Abstract
Developments in the field of glass research necessitate the mimicking of the optical properties of glass materials before melting the raw materials, as they are very expensive nowadays. An artificial neural network (ANN) was utilized during this work to train and predict the Judd–Ofelt parameters of various glasses, such as Ω2, Ω4 and Ω6, and the radiative lifetimes of many different types of rare-earth-doped glasses. The optimized ANN architecture for forecasting the Judd–Ofelt parameters were found to be very near to the experimentally measured parameters. Then, the conferred ANN model was employed to predict the Judd–Ofelt parameters of some newly prepared borosilicate glasses. Therein, a new glass system of 0.25 PbO–0.2 SiO2–(0.55 − x) B2O3–x Dy2O3, was prepared in order to employ the melt-quenching technique. The parameter results of the Judd–Ofelt theory, as well as the Ω2, Ω4 and Ω6 and radiative lifetimes showed that the supplementation of Dy2O3 switched the BO4 units to BO3 units with oxygens that were non-bridging atoms, thus weakening the glass frameworks. Therefore, it is very important to use an ANN to predict the Judd–Ofelt parameters of several rare-earth-doped glasses as luminescent materials.
1. Introduction
Silicate and Borate Glasses play a vital role in several technological applications, e.g., in creating chemically resistant containers due to the excessive steadiness of borosilicate glasses [1,2,3,4], as well as sealing glasses and fiber composites [5]. SiO2 conjunction in alkali borate glasses relies on the concentricity and allocation of a distinction of units involving triangles of BO3 and tetrahedra of BO4 that are bonded to various cations of modified oxides by BOs or NBOs [4]. Thermal control of the treatment process of silicate glasses yields a good fabrication of dominant physical properties like high-temperature strength, chemical resistance, and mechanical resistance [6,7]. These properties create such glasses that are utilized in the optical laser host matrix [4,8].
Even though there have been several studies, an emerging issue that demands adequate consideration is the high market cost of the rare-earth glass materials. Consequently, researchers often face obstacles in the synthesis of glass. Additionally, the fabrication processes are time consuming. These issues are motivating scientists to examine other effective approaches to mimicking the optical properties of glass materials before melting the raw materials in order to reduce the cost of the experimental work. Lately, the artificial neural network (ANN) has yielded outstanding performance in the modelling and simulation of the synthesis of various host glass systems with minimum experimental work [9,10]. The ability of ANNs in engineering domains has accomplished a powerful performance in simulating and forecasting many cases in different physical systems [11].
1.1. Literature Review
Jorge et al. [12] noted that when specific kinds of organic molecules are linked to a rare-earth ion, the resulting structure provides a high emission intensity when excited in the ultraviolet region. Trivalent dysprosium ions (Dy3+) as one of the rare-earth elements, have played an important role in optical applications among a variety of glass-doping materials. Until recently, Dy3+-doped glasses have been a material of great concern because they have intense emissions in the blue (4F9/2) and yellow (4F9/2) regions of 6H15/2 and 6H13/2, respectively. In particular, the relative intensity of 4F9/2 → 6H13/2 to 4F9/2 → 6H15/2 transitions, also known as the luminescence-intensity ratio (Y/B), indicates the local symmetry in the dysprosium-ion environment and the degree of covalence between Dy3+ and O2− ions. The intensity ratio (Y/B) can generally be modified by the variability of the glass system, its own chemical compositions, the concentration of dysprosium ions and thermal treatment [13]. Due to the high fluorescence emissions of the blue and yellow regions, Dy3+ ions have also been considered as promising luminescent centers for the development of white-light sources [14]. Among the doping products, the glass matrix also plays a significant role in these technologies.
Functionally, the artificial neural network (ANN) is a common machine-learning approach that aims to simulate the way the human brain processes information [15]. It offers a robust way to deal with regression and classification problems without directly defining the relationship between input and output variables. Therefore, artificial intelligence has verified its ability to mimic and predict the behavior of the various physical phenomena in most of the engineering domains [16]. This can be a result of employing AI, because it will efficiently solve several problems. There are different techniques for artificial intelligence, and these techniques have their own merits for selection. Some of these techniques include the artificial neural network (ANN), support-vector machine, random forest, k-nearest neighbor, and gradient-boost machine [17]. For this analysis, the ANN has been utilized in the investigation of the additional parameters that were implemented during this experiment.
The Dy3+-doped B2O3-SiO2-Gd2O3-CaO glasses were manufactured by Kaewkhao et al. [18] at different concentrations. Crafted glass material has been closely studied for its physical, mechanical, photoluminescent and scintillation properties. As a result, the glass densities were almost linearly proportional to the increased concentrations of Dy2O3. From spectrophotometry, nine absorption bands were observed, and from photoluminescence analysis, the glass sample showed a great excitement peak at 275 nm. At this wavelength of excitation, the emission bands of the created glasses showed the two greatest emission peaks at 577 nm (4F9/2 at 6H13/2) and 482 nm (4F9/2 at 6H15/2), with the highest emission intensity obtained from the sample prepared at 0.4 mol percent of Dy2O3.
Gaafar et al. [19] used the ANN technique to predict some substantial parameters such as density, ultrasonic-wave velocities, and moduli of elasticity of about 30 glass compositions, and the predicted results were in agreement with the experimentally measured parameters.
Gaafar et al. [20] also employed an AI model to mimic some parameters such as density, ultrasonic-wave velocities, and elastic moduli of various tellurite glasses. Consistency was observed between the experimental and predicted values. They used the model to manufacture four niobium-lead-tellurite glass systems. The experimental values suggest that the Nb2O5 acts as a framework modifier that supplies oxygen ions to form [TeO3] trigonal pyramids from [TeO4] trigonal bi-pyramids.
Deng [21] performed a detailed machine-learning analysis in order to predict the density of oxide glasses, Young’s modulus, shear modulus, and Poisson’s ratio of compositions, using a broad dataset. The results showed that the random forest, k-nearest neighbor and neural networks consistently delivered good performance, while support-vector machines consistently underperformed. The lasso linear regression works well for density prediction, although Poisson’s ratio is exceedingly difficult to predict using the R2 score that was determined by random forest to be about 0.7.
Arulmozhi & Sheelarani [22] attempted to determine ultrasonic velocity in tri-component glass-oxide systems and used the micro-structural properties of the system as the inputs to the ANN. They saw outstanding success in terms of their anticipated tests.
Krishnan et al. [23] assessed the ability of machine-learning data-driven models to predict the rate of dissolution of various aluminum-silicate glasses exposed to a wide range of pH values, from acidic to caustic. Four kinds of machine-learning techniques were studied, notably linear regression, support-vector-machine regression, random forest, and artificial neural network. They noticed though that while all of the linear methods failed to interpret the dissolution kinetics, the artificial-neural-network technique provided excellent predictions.
A neuro-computer-based stochastic numerical paradigm was developed by Ahmed et al. [24] to research the dynamics of temperature distribution in the porous-fin method by leveraging the power of the artificial-neural-network (ANN) processing, and then integrated it with the search query for genetic algorithms (GAs) and the powerful local-interior-point technique (IPT). The controlling porous-fin formula was converted into an analogous, nonlinear, second-order ordinary differential equation. The effect of heat on a rectangular fin with thermal conductivity and temperature-dependent internal heat generation was calculated by a stochastic solvent based on an ANN engineered with GA-IPT for two specific materials, Silicon Nitride Si3N4 and Aluminum Al. A developed ANN-GA-IPT methodology was employed in the transformed multi-time equation, and the precision, convergence and reliability of the model was verified by the assessment of the variance test.
The nonlinearity challenge of representing the heat distribution in the human head was resolved by the utilization of artificial-intelligence approaches including artificial neural networks (ANNs), genetic algorithms (GAs), and the active-set technique (AST) in [25]. The global function-approximation powers of unsupervised ANNs were used by Raja et al. [25] to construct a mathematical model of the problem by defining an error function in the mean-squared sense. The training of the design parameters of the ANN models was carried out with the global search ability of the GAs, a viable local search with the AST, and a hybrid approach of the GA-AST. The results of the proposed strategies were evaluated in terms of the temperature profiles by taking into account variations of problems with different Biot numbers, metabolic thermogenesis slope parameters and thermogenesis heat-production factors. The accuracy, efficacy and convergence of the developed models were also determined via statistics.
1.2. Innovative Contributions
- The ANN was utilized to train and predict the Judd–Ofelt parameters of Ω2, Ω4 and Ω6, and the radiative lifetimes of many various types of glasses doped with different types of rare-earth elements.
- The optimized ANN architecture of the outputs of such Judd–Ofelt parameters were found to be very near to their calculated values using the experimental approach.
- Then, the utilized ANN learning model was employed to forecast the values of the Judd–Ofelt parameters of a newly prepared glass system, 0.25 PbO–0.2 SiO2–(0.55 − x) B2O3–x Dy2O3.
- The predicted results of the new glass system were in good agreement with those obtained experimentally.
- The resolved ANN model yielded an outstanding performance for the realization of glass frameworks prior to designing the experiment.
1.3. Organization
The proposed work is organized as follows: Section 1 is the Introduction and is divided into three subsections (1.1 Related Works, 1.2 Innovative Contributions, 1.3 Organization). Section 2 is the Materials and Methods and is divided into three subsections (Experimental Setup, Numerical Model and Implementation). Section 3 is the Results and Discussion, and Section 4 is the Conclusion of the proposed work.
2. Materials & Methods
In this paper, an ANN was used to predict some important physical parameters, such as the Judd–Ofelt parameters of glass systems doped with Dy3+, which is one of the rare-earth elements that increases the luminescence properties of such glasses. Glass compositions of 0.25 PbO–0.2 SiO2–(0.55 − x) B2O3–x Dy2O3 with totally different Dy2O3 contents and fixed PbO and SiO2 contents were inspected. Judd–Ofelt-theory parameters, Ω2, Ω4 and Ω6, and radiative lifetimes were specified in order to investigate the results of introducing Dy2O3 to the neural-network model of the glasses. After that, a model using the ANN technique of artificial intelligence was employed to predict the Judd–Ofelt theory parameters, Ω2, Ω4 and Ω6, and radiative lifetimes of those glass compositions, as well as other glasses. This model allows for the direct mimicking of these important aspects while avoiding the use of expensive oxide materials.
2.1. Experimental Setup
2.1.1. Glasses Preparation
Glass compositions of 0.25 PbO–0.2 SiO2–(0.55 − x) B2O3–x Dy2O3 with completely different Dy2O3 contents and constant PbO and SiO2 contents were prepared by the melt-quenching technique. Table 1 depicts the data for each glass composition. Materials of analytical grades and 99.9% pureness B2O3, Dy2O3, PbO, and SiO2 chemicals were used to equip the glass pieces.

Table 1.
Glass compositions, density, molar volume, and concentration of Dy3+ of 0.25 PbO–0.2 SiO2–(0.55 − x) B2O3–x Dy2O3 glasses.
Suitable quantities of chemicals of each powder type were weighted using a digital balance (HR-200) with a sensitivity of ±0.0001 gm. The chemical mix’s homogeneity was achieved through continuous grinding with mortar agate. To remove water vapor and CO2, the mixture was heated to 727 K for 60 min, yielding a bubble-free liquid. The mixture was mixed in between to ensure consistency. When the required consistency was reached, the melt was pelted down in a stainless-steel mold, then treated in a furnace at 573 K for two hours to diminish the mechanical stresses. Bulk samples of glasses of 1 × 1 × 4 cm3 were thereby obtained.
On a glass plate, each glass sample was polished using fine aluminum-oxide abrasive and machine oil. Approximately ±20 μm of variation was discovered within the sample thickness. The amorphic quality of glasses was emphasized using X-ray diffraction (XRD). The absorption spectra of Dy3+-doped specimens were measured using a JAS-CO firm, V-570 dual-beam photometer in the 200–3000 nm range at room temperature. In the UV/VIS region, the fineness in wavelength was ±0.3 nm, while in the NIR area, the fineness was ±1.6 nm.
2.1.2. Density Measurements
The Density (ρ) of glasses was calculated using the Archimedes principle with toluene as the buoyant gas, and the relationship is as follows:
is density of toluene, and are the weights of the sample in the air and in toluene, respectively. We repeated the experiment 3 times, and the error was ±0.005 g/cm3.
2.2. Judd–Ofelt Analysis
The theory of Judd–Ofelt [26,27] was employed to work out the transition chances of Dy3+ excited levels. Computed oscillator strength for every transition is:
m, h, c, λ, n, and , are the electronic mass, Planck’s constant, the speed of light, the mean transition wavelength, the host-glass index of refraction, and the double-reduced matrix components of the unit tensor obtained from Weber [28]. Radial integrals, denominator of perturbations, and odd-dispute expressions of crystalline domain are all parameters of the Judd–Ofelt theory Ω2,4,6
The experimental strengths of the oscillator, which is the fexp of transitions, can be estimated by integrating every band of absorption as displayed in Figure 1 according to the equation:
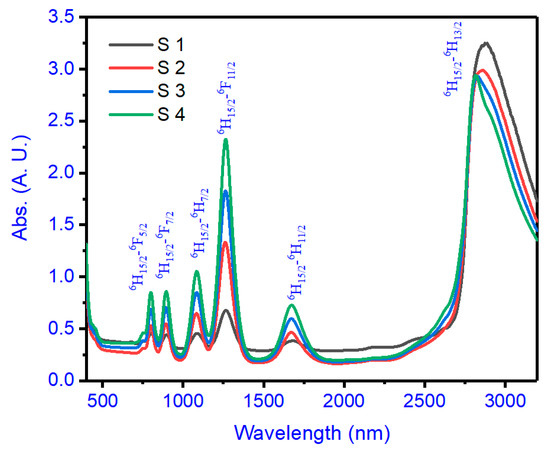
Figure 1.
Absorption spectra of glass compositions (S1–S4) with their transitions.
The electron charge, specimen thickness, active-ion-density number, and optical density are represented by the parameters e, l, N, and OD(λ).
The Judd–Ofelt-theory parameters including Ω2, Ω4 and Ω6 were estimated using the least-squares-match methodology as required by the Judd–Ofelt theory. The match fineness is acquired by the divergence of the extent of the root mean square (rms), which is set by:
where P is the number of different types of focused attention transitions throughout the spectrum. The following equation will be used to sample the Judd–Ofelt Ω2, Ω4 and Ω6 parameters in order to obtain the transition radiative possibilities for dipole transitions between excited states, and thus the minimum lie level of Dy3+.
The converse of the sum of A (k, k′) values computed through terminal levels is used to determine the radiative lifetimes of the corresponding excited degree level:
The radiative lifetimes were specified for all probable transitions from higher J-manifolds 6H13/2, 6H11/2, 6F11/2, 6H7/2, 6F7/2, and 6F5/2 to all minimum lie states of the Dy3+ in the host glass.
2.3. ANN-Regression Model
The ANN regressor is a supervised machine-learning approach that involves estimating the nonlinear relationship between the input features and the network outcomes. The outcomes have continuous values that can have any value between an interval. ANN-regression models have the power to learn the complex nonlinear relationship between the input features and its outcomes due to the existence of an activation function in each hidden layer.
In the present work, the ANN was applied to predict important parameters such as Ω2, Ω4 Ω6, and radiative lifetimes in order to save money and time. On the other hand, the ANN is limited to the availability of a subset of data for creating and training the network; it is sensitive to the accuracy of the data, which is often reflected in the accuracy of the predictions. This means that if the training data is incorrect, then the predicted outcomes will be incorrect as well, not because of the path but because of the information accuracy.
Within the ANN-regressor framework, the input layer is considered a signal dispenser and the hidden layers are considered signal detectors. At the same time, the output layer is considered a detected-feature collector and a response provider. Each output neuron is linked to its inputs (Xi). The outputs (Yj) of the jth neurons in a layer are prescribed in two equations as follows:
And
In the present work, the hyperbolic function is utilized and shaped as a sigmoid function utilizing a mathematical equation (see Equation (8)). This is because the hyperbolic function is considered a switch among the mediate domain as it is discriminated, in addition to the smoothness of it. Linear functions normally create outputs that are proportionate to the inputs up to the total output level. In addition, the steeping of the linear function at the transformation point from proportionate output to total output is very important and is also used due to the importance of the distinctive ability of the neuron-activation function to obtain consistent behavior in the back-propagation exercise (Shin, [29]). The property of suppression being close to zero is very important due to its domain from −1 to +1, rather than from 0 to 1 like the sigmoid function, while the values will be excited upon reaching the total level.
The final output (Yj) is either an input to the next layer or a restraint of the neural framework if it is the final layer.
The process of training is the second step in the neural-network process. The aim of such an operation is the tuning of the neural network until it produces an output. Through the distinction between the expected output and the real one, the error is calculated and propagated back throughout the network. The error is used by every neuron in the neural network to set the weights, and in the next reiteration the neural network’s error will be minimized for the same inputs. The process is repeated for a number of iterations as the errors between the desired and actual output is minimized until the desired tolerance is achieved. The moment that the neural network diminishes all errors and reaches the desired tolerance, the practicing operation might quit. The inaccuracy spread in the neural network begins at the output layer by the following:
And,
where wij is the corrected weight, w′ij is the prior worth of the weight, LR is the rate of learning, ej is the error code, Xi is the ith value of the input, Yj is the output, and dj is the desirable output.
To mimic and predict the Judd–Ofelt parameters of various glasses such as Ω2, Ω4 and Ω6 and the radiative lifetimes, a numerical model using the artificial-neural-network (ANN) technique was intended to mimic these necessary parameters. Then, to evolve a model of the neural network, the initial input and output variables must be specified. The input variables were chosen based on the character of the matter and thence the style of information that will be gathered, obviously to allocate the key variables of inputs with its linked output. Table 2 represents the key input and output variables of the neural network. Various neural networks are resolved and examined during this work in order to verify the simplest network model to ultimately mimic. Almost all engineering issues may be estimated using an ANN network with two hidden layers. Hence, two hidden layers have been utilized for the ANN architecture in this study. Figure 2 illustrates the utilized architecture for each outcome. Each hidden layer contained ten neurons as illustrated in Figure 2. The nonlinear logistic was utilized as an activation function. In the next stage of ANN design, the number of hidden node(s) in a hidden layer should be determined as they have a deep impact on the performance prediction of the ANN model [30,31].

Table 2.
Additional key input variables and output variables for all ANN models.
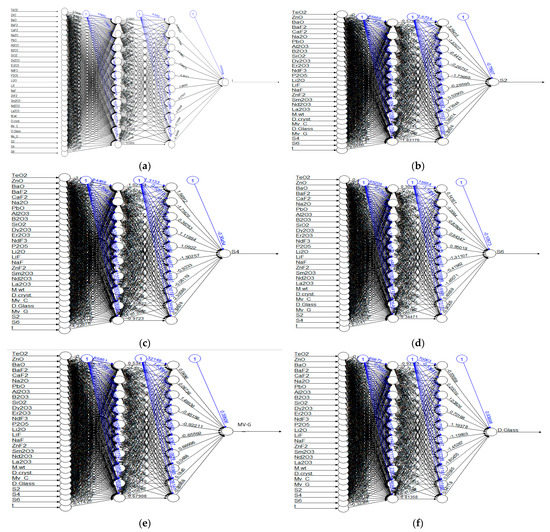
Figure 2.
ANN architecture for all outcomes. (a) ANN architecture for forecasting the radiative lifetime, T. (b) ANN architecture for forecasting S2. (c) ANN architecture for forecasting S4. (d) ANN architecture for forecasting S6. (e) ANN architecture for forecasting Mv_G. (f) ANN architecture for forecasting D.Glass.
The accuracy of the forecasting models is considerably influenced by the adequacy of the input features. We calculated the Pearson correlation coefficient between all input variables in order to verify the dependency of the outcomes on the feature space. As depicted by the correlation matrix in Figure 3, the outcomes are highly correlated with the input variables. Data-preprocessing approaches such as the principal-component analysis (PCA) [32] can enhance the performance of the forecasting model. The PCA reduces the feature space by inducing the key patterns that represent the input features. The output of the PCA is the principal components, eigen vectors, in the feature space. However, such a reduction in the feature space may yield worse accuracy in some applications [33]. In this study, the PCA was employed and investigated. The feature space was reduced and five principal components were selected to represent the entirety of features. Next, the ANN regressor was trained on the reduced set of features and the performance metrics were recorded for further comparison.
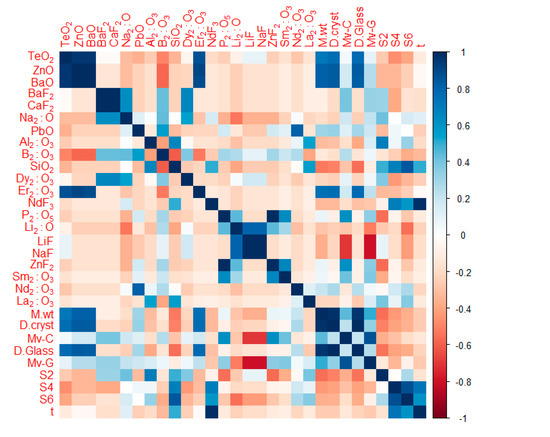
Figure 3.
The correlation matrix between the outcomes and input features.
The practicing operation of the sophisticated ANN model uses the experimental optical properties of some borosilicate glasses, allowing the model to predict the properties of other borosilicate glasses with no need to execute experiments.
The variables of the network model [19,20] that were built in the proposed model could be summarized as follows:
Learning rate (LR): Specifies the amount of the corrective word used to change the weights of each neuron during the training cycle = 1.
Momentum (M): used to speed up the convergence of the ANN.
Training tolerance (TRT): Defines the allowed error percentage when comparing the neural network’s output to the expected value (TRT = 0.001).
Testing tolerance (TST): is similar to training tolerance, but only applies to the neural-network outputs and the target values for the test data (TST = 0.003).
Input noise (IN): Allows for a little amount of random variation within each input for each training cycle (IN = 0).
Function gain (FG): Controls the width or scale of the selected element (FG = 1).
Scaling margin (SM): Adds extra headroom as a proportion of space (SM = 0.1).
Training epochs: The number of times a learning algorithm passes the entire dataset is measured in epochs = 231,940.
Percentage relative error (PRE): The proportion of percentage error between the estimated outputs and the real observed value, which is calculated as follows:
where is the predicted results using the developed ANN model, and is the actual values. The maximum percentage of the percentage error (MPRE) was set to 0.5%.
2.4. Data Splitting and Performance Metrics
To evaluate machine-learning models, the data were divided into training and validation sets to make sure that the prediction was performed using different data records that were not utilized in the training of the prediction model. In this study, the learning model was trained on 75% of the input data and validated on the remaining 25%.
There are numerous performance metrics with which to verify the loss in forecasting models such as the R squared (, and the root-mean-square error (RMSE). The measures the extent to which the forecasting model can measure the variability of the outcomes. Equation (13) describes the calculation of R squared. The second metric, RMSE, measures the differences between the actual and the predicted values, and it is calculated as described in Equation (14), where M, , , and are the number of samples, the actual values, the forecasted values, and the mean of the actual values, respectively.
2.5. Implementation
In order to implement the ANN, it is important to acquire the tools that are suitable for this type of labor, as there are several platforms such as Python, MATLAB, and R Studio. In this work, R Studio was used as it has several merits, such as its free obtainability and its integrated development environment that offers an alternate interface to R [34], regardless of whether the tool is run on Mac, PC, or Linux machines, because it offers a simple interface for users. R Studio was created by J.J. Allaire, creator of the artificial language ColdFusion. We utilized a library to implement the ANN-regression model, “neuralnet” package. The “neuralnet” R package was downloaded from Comprehensive R Archive Network (CRAN), which comprises several R libraries for different applications [35].
3. Results and Discussion
3.1. Density and Molar Volume
Table 1 displays the density as well as molar-volume data. Figure 4 displays the change in density after supplementation with various Dy2O3 mol percentages at the expense of B2O3. The density values were found to increase as the Dy2O3 mol percent increases. If we consider the additive rule of density, we can expect a rise in density values with the addition of Dy2O3. Dy2O3 has a density of 7.80 g/cm3, which is significantly higher than B2O3 (2.55 g/cm3) [36]. It is known that the structural unit of B2O3-based glasses gradually varies from BO4 units to BO3 with the rising the quantities of other components.
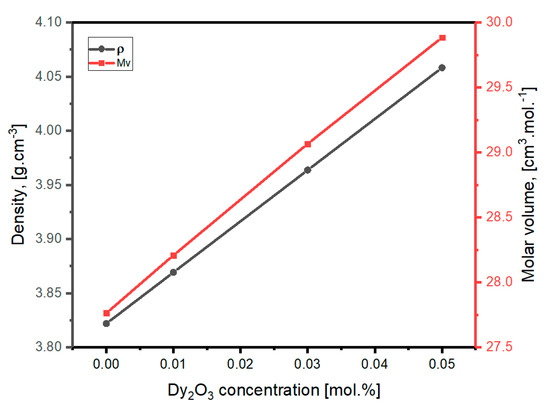
Figure 4.
Variation of density and molar volume with the Dy2O3 concentration.
Similarly, molar volume (Vm) was found to increase with rising Dy2O3 content as seen in Figure 4. This rise was anticipated because the atomic radii of dysprosium and boron atoms are 1.75 Å and 0.85 Å, respectively. Generally, the rise in molar volume points to the increase in spaces in the lattice framework of the glass. Therefore, the rise in molar volume with rising Dy2O3 content emphasizes the transference of BO3 units from BO4 units with oxygen atoms that are non-bridging, and thus means the framework becomes significantly more open.
3.2. Judd–Ofelt Analysis of Dy3+ Intensity
The estimated and experimentally specified oscillator strengths for Dy3+ in PbO–SiO2–B2O3 glasses were recorded at room temperature as displayed in Table 3. The values of experimentally specified fexp and computed fcal oscillator strengths are higher for 6H15/2 → 6H13/2 transition in every glass lattice. The fineness among the experimental and computed oscillator strengths gained by root mean square (rms) drifts. It is pointed attention that, for 6H15/2 → 6H13/2,6H15/2 → 6H11/2,6H15/2 → 6F11/2, 6H15/2 → 6H7/2, 6H15/2 → 6F7/2, and 6H15/2 → 6F5/2 transitions, the experimental oscillator stresses are in productive sensible consent with the computed values, pointing the legality of using the theory of Judd–Ofelt computations on the glass compositions.

Table 3.
Experimental (fexp) and calculated (fcal) oscillator strengths along (from the ground state, 6H15/2), with JO parameters of prepared glass samples.
Table 3 records the parameters gained from the Judd–Ofelt theory, including Ω2, Ω4 and Ω6. Ω2 is linked to the host-glass dispute, although Ω6 decreases as the covalence quality of the Dy-O bonds improves [37]. A distinctive merit of the Ω2 parameter is linked to the non-symmetry of the framework coordinates. The ligand polarizability of the molecules or ions, as well as the quality of bonding, is sensitive to the local circumference of the RE ions [37,38]. The other two Judd–Ofelt theoretical parameters, Ω4 and Ω6, include qualities such as the viscosity and hardness of the glass framework and dielectric media that inspire bonding between ligand atoms, as well as dysprosium ions [39,40]. Table 3 displays the Ω2 parameter of the Judd–Ofelt theory and shows that Ω2 rises from 3.402 × 10−20 to 5.268 × 10−20 with rising Dy2O3 mol percent. The Ω6 parameter also rises from 1.896 × 10−20 to 1.961 × 10−20 with rising of Dy2O3 content from 1 mol percent to 5 mol percent in PbO–SiO2–B2O3 glasses. Therefore, the rise in the parameters of the Judd–Ofelt theory emphasizes the decrease in the quality of covalent bonds. This decrease in covalence was in agreement with the decrease in the packing density, Vt, as displayed in Table 1.
The radiative lifetimes were specified for all the probable transitions from superior J-manifolds 6H13/2, 6H11/2, 6F11/2, 6H7/2, 6F7/2, and 6F5/2 to all minimum lie states of the Dy3+ in the borate host glass. The radiative lifetimes for the 6H13/2 state in glasses S2, S3, and S4 were 65.20, 67.08, and 66.93 ms, respectively, as displayed in Table 4. Other laser host glasses doped with Dy3+ were in agreement with these compositions [41,42,43].

Table 4.
Calculated lifetimes (τ, ms) of glass samples.
The model that exploited the ANN as an exercise model was resolved to be used for predicting the density, the parameters of Judd–Ofelt theory, Ω2, Ω4 and Ω6, and the radiative lifetimes of our inspected glass compositions, as listed in Tables S1 and S2. Over 35 glass compositions [44,45,46,47,48,49,50] were used in the learning model. The benefit of our model is that it allowed for the explicit mimicking of significant features of glasses without melting the raw oxide materials, relying on a number of theoretical parameters such as molar weights and oxide densities as inputs to the model to produce the expected outputs.
Table 5 demonstrates the results for the R squared and the RMSE for the ANN-regression model on the validation-data records. The results of the predicted values for the outcomes compromise the utilization of the PCA as a preprocessing step for the input variables. Nevertheless, the PCA yielded worse accuracy as illustrated by the results of the R squared and RMSE. Such performance is returned as it receives the linear latent feature in the feature space [51].

Table 5.
RMSE and R squared of the ANN forecasting models.
Figure 5, Figure 6 and Figure 7 display the variations of the experimentally gained values of density, molar volume, parameters of the Judd–Ofelt theory Ω2, Ω4 and Ω6, and radiative lifetimes with those that were predicted utilizing the resolved model in this work. The linear-regression process was utilized to display the agreement between the experimentally measured values and the values predicted using ANN. Solid straight lines display slopes that are within the unity domain, confirming the agreement between the experimental and predicted results. Every figure displays the slopes and correlation factors. Taking into account the uncertainties of the experimental data, the agreements in Figure 6 were very satisfactory. In all cases, the difference between the forecasted and experimentally obtained values was less than 0.5%, and in the inspected glasses, it was less than 5%. The proposed methodology can be a good alternative for problems in astrophysics, plasma physics, atomic physics, thermodynamics, electromagnetics, machines, nanotechnology, fluid mechanics, electrohydrodynamics, signal processing, power, energy, bioinformatics, economics and finance. Please see [24,25,52,53] and the citations therein.
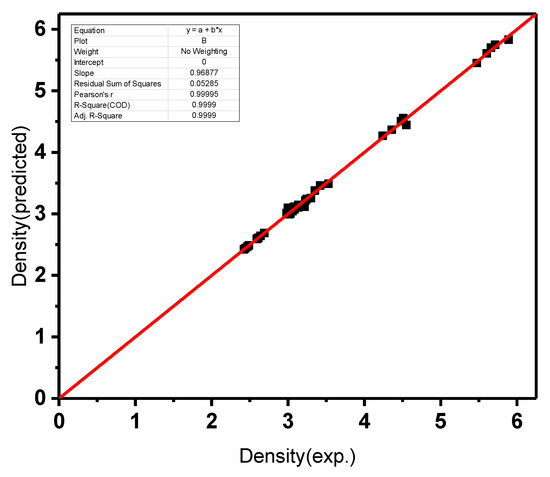
Figure 5.
Experimentally measured density against predicted density utilizing ANN.
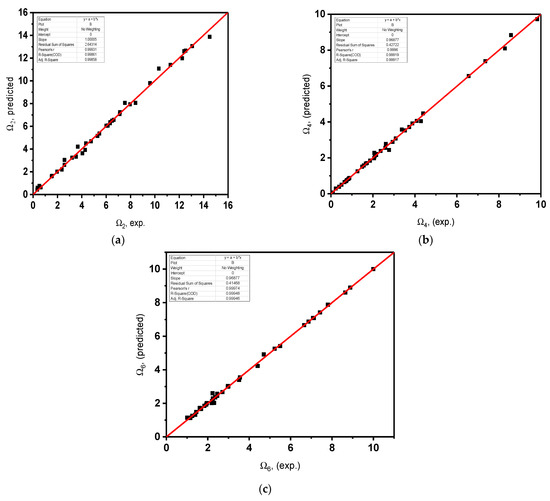
Figure 6.
Experimentally estimated Judd–Ofelt parameters against predicted utilizing ANN. (a) Estimated Judd–Ofelt parameter Ω against predicted Ω2 utilizing ANN. (b) Estimated Judd–Ofelt parameter Ω4 against predicted Ω4 utilizing ANN. (c) Estimated Judd–Ofelt parameter Ω6 against predicted Ω6 utilizing ANN.
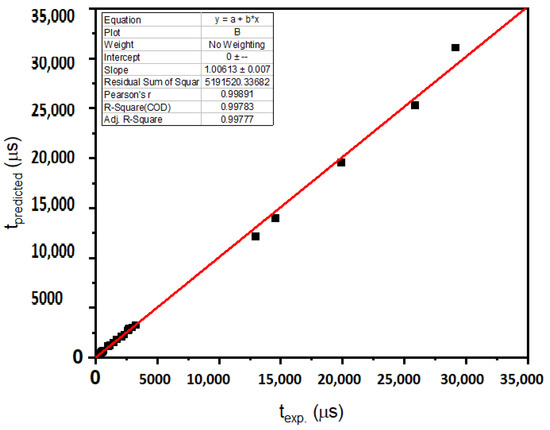
Figure 7.
Experimentally estimated lifetime t against predicted utilizing ANN.
The productive and sensitive approach for predicting our results shows that the resolved ANN model is a good tool for the simulation of the luminescence properties of rare-earth-doped glasses and can consequently lead to saving money from grants.
The adequacy of the results in this study using the ANN-regression models was compared with some recent ML-based research, such as the studies done by Deng [54], Abderrahmane [55], and Ahmed et al. [56]. In [54], three machine-learning techniques including the k-nearest neighbor (KNN), random forest (RF), and the ANN were utilized in forecasting the glass density. The result of the was 0.62 compared to 0.975 in our study. In [55], the Judd–Ofelt parameters (Ω2, Ω4 and Ω6) were estimated using the ANN technique. The retrieved results for the RMSE of the predicted values of Ω2, Ω4 and Ω6 were 1.4843, 0.5938, and 0.5136 compared to 0.102, 0.068, and 0.068, respectively, in this study. However, our results for the predicted glass density are as good as the results in [56], which yielded 0.983 for the using the RF-regression technique.
4. Conclusions
The density, Judd–Ofelt parameters Ω2, Ω4 and Ω6 and radiative lifetimes of oxide glasses were estimated using the ANN model. For over 35 distinct borate and silicate glasses, there was a high level of agreement between the measured and forecasted values. The rare-earth-ion concentrations, glass densities, molecular weights, and molar-volume values were used as inputs, and the outputs were their Judd–Ofelt parameters. By using the resolved model, the authors equipped a new glass composition of 0.25 PbO–0.2 SiO2–(0.55 − x) B2O3–x Dy2O3. The resolved model displayed a novel capability to determine the glass frameworks prior performing the experimental work. The experimental results of the Judd–Ofelt parameters for the glass system displayed a decrease in the bonds’ covalence quality. This decrease in the bond’s covalence nature is in good agreement with the decrease in the packing density, Vt. Therefore, Dy3+ ions have also been considered as promising luminescent centers.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/electronics11071045/s1. Table S1: Input parameters (Glass oxides); Table S2: Output parameters.
Author Contributions
Conceptualization, A.A.A., M.S.G., M.A., S.Y.M., S.A., H.E., M.S.M., H.N.A. and N.A.S.; methodology, A.A.A., M.S.G., M.A., S.Y.M., S.A., H.E., M.S.M., H.N.A. and N.A.S.; software, A.A.A., M.S.G., M.A., S.Y.M., S.A., H.E., M.S.M., H.N.A. and N.A.S.; validation, M.S.G., M.A., S.Y.M., S.A., H.E. and N.A.S.; formal analysis, A.A.A., M.S.G., M.A., S.Y.M., S.A., H.E., M.S.M., H.N.A. and N.A.S.; investigation, M.S.G., M.A., S.Y.M., S.A., H.E. and N.A.S.; resources, M.S.G., M.A., S.Y.M., S.A. and H.E.; data curation, M.S.G., M.A., S.Y.M., S.A. and H.E.; writing—original draft preparation, A.A.A., M.S.G., M.A., S.Y.M., S.A., H.E., M.S.M., H.N.A. and N.A.S.; writing—review and editing, A.A.A., M.S.G., M.A., S.Y.M., S.A., H.E., M.S.M., H.N.A. and N.A.S.; visualization, M.S.G., M.A., S.Y.M., S.A., H.E. and N.A.S.; supervision, M.S.G., M.A., S.Y.M., S.A. and H.E.; project administration, A.A.A., M.S.G., M.A., S.Y.M., S.A., H.E., M.S.M., H.N.A. and N.A.S.; funding acquisition, A.A.A., M.S.G., M.A., S.Y.M., S.A., H.E., M.S.M., H.N.A. and N.A.S. All authors have read and agreed to the published version of the manuscript.
Funding
Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R308), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Acknowledgments
Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R308), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. In addition, the authors express their gratitude’s to the Deanship of scientific research at Majmaah University, Saudi Arabia, for supporting this search work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Abd El-Fattah, Z.M.; Ahmad, F.; Hassan, M.A. Tuning the Structural and Optical Properties in Cobalt Oxide-Doped Borosilicate Glasses. J. Alloys Compd. 2017, 728, 773–779. [Google Scholar] [CrossRef]
- Wen, H.; Tanner, P.A. Optical Properties of 3d Transition Metal Ion-Doped Sodium Borosilicate Glass. J. Alloys Compd. 2015, 625, 328–335. [Google Scholar] [CrossRef]
- He, J.J.; Wu, S.Y.; Zhang, L.J.; Xu, Y.Q.; Ding, C.C. Theoretical Studies of the Concentration Dependences of g Factor and D-d Transition Band for Cr3+ in CdO-SrO-B2O3-SiO2 Glasses. J. Non-Cryst. Solids 2016, 437, 58–63. [Google Scholar] [CrossRef]
- Saddeek, Y.B.; Aly, K.A.; Bashier, S.A. Optical Study of Lead Borosilicate Glasses. Phys. B Condens. Matter 2010, 405, 2407–2412. [Google Scholar] [CrossRef]
- Zhao, P.; Kroeker, S.; Stebbins, J.F. Non-Bridging Oxygen Sites in Barium Borosilicate Glasses: Results from 11B and 17O NMR. J. Non-Cryst. Solids 2000, 276, 122–131. [Google Scholar] [CrossRef]
- ElBatal, F.H.; Selim, M.S.; Marzouk, S.Y.; Azooz, M.A. UV-Vis Absorption of the Transition Metal-Doped SiO2-B2O3-Na2O Glasses. Phys. B Condens. Matter 2007, 398, 126–134. [Google Scholar] [CrossRef]
- Abdel-Baki, M.; Salem, A.M.; Abdel-Wahab, F.A.; El-Diasty, F. Bond Character, Optical Properties and Ionic Conductivity of Li2O/B2O3/SiO2/Al2O3 Glass: Effect of Structural Substitution of Li2O for LiCl. J. Non-Cryst. Solids 2008, 354, 4527–4533. [Google Scholar] [CrossRef]
- Santhan Kumar, J.; Lakshmi Kumari, J.; Subba Rao, M.; Cole, S. EPR, Optical and Physical Properties of Chromium Ions in CdO-SrO-B2O3-SiO2 (CdSBSi) Glasses. Opt. Mater. 2013, 35, 1320–1326. [Google Scholar] [CrossRef]
- Effendy, N.; Ab Aziz, S.H.; Mohamed Kamari, H.; Mohd Zaid, M.H.; Abdul Wahab, S.A. Ultrasonic and Artificial Intelligence Approach: Elastic Behavior on the Influences of ZnO in Tellurite Glass Systems. J. Alloys Compd. 2020, 835, 155350. [Google Scholar] [CrossRef]
- Adamu, S.B.; Halimah, M.K.; Chan, K.T.; Muhammad, F.D.; Nazrin, S.N.; Scavino, E.; Kamaruddin, S.A.; Az’lina, A.H.; Ghani, N.A.M. Structural, prediction and simulation of elastic properties for tellurite-based glass systems doped with nano and micro Eu2O3 particles via artificial neural network model. J. Mater. Res. Technol. 2022, 17, 586–600. [Google Scholar] [CrossRef]
- Effendy, N.; Aziz, S.H.A.; Kamari, H.M.; Zaid, M.H.M.; Budak, C.E.A.; Shabdin, M.K.; Khiri, M.Z.A.; Wahab, S.A.A. Artificial Neural Network Prediction on Ultrasonic Performance of Bismuth-Tellurite Glass Compositions. J. Mater. Res. Technol. 2020, 9, 14082–14092. [Google Scholar] [CrossRef]
- Monteiro, J.H.S.K.; Mazali, I.O.; Sigoli, F.A. Determination of Judd-Ofelt Intensity Parameters of Pure Samarium(III) Complexes. J. Fluoresc. 2011, 21, 2237–2243. [Google Scholar] [CrossRef] [PubMed]
- Soltys, M.; Zur, L.; Pisarska, J.; Pisarski, W.A. Excitation and Luminescence of Dy3+ Ions in PbO-P2O5-Ga2O3 Glass System. J. Rare Earths 2014, 32, 213–216. [Google Scholar] [CrossRef]
- Wang, F.; Chen, B.; Pun, E.Y.B.; Lin, H. Dy3+ Doped Sodium-Magnesium-Aluminum-Phosphate Glasses for Greenish-Yellow Waveguide Light Sources. J. Non-Cryst. Solids 2014, 391, 17–22. [Google Scholar] [CrossRef]
- Rumelhart, D.E.; McClelland, J.L. A General Framework for Parallel Distributed Processing. In Parallel Distributed Processing: Explorations in the Microstructure of Cognition: Foundations; MIT Press: Boston, MA, USA, 1987; pp. 45–76. [Google Scholar]
- Russell, S.; Norvig, P. Artificial Intelligence: A Modern Approach, 4th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2016. [Google Scholar]
- Duda, R.; Hart, P.; Stork, G.D. Pattern Classification. In Wiley Interscience; Wiley: London, UK, 2001; Volume 20, ISBN 0-471-05669-3. [Google Scholar]
- Kaewkhao, J.; Wantana, N.; Kaewjaeng, S.; Kothan, S.; Kim, H.J. Luminescence Characteristics of Dy3+ Doped Gd2O3-CaO-SiO2-B2O3 Scintillating Glasses. J. Rare Earths 2016, 34, 583–589. [Google Scholar] [CrossRef]
- El-Mallawany, R.; Gaafar, M.; Azzam, Y. Prediction of Ultrasonic Parameters at Low Temperatures for Tellurite Glasses Using ANN. Chalcogenide Lett. 2014, 11, 227–232. [Google Scholar]
- Gaafar, M.S.; Abdeen, M.A.M.; Marzouk, S.Y. Structural Investigation and Simulation of Acoustic Properties of Some Tellurite Glasses Using Artificial Intelligence Technique. J. Alloys Compd. 2011, 509, 3566–3575. [Google Scholar] [CrossRef]
- Deng, B. Machine Learning on Density and Elastic Property of Oxide Glasses Driven by Large Dataset. J. Non-Cryst. Solids 2020, 529, 119768. [Google Scholar] [CrossRef]
- Arulmozhi, K.T.; Sheelarani, R. Prediction of Ultrasonic Velocities in Ternary Oxide Glasses Using Microstructural Properties of the Constituents as Predictor Variables; Artificial Neural Network (ANN) Approach. Sci. Iran. 2012, 19, 127–131. [Google Scholar] [CrossRef][Green Version]
- Anoop Krishnan, N.M.; Mangalathu, S.; Smedskjaer, M.M.; Tandia, A.; Burton, H.; Bauchy, M. Predicting the Dissolution Kinetics of Silicate Glasses Using Machine Learning. J. Non-Cryst. Solids 2018, 487, 37–45. [Google Scholar] [CrossRef]
- Ahmad, I.; Zahid, H.; Ahmad, F.; Raja, M.A.Z.; Baleanu, D. Design of Computational Intelligent Procedure for Thermal Analysis of Porous Fin Model. Chin. J. Phys. 2019, 59, 641–655. [Google Scholar] [CrossRef]
- Raja, M.A.Z.; Umar, M.; Sabir, Z.; Khan, J.A.; Baleanu, D. A New Stochastic Computing Paradigm for the Dynamics of Nonlinear Singular Heat Conduction Model of the Human Head. Eur. Phys. J. Plus 2018, 133, 364. [Google Scholar] [CrossRef]
- Opelt, G.S. Intensities of Crystal Spectra of Rare-Earth Ions. J. Chem. Phys. 1962, 37, 511–520. [Google Scholar] [CrossRef]
- Judd, B.R. Optical Absorption Intensities of Rare-Earth Ions. Phys. Rev. 1962, 127, 750–761. [Google Scholar] [CrossRef]
- Weber, M.J. Spontaneous Emission Probabilities and Quantum Efficiencies for Excited States of Pr3+ in LaF3. J. Chem. Phys. 1968, 48, 4774–4780. [Google Scholar] [CrossRef]
- Shih, Y. Neuralyst User’s Guide; Cheshire Engineering Corporation: Cheshire, UK, 1994; Volume 17. [Google Scholar]
- Mohamad, E.T.; Faradonbeh, R.S.; Armaghani, D.J.; Monjezi, M.; Majid, M.Z.A. An Optimized ANN Model Based on Genetic Algorithm for Predicting Ripping Production. Neural Comput. Appl. 2017, 28, 393–406. [Google Scholar] [CrossRef]
- Armaghani, D.J.; Mohamad, E.T.; Narayanasamy, M.S.; Narita, N.; Yagiz, S. Development of Hybrid Intelligent Models for Predicting TBM Penetration Rate in Hard Rock Condition. Tunn. Undergr. Space Technol. 2017, 63, 29–43. [Google Scholar] [CrossRef]
- Barshan, E.; Ghodsi, A.; Azimifar, Z.; Zolghadri Jahromi, M. Supervised Principal Component Analysis: Visualization, Classification and Regression on Subspaces and Submanifolds. Pattern Recognit. 2011, 44, 1357–1371. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, Y.; Yang, C.; Gui, W.; Ye, L. Probabilistic Density-Based Regression Model for Soft Sensing of Nonlinear Industrial Processes. J. Process Control 2017, 57, 15–25. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development for R; RStudio, PBC: Boston, MA, USA; Available online: http://www.rstudio.com/ (accessed on 18 February 2022).
- Fritsch, S.; Guenther, F.; Wright, M.N. Training of Neural Networks; R Package Neuralnet Version 1.44.2. 2019. Available online: https://CRAN.R-project.org/package=neuralnet (accessed on 18 February 2022).
- Lide, D.R. CRC Handbook of Chemistry and Physics, 8th ed.; CRC Press: London, UK, 2000. [Google Scholar]
- Sazali, E.S.; Sahar, R.; Ghoshal, S.K.; Rohani, S.; Arifin, R. Judd-Ofelt Intensity Parameters of Erbium Doped Lead Tellurite Glass. Mater. Sci. 2014, 6, 61–67. [Google Scholar]
- Khan, U.; Fanai, A.L.; Rai, S. Spectroscopic Properties of Sm3+ and CdS Co-Doped in Sol-Gel Silica Glass. Indian J. Pure Appl. Phys. 2020, 58, 157–163. [Google Scholar]
- Jayasimhadri, M.; Moorthy, L.R.; Saleem, S.A.; Ravikumar, R.V.S.S.N. Spectroscopic Characteristics of Sm3+-Doped Alkali Fluorophosphate Glasses. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2006, 64, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Inokuti, M.; Hirayama, F. Influence of Energy Transfer by the Exchange Mechanism on Donor Luminescence. J. Chem. Phys. 1965, 43, 1978–1989. [Google Scholar] [CrossRef]
- Marzouk, S.Y.; Zobaidi, S.; Okasha, A.; Gaafar, M.S. The Spectroscopic and Elastic Properties of Borosilicate Glasses Doped with NdF3. J. Non-Cryst. Solids 2018, 490, 22–30. [Google Scholar] [CrossRef]
- Gaafar, M.S.; Marzouk, S.Y. Judd–Ofelt Analysis of Spectroscopic Properties of Er3+ Doped TeO2-BaO-ZnO Glasses. J. Alloys Compd. 2017, 723, 1070–1078. [Google Scholar] [CrossRef]
- Makishima, A.; Mackenzie, J.D. Direct Calculation of Young’s Moidulus of Glass. J. Non-Cryst. Solids 1973, 12, 35–45. [Google Scholar] [CrossRef]
- Krishna, V.M.; Mahamuda, S.; Talewar, R.A.; Swapna, K.; Venkateswarlu, M.; Rao, A.S. Dy3+ Ions Doped Oxy-Fluoro Boro Tellurite Glasses for the Prospective Optoelectronic Device Applications. J. Alloys Compd. 2018, 762, 814–826. [Google Scholar] [CrossRef]
- Uma, V.; Maheshvaran, K.; Marimuthu, K.; Muralidharan, G. Structural and Optical Investigations on Dy3+ doped Lithium Tellurofluoroborate Glasses for White Light Applications. J. Lumin. 2016, 176, 15–24. [Google Scholar] [CrossRef]
- Wang, X.; Hu, L.; Meng, X.; Li, H.; Wang, S. Effect of Al2O3 and La2O3 on Structure and Spectroscopic Properties of Nd-Doped Sol–Gel Silica Glasses. J. Lumin. 2018, 204, 554–559. [Google Scholar] [CrossRef]
- Mohan, S.; Thind, K.S.; Sharma, G. Effect of Nd3+ Concentration on the Physical and Absorption Properties of Sodium-Lead-Borate Glasses. Braz. J. Phys. 2007, 37, 1306–1313. [Google Scholar] [CrossRef]
- Vijayakumar, M.; Marimuthu, K.; Sudarsan, V. Concentration Dependent Spectroscopic Behavior of Sm3+ Doped Leadfluoro-Borophosphate Glasses for Laser and LED Applications. J. Alloys Compd. 2015, 647, 209–220. [Google Scholar] [CrossRef]
- Kaur, S.; Vishwakarma, A.K.; Deopa, N.; Prasad, A.; Jayasimhadri, M.; Rao, A.S. Spectroscopic studies of Dy3+ doped borate glasses for cool white light generation. Mater. Res. Bull. 2018, 104, 77–82. [Google Scholar] [CrossRef]
- Vijaya Babu, K.; Cole, S. Luminescence Properties of Dy3+-Doped Alkali Lead Alumino Borosilicate Glasses. Ceram. Int. 2018, 44, 9080–9090. [Google Scholar] [CrossRef]
- Yang, Z.; Ge, Z. Monitoring and Prediction of Big Process Data with Deep Latent Variable Models and Parallel Computing. J. Process Control 2020, 92, 19–34. [Google Scholar] [CrossRef]
- Zahoor Raja, M.A.; Shah, Z.; Anwaar Manzar, M.; Ahmad, I.; Awais, M.; Baleanu, D. A New Stochastic Computing Paradigm for Nonlinear Painlevé II Systems in Applications of Random Matrix Theory. Eur. Phys. J. Plus 2018, 133, 254. [Google Scholar] [CrossRef]
- Sabir, Z.; Manzar, M.A.; Raja, M.A.Z.; Sheraz, M.; Wazwaz, A.M. Neuro-Heuristics for Nonlinear Singular Thomas-Fermi Systems. Appl. Soft Comput. J. 2018, 65, 152–169. [Google Scholar] [CrossRef]
- Ahmmad, S.K.; Jabeen, N.; Ahmed, S.T.U.; Ahmed, S.A.; Rahman, S. Artificial intelligence density model for oxide glasses. Ceram. Int. 2021, 47, 7946–7956. [Google Scholar] [CrossRef]
- Abderrahmane, B. Judd-Ofelt Parameters: Bayesian Inference and Deep Learning Approach. 2021. Available online: https://dspace.univ-ouargla.dz/jspui/bitstream/123456789/26371/1/Benhadjira.pdf (accessed on 18 February 2022).
- Ahmed, S.A.; Rajiya, S.; Samee, M.A.; Ahmmad, S.K.; Jaleeli, K.A. Density of Bismuth Boro Zinc Glasses Using Machine Learning Techniques. J. Inorg. Organomet. Polym. Mater. 2022, 32, 941–953. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).