Overview of Machine Learning and Deep Learning Approaches for Detecting Shockable Rhythms in AED in the Absence or Presence of CPR
Abstract
1. Introduction
2. Rhythms Annotation External Defibrillators
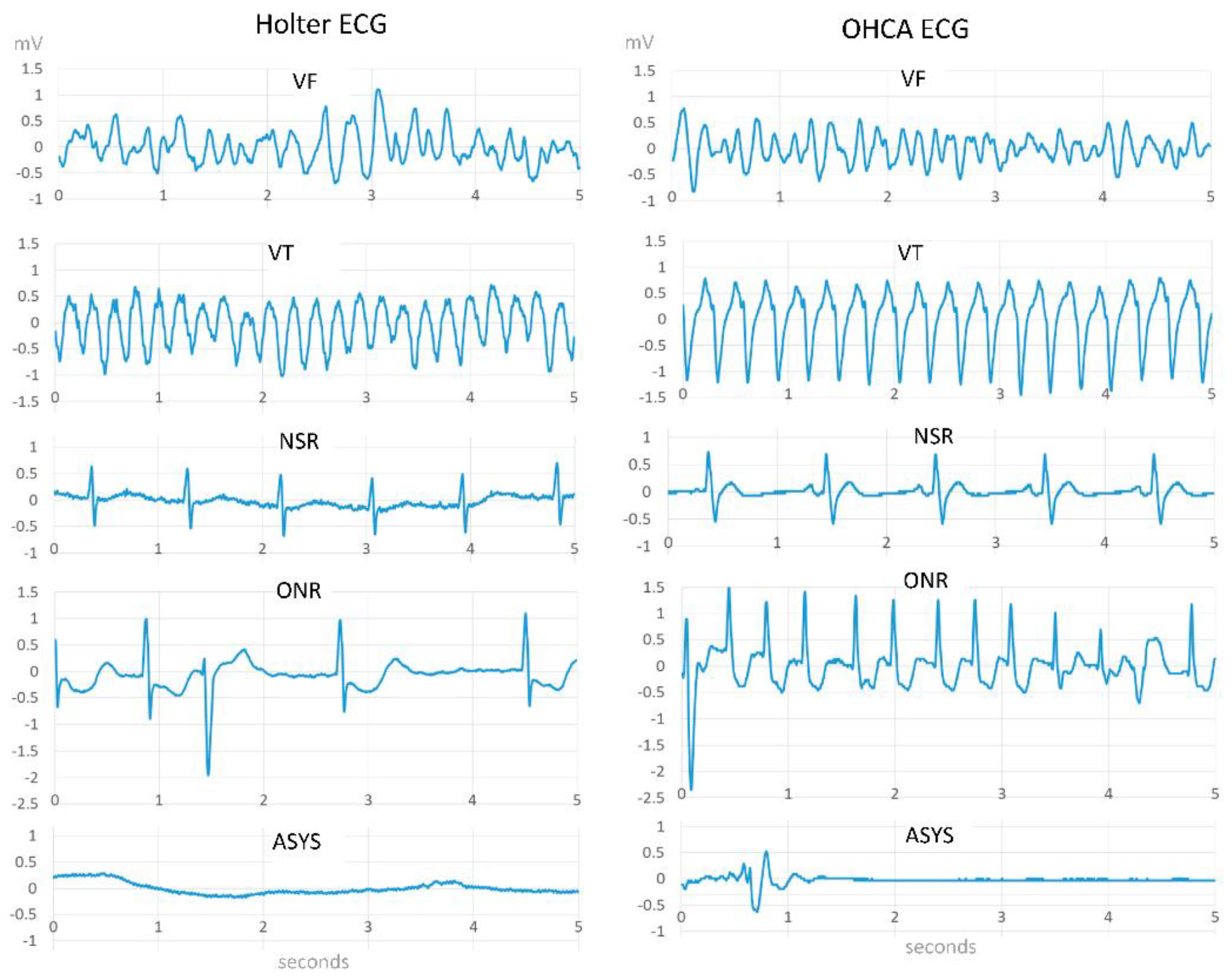
2.1. Shockable Rhythms
2.1.1. Ventricular Fibrillation (VF)
2.1.2. Ventricular Tachycardia (VT)
2.2. Non-Shockable Rhythms
2.2.1. Asystole (ASYS)
2.2.2. Pulseless Electrical Activity (PEA)
2.2.3. Other Non-Shockable Rhythms (ONR)
3. ECG Databases
3.1. Public Holter Databases
- AHA fibrillation database (AHADB) [18]: includes 30 min ECG recordings from 10 patients.
- Massachusetts Institute of Technology-Beth Israel Hospital (MIT-BIH) malignant ventricular ectopy database (VFDB) [19]: includes 22 half-hour ECG recordings of patients who experienced ventricular tachycardia, ventricular flutter, and ventricular fibrillation.
- Creighton University (CU) ventricular tachyarrhythmia database (CUDB) [20]: includes 35 eight-minute ECG recordings of people who have undergone sustained ventricular tachycardia, ventricular flutter, and ventricular fibrillation episodes.
- MIT-BIH Normal Sinus Rhythm Database ((NSRDB) [21]: includes 18 long-term ECG recordings of 18 subjects.
- MIT-BIH AF Database (AFDB) [22]: includes 25 long-term ECG recordings (of human subjects with AF.
- Sudden Cardiac Death Holter Database (SDBB) [23]: includes 18 patients with underlying sinus rhythm.
3.2. Out-of-Hospital Cardiac Arrests (OHAC) Databases
4. Performances Metrics
- True positive (TP): shock is correctly advised for a shockable rhythm
- False positive (FP): shock incorrectly advised for a non-shockable rhythm
- False negative (FN): no shock is advised for a shockable rhythm
- True negative (TN): no shock is advised for a non-shockable rhythm
5. Deep Learning and Machine Learning Techniques for Detecting Shockable Rhythms in AED While CPR Is Not Being Applied
5.1. Support Vector Machine (SVM)
5.2. Random Forest (RF)
5.3. Boosting and Logistic Regression (B-LR)
5.4. Convolution Neural Network (CNN)
5.5. Convolution Neural Network and Boosting Algorithm (CNN-BS)
5.6. Convolution Neural Network and Support Vector Machine (CNN-SVM)
5.7. CNN and Long Short-Term Memory (CNN-LSTM)
5.8. Optimized CNN
5.9. CNN and Recurrent Neural Network (CNN-RNN)
6. Deep Learning and Machine Learning Techniques for Detecting Shockable Rhythms in AED during Chest Compression
6.1. SVM
6.2. CNN
6.3. CNN with Bidirectional LSTM and Residual Networks
6.4. Backpropagation Neural Network (BP-NN)
7. Discussion
8. Limitations of Surveyed Works and Recommendations for Future Research
8.1. Limitations of Surveyed Works
8.2. Limitations of Surveyed Works
8.2.1. False Alarm Rate
8.2.2. Lack of Databases with a Higher Number of Patient ECG Recordings
8.2.3. Imbalanced Datasets
8.2.4. Lack of Standard Datasets
8.2.5. Lacks the Application of Unsupervised and Reinforcement Learning
9. Conclusions
- From the literature review, the optimized CNN is the best-known algorithm with the shortest detection time and higher specificity and sensitivity when CPR is stopped than other DL and ML algorithms. Similarly, during CPR, DL gives better performance than ML algorithms.
- DL/ML-based algorithms are data-driven approaches; therefore, data preprocessing impacts the algorithm’s performance.
- There is a considerable research gap in reducing the false alarm rate, standardization of algorithms and datasets, balancing the datasets, collecting large datasets from many patients, and implementing less tedious learning algorithms, such as unsupervised and reinforcement learning.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferretti, J.; di Pietro, L.; de Maria, C. Open-source automated external defibrillator. HardwareX 2017, 2, 61–70. [Google Scholar] [CrossRef]
- Patil Kaustubha, D.; Halperin Henry, R.; Becker Lance, B. Cardiac Arrest. Circ. Res. 2015, 116, 2041–2049. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.heart.org/-/media/files/health-topics/answers-by-heart/pe-abh-what-is-an-automated-external-defibrillator-ucm_300340.pdf (accessed on 12 January 2021).
- Sudden Cardiac Arrest (SCA). Available online: https://www.rqhealth.ca/department/cardiosciences/sudden-cardiac-arrest-sca (accessed on 9 May 2022).
- Fabbri, A.; Marchesini, G.; Spada, M.; Iervese, T.; Dente, M.; Galvani, M.; Vandelli, A. Monitoring intervention programmes for out-of-hospital cardiac arrest in a mixed urban and rural setting. Resuscitation 2006, 71, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Kennedy Space Center Automated External Defibrillator (AED) Program—Home Page. Available online: https://aed.ksc.nasa.gov/FAQs/AED%20FAQs (accessed on 12 January 2021).
- Sleightholm, K. Non-Shockable Rhythms. 2019. Available online: https://www.firstaidshow.com/non-shockable-rhythms/ (accessed on 12 January 2021).
- Delgado, H.; Toquero, J.; Mitroi, C.; Castro, V.; Lozano, I.F. Principles of External Defibrillators; IntechOpen: London, UK, 2013. [Google Scholar] [CrossRef]
- Cummins, R.O.; Hazinski, M.F.; Kerber, R.E.; Kudenchuk, P.; Becker, l.; Nichol, G.; Malanga, B.; Aufderheide, P.T.; Stapleton, E.M.; Kern, K.; et al. Low-Energy Biphasic Waveform Defibrillation: Evidence-Based Review Applied to Emergency Cardiovascular Care Guidelines. Circulation 1998, 97, 1654–1667. [Google Scholar] [CrossRef] [PubMed]
- P. 27 F. 2012|20:17 GMT, The Shocking Truth About Defibrillators—IEEE Spectrum. IEEE Spectrum: Technology, Engineering, and Science News. 2012. Available online: https://spectrum.ieee.org/biomedical/devices/the-shocking-truth-about-defibrillators (accessed on 12 January 2021).
- Shah, J.; Maisel, W.H. Recalls and safety alerts affecting automated external defibrillators. JAMA 2006, 296, 655–660. [Google Scholar] [CrossRef]
- Figuera, C.; Irusta, U.; Morgado, E.; Aramendi, E.; Ayala, U.; Wik, L.; Kramer-Johansen, J.; Eftestøl, T.; Alonso-Atienza, F. Machine Learning Techniques for the Detection of Shockable Rhythms in Automated External Defibrillators. PLoS ONE 2016, 11, e0159654. [Google Scholar] [CrossRef]
- De Gauna, S.R.; Irusta, U.; Ruiz, J.; Ayala, U.; Aramendi, E.; Eftestøl, T. Rhythm Analysis during Cardiopulmonary Resuscitation: Past, Present, and Future. BioMed. Res. Int. 2014, 2014, e386010. [Google Scholar] [CrossRef]
- Ibrahim, W.H. Recent advances and controversies in adult cardiopulmonary resuscitation. Postgrad. Med. J. 2007, 83, 649–654. [Google Scholar] [CrossRef]
- Shirin, H.; Alicia, C.; Matt, V.; Chon, K.H. Deep Neural Network Approach for Continuous ECG-Based Automated External Defibrillator Shock Advisory System During Cardiopulmonary Resuscitation. J. Am. Heart Assoc. 2021, 10, e019065. [Google Scholar] [CrossRef]
- Krasteva, V.; Ménétré, S.; Didon, J.-P.; Jekova, I. Fully Convolutional Deep Neural Networks with Optimized Hyperparameters for Detection of Shockable and Non-Shockable Rhythms. Sensors 2020, 20, 2875. [Google Scholar] [CrossRef]
- Jekova, I. Real time detection of ventricular fibrillation and tachycardia. Physiol. Meas. Available online: https://www.academia.edu/3331968/Real_time_detection_of_ventricular_fibrillation_and_tachycardia (accessed on 16 March 2021).
- Association, T.A.H. AHA Database Sample Excluded Record. Physionet.Org. 2003. Available online: https://physionet.org/content/ahadb/1.0.0/ (accessed on 23 October 2022).
- Greenwald, S.D. The MIT-BIH Malignant Ventricular Arrhythmia Database. Physionet.Org. 1992. Available online: https://physionet.org/content/vfdb/1.0.0/ (accessed on 23 October 2022).
- Nolle, F.M.; Bowser, R.W. Creighton University Ventricular Tachyarrhythmia Database. Physionet.Org. 1992. Available online: https://physionet.org/content/cudb/1.0.0/ (accessed on 23 October 2022).
- Moody, G.B. The Beth Israel Deaconess Medical Center, The MIT-BIH Normal Sinus Rhythm Database. Physionet.Org. 1990. Available online: https://physionet.org/content/nsrdb/1.0.0/ (accessed on 23 October 2022).
- Moody, G.B.; Mark, R.G. MIT-BIH Atrial Fibrillation Database. Physionet.Org . 1992. Available online: https://physionet.org/content/afdb/1.0.0/ (accessed on 23 October 2022).
- Greenwald, S.D. Sudden Cardiac Death Holter Database. Physionet.Org . 1984. Available online: https://physionet.org/content/sddb/1.0.0/ (accessed on 23 October 2022).
- Picon, A.; Irusta, U.; Álvarez-Gila, A.; Aramendi, E.; Alonso-Atienza, F.; Figuera, C.; Ayala, U.; Garrote, E.; Wik, L.; Kramer-Johansen, J.; et al. Mixed convolutional and long short-term memory network for the detection of lethal ventricular arrhythmia. PLoS ONE 2019, 14, e0216756. [Google Scholar] [CrossRef]
- Nishiyama, T.; Nishiyama, A.; Negishi, M.; Kashimura, S.; Katsumata, Y.; Kimura, T.; Nishiyama, N.; Tanimoto, Y.; Aizawa, Y.; Mitamura, H.; et al. Diagnostic Accuracy of Commercially Available Automated External Defibrillators. J. Am. Heart Assoc. 2015, 4, e002465. [Google Scholar] [CrossRef]
- Jekova, I.; Krasteva, V. Optimization of End-to-End Convolutional Neural Networks for Analysis of Out-of-Hospital Cardiac Arrest Rhythms during Cardiopulmonary Resuscitation. Sensors 2021, 21, 4105. [Google Scholar] [CrossRef]
- Isasi, I.; Irusta, U.; Aramendi, E.; Eftestøl, T.; Kramer-Johansen, J.; Wik, L. Rhythm Analysis during Cardiopulmonary Resuscitation Using Convolutional Neural Networks. Entropy 2020, 22, 595. [Google Scholar] [CrossRef]
- Amarappa, S.; Sathyanarayana, D.S.V. Data classification using Support vector Machine (SVM), a simplified approach. Int. J. Electron. Comput. Sci. Eng. 2014, 3, 435–445. [Google Scholar]
- Support Vector Machine—An Overview|ScienceDirect Topics. Available online: https://www.sciencedirect.com/topics/computer-science/support-vector-machine (accessed on 17 March 2021).
- Li, Q.; Rajagopalan, C.; Clifford, G.D. Ventricular Fibrillation and Tachycardia Classification Using a Machine Learning Approach. IEEE Trans. Biomed. Eng. 2014, 61, 1607–1613. [Google Scholar] [CrossRef]
- Nam, D.H.; Kang, D.W.; Myoung, H.S.; Lee, K.J. Detection method for shockable rhythm based on a single feature. Electron. Lett. 2016, 52, 686–688. [Google Scholar] [CrossRef]
- Boulesteix, A.-L.; Janitza, S.; Kruppa, J.; König, I.R. Overview of random forest methodology and practical guidance with emphasis on computational biology and bioinformatics. WIREs Data Min. Knowl. Discov. 2012, 2, 493–507. [Google Scholar] [CrossRef]
- Tripathy, R.K.; Sharma, L.N.; Dandapat, S. Detection of Shockable Ventricular Arrhythmia using Variational Mode Decomposition. J. Med. Syst. 2016, 40, 79. [Google Scholar] [CrossRef]
- Redpath, D.B.; Lebart, K. Boosting feature selection. In Proceedings of the Pattern Recognition and Data Mining: Third International Conference on Advances in Pattern Recognition, ICAPR 2005, Bath, UK, 22–25 August 2005; pp. 305–314. [Google Scholar] [CrossRef]
- Zhang, Y.; Qi, J.; Shu, H.; Cao, J. A Hybrid KNN-LR Classifier and its Application in Customer Churn Prediction. In Proceedings of the 2007 IEEE International Conference on Systems, Man and Cybernetics, Montreal, QC, Canada, 7–10 October 2007. [Google Scholar] [CrossRef]
- Yamashita, R.; Nishio, M.; Do, R.K.G.; Togashi, K. Convolutional neural networks: An overview and application in radiology. Insights Imaging 2018, 9, 611–629. [Google Scholar] [CrossRef]
- Ebrahimi, Z.; Loni, M.; Daneshtalab, M.; Gharehbaghi, A. A review on deep learning methods for ECG arrhythmia classification. Expert Syst. Appl. X 2020, 7, 100033. [Google Scholar] [CrossRef]
- Al-Saffar, A.A.M.; Tao, H.; Talab, M.A. Review of deep convolution neural network in image classification. In Proceedings of the 2017 International Conference on Radar, Antenna, Microwave, Electronics, and Telecommunications (ICRAMET), Jakarta, Indonesia, 22–23 October 2017; pp. 26–31. [Google Scholar] [CrossRef]
- Acharya, U.R.; Fujita, H.; Oh, S.L.; Raghavendra, U.; Tan, J.H.; Adam, M.; Gertych, A.; Hagiwara, Y. Automated identification of shockable and non-shockable life-threatening ventricular arrhythmias using convolutional neural network. Future Gener. Comput. Syst. 2018, 79, 952–959. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Nguyen, B.V.; Kim, K. Deep Feature Learning for Sudden Cardiac Arrest Detection in Automated External Defibrillators. Sci. Rep. 2018, 8, 17196. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.; Kiseon, K. Feature Learning Using Convolutional Neural Network for Cardiac Arrest Detection. In Proceedings of the 2018 International Conference on Smart Green Technology in Electrical and Information Systems (ICSGTEIS), Bali, Indonesia, 25–27 October 2018; pp. 39–42. [Google Scholar] [CrossRef]
- Basnet, M.; Ali, M.H. Deep Learning-Based Intrusion DETECTION system For Electric Vehicle charging station. In Proceedings of the 2020 2nd International Conference on Smart Power & Internet Energy Systems (SPIES), Bangkok, Thailand, 2–4 June 2020; 2020; pp. 408–413. [Google Scholar]
- Basnet, M.; Poudyal, S.; Ali, M.H.; Dasgupta, D. Ransomware Detection Using Deep Learning in the SCADA System of Electric Vehicle Charging Station. In Proceedings of the 2021 IEEE PES Innovative Smart Grid Technologies Conference—Latin America (ISGT Latin America), Lima, Peru, 15–17 September 2021; pp. 1–5. [Google Scholar] [CrossRef]
- Shewalkar, A.; Nyavanandi, D.; Ludwig, S. Performance Evaluation of Deep neural networks Applied to Speech Recognition: Rnn, LSTM and GRU. J. Artif. Intell. Soft Comput. Res. 2019, 9, 235–245. [Google Scholar] [CrossRef]
- Performance Aspects of Automated Rhythm Detection Capabilities for AEDs. Available online: https://www.eplabdigest.com/articles/Performance-Aspects-Automated-Rhythm-Detection-Capabilities-AEDs (accessed on 12 January 2021).
- Andersen, R.S.; Peimankar, A.; Puthusserypady, S. A deep learning approach for real-time detection of atrial fibrillation. Expert Syst. Appl. 2019, 115, 465–473. [Google Scholar] [CrossRef]
- Eilevstjønn, J.; Eftestøl, T.; Aase, S.O.; Myklebust, H.; Husøy, J.H.; Steen, P.A. Feasibility of shock advice analysis during CPR through removal of CPR artefacts from the human ECG. Resuscitation 2004, 61, 131–141. [Google Scholar] [CrossRef]
- Ayala, U.; Irusta, U.; Ruiz, J.; Eftestøl, T.; Kramer-Johansen, J.; Alonso-Atienza, F.; Alonso, E.; González-Otero, D. A reliable method for rhythm analysis during cardiopulmonary resuscitation. BioMed. Res. Int. 2014, 2014, 872470. [Google Scholar] [CrossRef]
- Isasi, I.; Irusta, U.; Aramendi, E.; Olsen, J.-Å.; Wik, L. Detection of Shockable Rhythms Using Convolutional Neural Networks During Chest Compressions Provided by a Load Distributing Band. Comput. Cardiol. 2020, 47, 1–4. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, P.; Yan, R.; Gao, R.X. Long short-term memory for machine remaining life prediction. J. Manuf. Syst. 2018, 48, 78–86. [Google Scholar] [CrossRef]
- Veit, A.; Wilber, M.; Belongie, S. Residual Networks Behave Like Ensembles of Relatively Shallow Networks. arXiv 2016, arXiv:160506431. Available online: http://arxiv.org/abs/1605.06431 (accessed on 17 March 2021).
- Jin, W.; Li, Z.J.; Wei, L.S.; Zhen, H. The improvements of BP neural network learning algorithm. In Proceedings of the WCC 2000—ICSP 2000. 2000 5th International Conference on Signal Processing Proceedings. 16th World Computer Congress 2000, Beijing, China, 21–25 August 2000; pp. 1647–1649. [Google Scholar] [CrossRef]
- Ming, Y.; Wu, T.; Yang, P.; Lv, M.; Hou, F.; Zhang, G.; Feng, C. Detection of Shockable Rhythm during Chest Compression based on Machine Learning. In Proceedings of the 2019 IEEE 8th Joint International Information Technology and Artificial Intelligence Conference (ITAIC), Chongqing, China, 24–26 May 2019; pp. 365–370. [Google Scholar] [CrossRef]
- Krasteva, V.; Jekova, I.; Dotsinsky, I.; Didon, J.-P. Shock Advisory System for Heart Rhythm Analysis During Cardiopulmonary Resuscitation Using a Single ECG Input of Automated External Defibrillators. Ann. Biomed. Eng. 2010, 38, 1326–1336. [Google Scholar] [CrossRef]
- Association, A.H. Compression-only CPR Increases Survival of Out-Of-Hospital Cardiac Arrest. Available online: https://medicalxpress.com/news/2019-04-compression-only-cpr-survival-out-of-hospital-cardiac.html (accessed on 16 March 2021).
- Haque, A. EC-GAN: Low-Sample Classification using Semi-Supervised Algorithms and GANs (Student Abstract). Proc. AAAI Conf. Artif. Intell. 2021, 35, 15797–15798. [Google Scholar] [CrossRef]
- Chatziagapi, A.; Paraskevopoulos, G.; Sgouropoulos, D.; Pantazopoulos, G.; Nikandrou, M.; Giannakopoulos, T.; Katsamanis, A.; Potamianos, A.; Narayanan, S. Data Augmentation Using GANs for Speech Emotion Recognition. Interspeech 2019, 171–175. [Google Scholar] [CrossRef]
- Walia, M.; Tierney, B.; McKeever, S. Synthesising Tabular Data using Wasserstein Conditional GANs with Gradient Penalty. AICS 2020, 12, 325–336. [Google Scholar]
- Engelmann, J.; Lessmann, S. Conditional Wasserstein GAN-based oversampling of tabular data for imbalanced learning. Expert Syst. Appl. 2021, 174, 114582. [Google Scholar] [CrossRef]
- Zhu, B.; Pan, X.; Broucke, S.V.; Xiao, J. A GAN-based hybrid sampling method for imbalanced customer classification. Inf. Sci. 2022, 609, 1397–1411. [Google Scholar] [CrossRef]
| Shockable (Sh) | Non-Shockable (NSh) | ||
|---|---|---|---|
| AED algorithm decision | Shock | True Positive (TP) | False Positive (FP) |
| No-shock | False Negative (FN) | True Negative (TN) | |
| Ref | Type of Methods | Approach | Segment | Se (%) | Sp (%) | Databases |
|---|---|---|---|---|---|---|
| [30] | ML | SVM, Genetic algorithm | 5 s | 96.2 | 96.2 | AHADB, CUDB, VFDB |
| [31] | ML | SVM, DWT | 3 s 4 s 5 s | 97.8 97.7 98.8 | 98.0 98.3 98.4 | MITDB, AHADB, CUDB |
| [33] | ML | RF, VMD | N/A | 95.2 | 91.04 | N/A |
| [12] | ML | LR, BS | N/A | 96.6 94.7 | 98.8 96.5 | Public OCHA |
| [39] | DL | CNN | 2 s | 95.32 | 91.04 | MITDB, VFDB, CUDB |
| [40] | DL and ML | CNN, BS, MVMD | 8 s | 97.0 | 99.44 | VFDB, CUDB |
| [41] | DL and ML | CNN, SVM, MVMD | 5 s | 95.2 | 99.31 | VFDB, CUDB |
| [24] | DL and ML | CNN, LSTM | 4 s 4 s 2 s 2 s | 99.7 99.2 97.5 97.5 | 98.9 96.7 93.6 97.5 | Public OHCA OHCA Public |
| [26] | DL and ML | DCNN, HP optimization | 5 s 2 s | 96.6 97.6 | 99.4 98.7 | OCHA OCHA |
| [46] | DL and ML | CNN, RNN | N/A | 98.98 98.96 N/A | 96.95 86.04 95.01 | AFDB MITDB NSRDB |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dahal, K.; Ali, M.H. Overview of Machine Learning and Deep Learning Approaches for Detecting Shockable Rhythms in AED in the Absence or Presence of CPR. Electronics 2022, 11, 3593. https://doi.org/10.3390/electronics11213593
Dahal K, Ali MH. Overview of Machine Learning and Deep Learning Approaches for Detecting Shockable Rhythms in AED in the Absence or Presence of CPR. Electronics. 2022; 11(21):3593. https://doi.org/10.3390/electronics11213593
Chicago/Turabian StyleDahal, Kamana, and Mohd. Hasan Ali. 2022. "Overview of Machine Learning and Deep Learning Approaches for Detecting Shockable Rhythms in AED in the Absence or Presence of CPR" Electronics 11, no. 21: 3593. https://doi.org/10.3390/electronics11213593
APA StyleDahal, K., & Ali, M. H. (2022). Overview of Machine Learning and Deep Learning Approaches for Detecting Shockable Rhythms in AED in the Absence or Presence of CPR. Electronics, 11(21), 3593. https://doi.org/10.3390/electronics11213593







