Segmentation of Echocardiography Based on Deep Learning Model
Abstract
:1. Introduction
2. Methods
2.1. Dataset
2.2. Model Construction
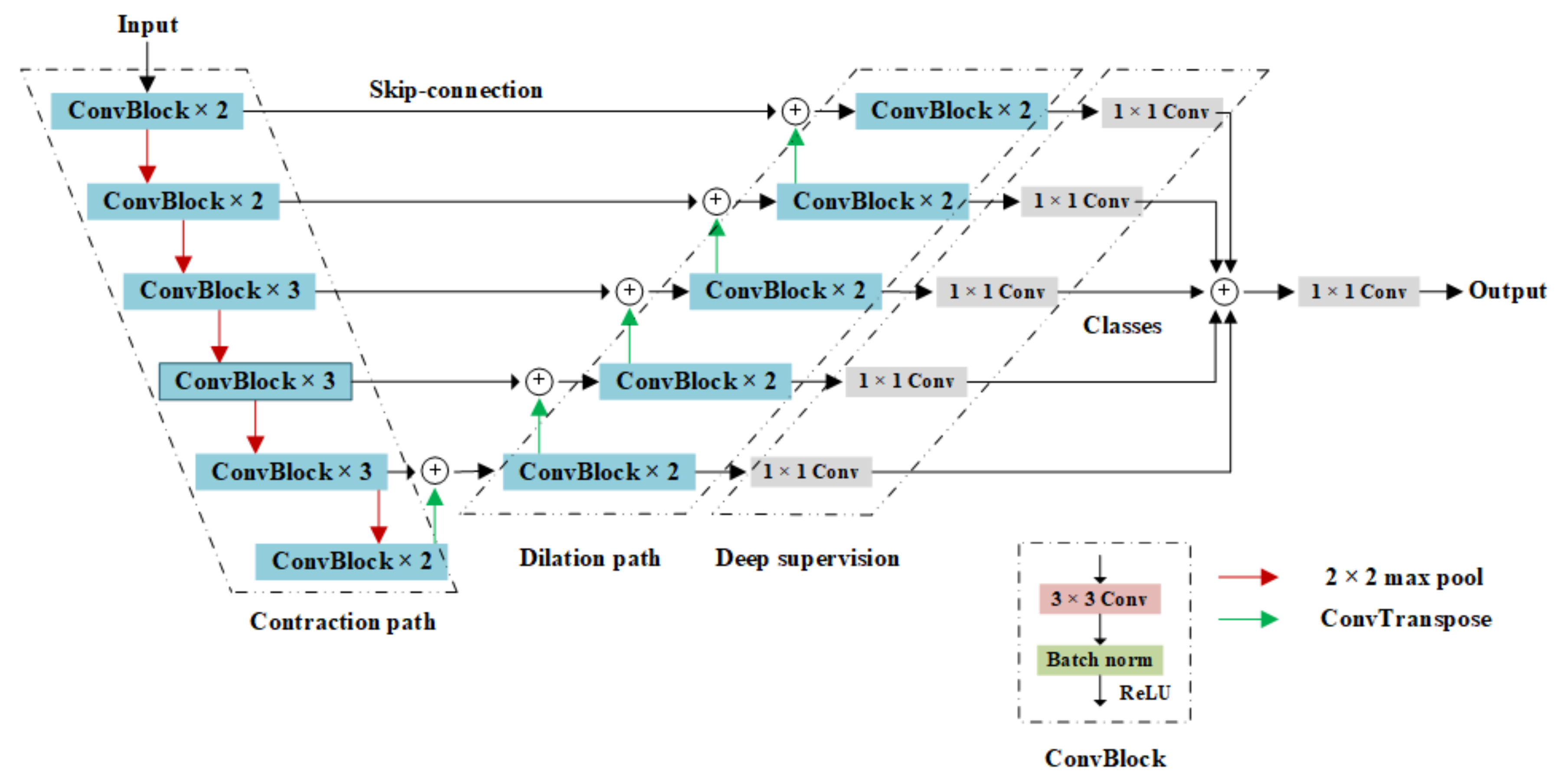
2.2.1. Overall Architecture of the Model
- The contraction path used the VGG16 network for image feature extraction;
- The expanded path restored the image by layer hopping and transpose convolution;
- The deep supervision mechanism supplemented multi-scale information.
2.2.2. Contraction Path
2.2.3. Expansion Path
2.2.4. Deep Supervision Structure
2.3. Loss Function
3. Results
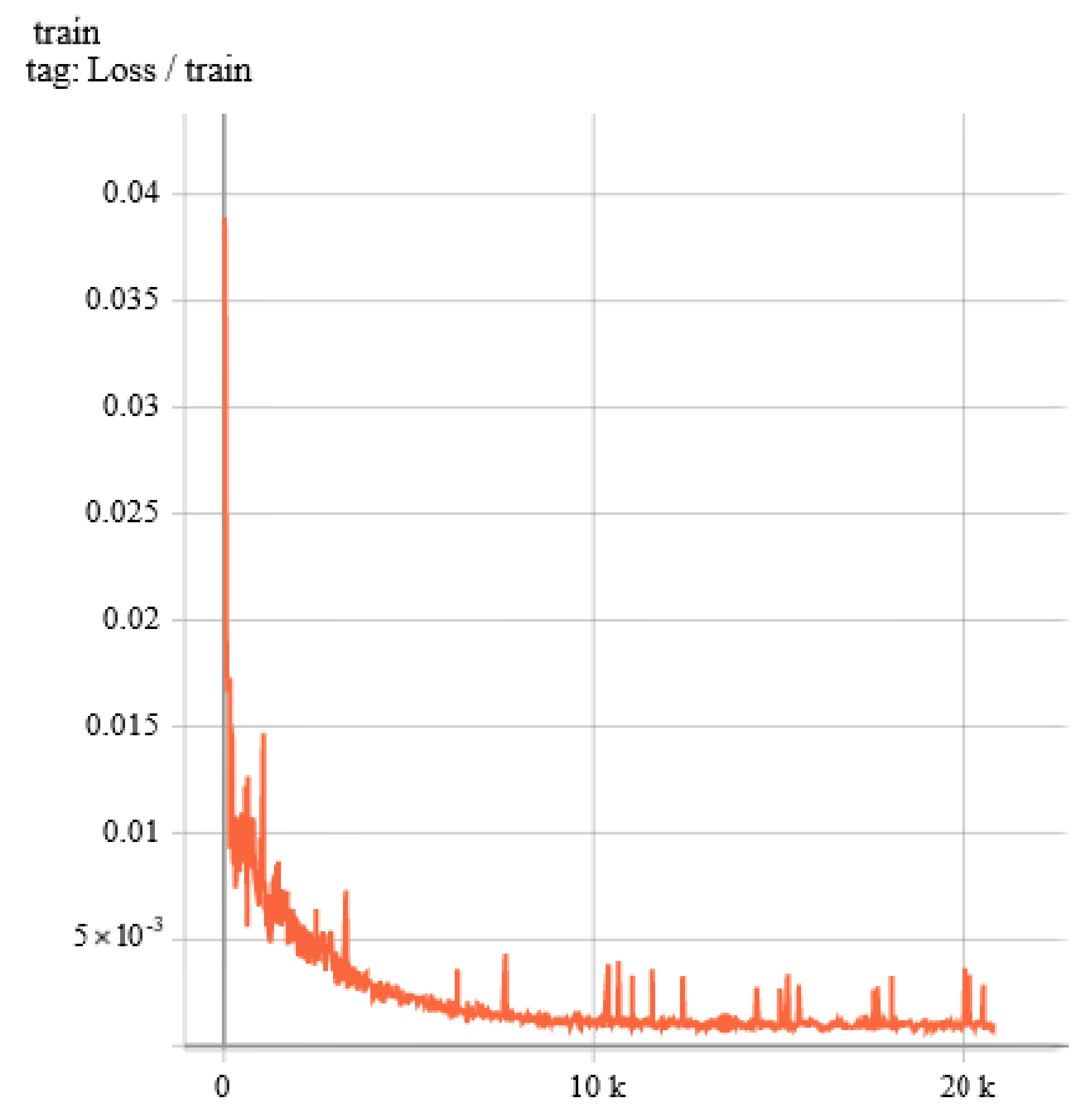
3.1. Details of Train
3.2. Segmentation Results
3.3. Result Evaluation
4. Discussion
4.1. Comparison and Analysis
4.2. Effectiveness of Model Construction
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nkomo, V.T.; Gardin, J.M.; Skelton, T.N.; Gottdiener, J.S.; Scott, C.G.; Enriquez-Sarano, M. Burden of valvular heart diseases: A population-based study. Lancet 2006, 368, 1005–1011. [Google Scholar] [CrossRef]
- Enriquez-Sarano, M.; Avierinos, J.F.; Messika-Zeitoun, D.; Detaint, D.; Capps, M.; Nkomo, V.; Scott, C.; Schaff, H.V.; Tajik, A.J. Quantitative determinants of the outcome of asymptomatic mitral regurgitation. N. Engl. J. Med. 2005, 352, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Zoghbi, W.A.; Adams, D.; Bonow, R.O.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A.; Hahn, R.T.; Han, Y.; Hung, J.; Lang, R.M.; et al. Recommendations for noninvasive evaluation of native valvular regurgitation: A report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Magnetic Resonance. J. Am. Soc. Echocardiogr. 2017, 30, 303–371. [Google Scholar] [CrossRef] [PubMed]
- Yiu, S.F.; Enriquez-Sarano, M.; Tribouilloy, C.; Seward, J.B.; Tajik, A.J. Determinants of the degree of functional mitral regurgitation in patients with systolic left ventricular dysfunction: A quantitative clinical study. Circulation 2000, 102, 1400–1406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kagiyama, N.; Mondillo, S.; Yoshida, K.; Mandoli, G.E.; Cameli, M. Subtypes of atrial functional mitral regurgitation: Imaging insights into their mechanisms and therapeutic implications. JACC Cardiovasc. Imaging 2020, 13, 820–835. [Google Scholar] [CrossRef] [PubMed]
- Barreiro-Perez, M.; Caneiro-Queija, B.; Puga, L.; Gonzalez-Ferreiro, R.; Alarcon, R.; Parada, J.A.; Iñiguez-Romo, A.; Estevez-Loureiro, R. Imaging in Transcatheter Mitral Valve Replacement: State-of-Art Review. J. Clin. Med. 2021, 10, 5973. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, S.; Berardini, A.; Statuto, G.; Angeletti, A.; Massaro, G.; Capobianco, C.; Piemontese, G.P.; Spadotto, A.; Toniolo, S.; Caponetti, A.G.; et al. Percutaneous Mitral Valve Repair with the MitraClip System in the Current Clinical Practice. Hearts 2021, 2, 74–86. [Google Scholar] [CrossRef]
- Stone, G.W.; Vahanian, A.S.; Adams, D.H.; Abraham, W.T.; Borer, J.S.; Bax, J.J.; Schofer, J.; Cutlip, D.E.; Krucoff, M.W.; Blackstone, E.H.; et al. Clinical trial design principles and endpoint definitions for transcatheter mitral valve repair and replacement: Part 1: Clinical trial design principles: A consensus document from the mitral valve academic research consortium. Eur. Heart J. 2015, 36, 1851–1877. [Google Scholar] [CrossRef] [PubMed]
- O’Gara, P.T.; Grayburn, P.A.; Badhwar, V.; Afonso, L.C.; Carroll, J.D.; Elmariah, S.; Kithcart, A.P.; Nishimura, R.A.; Ryan, T.J.; Schwartz, A.; et al. 2017 ACC expert consensus decision pathway on the management of mitral regurgitation: A report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J. Am. Coll. Cardiol. 2017, 70, 2421–2449. [Google Scholar] [CrossRef] [PubMed]
- Bonow, R.O.; O’Gara, P.T.; Adams, D.H.; Badhwar, V.; Bavaria, J.E.; Elmariah, S.; Hung, J.W.; Lindenfeld, J.; Morris, A.A.; Satpathy, R.; et al. 2020 focused update of the 2017 ACC expert consensus decision pathway on the management of mitral regurgitation: A report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2020, 75, 2236–2270. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, J.; Queirós, S.; Bernard, O.; Engvall, J.; Edvardsen, T.; Nagel, E.; D’hooge, J. Fast and fully automatic left ventricular segmentation and tracking in echocardiography using shape-based b-spline explicit active surfaces. IEEE Trans. Med. Imaging 2017, 36, 2287–2296. [Google Scholar] [CrossRef] [PubMed]
- Ali, Y.; Janabi-Sharifi, F.; Beheshti, S. Echocardiographic image segmentation using deep Res-U network. Biomed. Signal Processing Control 2021, 64, 102248. [Google Scholar] [CrossRef]
- Liu, F.; Wang, K.; Liu, D.; Yang, X.; Tian, J. Deep pyramid local attention neural network for cardiac structure segmentation in two-dimensional echocardiography. Med. Image Anal. 2021, 67, 101873. [Google Scholar] [CrossRef] [PubMed]
- Zyuzin, V.; Mukhtarov, A.; Neustroev, D.; Chumarnaya, T. Segmentation of 2D Echocardiography Images using Residual Blocks in U-Net Architectures. In Proceedings of the 2020 Ural Symposium on Biomedical Engineering, Radioelectronics and Information Technology (USBEREIT), IEEE, Yekaterinburg, Russia, 14–15 May 2020; pp. 499–502. [Google Scholar]
- Zhao, C.; Xia, B.; Chen, W.; Guo, L.; Du, J.; Wang, T.; Lei, B. Multi-scale Wavelet Network Algorithm for Pediatric Echocardiographic Segmentation via Feature Fusion. In Proceedings of the 2021 IEEE 18th International Symposium on Biomedical Imaging (ISBI), IEEE, Nice, France, 13–16 April 2021; pp. 1402–1405. [Google Scholar]
- Sultan, M.S.; Martins, N.; Costa, E.; Veiga, D.; Ferreira, M.J.; Mattos, S.; Coimbra, M.T. A new method for the anterior mitral leaflet segmentation in echocardiography videos using the virtual m-mode space. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), IEEE, Honolulu, HI, USA, 18-21 July 2018; pp. 3120–3123. [Google Scholar]
- Costa, E.; Martins, N.; Sultan, M.S.; Veiga, D.; Ferreira, M.; Mattos, S.; Coimbra, M. Mitral valve leaflets segmentation in echocardiography using convolutional neural networks. In Proceedings of the 2019 IEEE 6th Portuguese Meeting on Bioengineering (ENBENG), IEEE, Lisbon, Portugal, 22–23 February 2019; pp. 1–4. [Google Scholar]
- Corinzia, L.; Laumer, F.; Candreva, A.; Taramasso, M.; Maisano, F.; Buhmann, J.M. Neural collaborative filtering for unsupervised mitral valve segmentation in echocardiography. Artif. Intell. Med. 2020, 110, 101975. [Google Scholar] [CrossRef] [PubMed]
- Abdi, A.H.; Luong, C.; Tsang, T.; Allan, G.; Nouranian, S.; Jue, J.; Hawley, D.; Fleming, S.; Gin, K.; Swift, J.; et al. Automatic Quality Assessment of Echocardiograms Using Convolutional Neural Networks: Feasibility on the Apical Four-Chamber View. IEEE Trans. Med. Imaging 2017, 36, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Ronneberger, O.; Fischer, P.; Brox, T. U-net: Convolutional networks for biomedical image segmentation. In Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention; Springer: Cham, Switzerland, 2015; pp. 234–241. [Google Scholar]
- Haak, A.; Mulder, H.W.; Ren, B.; Vegas-Sánchez-Ferrero, G.; van Burken, G.; van der Steen, A.F.; van Stralen, M.; Pluim, J.P.; van Walsum, T.; Bosch, J.G. Segmentation of multiple heart cavities in wide-view fused 3D transesophageal echocardiograms. In Proceedings of the 2014 IEEE International Ultrasonics Symposium, IEEE, Chicago, IL, USA, 3–6 September 2014; pp. 691–694. [Google Scholar]



| NM | DMR | FMR | aFMR | Total | |
|---|---|---|---|---|---|
| 2CH | 110 | 265 | 129 | 46 | 550 |
| 3CH | 206 | 225 | 141 | 39 | 611 |
| 4CH | 542 | 284 | 164 | 32 | 1022 |
| Total | 858 | 774 | 434 | 117 | 2183 |
| LA | LV | MV | |
|---|---|---|---|
| PA | 0.991 ± 0.001 | ||
| CPA | 0.934 ± 0.002 | 0.925 ± 0.003 | 0.794 ± 0.007 |
| IoU | 0.880 ± 0.002 | 0.847 ± 0.001 | 0.615 ± 0.003 |
| Dice | 0.935 ± 0.002 | 0.915 ± 0.002 | 0.757 ± 0.003 |
| Author | Method | Dataset | Dice (LA) | Dice (LV) | Dice (MV) |
|---|---|---|---|---|---|
| Sultanl, M.S., et al. [16] | M-mode, LAC | 62 videos | / | / | 0.63 |
| Costa, E., et al. [17] | U-Net | 101 videos | / | / | 0.742 (PLAX) 0.795 (A4C) |
| Corinzia, L., et al. [18] | NN-MitralSeg | 39 videos | / | / | 0.495 |
| Pedrosa, J., et al. [11] | SSM, lAAOF | CETUS | / | 0.909 (ED), 0.875 (ES) | / |
| Ali, Y., et al. [12] | Res-U | CAMUS | / | 0.975 (ED), 0.972 (ES) | / |
| Liu, F., et al. [13] | PLANet | CAMUS & sub-EchoNet-Dynamic | / | 0.942 (ED), 0.918 (ES) | / |
| Alexander Haak, et al. [21] | ASM | 63D TEE volumes | 0.92 | / | / |
| Zyuzin, V., et al. [14] | Res-U | CAMUS | 0.904 | / | / |
| Zhao, C., et al. [15] | MS-Net | CAMUS & 127 videos | 0.98 | / | / |
| Proposed | VDS-UNet | 153 videos | 0.935 | 0.915 | 0.757 |
| Dice (LA) | Dice (LV) | Dice (MV) | |
|---|---|---|---|
| UNet | 0.917 | 0.913 | 0.736 |
| UNet (VGG16) | 0.917 | 0.909 | 0.726 |
| UNet (ds) | 0.93 | 0.909 | 0.72 |
| Propoesd | 0.935 | 0.915 | 0.757 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.; Ge, Z.; Wang, H.; Wu, J.; Hu, C.; Li, N.; Wu, X.; Pan, C. Segmentation of Echocardiography Based on Deep Learning Model. Electronics 2022, 11, 1714. https://doi.org/10.3390/electronics11111714
Huang H, Ge Z, Wang H, Wu J, Hu C, Li N, Wu X, Pan C. Segmentation of Echocardiography Based on Deep Learning Model. Electronics. 2022; 11(11):1714. https://doi.org/10.3390/electronics11111714
Chicago/Turabian StyleHuang, Helin, Zhenyi Ge, Hairui Wang, Jing Wu, Chunqiang Hu, Nan Li, Xiaomei Wu, and Cuizhen Pan. 2022. "Segmentation of Echocardiography Based on Deep Learning Model" Electronics 11, no. 11: 1714. https://doi.org/10.3390/electronics11111714
APA StyleHuang, H., Ge, Z., Wang, H., Wu, J., Hu, C., Li, N., Wu, X., & Pan, C. (2022). Segmentation of Echocardiography Based on Deep Learning Model. Electronics, 11(11), 1714. https://doi.org/10.3390/electronics11111714






