The Influence of a High-Voltage Discharge in a Helicoidal Twisted-Pair Structure on Enzyme Adsorption
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Enzyme
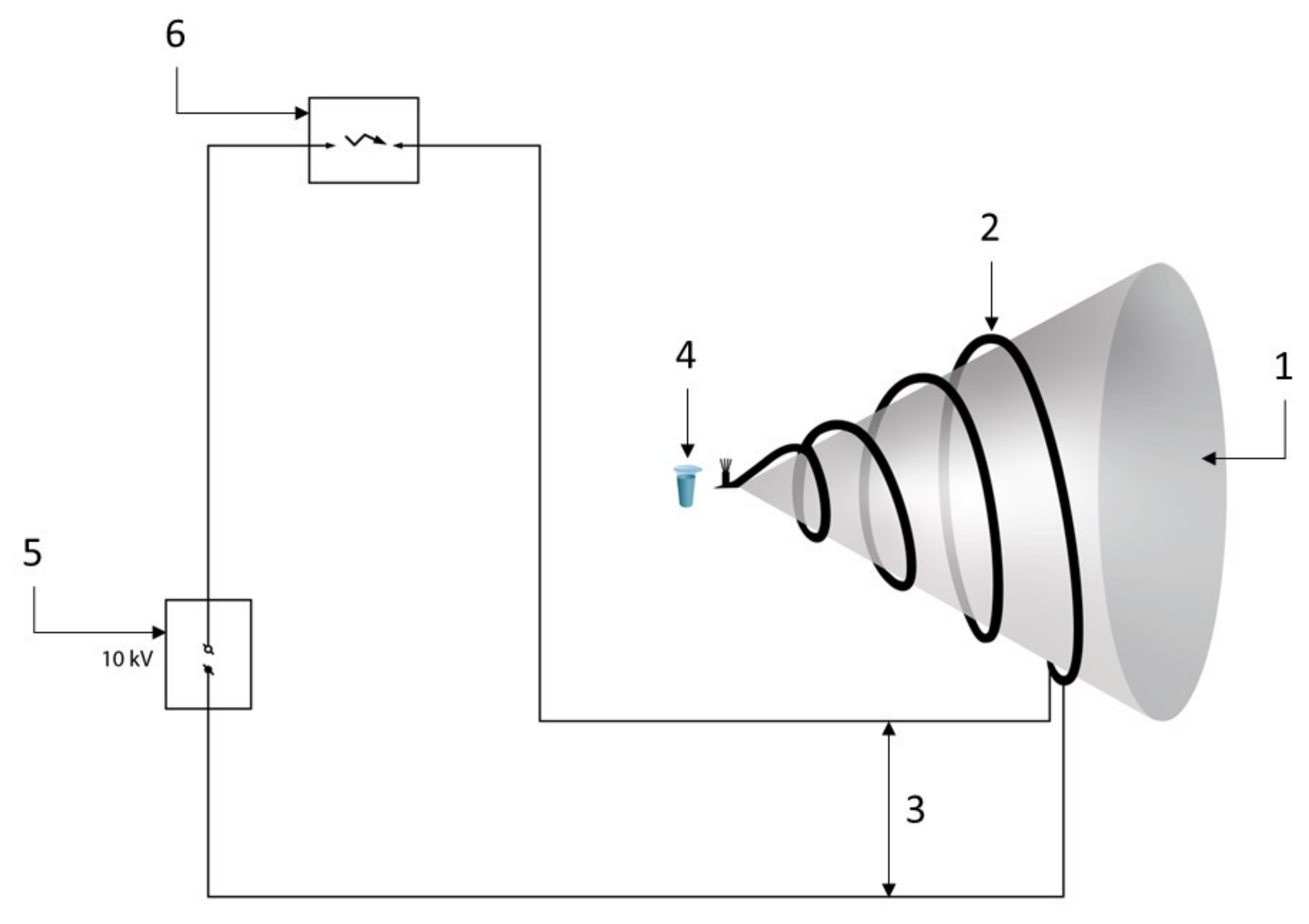
2.2. Experimental Setup
2.3. AFM Experiments
2.4. Spectrophotometry
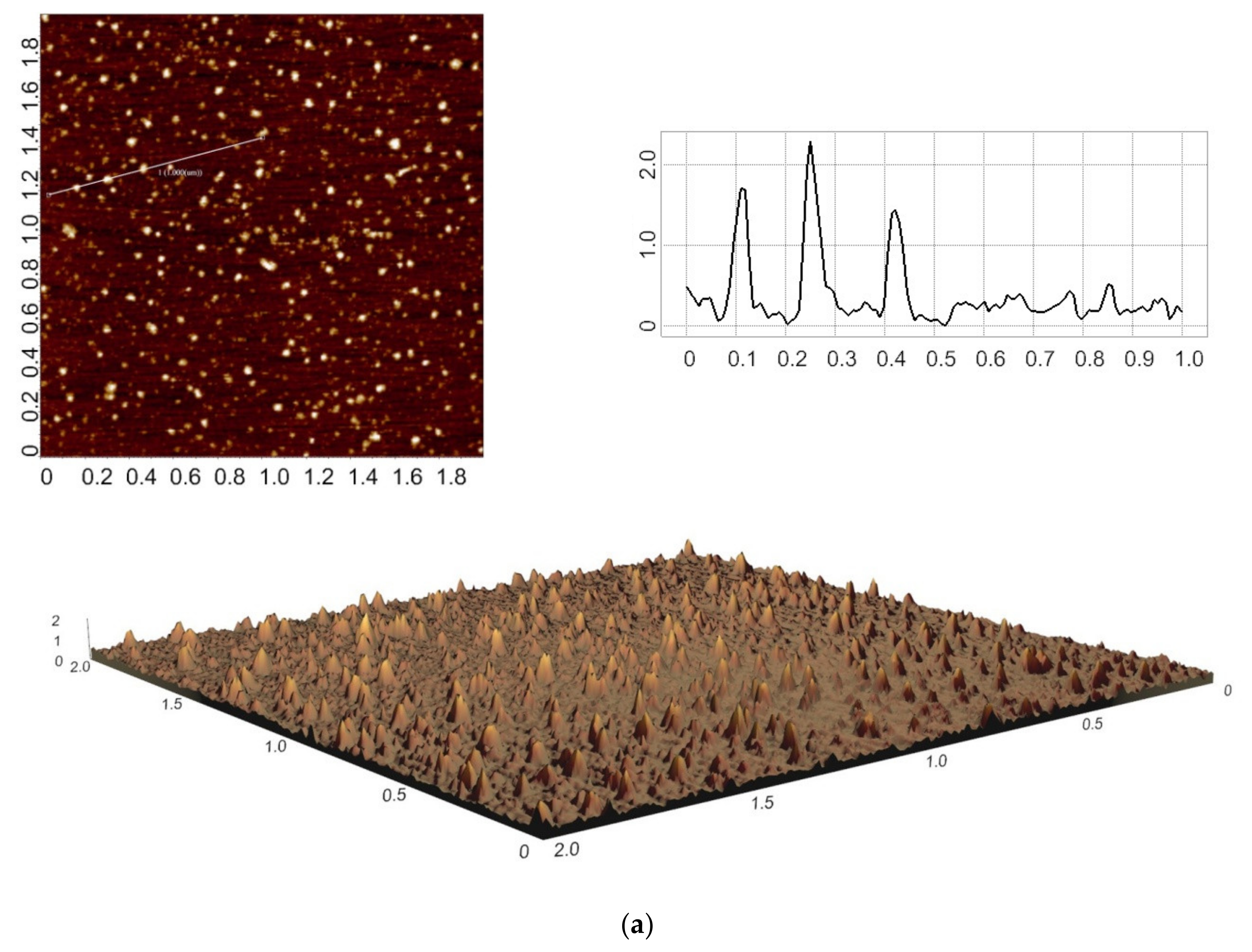
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sopova, J.V.; Koshel, E.I.; Belashova, T.A.; Zadorsky, S.P.; Sergeeva, A.V.; Siniukova, V.A.; Shenfeld, A.A.; Velizhanina, M.E.; Volkov, K.V.; Nizhnikov, A.A.; et al. RNA-binding protein FXR1 is presented in rat brain in amyloid form. Sci. Rep. 2019, 9, 18983. [Google Scholar] [CrossRef] [PubMed]
- Benseny-Cases, N.; Álvarez-Marimon, E.; Aso, E.; Carmona, M.; Klementieva, O.; Appelhans, D.; Ferrer, I.; Cladera, J. In situ structural characterization of early amyloid aggregates in Alzheimer’s disease transgenic mice and Octodon degus. Sci. Rep. 2020, 10, 5888. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Fu, Z.; Meng, L.; He, M.; Zhang, Z. The early events that initiate β-amyloid aggregation in Alzheimer’s disease. Front. Aging Neurosci. 2018, 10, 359. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, M.; Xia, K.; Colón, W.; Vieira, S.I.; Ribeiro, F. Protein aggregation, cardiovascular diseases, and exercise training: Where do we stand? Ageing Res. Rev. 2017, 40, 1–10. [Google Scholar] [CrossRef]
- Xu, J.; Reumers, J.; Couceiro, J.R.; De Smet, F.; Gallardo, R.; Rudyak, S.; Cornelis, A.; Rozenski, J.; Zwolinska, A.; Marine, J.C.; et al. Gain of function of mutant p53 by coaggregation with multiple tumor suppressors. Nat. Chem. Biol. 2011, 7, 285–295. [Google Scholar] [CrossRef]
- Gavrilenko, T.I.; Ryzhkova, N.A.; Parkhomenko, A.N. Myeloperoxidase and its role in development of ischemic heart disease. Ukr. J. Cardiol. 2014, 4, 119–126. [Google Scholar]
- Ahibom, A.; Day, N.; Feychting, M.; Roman, E.; Skinner, J.; Dockerty, J.; Linet, M.; McBride, M.; Michaelis, J.; Olsen, J.H.; et al. A pooled analysis of magnetic fields and childhood Ieukaemia. Br. J. Cancer 2000, 83, 692–698. [Google Scholar] [CrossRef]
- Dowson, D.I.; Lewith, G.T.; Campbell, M.; Mullee, M.A.; Brewster, L.A. Overhead high-voltage cables and recurrent headache and depressions. Practitioner 1988, 232, 435–436. [Google Scholar]
- Greenland, S.; Sheppard, A.R.; Kaune, W.T.; Poole, C.; Kelsh, M.A. A pooled analysis of magnetic fields, wire codes, and childhood leukemia. Epidemiology 2000, 11, 624–634. Available online: http://www.jstor.org/stable/3703814 (accessed on 14 September 2022). [CrossRef]
- Perry, S.; Pearl, L.; Binns, R. Power frequency magnetic field; depressive illness and myocardial infarction. Public Health 1989, 103, 177–180. [Google Scholar] [CrossRef]
- El-Kaliuoby, M.I.; El-Khatib, A.; Mohamed, M.; Roston, G.; Khalil, A.M.; Ahmed, M.M.; AbdulSalam, H. Monitoring of Rat’s Blood Rheology after Exposure to 50-Hz Electric Fields (Occupational Study). OSR J. Environ. Sci. Toxicol. Food Technol. 2016, 10, 4–8. [Google Scholar] [CrossRef]
- Korpinen, L.; Partanen, J. Influence of 50-Hz electric and magnetic fields on human blood pressure. Radiat. Environ. Biophys. 1996, 35, 199–204. [Google Scholar] [CrossRef]
- Mahfouz, A.; Hassan, S.A.; Arisha, A. Practical simulation application: Evaluation of process control parameters in Twisted-Pair Cables manufacturing system. Simul. Model. Pract. Theory 2010, 18, 471–482. [Google Scholar] [CrossRef]
- Ivanov, Y.D.; Pleshakova, T.O.; Shumov, I.D.; Kozlov, A.F.; Ivanova, I.A.; Valueva, A.A.; Tatur, V.Y.; Smelov, M.V.; Ivanova, N.D.; Ziborov, V.S. AFM imaging of protein aggregation in studying the impact of knotted electromagnetic field on a peroxidase. Sci. Rep. 2020, 10, 9022. [Google Scholar] [CrossRef]
- Morelli, A.; Ravera, S.; Panfoli, I.; Pepe, I.M. Effects of extremely low frequency electromagnetic fields on membrane-associated enzymes. Arch. Biochem. Biophys. 2005, 441, 191–198. [Google Scholar] [CrossRef]
- Thumm, S.; Löschinger, M.; Glock, S.; Hämmerle, H.; Rodemann, H.P. Induction of cAMP-dependent protein kinase A activity in human skin fibroblasts and rat osteoblasts by extremely low-frequency electromagnetic fields. Radiat. Environ. Biophys. 1999, 38, 195–199. [Google Scholar] [CrossRef]
- Caliga, R.; Maniu, C.L.; Mihăşan, M. ELF-EMF exposure decreases the peroxidase catalytic efficiency in vitro. Open Life Sci. 2016, 11, 71–77. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, B.; Pang, H.; Wang, Y.; Fu, H.; Chen, X.; Wang, Y. The effect of radio frequency heating on the inactivation and structure of horseradish peroxidase. Food Chem. 2023, 398, 133875. [Google Scholar] [CrossRef]
- Fortune, J.A.; Wu, B.-I.; Klibanov, A.M. Radio Frequency Radiation Causes No Nonthermal Damage in Enzymes and Living Cells. Biotechnol. Prog. 2010, 26, 1772–1776. [Google Scholar] [CrossRef]
- Lopes, L.C.; Barreto, M.T.; Gonçalves, K.M.; Alvarez, H.M.; Heredia, M.F.; De Souza, R.O.M.; Cordeiro, Y.; Dariva, C.; Fricks, A.T. Stability and structural changes of horseradish peroxidase: Microwave versus conventional heating treatment. Enzym. Microb. Technol. 2015, 69, 10–18. [Google Scholar] [CrossRef]
- Latorre, M.E.; Bonelli, P.R.; Rojas, A.M.; Gerschenson, L.N. Microwave inactivation of red beet (Beta vulgaris L. var. conditiva) peroxidase and polyphenoloxidase and the effect of radiation on vegetable tissue quality. J. Food Eng. 2012, 109, 676–684. [Google Scholar] [CrossRef]
- Available online: https://electricps.ru/razriad (accessed on 14 September 2022).
- Ivanov, Y.D.; Tatur, V.Y.; Pleshakova, T.O.; Shumov, I.D.; Kozlov, A.F.; Valueva, A.A.; Ivanova, I.A.; Ershova, M.O.; Ivanova, N.D.; Stepanov, I.N.; et al. The Effect of Incubation near an Inversely Oriented Square Pyramidal Structure on Adsorption Properties of Horseradish Peroxidase. Appl. Sci. 2022, 12, 4042. [Google Scholar] [CrossRef]
- Ignatenko, O.V.; Sjölander, A.; Hushpulian, D.M.; Kazakov, S.V.; Ouporov, I.V.; Chubar, T.A.; Poloznikov, A.A.; Ruzgas, T.; Tishkov, V.I.; Gorton, L.; et al. Electrochemistry of chemically trapped dimeric and monomeric recombinant horseradish peroxidase. Adv. Biosens. Bioelectron. 2013, 2, 25–34. [Google Scholar]
- Ziborov, V.S.; Pleshakova, T.O.; Shumov, I.D.; Kozlov, A.F.; Valueva, A.A.; Ivanova, I.A.; Ershova, M.O.; Larionov, D.I.; Evdokimov, A.N.; Tatur, V.Y.; et al. The Impact of Fast-Rise-Time Electromagnetic Field and Pressure on the Aggregation of Peroxidase upon Its Adsorption onto Mica. Appl. Sci. 2021, 11, 11677. [Google Scholar] [CrossRef]
- Ivanov, Y.D.; Tatur, V.Y.; Pleshakova, T.O.; Shumov, I.D.; Kozlov, A.F.; Valueva, A.A.; Ivanova, I.A.; Ershova, M.O.; Ivanova, N.D.; Repnikov, V.V.; et al. Effect of Spherical Elements of Biosensors and Bioreactors on the Physicochemical Properties of a Peroxidase Protein. Polymers 2021, 13, 1601. [Google Scholar] [CrossRef] [PubMed]
- Kiselyova, O.I.; Yaminsky, I.V.; Ivanov, Y.D.; Kanaeva, I.P.; Kuznetsov, V.Y.; Archakov, A.I. AFM study of membrane proteins, cytochrome P450 2B4, and NADPH–Cytochrome P450 reductase and their complex formation. Arch. Biochem. Biophys. 1999, 371, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, Y.D.; Pleshakova, T.O.; Shumov, I.D.; Kozlov, A.F.; Ivanova, I.A.; Valueva, A.A.; Ershova, M.O.; Tatur, V.Y.; Stepanov, I.N.; Repnikov, V.V.; et al. AFM study of changes in properties of horseradish peroxidase after incubation of its solution near a pyramidal structure. Sci. Rep. 2021, 11, 1–9. [Google Scholar] [CrossRef]
- Ivanov, Y.D.; Pleshakova, T.O.; Shumov, I.D.; Kozlov, A.F.; Romanova, T.S.; Valueva, A.A.; Tatur, V.Y.; Stepanov, I.N.; Ziborov, V.S. Investigation of the Influence of Liquid Motion in a Flow-based System on an Enzyme Aggregation State with an Atomic Force Microscopy Sensor: The Effect of Water Flow. Appl. Sci. 2020, 10, 4560. [Google Scholar] [CrossRef]
- Ziborov, V.S.; Pleshakova, T.O.; Shumov, I.D.; Kozlov, A.F.; Ivanova, I.A.; Valueva, A.A.; Tatur, V.Y.; Negodailov, A.N.; Lukyanitsa, A.A.; Ivanov, Y.D. Investigation of the Influence of Liquid Motion in a Flow-Based System on an Enzyme Aggregation State with an Atomic Force Microscopy Sensor: The Effect of Glycerol Flow. Appl. Sci. 2020, 10, 4825. [Google Scholar] [CrossRef]
- Ivanov, Y.D.; Pleshakova, T.O.; Shumov, I.D.; Kozlov, A.F.; Valueva, A.A.; Ivanova, I.A.; Ershova, M.O.; Larionov, D.I.; Repnikov, V.V.; Ivanova, N.D.; et al. AFM and FTIR Investigation of the Effect of Water Flow on Horseradish Peroxidase. Molecules 2021, 26, 306. [Google Scholar] [CrossRef]
- Pleshakova, T.O.; Kaysheva, A.L.; Shumov, I.D.; Ziborov, V.S.; Bayzyanova, J.M.; Konev, V.A.; Uchaikin, V.F.; Archakov, A.I.; Ivanov, Y.D. Detection of hepatitis C virus core protein in serum using aptamer-functionalized AFM chips. Micromachines 2019, 10, 129. [Google Scholar] [CrossRef]
- Sanders, S.A.; Bray, R.C.; Smith, A.T. pH-Dependent properties of a mutant horseradish peroxidase isoenzyme C in which Arg38 has been replaced with lysine. Eur. J. Biochem. 1994, 224, 1029–1037. [Google Scholar] [CrossRef]
- Kiselyov, V.F.; Saletskii, A.M.; Semikhina, L.P. Structural changes in water after exposure to weak alternating magnetic fields. Vestn. MSU. Series 3 Physics. Astron. 1990, 31, 53–58. [Google Scholar]
- Pershin, S.M. The Physical Basis of the Weak Fields Interaction with Bio Objects Is the Quantum Differences of H2O Spin-Isomers. Online Biophysical Blog. Available online: http://www.biophys.ru/archive/congress2012/proc-p27.htm (accessed on 14 September 2022).
- Pershin, S.M. Conversion of ortho-para H2O isomers in water and a jump in erythrocyte fluidity through a microcapillary at a temperature of 36.6 ± 0.3 °C. Phys. Wave Phenom. 2009, 17, 241–250. [Google Scholar] [CrossRef]
- Morón, M.C. Protein hydration shell formation: Dynamics of water in biological systems exhibiting nanoscopic cavities. J. Mol. Liq. 2021, 337, 116584. [Google Scholar] [CrossRef]
- Fogarty, A.C.; Laage, D. Water Dynamics in Protein Hydration Shells: The Molecular Origins of the Dynamical Perturbation. J. Phys. Chem. B 2014, 118, 7715–7729. [Google Scholar] [CrossRef]
- Verma, P.K.; Rakshit, S.; Mitra, R.K.; Pal, S.K. Role of hydration on the functionality of a proteolytic enzyme α-chymotrypsin under crowded environment. Biochimie 2011, 93, 1424–1433. [Google Scholar] [CrossRef]
- Laage, D.; Elsaesser, T.; Hynes, J.T. Water Dynamics in the Hydration Shells of Biomolecules. Chem. Rev. 2017, 117, 10694–10725. [Google Scholar] [CrossRef]
- Poojary, M.M.; Roohinejad, S.; Koubaa, M.; Barba, F.J.; Passamonti, P.; Jambrak, A.R.; Oey, I.; Greiner, R. Impact of Pulsed Electric Fields on Enzymes. In Handbook of Electroporation; Miklavčič, D., Ed.; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Zhong, K.; Hu, X.; Zhao, G.; Chen, F.; Liao, X. Inactivation and conformational change of horseradish peroxidase induced by pulsed electric field. Food Chem. 2005, 92, 473–479. [Google Scholar] [CrossRef]
- Zhong, K.; Wu, J.; Wang, Z.; Chen, F.; Liao, X.; Hu, X.; Zhang, Z. Inactivation kinetics and secondary structural change of PEF-treated POD and PPO. Food Chem. 2007, 100, 115–123. [Google Scholar] [CrossRef]
- Ohshima, T.; Tamura, T.; Sato, M. Influence of pulsed electric field on various enzyme activities. J. Electrost. 2007, 65, 156–161. [Google Scholar] [CrossRef]
- Vanga, S.K.; Wang, J.; Jayaram, S.; Raghavan, V. Effects of Pulsed Electric Fields and Ultrasound Processing on Proteins and Enzymes: A Review. Processes 2021, 9, 722. [Google Scholar] [CrossRef]
- Jeroish, Z.E.; Bhuvaneshwari, K.S.; Samsuri, F.; Narayanamurthy, V. Microheater: Material, design, fabrication, temperature control, and applications—A role in COVID-19. Biomed Microdevices 2022, 24, 3. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.; Lee, S.; Hwang, Y. Truly 3D microfluidic heating system with iterative structure of coil heaters and fluidic channels. Smart Mater. Struct. 2022, 31, 035016. [Google Scholar] [CrossRef]
- Available online: https://www.fermentador-bioreactor.com/en/features/precise-temperature-regulation-with-soft-ir-heating/ (accessed on 14 September 2022).



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanov, Y.D.; Tatur, V.Y.; Shumov, I.D.; Kozlov, A.F.; Valueva, A.A.; Ivanova, I.A.; Ershova, M.O.; Ivanova, N.D.; Stepanov, I.N.; Lukyanitsa, A.A.; et al. The Influence of a High-Voltage Discharge in a Helicoidal Twisted-Pair Structure on Enzyme Adsorption. Electronics 2022, 11, 3276. https://doi.org/10.3390/electronics11203276
Ivanov YD, Tatur VY, Shumov ID, Kozlov AF, Valueva AA, Ivanova IA, Ershova MO, Ivanova ND, Stepanov IN, Lukyanitsa AA, et al. The Influence of a High-Voltage Discharge in a Helicoidal Twisted-Pair Structure on Enzyme Adsorption. Electronics. 2022; 11(20):3276. https://doi.org/10.3390/electronics11203276
Chicago/Turabian StyleIvanov, Yuri D., Vadim Yu. Tatur, Ivan D. Shumov, Andrey F. Kozlov, Anastasia A. Valueva, Irina A. Ivanova, Maria O. Ershova, Nina D. Ivanova, Igor N. Stepanov, Andrei A. Lukyanitsa, and et al. 2022. "The Influence of a High-Voltage Discharge in a Helicoidal Twisted-Pair Structure on Enzyme Adsorption" Electronics 11, no. 20: 3276. https://doi.org/10.3390/electronics11203276
APA StyleIvanov, Y. D., Tatur, V. Y., Shumov, I. D., Kozlov, A. F., Valueva, A. A., Ivanova, I. A., Ershova, M. O., Ivanova, N. D., Stepanov, I. N., Lukyanitsa, A. A., & Ziborov, V. S. (2022). The Influence of a High-Voltage Discharge in a Helicoidal Twisted-Pair Structure on Enzyme Adsorption. Electronics, 11(20), 3276. https://doi.org/10.3390/electronics11203276





