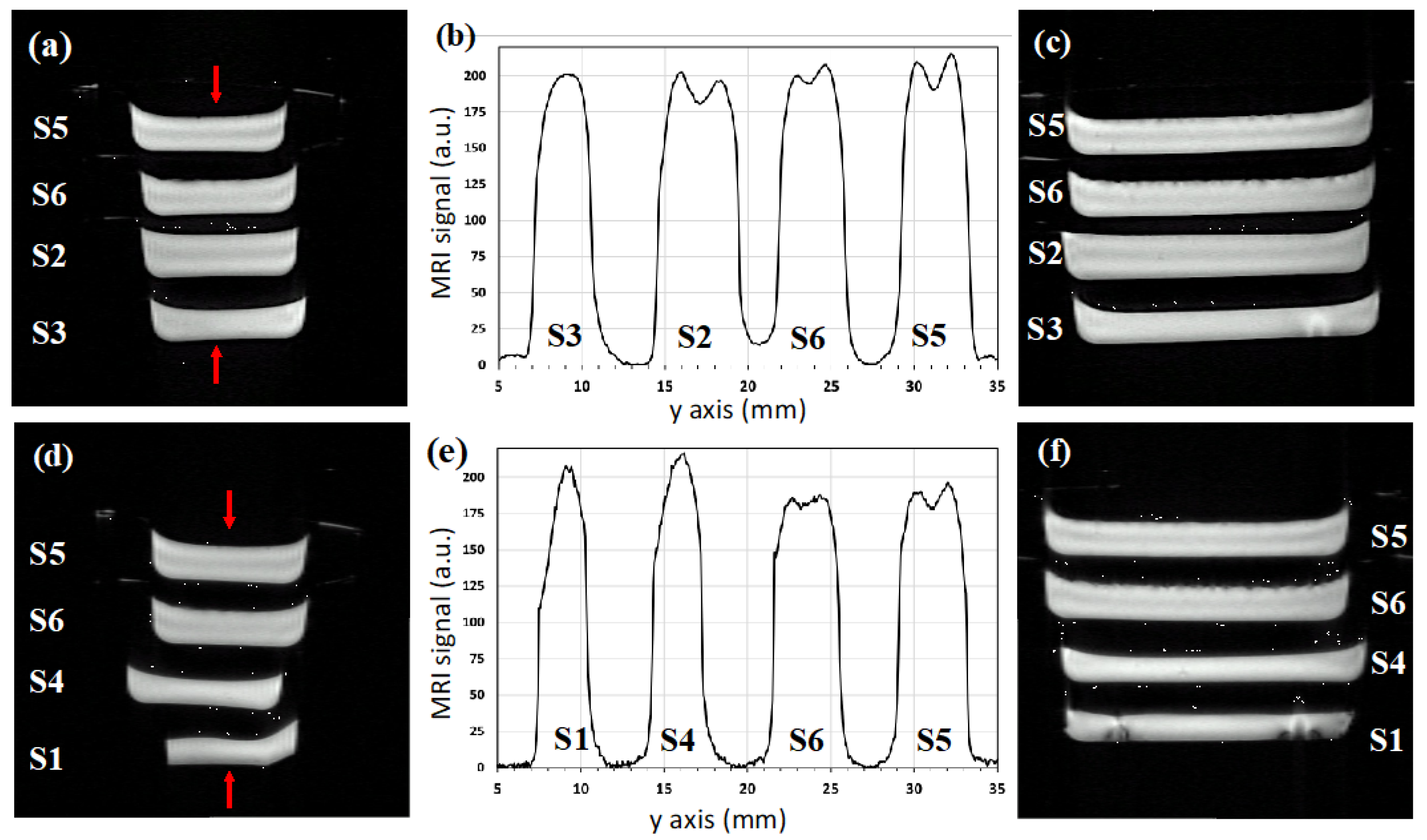
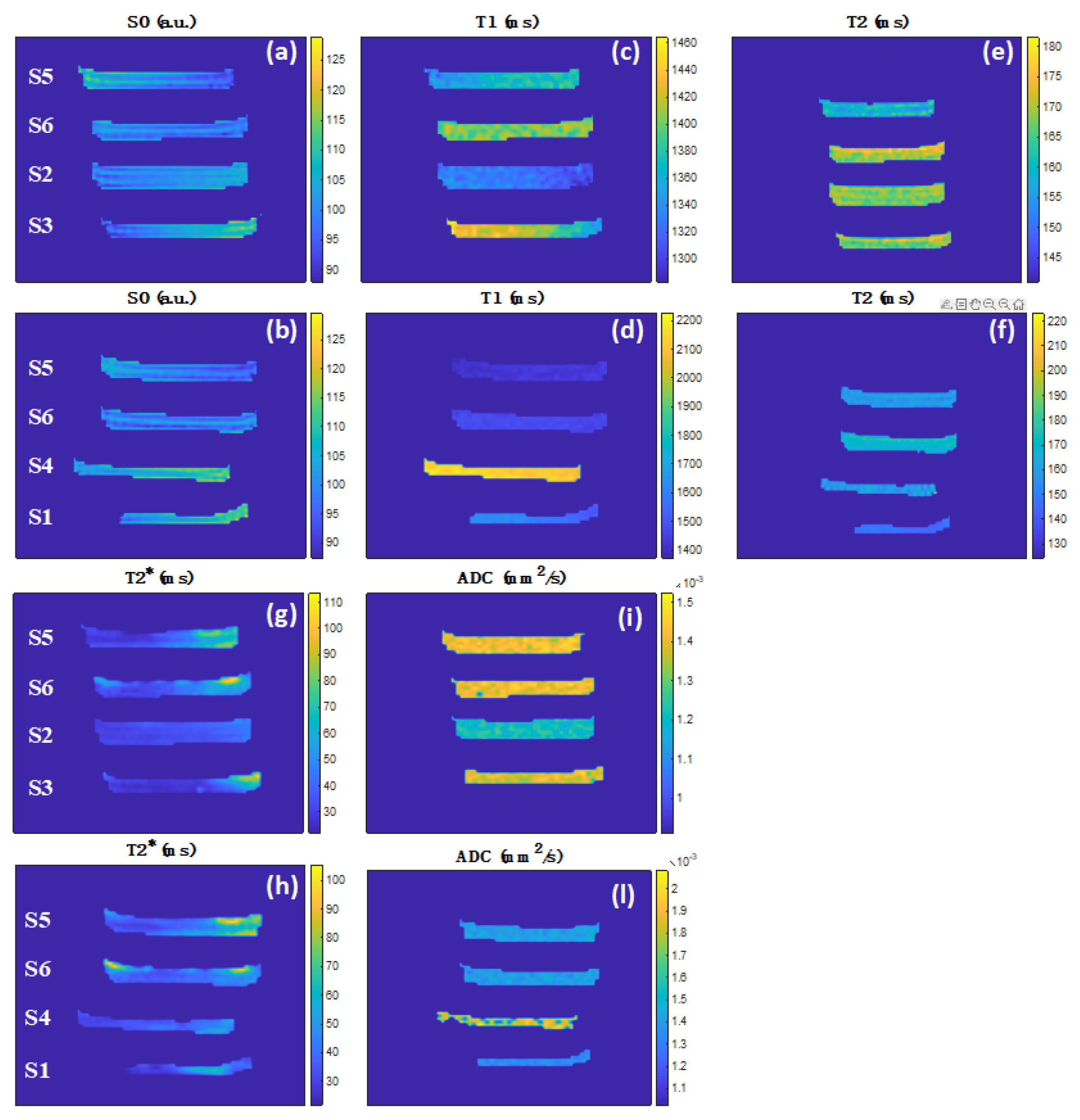
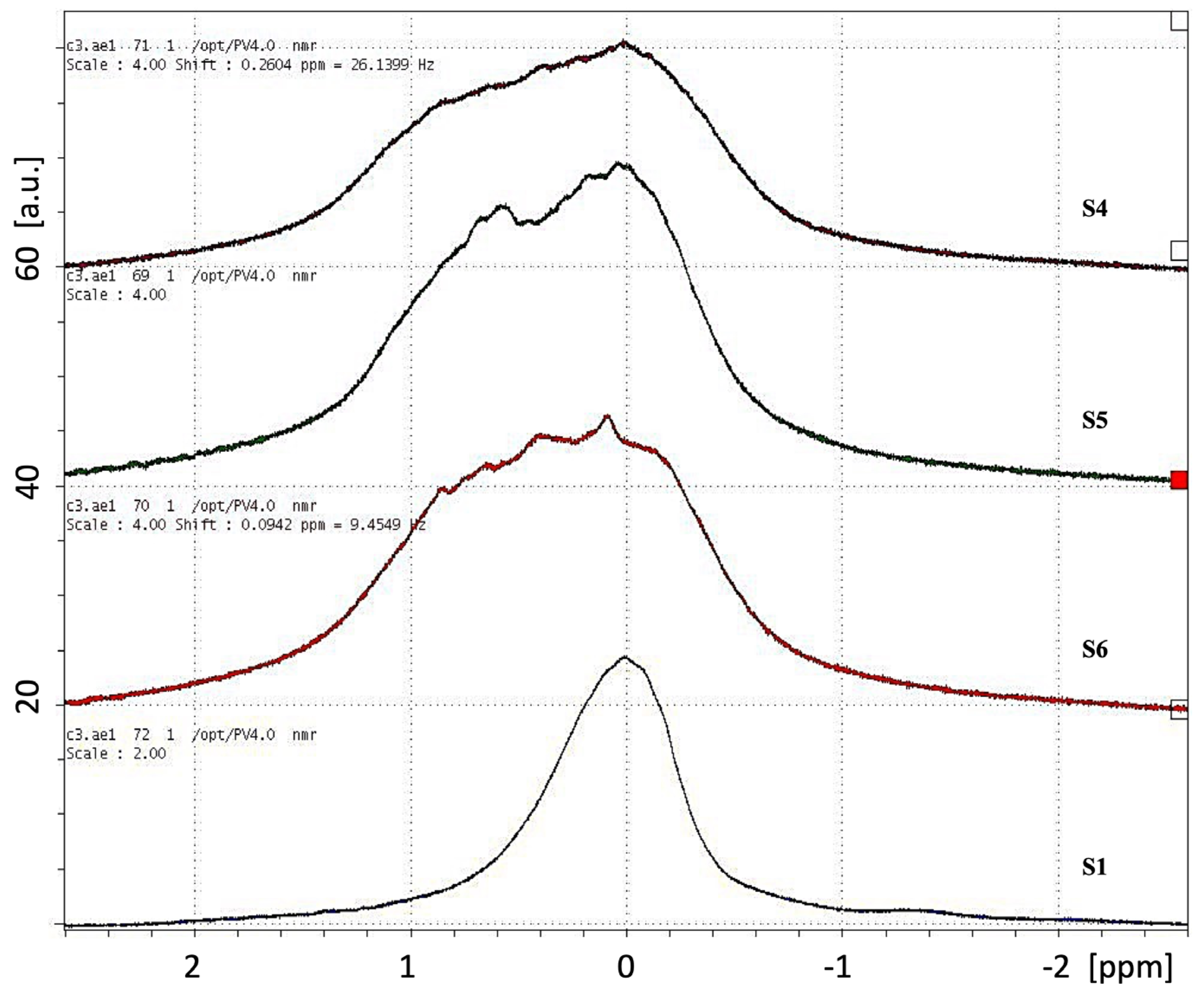
| Sample | Hydrogels | Agar (% w/v) | Sodium Alginate (% w/v) | CaCl2 (% w/v) | PEG (% w/v) | PVP (% w/v) | γ-Irradiation 25 kGy |
|---|---|---|---|---|---|---|---|
| S1 * | Neoheal® | 1 | No | No | 2 | 10 | Yes |
| S2 | H2O-AG-PEG-PVPγ | 1 | No | No | 2 | 10 | Yes |
| S3 | H2O-AG-PVPγ | 1 | No | No | No | 10 | Yes |
| S4 | H2O-AG-CAL | 1 | 0.2 | 1 | No | No | No |
| S5 | H2O-AG-CAL-PVP | 1 | 0.2 | 1 | No | 10 | No |
| S6 | H2O-AG-CAL-PVPγ | 1 | 0.2 | 1 | No | 10 | Yes |
| Sample | Hydrogels | PD ± 3 (a.u.) | T1 ± 30 (ms) | T2 ± 1 (ms) | T2* ± 1 (ms) | ADC ± 0.01 (×10−3 mm2 s−1) |
|---|---|---|---|---|---|---|
| S1 | Neoheal® | 112 | 1580 | 146 | 33 | 1.34 |
| S2 | H2O-AG-PEG-PVPγ | 104 | 1320 | 168 | 34 | 1.21 |
| S3 | H2O-AG-PVPγ | 106 | 1400 | 169 | 29 | 1.39 |
| S4 | H2O-AG-CAL | 111 | 2130 | 159 | 37 | 1.67 |
| S5 | H2O-AG-CAL-PVP | 103 | 1400 | 160 | 37 | 1.42 |
| S6 | H2O-AG-CAL-PVPγ | 100 | 1440 | 170 | 39 | 1.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corradini, V.; Pajewski, L.A.; Di Censo, D.; Alecci, M.; Galante, A. Characterization of a Novel Packaged Hydrogel Wound Dressing by 2.35 T Magnetic Resonance Imaging. Electronics 2023, 12, 188. https://doi.org/10.3390/electronics12010188
Corradini V, Pajewski LA, Di Censo D, Alecci M, Galante A. Characterization of a Novel Packaged Hydrogel Wound Dressing by 2.35 T Magnetic Resonance Imaging. Electronics. 2023; 12(1):188. https://doi.org/10.3390/electronics12010188
Chicago/Turabian StyleCorradini, Valentina, Leonardo A. Pajewski, Davide Di Censo, Marcello Alecci, and Angelo Galante. 2023. "Characterization of a Novel Packaged Hydrogel Wound Dressing by 2.35 T Magnetic Resonance Imaging" Electronics 12, no. 1: 188. https://doi.org/10.3390/electronics12010188
APA StyleCorradini, V., Pajewski, L. A., Di Censo, D., Alecci, M., & Galante, A. (2023). Characterization of a Novel Packaged Hydrogel Wound Dressing by 2.35 T Magnetic Resonance Imaging. Electronics, 12(1), 188. https://doi.org/10.3390/electronics12010188







