A Quasi-Wireless Intraoperatory Neurophysiological Monitoring System
Abstract
1. Introduction
2. Theoretical Framework
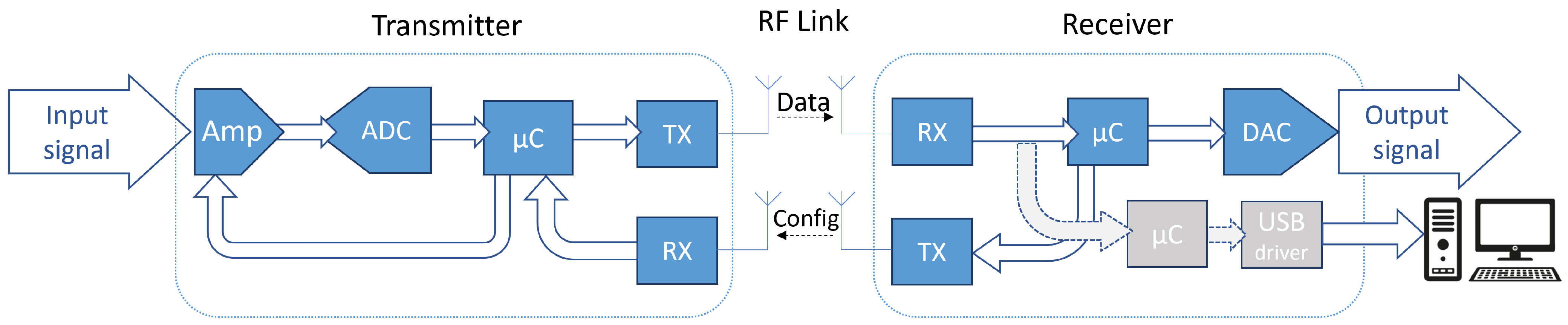
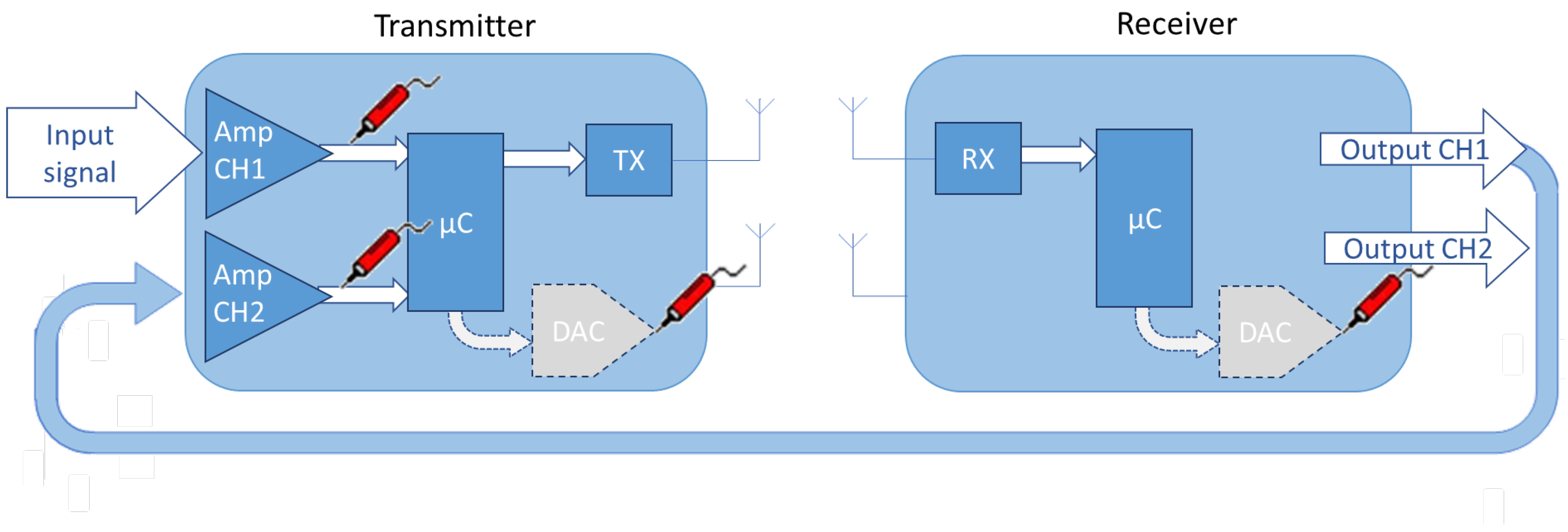
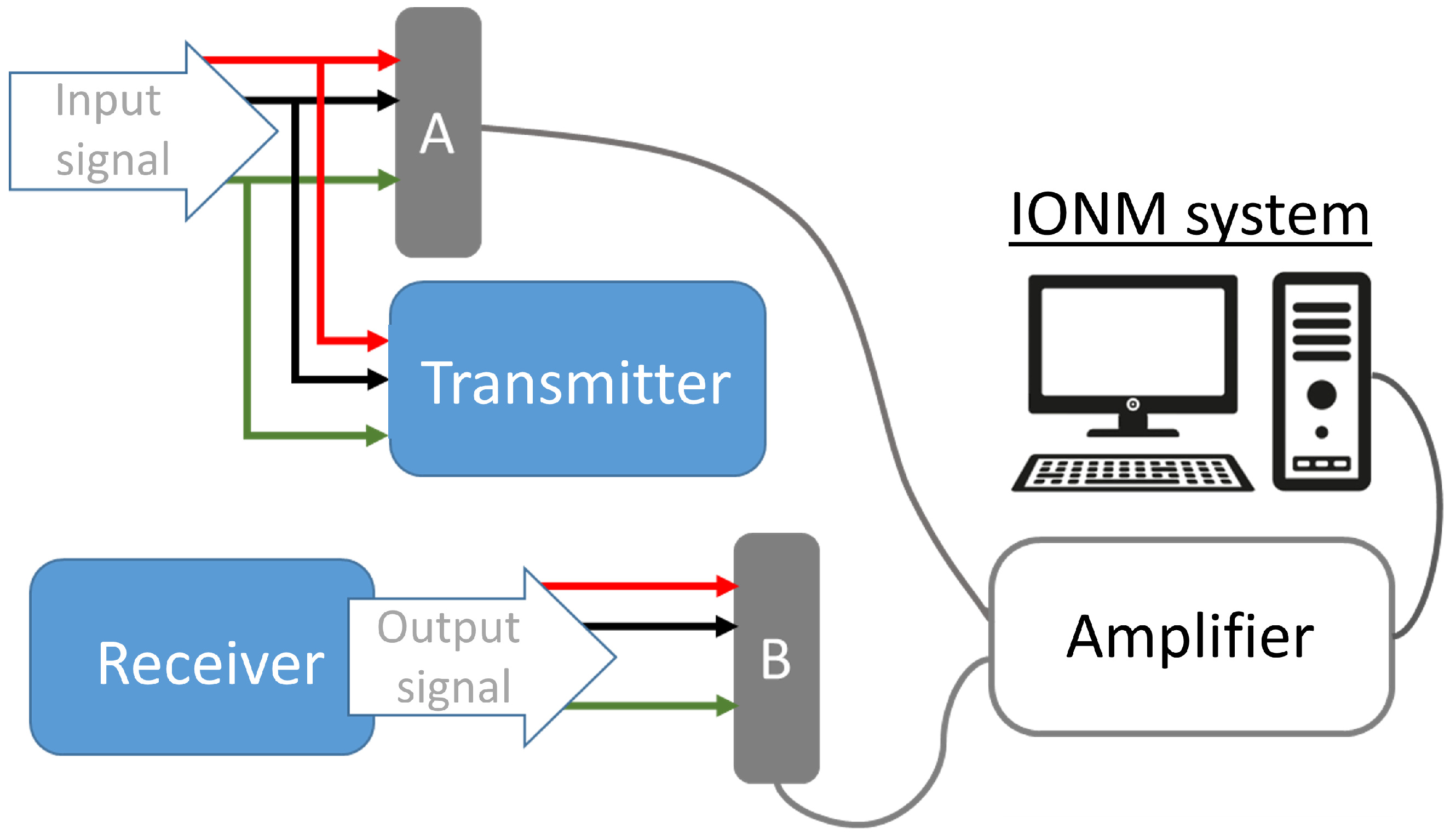
3. WIONM Prototype
3.1. Transmitter
3.2. Receiver
3.3. USB Tracker
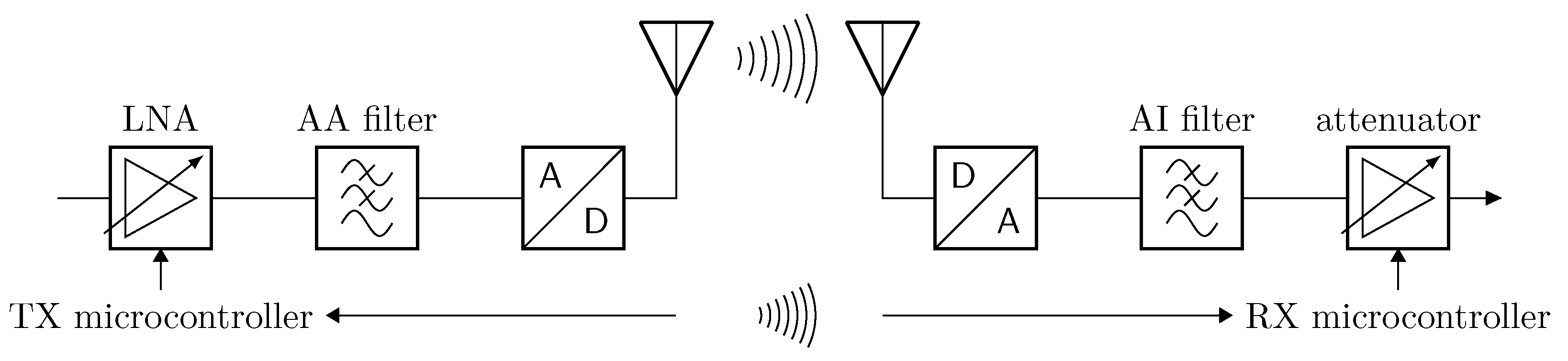
3.4. Transmission Wireless Link
3.4.1. Data Transmission
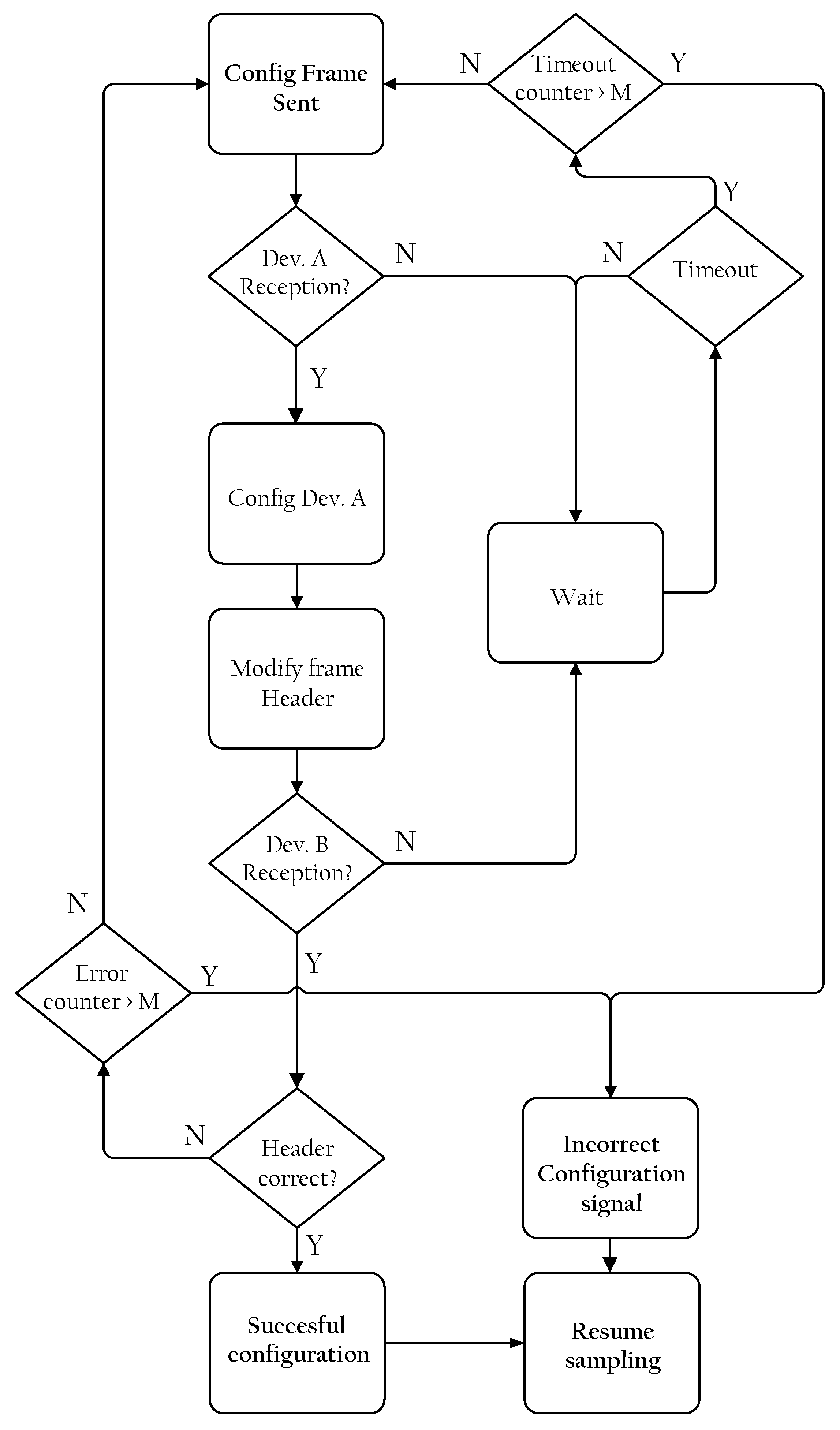
3.4.2. Configuration Frame Transmission
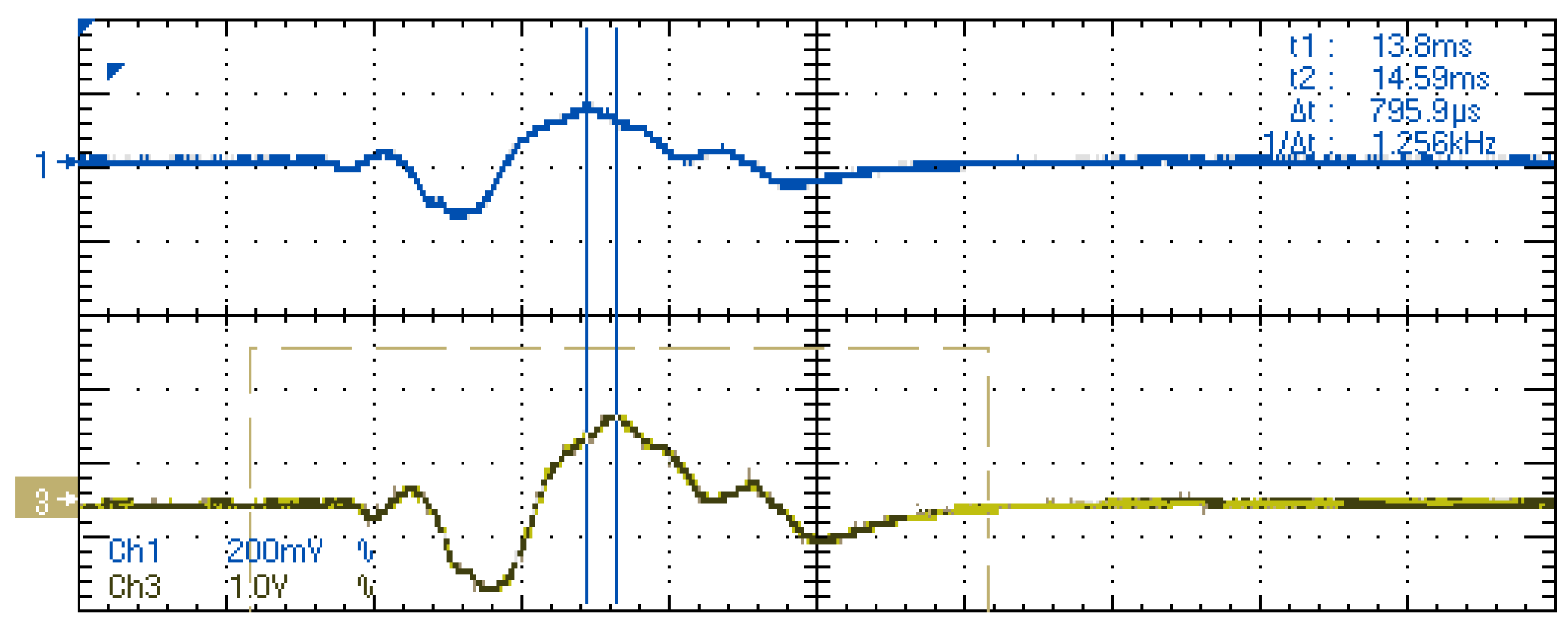
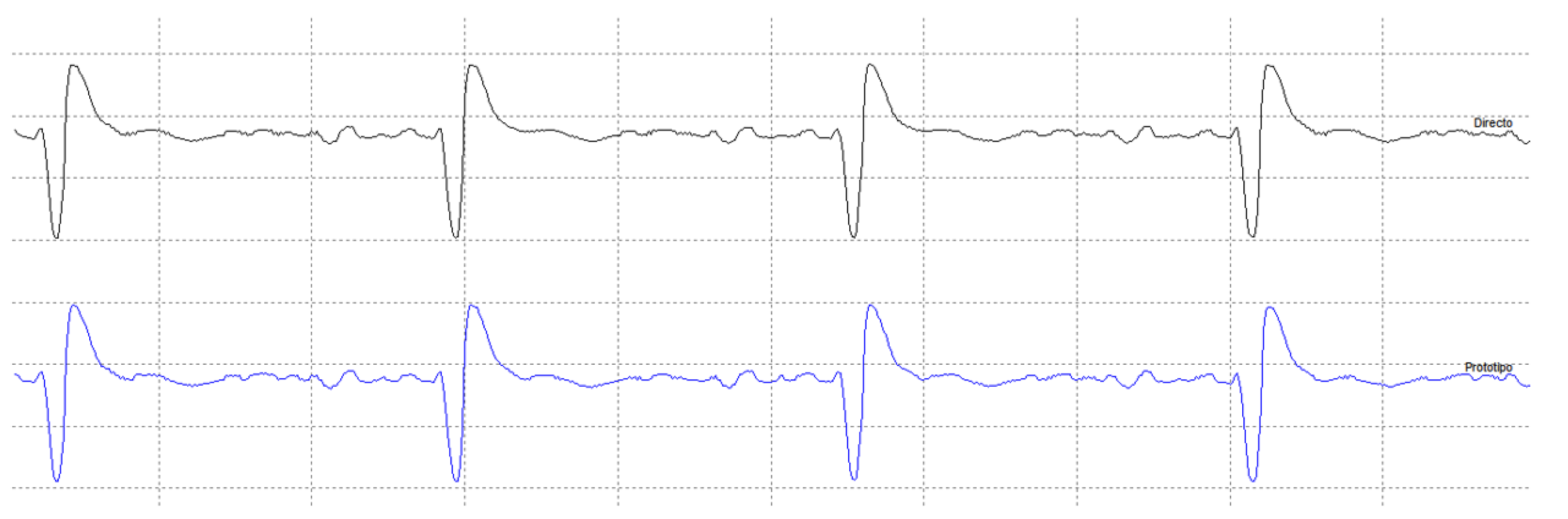
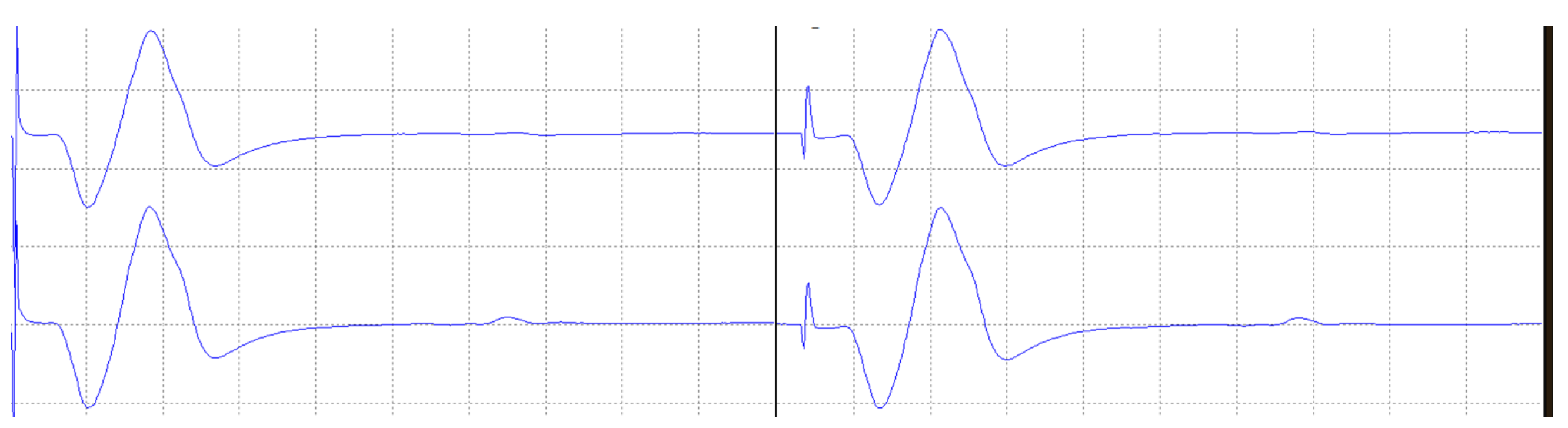
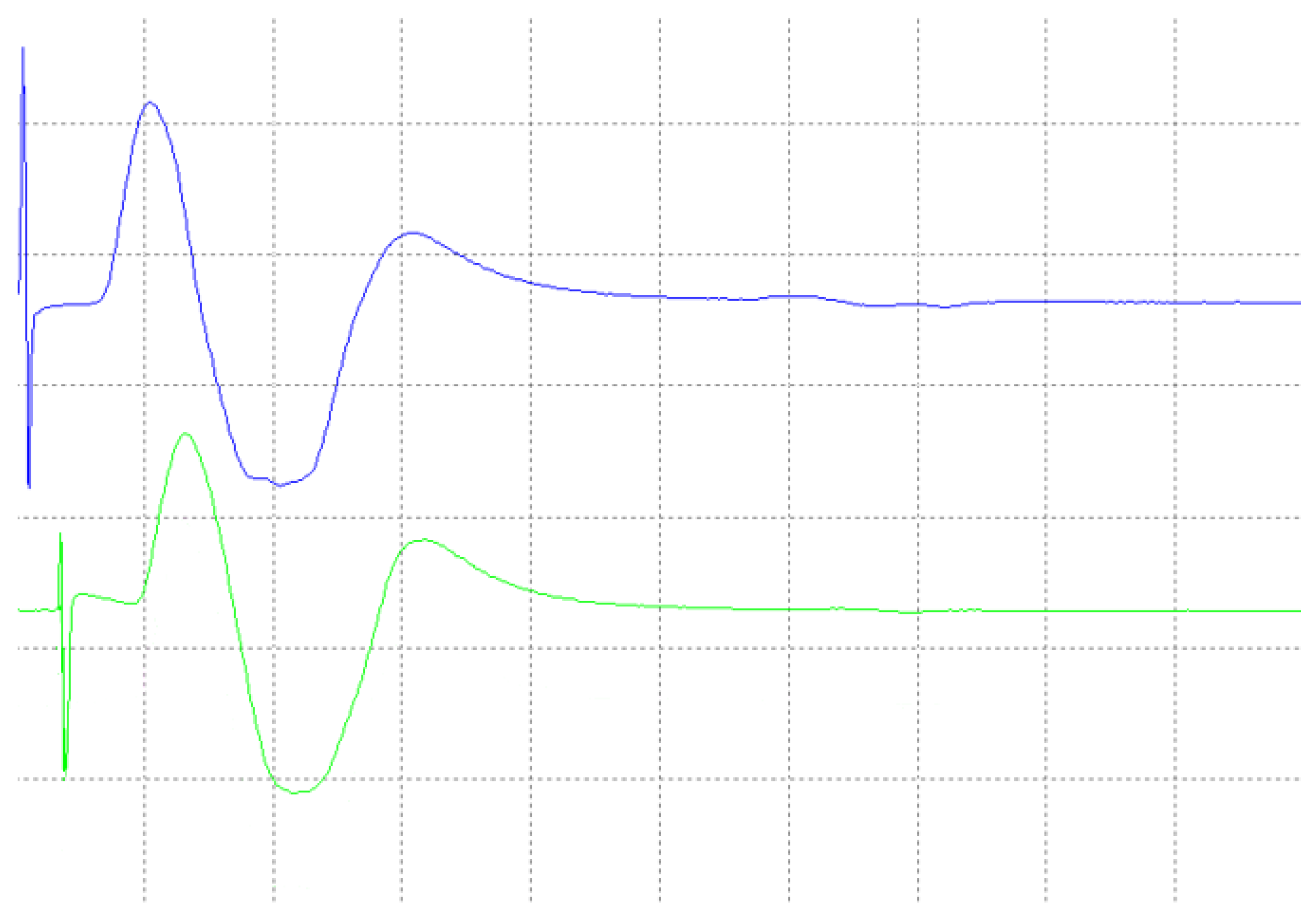
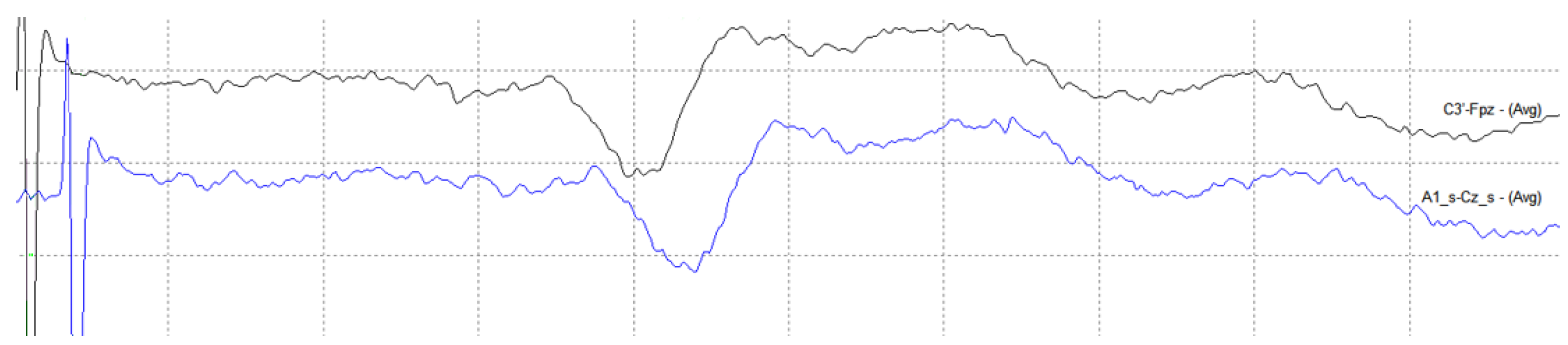
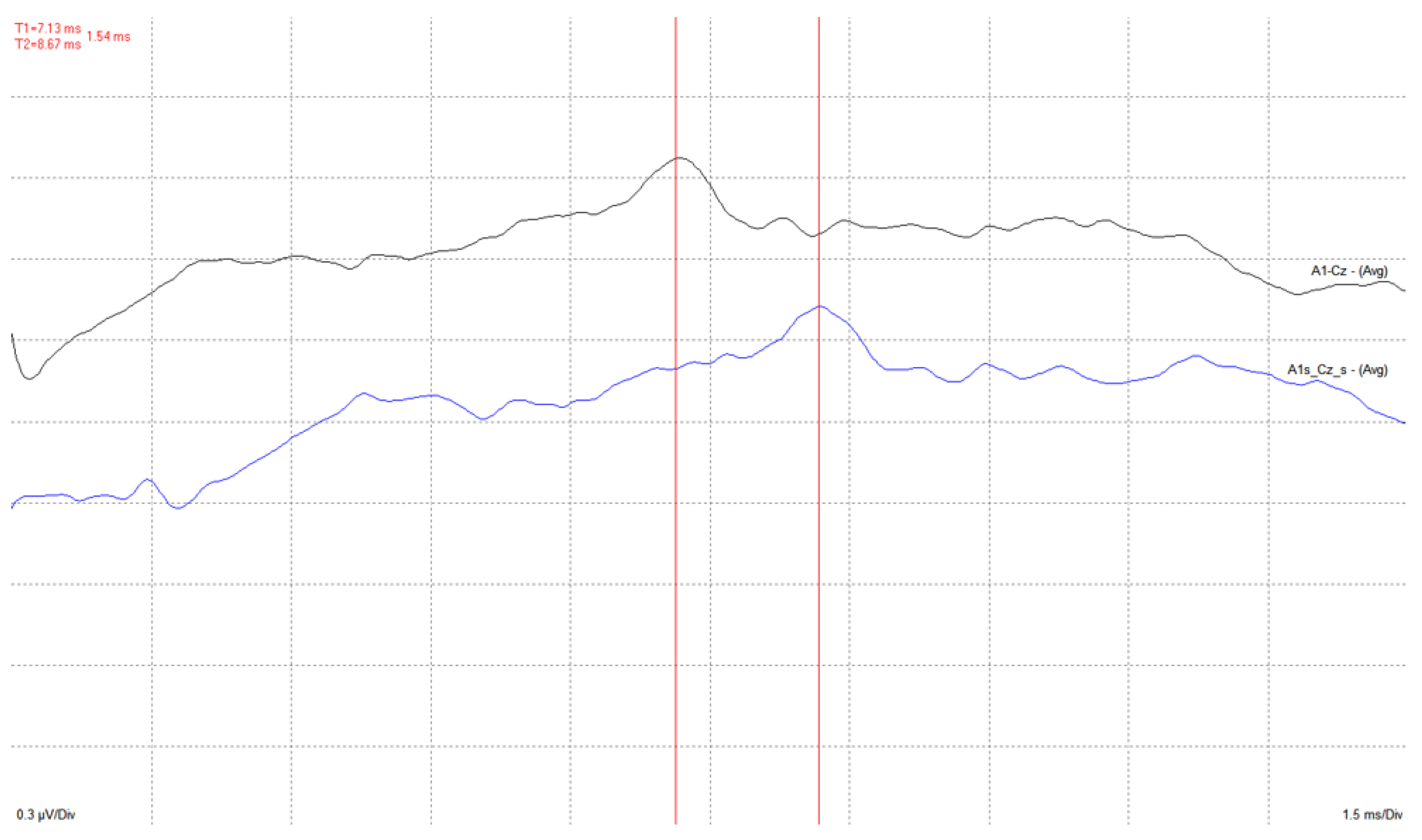
4. Results
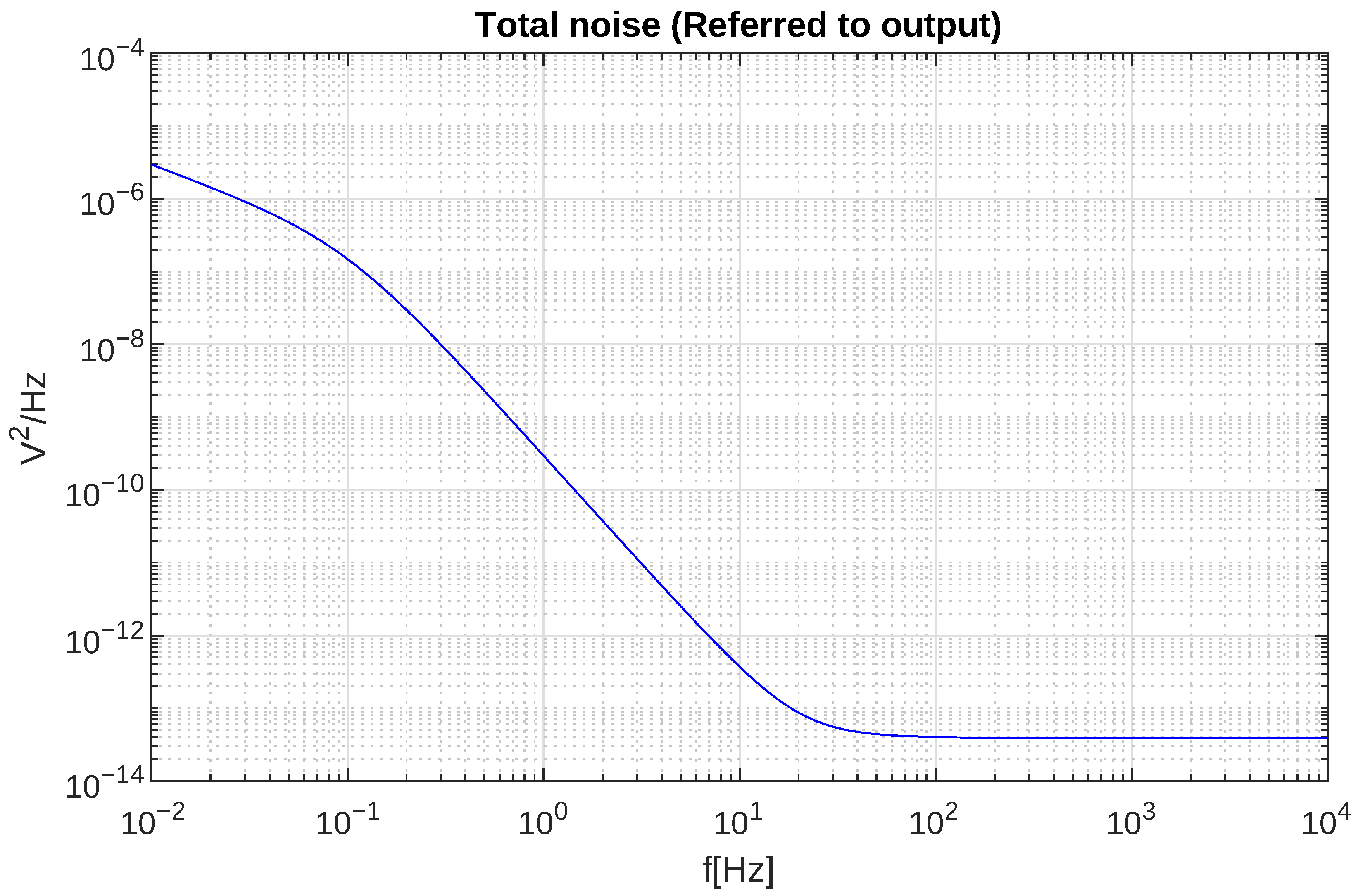
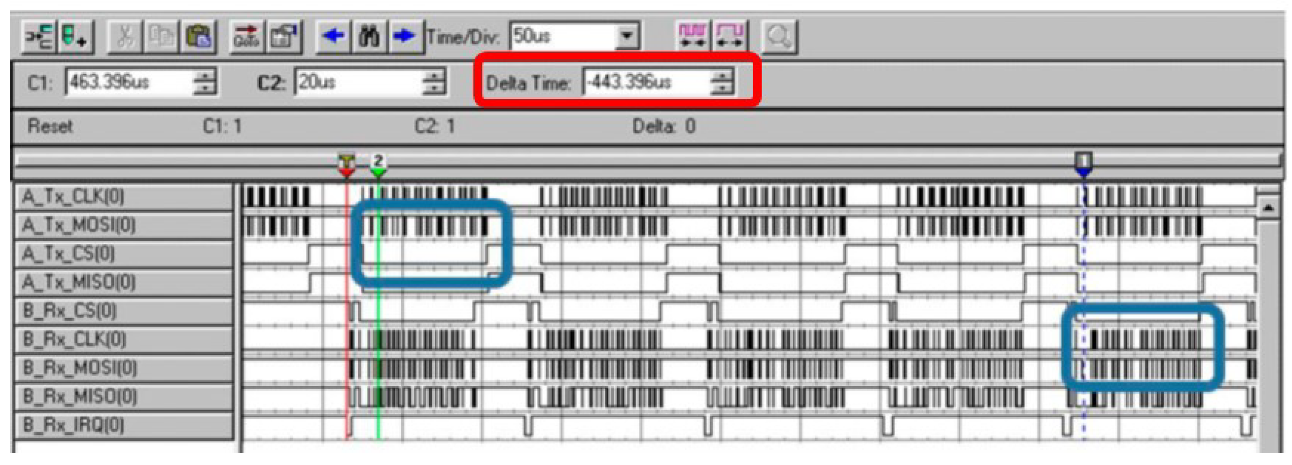
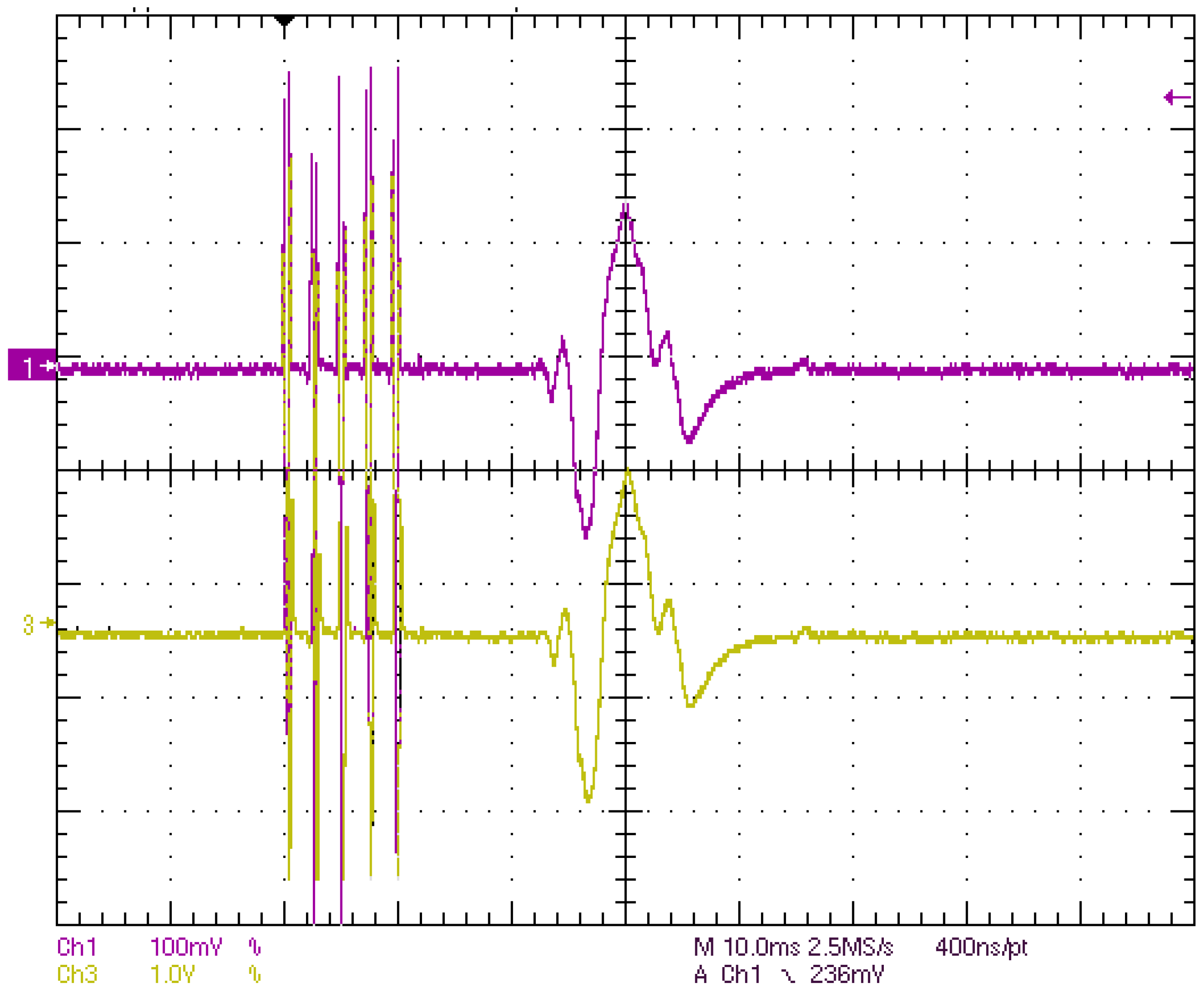
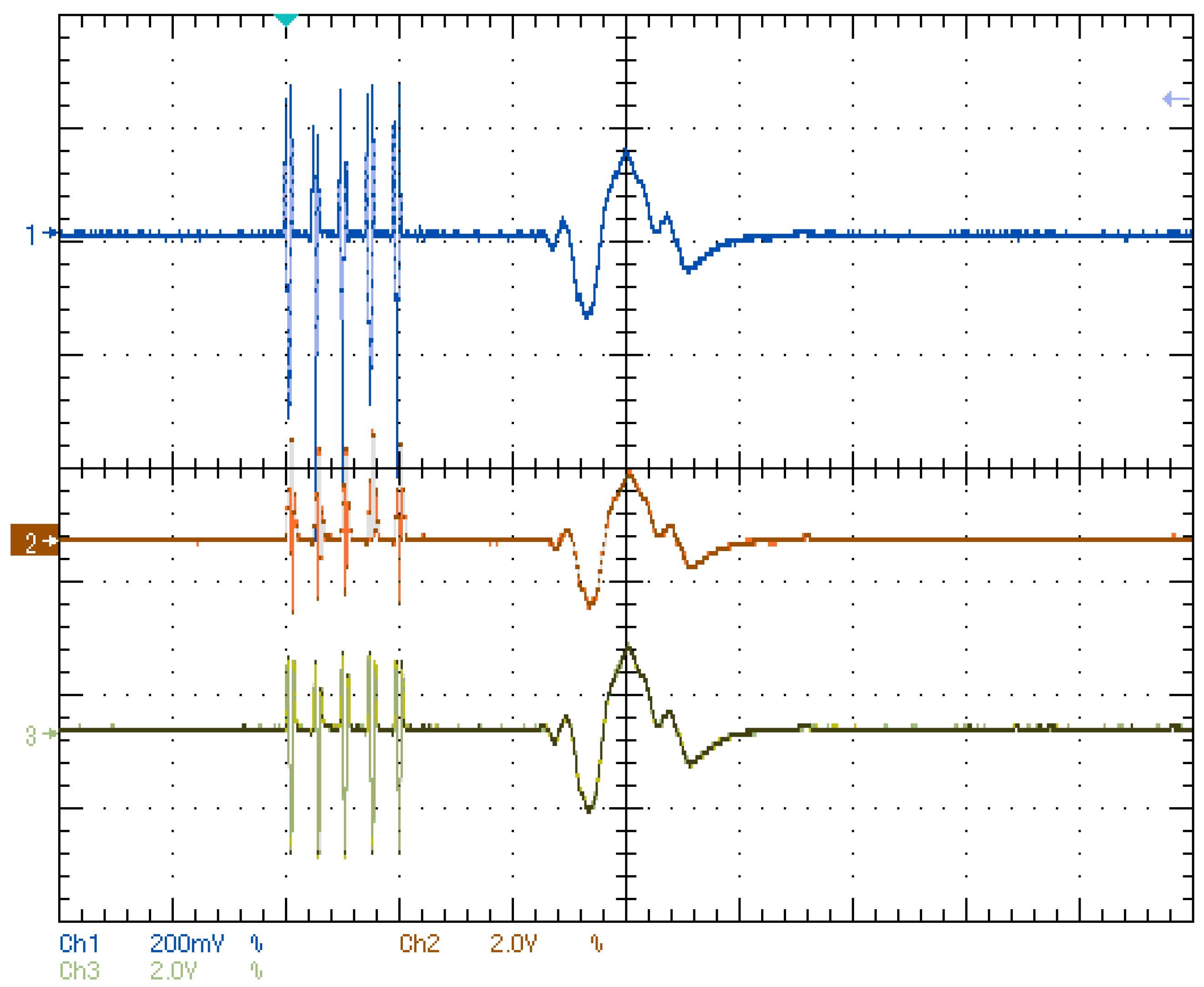
4.1. Laboratory Results
4.2. Medical Site Results
5. Discussion
- Due to all the stages of the system from signal acquisition to reconstruction, a latency time is introduced. Although this feature is critical in the medical evaluation of the recorded signals, test measurements show that this delay is constant and acceptably low for the intended application.
- Several wireless technologies and protocols were considered, and an ISM RF radio module was selected as the most adequate one. A custom protocol was developed in order to comply with the sampling frequency and low latency requested, including data transmission/reception and configuration commands dialog.
- The system developed is versatile, and input channels can be configured to work as single-ended (referential) or differential inputs. The user has the possibility to change the configuration as well as the channel utilized as the reference for the measurements.
- The system includes a novel impedance measurement method, necessary to assess the correct connection of the electrodes, similarly to what is installed in current commercial monitoring devices. This method is implemented in the analog front end of the system and has been thoroughly described in [16].
- Currently available commercial monitoring systems present a software interface that allows the user to adjust the amplification gain depending on the voltage range of the signal. In our system an algorithm is implemented that analyzes the amplitude of the signal recorded on each channel and automatically changes the setting of the programmable gain amplifiers in the analog front-end.
- The validation of the system has been carried out through laboratory and field tests. The results of the experiments demonstrate sufficient accuracy in signal regeneration across the full range of the expected input signals, as well as an acceptable delay between actual and regenerated signal.
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| WIONM | Wireless Intraoperative Neurophysiological Monitoring |
| IONM | Intraoperative Neurophysiological Monitoring |
| EEG | Electroencephalography |
| EMG | Electromyography |
| ECoG | Electrocorticography |
| ADC | Analog-to-Digital Converter |
| DAC | Digital-to-Analog Converter |
| RF | Radio-Frequency |
| CRC | Cyclic Redundancy Check |
| SSEP | Somato-Sensory Evoked Potentials |
| EP | Evoked Potential |
| MEP | Motor Evoked Potential |
| AEP | Auditory Evoked Potential |
References
- Howick, J.; Cohen, B.A.; McCulloch, P.; Thompson, M.; Skinner, S.A. Foundations for evidence-based intraoperative neurophysiological monitoring. Clin. Neurophysiol. 2016, 127, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Vega-Zelaya, L.; Sola, R.G.; Pastor, J. Intraoperative Neurophysiological Monitoring in Neuro-oncology. In Neurooncology—Newer Developments; Agrawal, A., Ed.; IntechOpen: Rijeka, Croatia, 2016; Chapter 9. [Google Scholar]
- Liao, L.D.; Chen, C.Y.; Wang, I.J.; Chen, S.F.; Li, S.Y.; Chen, B.W.; Chang, J.Y.; Lin, C.T. Gaming control using a wearable and wireless EEG-based brain-computer interface device with novel dry foam-based sensors. J. Neuroeng. Rehabil. 2012, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-T.; Ko, L.-W.; Chang, M.-H.; Duann, J.-R.; Chen, J.-Y.; Su, T.-P.; Jung, T.-P. Review of Wireless and Wearable Electroencephalogram Systems and Brain-Computer Interfaces—A Mini-Review. Gerontology 2010, 56, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.M.; Cauwenberghs, G. Wireless Non-contact EEG/ECG Electrodes for Body Sensor Networks. In Proceedings of the 2010 International Conference on Body Sensor Networks, Singapore, 7–9 June 2010; pp. 297–301. [Google Scholar]
- Sodagar, A.M.; Wise, K.D.; Najafi, K. A Wireless Implantable Microsystem for Multichannel Neural Recording. IEEE Trans. Microw. Theroy Tech. 2009, 57, 2565–2573. [Google Scholar] [CrossRef]
- Vetter, R.J.; Williams, J.C.; Hetke, J.F.; Nunamaker, E.A.; Kipke, D.R. Chronic Neural Recording Using Silicon-Substrate Microelectrode Arrays Implanted in Cerebral Cortex. IEEE Trans. Biomed. Eng. 2004, 51, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Casson, A.J.; Yates, D.C.; Smith, S.J.; Duncan, J.S.; Rodriguez-villegas, E. Weareable Electroenchepalography. IEEE Eng. Med. Biol. Mag. 2010, 29, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Patki, S.; Grundlehner, B.; Verwegen, A.; Mitra, S.; Xu, J.; Matsumoto, A.; Yazicioglu, R.F.; Penders, J. Wireless EEG system with real time impedance monitoring and active electrodes. In Proceedings of the 2012 IEEE Biomedical Circuits and Systems Conference (BioCAS), Hsinchu, Taiwan, 28–30 November 2012; pp. 108–111. [Google Scholar]
- Sagha, H.; Perdikis, S.; Chavarriaga, R. Quantifying Electrode Reliability during Brain—Computer Interface Operation. IEEE Trans. Biomed. Eng. 2015, 62, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Buxi, D.; Kim, S.; Van Helleputte, N.; Altini, M.; Wijsman, J.; Yazicioglu, R.F.; Penders, J.; Van Hoof, C. Correlation between electrode-tissue impedance and motion artifact in biopotential recordings. IEEE Sens. J. 2012, 12, 3373–3383. [Google Scholar] [CrossRef]
- Ferree, T.C.; Luu, P.; Russell, G.S.; Tucker, D.M. Scalp electrode impedance, infection risk, and EEG data quality. Clin. Neurophysiol. 2001, 112, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Shannon, C. Communication in the Presence of Noise. Proc. IRE 1949, 37, 10–21. [Google Scholar] [CrossRef]
- Webster, J. Medical Instrumentation Application and Design, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2009; Available online: https://books.google.es/books?id=CQ4cAAAAQBAJ (accessed on 15 October 2022).
- AD8429 1nV/ Low Noise Instrumentation Amplifier. 2017. Available online: https://www.analog.com/media/en/technical-documentation/data-sheets/ad8429.pdf (accessed on 15 October 2022).
- Alonso, E.; Giannetti, R.; Rodríguez-Morcillo, C.; Matanza, J.; Muñoz-Frías, J.D. A Novel Passive Method for the Assessment of Skin-Electrode Contact Impedance in Intraoperative Neurophysiological Monitoring Systems. Sci. Rep. 2020, 10, 2819. [Google Scholar] [CrossRef] [PubMed]
- Scandurra, G.; Cannatà, G.; Giusi, G.; Ciofi, C. A new approach to DC removal in high gain, low noise voltage amplifiers. In Proceedings of the 2017 International Conference on Noise and Fluctuations (ICNF), Vilnius, Lithuania, 20–23 June 2017; pp. 1–4. [Google Scholar]
- Huang, C.c.; Hung, S.H.; Chung, J.F.; Van, L.D.; Lin, C.T. Front-End Amplifier of Low-Noise and Tunable BW/Gain for Portable Biomedical Signal Acquisition. In Proceedings of the 2008 IEEE International Symposium on Circuits and Systems (ISCAS), Seattle, WA, USA, 18–21 May 2008; pp. 2717–2720. [Google Scholar]
- Harrison, R.R.; Charles, C. A Low-Power Low-Noise CMOS Amplifier for Neural Recording Applications. IEEE J. Solid-State Circuits 2003, 38, 958–965. [Google Scholar] [CrossRef]
- Müller-Putz, G.R.; Scherer, R.; Neuper, C.; Pfurtscheller, G. Steady-State Somatosensory Evoked Potentials: Suitable Brain Signals for Brain—Computer Interfaces? IEEE Trans. Neural Syst. Rehabil. Eng. 2006, 14, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Chestek, C.A.; Gilja, V.; Nuyujukian, P.; Kier, R.J.; Solzbacher, F.; Ryu, S.I.; Harrison, R.R.; Shenoy, K.V. HermesC: Low-Power Wireless Neural Recording System for Freely Moving Primates. IEEE Trans. Neural Syst. Rehabil. Eng. 2009, 17, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Farajidavar, A.; Seifert, J.L.; Bell, J.E.S.; Seo, Y.S.; Delgado, M.R.; Sparagana, S.; Romero, M.I.; Chiao, J.C. A wireless system for monitoring transcranial motor evoked potentials. Ann. Biomed. Eng. 2011, 39, 517–523. [Google Scholar] [CrossRef] [PubMed]
- IGlobal Intraoperative Neuromonitoring (IONM) Market to Reach USD 9.03 Billion by 2028: Vantage Market Research. 2021. Available online: https://www.globenewswire.com/en/news-release/2021/11/18/2337755/0/en/Global-Intraoperative-Neuromonitoring-IONM-Market-to-Reach-USD-9-03-Billion-by-2028-Vantage-Market-Research.html (accessed on 15 October 2022).
- Jiménez-Carles Gil-Delgado, E. Intraoperative Monitoring System. Patent Application WO 2018/011439 Al, 18 January 2018. Available online: https://patentscope.wipo.int/search/es/detail.jsf?docId=WO2018011439 (accessed on 15 October 2022).


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alonso Rivas, E.; Giannetti, R.; Rodríguez-Morcillo García, C.; Matanza Domingo, J.; Muñoz Frías, J.D.; Scandurra, G.; Ciofi, C.; Vega-Zelaya, L.; Pastor, J. A Quasi-Wireless Intraoperatory Neurophysiological Monitoring System. Electronics 2022, 11, 3918. https://doi.org/10.3390/electronics11233918
Alonso Rivas E, Giannetti R, Rodríguez-Morcillo García C, Matanza Domingo J, Muñoz Frías JD, Scandurra G, Ciofi C, Vega-Zelaya L, Pastor J. A Quasi-Wireless Intraoperatory Neurophysiological Monitoring System. Electronics. 2022; 11(23):3918. https://doi.org/10.3390/electronics11233918
Chicago/Turabian StyleAlonso Rivas, Eduardo, Romano Giannetti, Carlos Rodríguez-Morcillo García, Javier Matanza Domingo, José Daniel Muñoz Frías, Graziella Scandurra, Carmine Ciofi, Lorena Vega-Zelaya, and Jesús Pastor. 2022. "A Quasi-Wireless Intraoperatory Neurophysiological Monitoring System" Electronics 11, no. 23: 3918. https://doi.org/10.3390/electronics11233918
APA StyleAlonso Rivas, E., Giannetti, R., Rodríguez-Morcillo García, C., Matanza Domingo, J., Muñoz Frías, J. D., Scandurra, G., Ciofi, C., Vega-Zelaya, L., & Pastor, J. (2022). A Quasi-Wireless Intraoperatory Neurophysiological Monitoring System. Electronics, 11(23), 3918. https://doi.org/10.3390/electronics11233918









