Abstract
Counterfeit drugs are forgery-tagged medicines that are considered to be drugs without vigorous active pharmaceutical ingredients (API). India, being the world’s largest producer of drugs, faces a crucial issue of counterfeits. Moreover, counterfeits identify their path into the pharmaceutical supply chain (PSC) effortlessly owing to the dearth of security and traceability in the prevailing system. This is because the software applications currently in use stockpile the information about drugs on centralized servers and are accessed by manufacturers, distributors and retailers via the internet. The security of such systems is found to be weak. To address these issues, in this work, a novel method called Supersingular Isogeny and Hosmer–Lemeshow Logistic Regression-based (SI-HLLR) secured information sharing for the pharmaceutical supply chain is proposed. The SI-HLLR method is split into two sections, block validation and authentication. First, with the pharmaceutical sales data provided as input, the supersingular isogeny Diffie–Hellman key exchange model is applied for block validation and then is implemented using a blockchain. Next, with the validated blocks, the authentication mechanism is performed using Hosmer–Lemeshow logistic regression-based authentication that in turn eliminates the counterfeit drugs from the pharmaceutical supply chain. The hyperledger fabric blockchain solution using SI-HLLR leads to improved security ensuring data integrity and better authentication accuracy in the proposed method.
1. Introduction
Every business must concern itself with supply chain management. However, as far as the pharmaceutical industry is concerned, possible features have a substantial influence on them all. This is due to the reason that sending medical products where they must go means life or death. Significant problems are encountered when this supply chain is said to be falsified or manipulated. Hence, a secured information-sharing protocol for the pharmaceutical supply chain remains the only solution for the smooth flow of information among users.
A blockchain-based pharmaceutical supply chain management system (blockchain-based PSCM) for information sharing was proposed in [1]. Here, the authors have shown how the blockchain mechanism can be combined with the traditional pharmaceutical supply chain in order to achieve a secured way of sharing information. The proposed system shares cryptographic keys with all the network participants securely with help of smart contract and consensus mechanism.
The consensus algorithm plays a vital role in the blockchain-based system. It is designed to perform block validation. In addition, both transaction and block validation were followed, by which the security analysis was performed, to secure against all probable security threats. With this, a reasonable performance in terms of both communication and computation overheads was attained. As a result, confidentiality, integrity, and authentication were improved to a greater extent. However, the IoT and blockchain were not combined to enhance the security and block the verification process.
To overcome the issue, distinct from conventional supply chains in [2] that were designed on the basis of the centralized systems, a fully distributed method based on blockchain technology was proposed. With this fully distributed mechanism, the supply chain management system was able to bestow quality, integrity, and traceability.
In this paper, the proposed system was built using permissioned blockchain hyperledger fabric, which allows only limited number of authorized members. With this feature, the proposed system automates the supply chain management operations and makes use of smart contract. Hence, the proposed system achieves an information traceability in more secured, transparent, and immutable way. Moreover, the proposed system implements a quality-control mechanism by adding and modifying the rules at runtime.
Supply chain management systems have issues, and nearly all of them require immediate attention and action. The extent and complexity of these issues may be different. Security and privacy were not improved, with higher latency, lesser data integrity, and authentication accuracy being some of the challenges. The challenges of the supply chain management system are increasing day by day, and with the emphasis on stability, these concerns are the focus of this paper.
The supply chain is a significant role in any industry. The security, privacy, and transparency of the products are demanding issues in SCM. Small disorders in the supply chain can dislocate the whole market and can cause vast financial loss to the business involved. Several blockchain methods were developed to discover the products in the SCM process. To discover the origin of counterfeit products that somehow have arrived to the customer, a well-maintained and unchallengeable supply chain is essential. In the existing supply chain management system, there are numerous issues such as tampering with products, delay, third party, etc. In addition, proper authentication between the participants, data management, and integrity of the data are very imperative and necessary. Based on this insight, the blockchain-enabled pharma supply chain is capable of addressing the above-mentioned issues.
The main contributions of this paper are summarized as below:
- To propose a machine learning-enabled blockchain method called supersingular isogeny and Hosmer–Lemeshow logistic regression-based (SI-HLLR) blockchain-enabled pharma supply chain, which aims to improve smart contract accuracy with minimum latency.
- To ensure robust block validation using the supersingular isogeny Diffie–Hellman key exchange algorithm with separate keys and signature for the distributor and the retailer, thus ensuring privacy and the integrity of data in the block.
- To present Hosmer–Lemeshow logistic regression-based authentication for our method with the objective of improving authentication accuracy for minimum latency.
The remainder of this paper is organized as follows. In Section 2, we review the literature related to blockchain-secured information sharing methods and machine learning-based methods. In Section 3, the block validation and authentication model with the aid of algorithms and diagrammatic representations are defined. In Section 4, several metrics are evaluated to show the efficiency of the proposed method, supersingular isogeny and Hosmer–Lemeshow logistic regression-based (SI-HLLR) blockchain-enabled pharma supply chain. Finally, conclusions are drawn in Section 5.
2. Related Work
Counterfeit drugs have been considered as a significant issue globally with major influence on health and economies over the past few years. Since then, the issue of counterfeit drugs has continued to evolve with developing countries being the most affected. Over the recent few years, with the global division of labor, the supply chain has been ceaselessly evolving, bringing great challenges to supply chain management. The supply chain’s operations in turn not only influence the objectives of the core businesses but also bring negative aspects to the relevant interests of those businesses, thus making it paramount to propose a secure and effective supply chain system.
The significance of and influence on pharmaceutical companies concerning counterfeit drugs in India was investigated in [3]. Nevertheless, the vigorous complementary aspect of these domains has been discussed with only a little success. However, with high number of digital health an interactive feedback loop between requirements and processing has to be analyzed. In [4], an overview of advanced solutions for new products interacting with the pharmaceutical and digital worlds were provided along with an in-depth investigation. Additionally, the application of blockchains in pharmaceutical industries has received great attention. Certain major consensus protocols along with their advantages and drawbacks were included in [5]. However, with the involvement of third-party security and privacy, those protocols have been said to be compromised. A peer-to-peer encryption mechanism was designed in [6] in conjunction with smart contract, thus ensuring a maximum level of safety.
A novel blockchain-based supply chain framework (SESCF) was proposed in [7] that solved the supply chain issues in a secure and effective manner. Yet another architecture employing semantic web to improve representation potentiality was presented in [8]. With this representation, drug counterfeiting was not only prevented but also patient satisfaction was enhanced to a greater extent. A detailed review on health operations and the supply chain was investigated in [9]. However, there were flaws observed in coordination, resulting in security being compromised. To address this aspect, a drug distribution process maintaining market equilibrium and also tampering using a blockchain was designed in [10].
An article concerning a UN questionnaire on supply chain sustainability was proposed in [11] from numerous social networks such as Facebook, Twitter, and Instagram. Moreover, an in-depth comparison made for pharmaceutical industry was more accessible on Twitter, in comparison with other social networks. In [12], a review of architecture involving blockchain and various consensus protocols applied to healthcare and smart city were designed. A digital twin concept supporting blockchain for the smart supply chain by China’s largest retailer was investigated in [13]. In [14], yet another smart supply chain management with information and communication technologies for strategies applied in North America was investigated in detail. Healthcare supply chains are complicated compositions stretching across several organizational boundaries, bestowing an evaluative cornerstone to services essential for day-to-day life. The intrinsic convolution of systems introduces inaccurate information.
One of the repercussions of such drawbacks is that the counterfeit drugs within the prevailing supply chains not only have a severe negative influence on human health but also result in acute economic loss to the healthcare industry also. Hence, an end-to-end product tracking system covering the pharmaceutical supply chain is essential to eliminate counterfeits. In [15], an Ethereum blockchain-based method including smart contracts and decentralized off-chain storage for effective product traceability in the healthcare supply chain was proposed. With the aid of smart contract, data provenance was guaranteed along with the elimination of intermediaries, thus ensuring security to all stakeholders. However, the total production cost involved in ensuring security was not addressed. To address on this issue, benders decomposition was employed in [16] and feasibility cuts were introduced in addition to risk-adjusted production, thus drastically reducing the cost involved. Related reviews on pharmaceutical sciences and education aspects from a bibliometric angle were designed in [17].
A viable supply chain model discussing the relationships between viability and reliability for decision makers to design the supply chain was discussed in [18]. In [19], fuzzy interpretive structural modeling to identify the relationship between inhibitors with the objective of minimizing the operative cost and overhead was proposed. Conflicts of interests arising between the pharmaceutical industry and health-oriented parliamentary group were discussed in [20]. The ever-increasing pollution and waste creation have made industries globally seek to design the formulation of a circular economy (CE) in supply chains. In [21], a significant method for the application of circular supply chain management (CSCSM) was proposed to assist the pharmaceutical industries to apply CSCM in their organizations. A review and future perspective on supply chain interferences and resilience was discussed in detail in [22]. Positive and negative influences on information sharing in supply chain were investigated in [23].
A summary of the working rules and regulations and the application research status concerning blockchain smart contract, along with the analysis and development and challenges of smart contract, were discussed in [24]. A traditional Chinese medicine supply chain for analyzing the business prospective, data transparency and safety issues was detailed in [25]. A blockchain-based framework was investigated in [26] for handling security issues. However, it failed to consider authentication accuracy. A smart contract-based healthcare management system was introduced in [27] for enhancing privacy and security. A blockchain and machine learning-based drug supply chain management and recommendation system (DSCMR) was developed in [28] for improving the supply chain process. However, data integrity was not achieved.
Motivated by the above materials, in this work, a novel method called supersingular isogeny and Hosmer–Lemeshow logistic regression-based (SI-HLLR) blockchain-enabled pharma supply chain is proposed. While performing secured information sharing for the pharma supply chain, several performance indicators are presented. In our work, we have considered two predominant performance factors, namely data integrity and authentication accuracy, for secured information sharing. The reason behind considering these two performance factors are first, the validation of the block should be performed for secured information sharing. This we have performed by utilizing the Hosmer–Lemeshow logistic regression-based authentication algorithm. The second reason for checking the data integrity is that not only the block validation should be performed but also the data (i.e., the product information) written into the block should be verified so that the data should not be changed by unintended recipients. This we have performed by means of the supersingular isogeny Diffie–Hellman key exchange algorithm. Therefore, these two performance factors along with latency are taken into consideration.
3. Supersingular Isogeny and Hosmer–Lemeshow Logistic Regression-Based (SI-HLLR) Blockchain-Enabled Pharma Supply Chain
At first, the application area in which the blockchain was presented is that of transactional systems, in exacting electronic payment systems. Blockchain technology is a new paradigm that can present important profit to the industry. Blockchain technology may be used to record transactions across many computers or edge IoT devices. Its features as a shared, synchronized ledger can help organizations to manage complexity across their value chains. The high level of benefits such as minimizing the cost, time, and risk associated with complex business processes can be achieved with the help of blockchain technology. Owing to its decentralized nature, blockchains have become one of the most trusted technologies for healthcare systems. Here, the information pertaining to drugs are created, updated, controlled, verified and stored in a digital ledger by several actors such as manufacturer ‘’, distributors ‘’ and retailers ‘’. These three actors form the responsibility for storing drug details in the respectively digital ledger, with the digital ledger being maintained as separate database. As a result, authenticity verification, identification, and elimination of fake drugs are said to be facilitated. Moreover, each peer possessing a certain number of access permissions ensures data privacy and data integrity. Moreover, data consistency is also said to be arrived by implementing a consensus algorithm, thus entailing the correctness and consistency of the data stored on the digital ledger. Figure 1 shows the block diagram of the proposed method, supersingular isogeny and Hosmer–Lemeshow logistic regression-based (SI-HLLR) blockchain-enabled pharma supply chain. At first, the pharmaceutical sales data is taken from the pharma sales dataset. The major stakeholders of the pharmaceutical supply chain network are manufacturers, distributors, and retailers. Then, the supersingular isogeny Diffie–Hellman key exchange model uses a blockchain to perform block validation. After the validated blocks, Hosmer–Lemeshow logistic regression-based authentication is used to perform an authentication mechanism for removing the counterfeit drugs from the pharmaceutical supply chain. As a result, SI-HLLR provides superior authentication accuracy and enhanced security and data integrity.
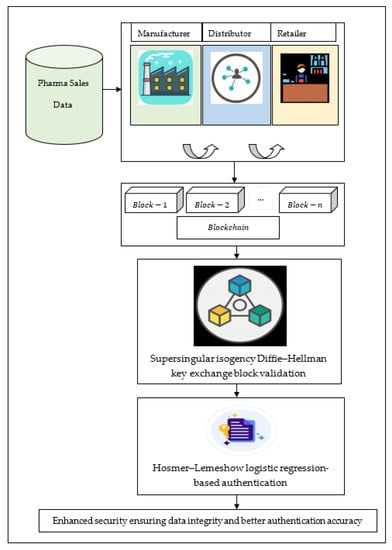
Figure 1.
Block diagram of supersingular isogeny and Hosmer–Lemeshow logistic regression-based (SI-HLLR).
As shown in the above figure, to start with, the list of medicines ‘’ are prepared by manufacturer ‘’ and the medicine details of the selected group of drugs from the dataset (57 drugs) are entered into the decentralized distributed ledger ‘’.
Upon successful data entry of the corresponding medicine details by the manufacturer, the medicines are said to be ready for shipment to distributor locations or distributors ‘’. Those lists of medicine ‘’ details along with the list of distributors ‘’ are stored in the form of blocks.
Then, from the above Equation (2), each block ‘’ holds the medicine details and the distributor details stored in the form of a block vector for the corresponding distributor and the retailer details stored in the form of a block vector for the respective retailers as given below.
Now the retailer ‘’ receives and validates the received drug shipment details, where the source and destination shipment route of the drug are validated by reading the decentralized distributed ledger ‘’. Upon successful storage of the above blocks in the form of the distinct vectors for the distributors ‘’ and retailers ‘’, the next process remains in the validation of blocks.
3.1. Supersingular Isogeny Diffie–Hellman Key Exchange Block Validation
The block validations in our work are performed by means of a read (i.e., read from the distributed ledger) and write (i.e., write into the distributed ledger) operation via smart contract by the blockchain service provider ‘’. The task of smart contract remains in maintaining the pharmaceutical transaction data in the same order as it receives them. The smart contract via the blockchain service provider ‘’ verifies and validates the transaction proposal for approval and stores them as blocks in the blockchain.
The smart contract employed in our proposed method performs several tasks such as node interaction with the ledger. To be more specific, the manufacturers who have obtained the drug details from Pharma Sales Data dataset in a span of six years for a period between 2014 and 2019, indicating the date and the time of sale, the pharmaceutical drug brand name and the quantity sold, exported from the point-of-sale system in the individual pharmacy, are entered with help of smart contract. Smart contract along with the block validation performed using supersingular isogeny Diffie–Hellman key exchange allows the client (i.e., the distributor or retailer) to view only relevant information such as the name of the drug and the serial number from the ledger. A client (i.e., the distributor or retailer) in turn can obtain that information by sending a request to smart contract via read operation ‘’ or write operation ‘’, respectively. In case of read operation ‘’, the distributors ‘’ verifies all the drug shipments received from manufacturer ‘’ by performing read operation in the decentralized ledger (i.e., obtained from decentralized distributed ledger ‘’). On the other hand, in case of write operation ‘’, upon successful verification by the distributors obtained from the manufacturer, the distributor enters the received drug shipment details into the decentralized distributed ledger ‘’, via write operation. Figure 2 shows the structure of the supersingular isogeny Diffie–Hellman key exchange block validation model.
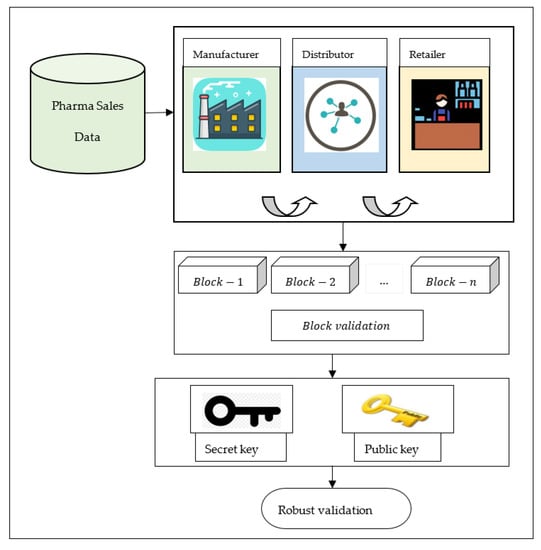
Figure 2.
Structure of supersingular isogeny Diffie–Hellman key exchange block validation model.
As shown in Figure 2 above, the supersingular isogeny Diffie–Hellman key exchange works with the graph whose vertices are supersingular elliptic curves with edges being isogenies between those elliptic curves. The setup for supersingular isogeny Diffie–Hellman key exchange is a prime of the form given as below.
From the above Equation (5), the setup is established for distinct small primes, ‘’, ‘’ and large exponents ‘’, ‘’, a small cofactor ‘’, with a supersingular elliptic curve ‘’. In our work, the curve has two large torsion subgroups, ‘’ and ‘’, that are assigned to distributor ‘’ and retailer ‘’, respectively. Each party (i.e., the distributor or retailer) initiates the process for block validation by the smart contract via the blockchain service provider by selecting a (secret) random cyclic subgroup of their corresponding torsion subgroup and computing the corresponding (secret) isogeny. With this, the system settings for the distributor and retailer in our work is mathematically stated as given below.
After successfully completing the setup process, as given in the above Equation (6), the secret key ‘’ and public key ‘’ are generated using the above ‘’. To start with two entities, ‘’ and ‘’ are generated in an arbitrary manner and evaluate ‘’ and ‘’, and with these two functions, the secret key is obtained as given below.
Next, the public key is mathematically stated as given below.
From the above secret key ‘’, the public key ‘’ and the block ‘’, the signature is computed as given below.
Given the signature ‘’ of the corresponding block ‘’, Sig = ¹S; rº of the block B, the smart contract via the blockchain service provider sends ‘’. On the other hand, the receiver on the receiving side checks the following formulation.
Upon successful check, the corresponding block is said to be validated or vice versa.
A flowchart of the block validation model using supersingular isogeny Diffie–Hellman key exchange is depicted in Figure 3. The pseudo-code representation of supersingular isogeny Diffie–Hellman key exchange is given in Algorithm 1 below.
| Algorithm 1. Supersingular isogeny Diffie–Hellman key exchange. |
| Input: Dataset ‘’, manufacturer ‘’, medicines ‘’, distributors ‘’, Blockchain Service Provider ‘’ |
| Output: Robust block validation |
| 1: Initialize time ‘’, small primes, ‘’, ‘’ 2: Begin 3: For each dataset ‘’ with manufacturer ‘’ and distributors ‘’ 4: Formulate block vector as in Equations (3) and (4) 5: Formulate setup for key exchange as in Equation (5) 6: Generate signature for parameter settings as in Equation (6) 7: Obtain the secret key and public key as in Equations (7) and (8) 8: Generate actual signature as in Equation (9) 9: If ‘’ 10: Then block validation is successful 11: Write the data in the distributed ledger ‘’ 12: Proceed with the authentication process 13: End if 14: If ‘’ 15: Then block validation is not said to be successful 16: End if 17: End |
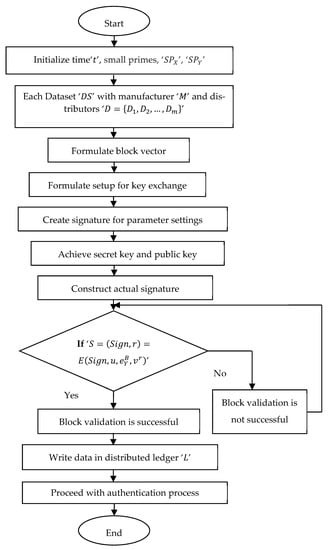
Figure 3.
Flow chart of supersingular isogeny Diffie–Hellman key exchange block validation model.
As given above in the supersingular isogeny Diffie–Hellman key exchange Algorithm 1, separate keys and signature are generated for distributor and reseller. Accordingly, the corresponding block vector validation is performed using supersingular isogeny function. The advantage of this function is the security aspect where only upon the successful validation of each block further processing is continued. This in turn assists in ensuring the privacy and integrity of data in the block. As a result, only the authorized node or user can send a request to the distributed ledger by invoking smart contract functions.
3.2. Hosmer–Lemeshow Logistic Regression-Based Authentication
Upon successful block validation, every successful block transaction is converted into immutable records of data and recorded as transaction blocks into the digital ledger with the aid of smart contract. Next, the client (i.e., distributor or retailer) checks for the user credentials in order to authorize the sending transaction proposals of either the distributor or retailer by employing the Hosmer–Lemeshow logistic regression test. The blockchain service provider now authenticates the results in response to the transaction using this Hosmer–Lemeshow logistic regression test and writes the results of the transaction block (i.e., either authenticated or not authenticated) into the ledger. The blockchain service provider via smart contract then signs the authenticated transaction proposal and sends the response to the client application (i.e., either distributor or retailer) for further processing. The Hosmer–Lemeshow logistic regression test, being a statistical test for logistic regression model tests, assesses whether or not the observed event rates (i.e., observed block validated) match the expected event rates (i.e., expected block validated) in the subgroups of the model population. The expected probability of success (for distributor ‘’) for the logistic regression model is mathematically formulated as given below.
In a similar manner, the expected probability of success (for retailer ‘’) for the logistic regression model is mathematically formulated as given below.
From the above Equations (11) and (12), ‘’ and ‘’ specify the intercept for distributor ‘’, retailer ‘’ whereas ‘’ and ‘’ represent the coefficient for distributor ‘’, retailer ‘’, respectively. Finally, the Hosmer–Lemeshow logistic regression test for the authentication test is mathematically stated as given below.
From the above Equation (13), ‘’, ‘’, ‘’, ‘’ denote the observed ‘’, the expected ‘’, the total observations and the predicted risk for block ‘’ being compromised.
A flowchart of block authentication using Hosmer–Lemeshow logistic regression-based authentication is depicted in Figure 4. The pseudo-code representation for Hosmer–Lemeshow logistic regression-based authentication is given in Algorithm 2.
| Algorithm 2. Hosmer–Lemeshow logistic regression-based authentication. |
| Input: Dataset ‘’, manufacturer ‘’, medicines ‘’, distributors ‘’, Blockchain Service Provider ‘’ |
| Output: Accurate movement of pharma supply chain |
| 1: Begin 2: For each dataset ‘’ with manufacturer ‘’ and distributors ‘’ 3: Formulate expected probability of success (for distributor ‘’) as in Equation (11) 4: Formulate expected probability of success (for retailer ‘’) as in Equation (12) 5: Perform authentication test as in Equation (13) 6: If ‘’ 7: Then authentication is successful 8: Proceed with either read or write operation 9: End if 10: If ‘’ 11; Then authentication is not successful 12: End if 13: End for 14: End |
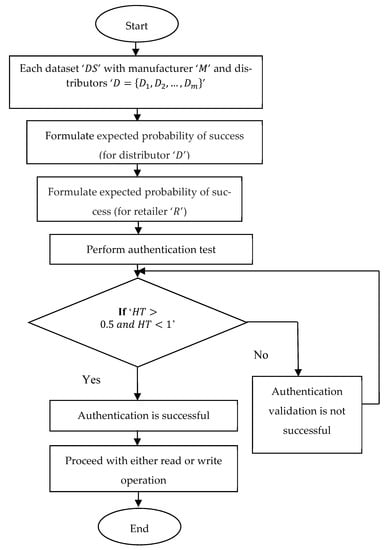
Figure 4.
Flow chart of Hosmer–Lemeshow logistic regression-based authentication.
As given in Algorithm 2 above with the objective of improving latency and authentication accuracy, the Hosmer–Lemeshow test along with logistic regression function is designed to ensure authentication. First, the logistic regression function is applied for each distributor and retailer. This is followed by the Hosmer–Lemeshow test, with which either successful authentication is generated or vice versa. This in turn improves the authentication accuracy with minimum latency.
4. Experimental Setup
In this section, the performance of supersingular isogeny and Hosmer–Lemeshow logistic regression-based (SI-HLLR) secured information sharing for the pharmaceutical supply chain is measured using Python high-level general-purpose programming language. To measure and validate the SI-HLLR method, the pharma sales dataset extracted from Kaggle are used. In the healthcare environment, the input pharmaceutical sales data is collected from the pharma sales dataset. Afterwards, block validation is executed with the aid of the supersingular isogeny Diffie–Hellman key exchange model in the blockchain with higher privacy and integrity of data. Only an authorized node or user can transmit a request to the distributed ledger with the aid of smart contract functions. Accurate movement of the pharma supply chain is provided, by applying Hosmer–Lemeshow logistic regression-based authentication with eradicating counterfeit drugs. The logistic regression function is used with all distributors and retailers. The Hosmer–Lemeshow test is used to create authentication for finding authenticated or non-authenticated persons. In the experimental setup, the blockchain-enabled pharma supply chain is used in the healthcare environment for conducting the experiments. In addition, the experiments were performed with the hardware and software specifications of the Windows 10 operating machine, core i3-4130 3.40 GHZ processor, 4GB RAM, 1TB (1000 GB) hard disk. The number of products in the range from 500 to 5000 are used to validate the simulation. In this work, experiments are performed on factors such as data integrity, latency, and authentication accuracy with respect to distinct products.
4.1. Performance Analysis of Latency
The first parameter of significance for pharmaceutical supply chain management is the latency rate. To be more specific, the latency rate refers to the time at which the actual block validation is performed. The latency is mathematically expressed as given below.
From the above Equation (14), latency ‘’ is measured based on the products involved in the simulation ‘’ and the actual time involved in the process of validating the blocks ‘’ by the blockchain service provider via smart contract. It is measured in terms of milliseconds (ms).
Figure 5 given above shows the graphical representation of the latency with respect to different products ranging between 500 and 5000. From the above graph, increasing the number of products increases the latency using all the three methods. The reason behind the increase in the number of products causing a subsequent increase in the latency is due to the increase in the product size also. As all product lists are stored in a block that are consecutively attached to a blockchain that needs to be validated to perform secured information sharing for the pharma supply chain, this results in an increase in the validation of block and thus also increasing the latency rate. Comparative analysis showed better results when applied with the SI-HLLR method upon comparison to [1,2]. The reason was due to the logistic regression function applied separately for each distributor and retailer. Here, the statistical test using Hosmer–Lemeshow is conducted to check the validity of the observed event rates with that of the expected event rates. Only with the expected probability of success is the validity ensured. Therefore, the latency using the SI-HLLR method is said to be reduced by 21% compared to [1] and 38% compared to [2], respectively.
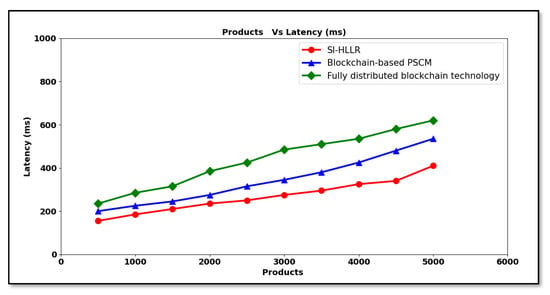
Figure 5.
Latency versus products.
4.2. Performance Analysis of Data Integrity
Data integrity is one of the security parameters that is measured as the percentage ratio of the number of products that are not altered by any intruders to the number of products ready for simulation. The data integrity rate is formulated as given below,
From the above Equation (15), the data integrity rate ‘’ is measured based on the number of products that are not changed by unintended recipients ‘’ and the total number of products ‘’ involved in simulation. The data integrity is measured in terms of percentage (%).
Figure 6 above shows the graphical representation of data integrity for 5000 different products observed at different time intervals. From the above figure, the data integrity using SI-HLLR is said to be comparatively better than [1] and [2]. This is owing to the reason that, with the frequency increases in the product size, there are a small number of products being unnoticed during the corresponding block vector validation. With this, the frequency of the products that are not changed by the unintended recipients also increases slightly. Though a decreasing trend was found using all the three methods, a higher marginal difference was found using [1] and [2]. This is because of the Hosmer–Lemeshow logistic regression-based authentication algorithm applied in the SI-HLLR method. By applying this algorithm, two different functions, the Hosmer–Lemeshow test and the logistic regression function, were applied in the SI-HLLR method to find whether the distributors and retailers were authenticated persons or not. Here, the logistic regression function was initially applied for each distributor and retailer. Next, to the resultant regression, the Hosmer–Lemeshow test was applied for observing authenticated or non-authenticated persons. This in turn increased the authentication accuracy using the SI-HLLR method by 4% compared to [1] and 9% compared to [2].
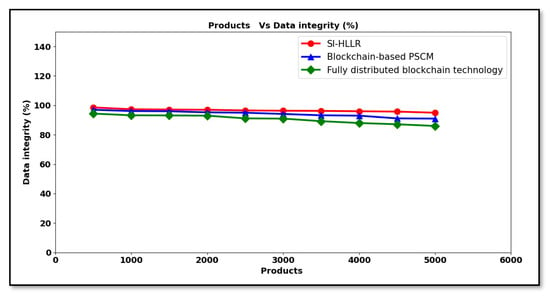
Figure 6.
Data integrity versus products.
4.3. Performance Analysis of Authentication Accuracy
Finally, authentication accuracy involved in pharmaceutical supply chain based on blockchain is mathematically stated as given below.
From the above Equation (16), the authentication accuracy ‘’ is measured based on the sample products involved in the simulation process ‘’ and the products correctly authenticated ‘’. It is measured in terms of percentage (%).
Finally, Figure 7 shows the authentication accuracy for 5000 distinct products collected to be ready for shipment at different time intervals. A steep trend is observed in authentication accuracy using all the three methods, as observed in the above figure. The reason behind the decreasing trend was that, with the increase in product size, a greater numbers of products are said to be in the queue for authentication checking. Here, the blockchain service provider has to check many products via the blockchain for authentication validation, where a small portion is said to be unnoticed. As a result, some of the products, though not authenticated ones, are found to be authenticated during the transaction validation process. However, the accuracy for authentication is found to be comparatively better when applied with SI-HLLR than [1,2]. The reason behind the improvement was due to the application of the Hosmer–Lemeshow logistic regression-based authentication algorithm. By applying this algorithm, the Hosmer–Lemeshow test and the logistic regression function were applied to the validated blocks. Initially, for each distributor and retailer requirements, a separate logistic regression function was applied. With the regression results, the Hosmer–Lemeshow test for the hypothesis was performed, with which only an authenticated distributor or retailer were provided with their required medical data by the blockchain service provider. Accordingly, the distributor ledger information was either inserted with new medical data or given to the distributor or retailer for further processing. This in turn improved the authentication accuracy by 4% and 9% compared to [1,2] respectively.
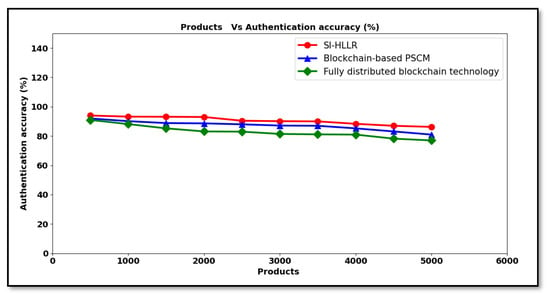
Figure 7.
Authentication accuracies versus product.
5. Conclusions
In this paper, we proposed a machine learning-enabled blockchain method called the supersingular isogeny and Hosmer–Lemeshow logistic regression-based method (SI-HLLR), which aims to perform secured information sharing in the pharmaceutical supply chain. First of all, the distributed ledger structure is optimized, where block bodies are optionally stored and retrieved separately with a block validation mechanism called the supersingular isogeny Diffie–Hellman key exchange model. This model enables distributors or retailers to get validated by the blockchain service provider and achieve robust block validation faster, thus ensuring the privacy and data integrity of the medical data information in each block. Focusing on the characteristics of the authenticator for each distributor and retailer, prior to the medical data storage or retrieval via smart contract and distributed ledger, the Hosmer–Lemeshow logistic regression test is applied to each entity. The extensive simulation results show that our proposed SI-HLLR method can efficiently minimize the latency by 30% and aid in improving the authentication accuracy by 7% and the data integrity by 7%, thus paving the way for secured information sharing for the pharmaceutical supply chain. In the future, the proposed method will be further extended to enhance the authentication accuracy and ensure security by using a deep learning-based pharmaceutical supply chain method.
Author Contributions
Conceptualization, A.P.; methodology, A.P.; software, A.P.; validation, S.C.; formal analysis, S.C.; investigation, A.P. and S.C.; resources; A.P.; Data curation, A.P.; writing—original draft preparation, A.P.; writing-review and editing, A.P. and S.C.; visualization, A.P.; supervision, S.C. project administration, A.P.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| PSC | Pharmaceutical Supply Chain |
| API | Active Pharmaceutical Ingredients |
| SI-HLLR | Supersingular Isogeny and Hosmer–Lemeshow Logistic Regression-based |
| PSCM | Pharmaceutical Supply Chain Management |
References
- Dwivedi, S.K.; Amin, R.; Vollala, S. Blockchain based secured information sharing protocol in supply chain management system with key distribution mechanism. J. Inf. Secur. Appl. 2020, 54, 102554. [Google Scholar] [CrossRef]
- Marchese, A.; Tomarchio, O. A Blockchain-Based System for Agri-Food Supply Chain Traceability Management. SN Comput. Sci. 2022, 3, 279. [Google Scholar] [CrossRef]
- Shah, N.A.; Sattigeri, B.M.; Patel, N.N.; Desai, H.A. Counterfeit drugs in India: Significance and impact on pharmacovigilance. Int. J. Res. Med. Sci. 2015, 3, 2156–2160. [Google Scholar] [CrossRef]
- Raijada, D.; Wac, K.; Greisen, E.; Rantanen, J.; Genina, N. Integration of personalized drug delivery systems into digital health. Adv. Drug Deliv. Rev. 2021, 176, 113857. [Google Scholar] [CrossRef]
- Zhang, S.; Lee, J.H. Analysis of the main consensus protocols of blockchain. Korean Inst. Commun. Inf. Sci. 2020, 6, 93–97. [Google Scholar] [CrossRef]
- Turjo, M.D.; Khan, M.M.; Kaur, M.; Zaguia, A. Smart Supply Chain Management Using the Blockchain and Smart Contract. Sci. Program. 2021, 2021, 6092792. [Google Scholar] [CrossRef]
- Lou, M.; Dong, X.; Cao, Z.; Shen, J. SESCF: A Secure and Efficient Supply Chain Framework via Blockchain-Based Smart Contracts. Secur. Commun. Netw. 2021, 2021, 8884478. [Google Scholar] [CrossRef]
- Ouf, S. A Proposed Architecture for Pharmaceutical Supply Chain Based Semantic Blockchain. Int. J. Intell. Eng. Syst. 2021, 14, 31–42. [Google Scholar] [CrossRef]
- Ali, I.; Kannan, D. Mapping research on healthcare operations and supply chain management: A topic modelling-based literature review. Ann. Oper. Res. 2022, 315, 29–55. [Google Scholar] [CrossRef]
- Humayun, M.; Jhanjhi, N.Z.; Niazi, M.; Amsaad, F.; Masood, I. Securing Drug Distribution Systems from Tampering Using Blockchain. Electronics 2022, 11, 1195. [Google Scholar] [CrossRef]
- Seddigh, M.R.; Shokouhyar, S.; Loghmani, F. Approaching towards sustainable supply chain under the spotlight of business intelligence. Ann. Oper. Res. 2022, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ismail, L.; Materwala, H. A Review of Blockchain Architecture and Consensus Protocols: Use Cases, Challenges, and Solutions. Symmetry 2019, 11, 1198. [Google Scholar] [CrossRef]
- Wang, L.; Deng, T.; Shen, Z.J.M.; Hu, H.; Qi, Y. Digital twin-driven smart supply chain. Front. Eng. Manag. 2022, 9, 56–70. [Google Scholar] [CrossRef]
- Zhang, G.; Yang, Y.; Yang, G. Smart supply chain management in Industry 4.0: The review, research agenda and strategies in North America. Ann. Oper. Res. 2022, 1–43. [Google Scholar] [CrossRef] [PubMed]
- Musamih, A.; Salah, K.; Jayaraman, R.; Arshad, J.; Debe, M.; Al-Hammadi, Y.; Ellahham, S. A Blockchain-Based Approach for Drug Traceability in Healthcare Supply Chain. IEEE Access 2021, 9, 9728–9743. [Google Scholar] [CrossRef]
- Glogg, R.Y.; Timonina-Farkas, A.; Seifert, R.W. Modeling and mitigating supply chain disruptions as a bilevel network flow problem. Comput. Manag. Sci. 2021, 19, 395–423. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, X.; Wu, L.; Hu, P.; Huang, Y.; Pan, X.; Wu, C. Progress on Pharmaceutical Sciences/Pharmacy Postgraduate Education: A Bibliometric Perspective. J. Pharm. Innov. 2021, 1–13. [Google Scholar] [CrossRef]
- Ivanov, D. Viable supply chain model: Integrating agility, resilience and sustainability perspectives—Lessons from and thinking beyond the COVID-19 pandemic. Ann. Oper. Res. 2020, 1–21. [Google Scholar] [CrossRef]
- Sharma, A.; Abbas, H.; Siddiqui, M.Q. Modelling the inhibitors of cold supply chain using fuzzy interpretive structural modeling and fuzzy MICMAC analysis. PLoS ONE 2021, 16, e0249046. [Google Scholar] [CrossRef]
- Rickard, E.; Ozieranski, P. A hidden web of policy influence: The pharmaceutical industry’s engagement with UK’s All-Party Parliamentary Groups. PLoS ONE 2021, 16, e0252551. [Google Scholar] [CrossRef]
- Khan, F.; Ali, Y. Implementation of the circular supply chain management in the pharmaceutical industry. Environ. Dev. Sustain. 2022, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Katsaliaki, K.; Galetsi, P.; Kumar, S. Supply chain disruptions and resilience: A major review and future research agenda. Ann. Oper. Res. 2021, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Diem Le, C.T.; Pakurár, M.; Kun, I.A.; Oláh, J. The impact of factors on information sharing: An application of meta-analysis. PLoS ONE 2021, 16, e0260653. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.Y.; Zhang, L.; Li, J.; Ji, L.L.; Sun, Y. A survey of application research based on blockchain smart contract. Wirel. Netw. 2022, 28, 635–690. [Google Scholar] [CrossRef]
- Li, D.; Gong, Y.; Zhang, X.; Huang, M. An Exploratory Study on the Design and Management Model of Traditional Chinese Medicine Quality Safety Traceability System Based on Blockchain Technology. Secur. Commun. Netw. 2022, 2022, 7011145. [Google Scholar] [CrossRef]
- Idrees, S.M.; Nowostawski, M.; Jameel, R.; Mourya, A.K. Security Aspects of Blockchain Technology Intended for Industrial Applications. Electronics 2021, 10, 951. [Google Scholar] [CrossRef]
- Khatoon, A. A Blockchain-Based Smart Contract System for Healthcare Management. Electronics 2020, 9, 94. [Google Scholar] [CrossRef]
- Abbas, K.; Afaq, M.; Ahmed Khan, T.; Song, W.C. A Blockchain and Machine Learning-Based Drug Supply Chain Management and Recommendation System for Smart Pharmaceutical Industry. Electronics 2020, 9, 852. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).