Sodium Radiofrequency Coils for Magnetic Resonance: From Design to Applications
Abstract
1. Introduction
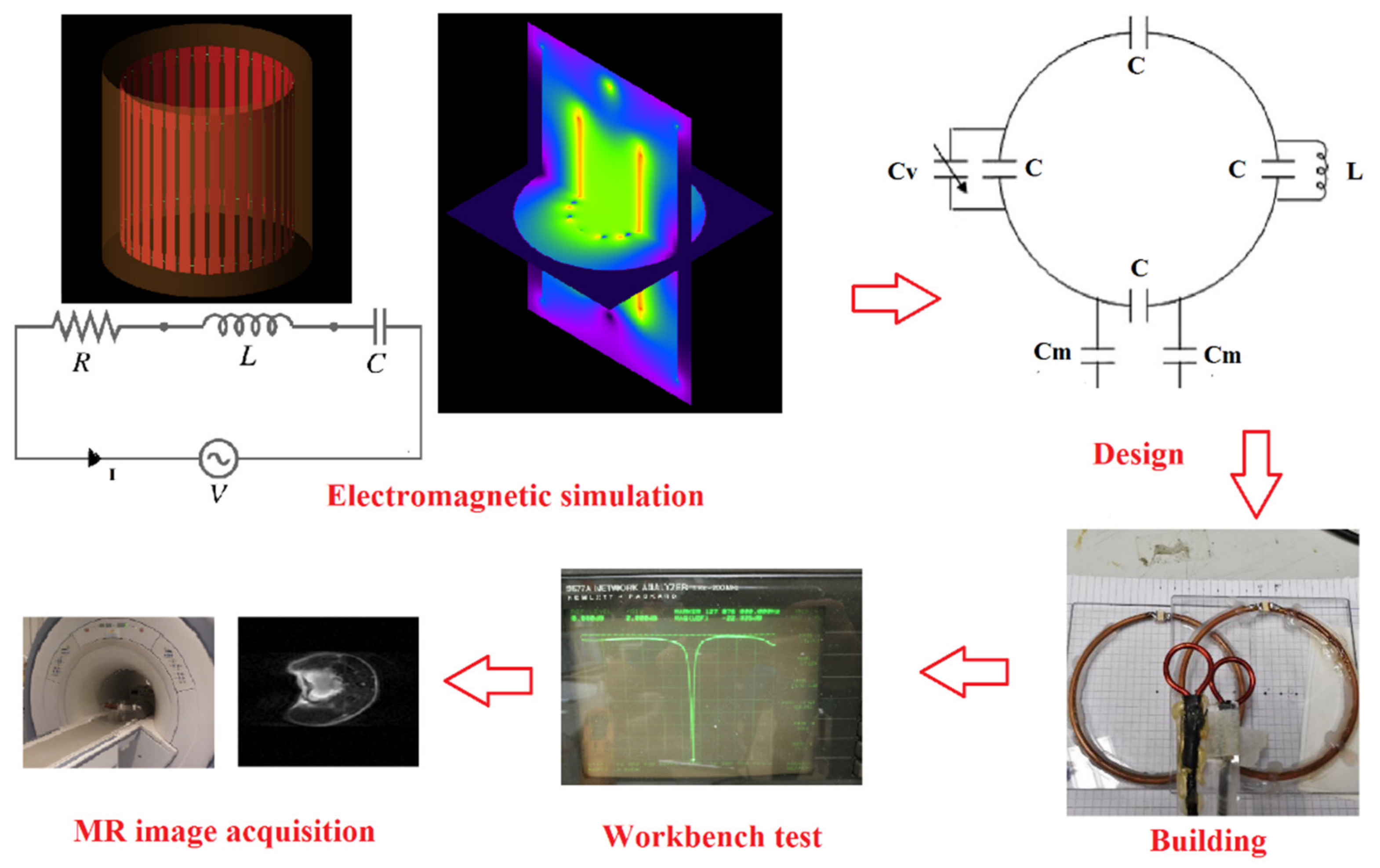
2. Coil Theory
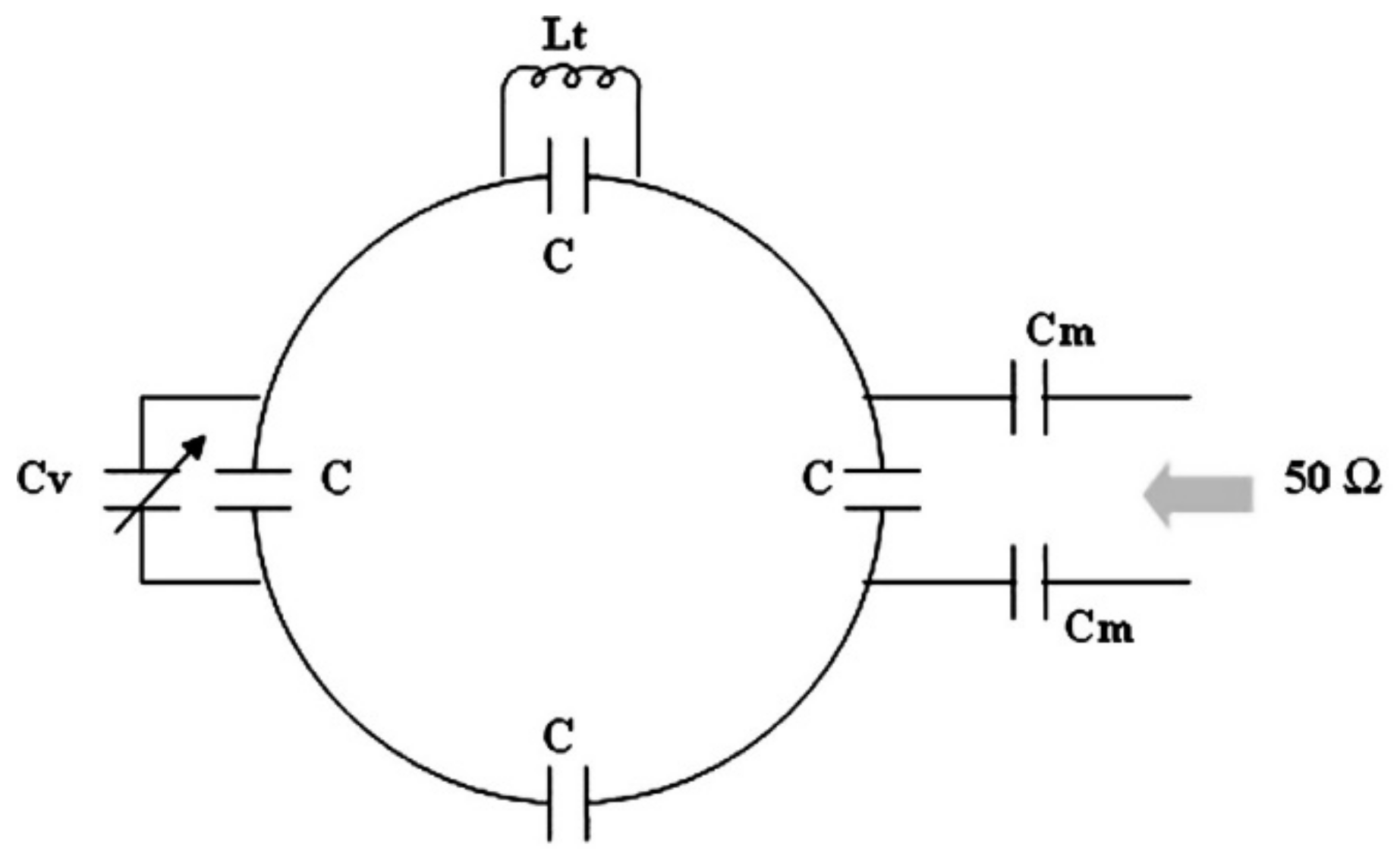
2.1. Coil Design
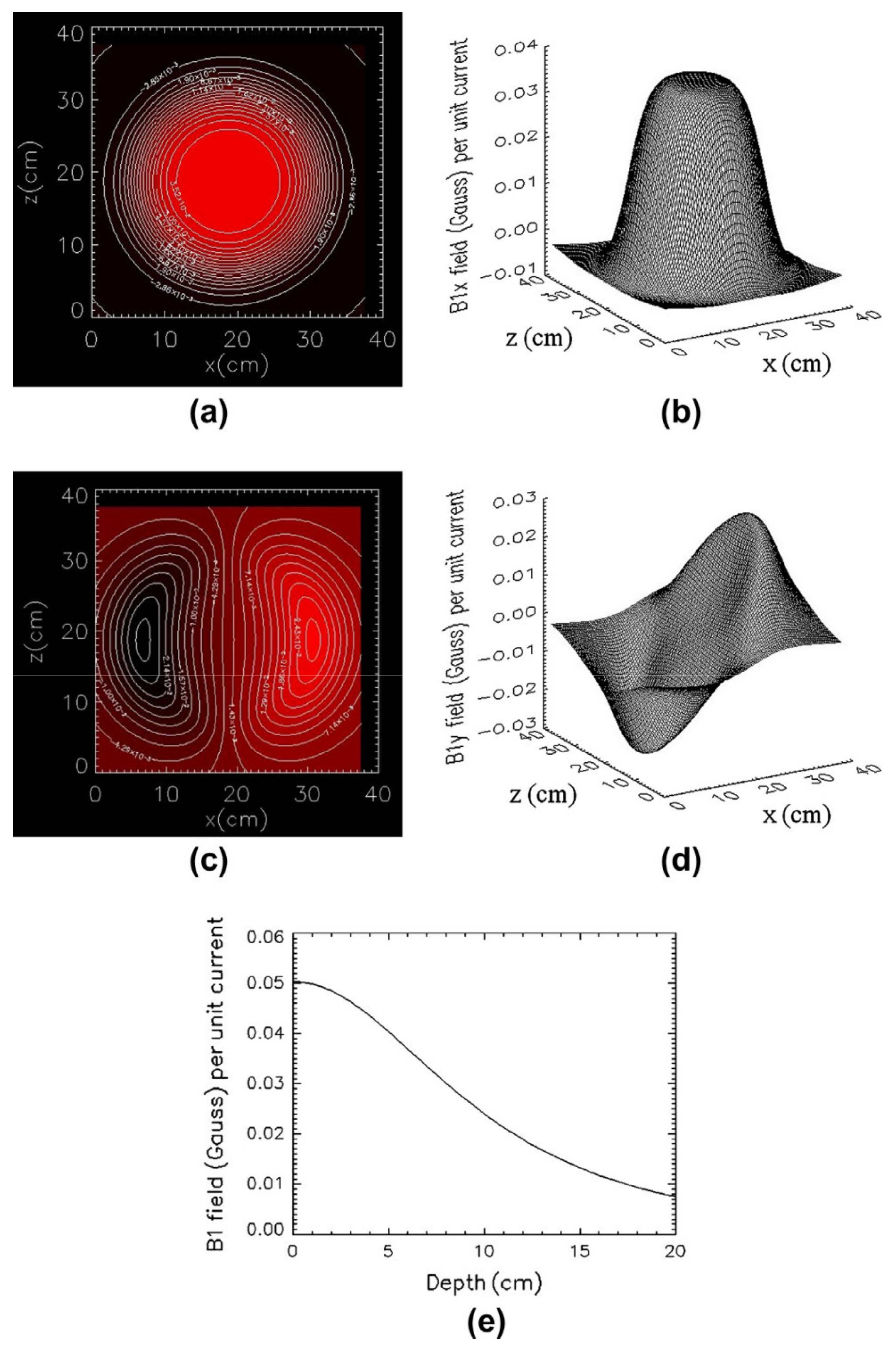
2.2. Coil Simulation
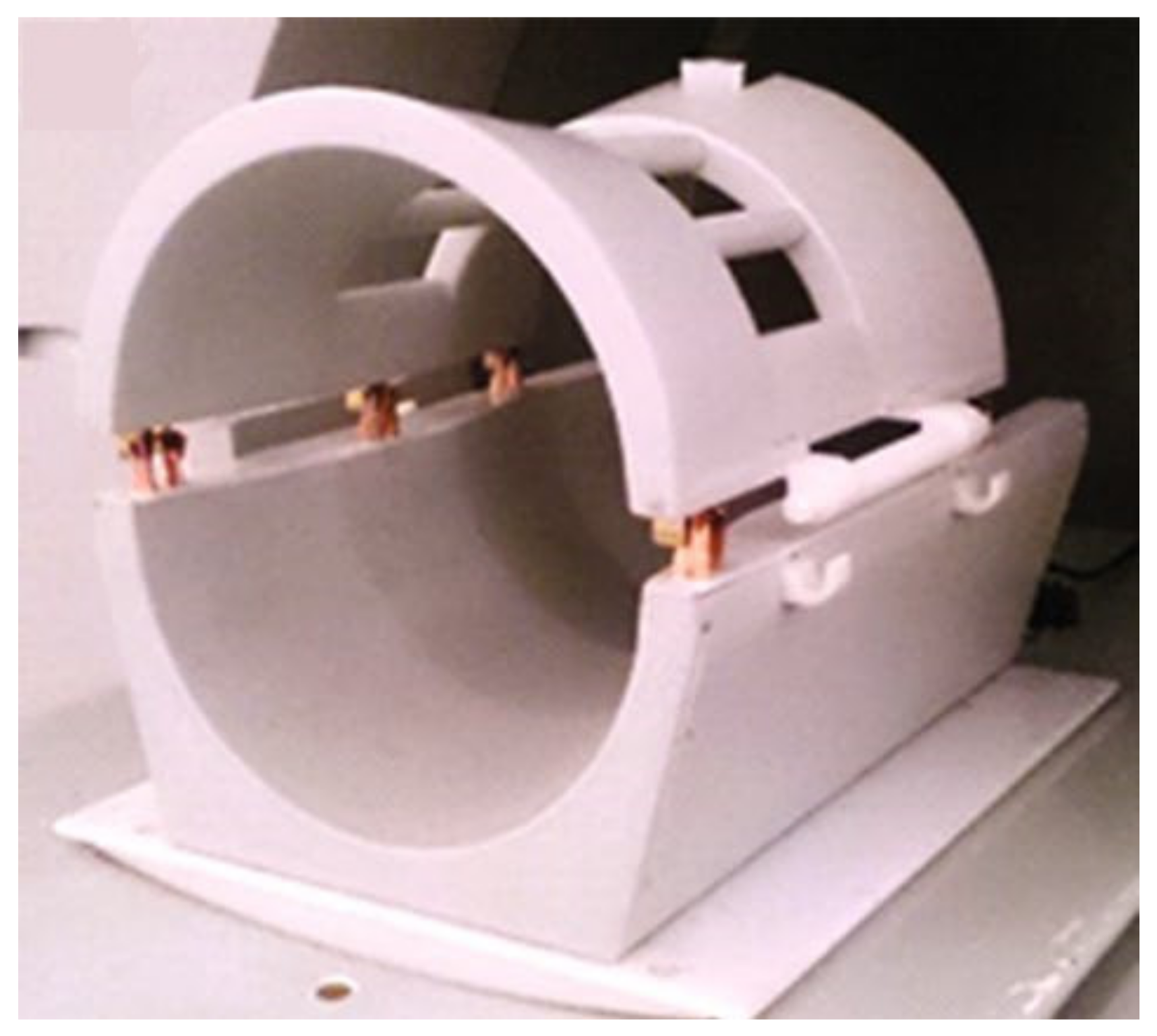
2.3. Coil Test
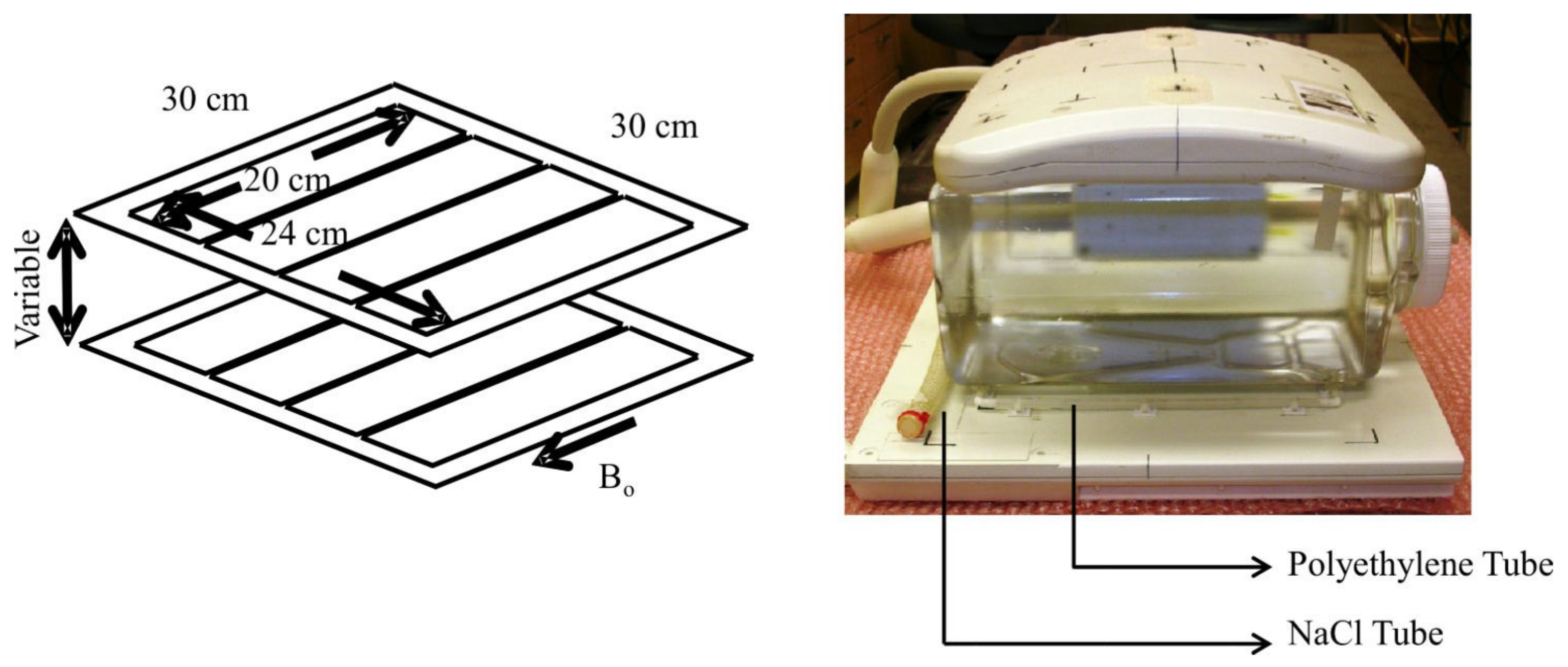
3. Sodium Coils
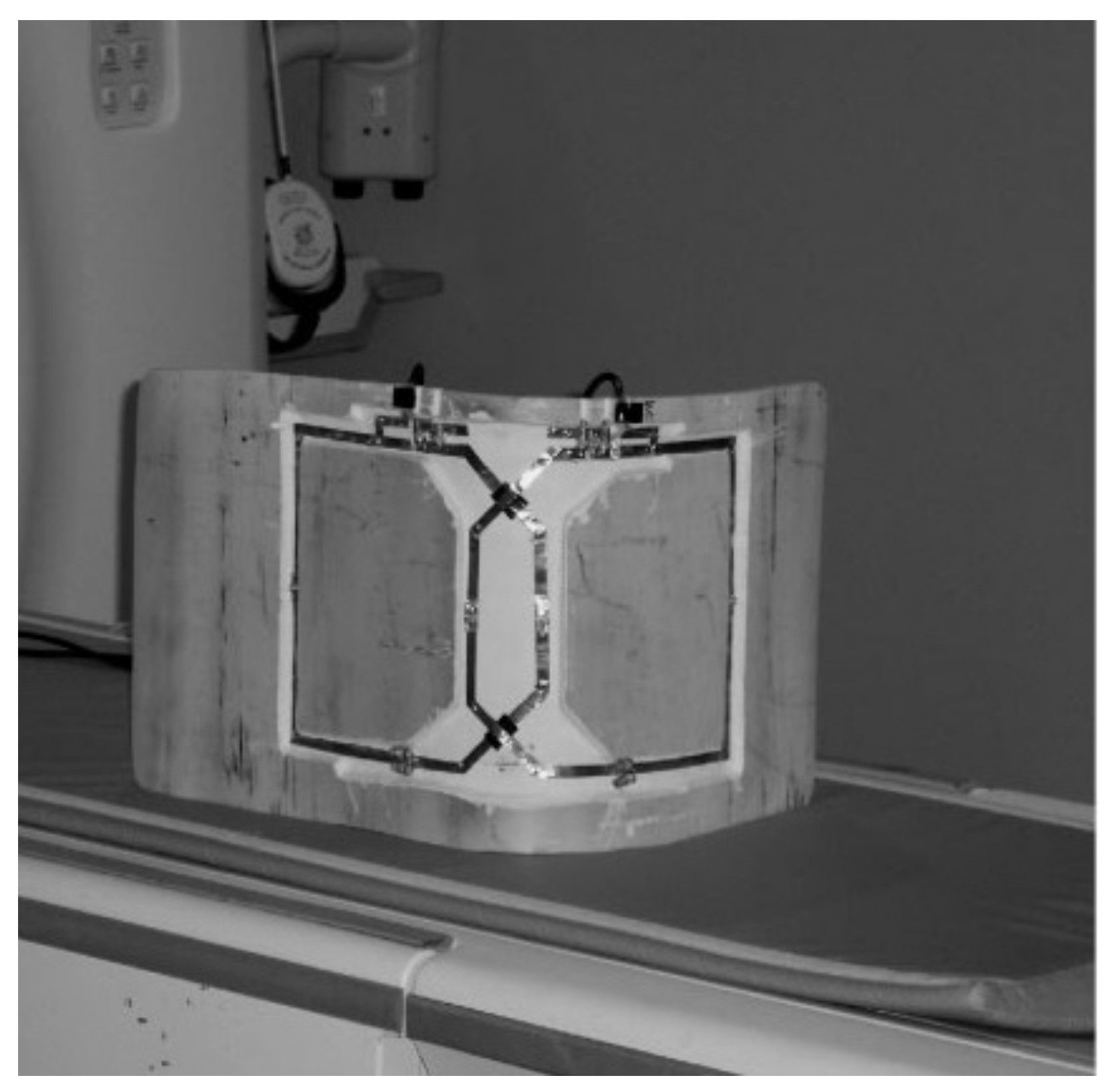
3.1. Surface and Volume Sodium-Only Coils
3.2. Surface and Volume Dual-Tuned Sodium/Hydrogen Coils
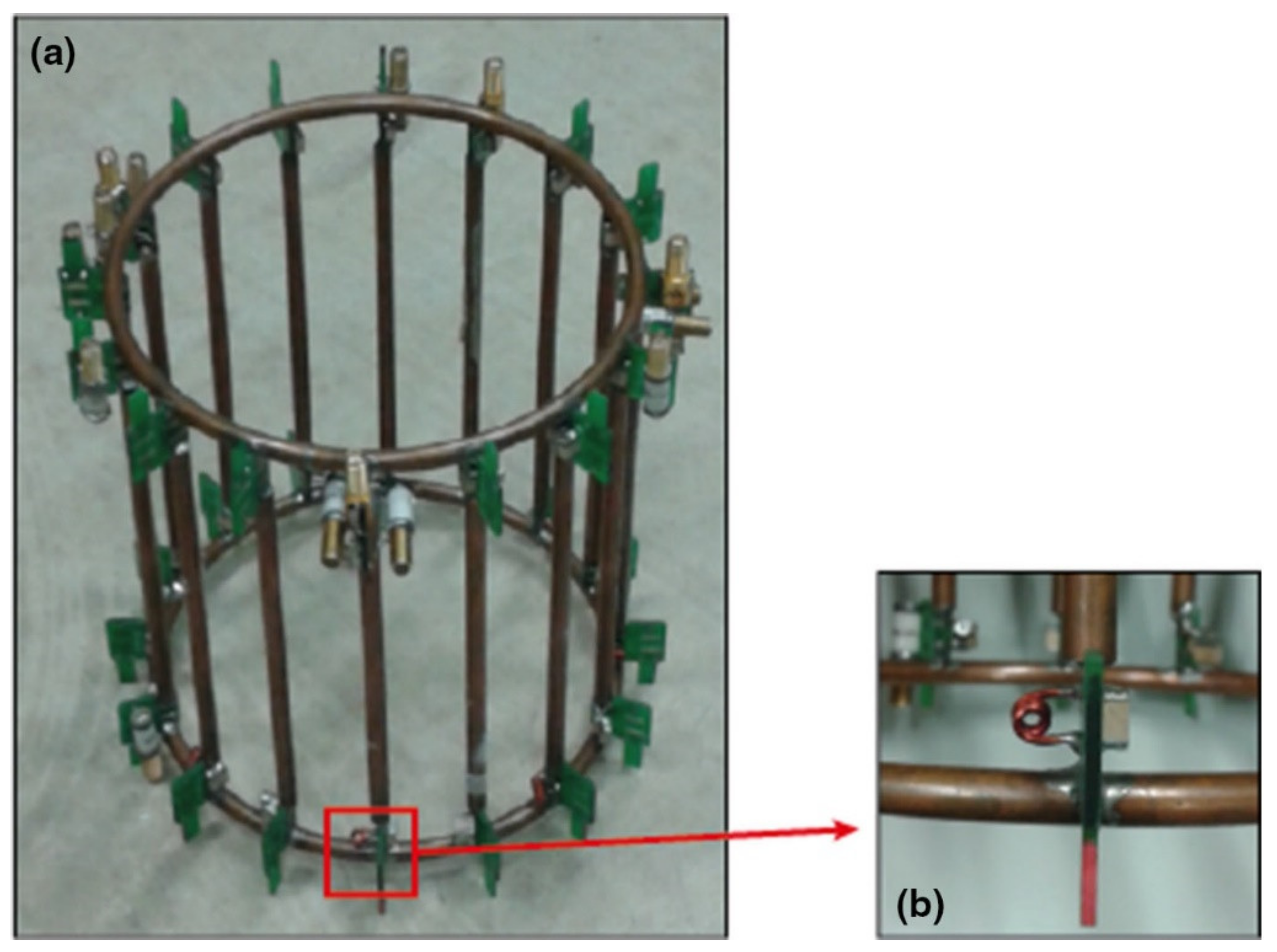
3.3. Phased-Array Sodium-Only Coils
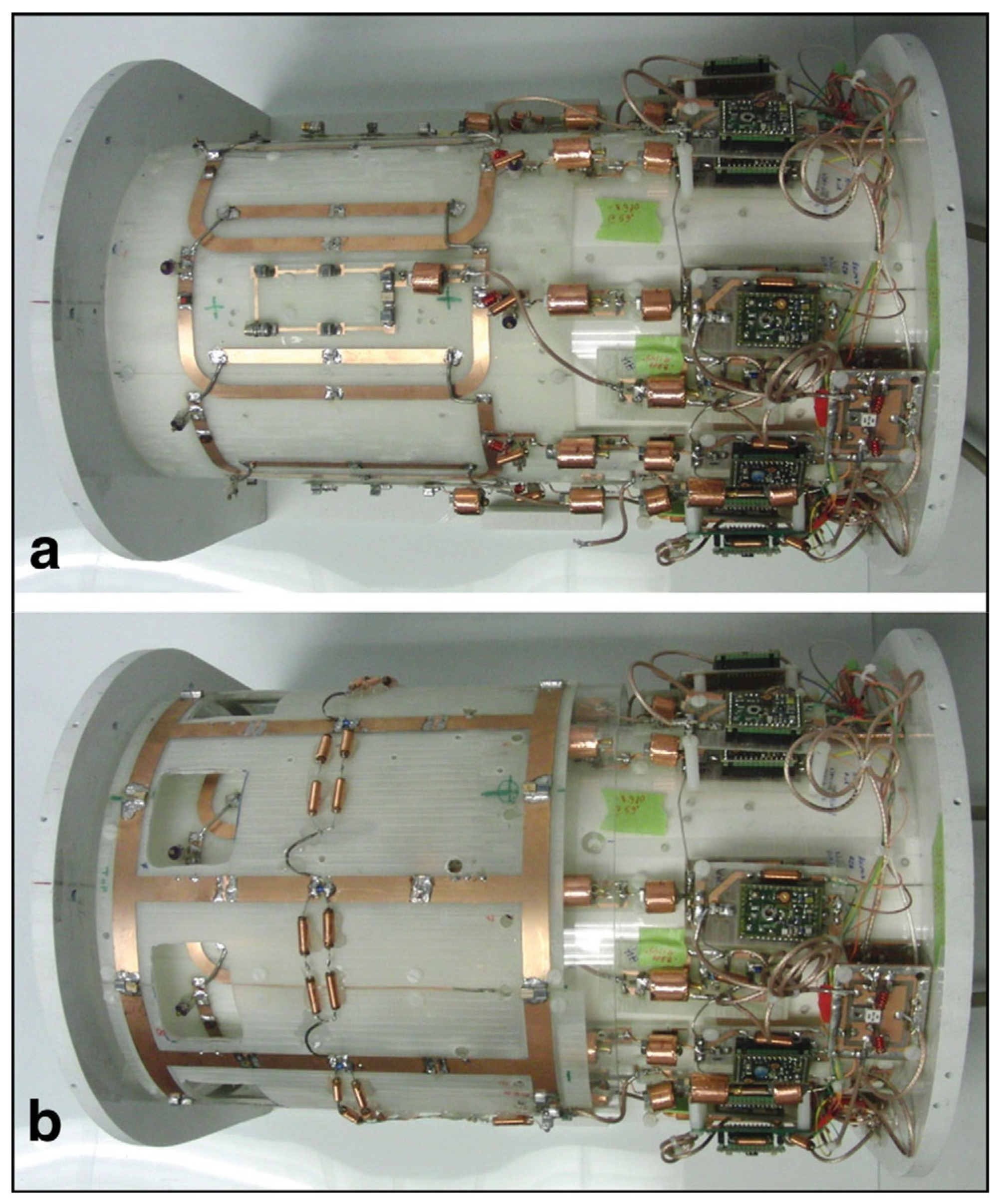
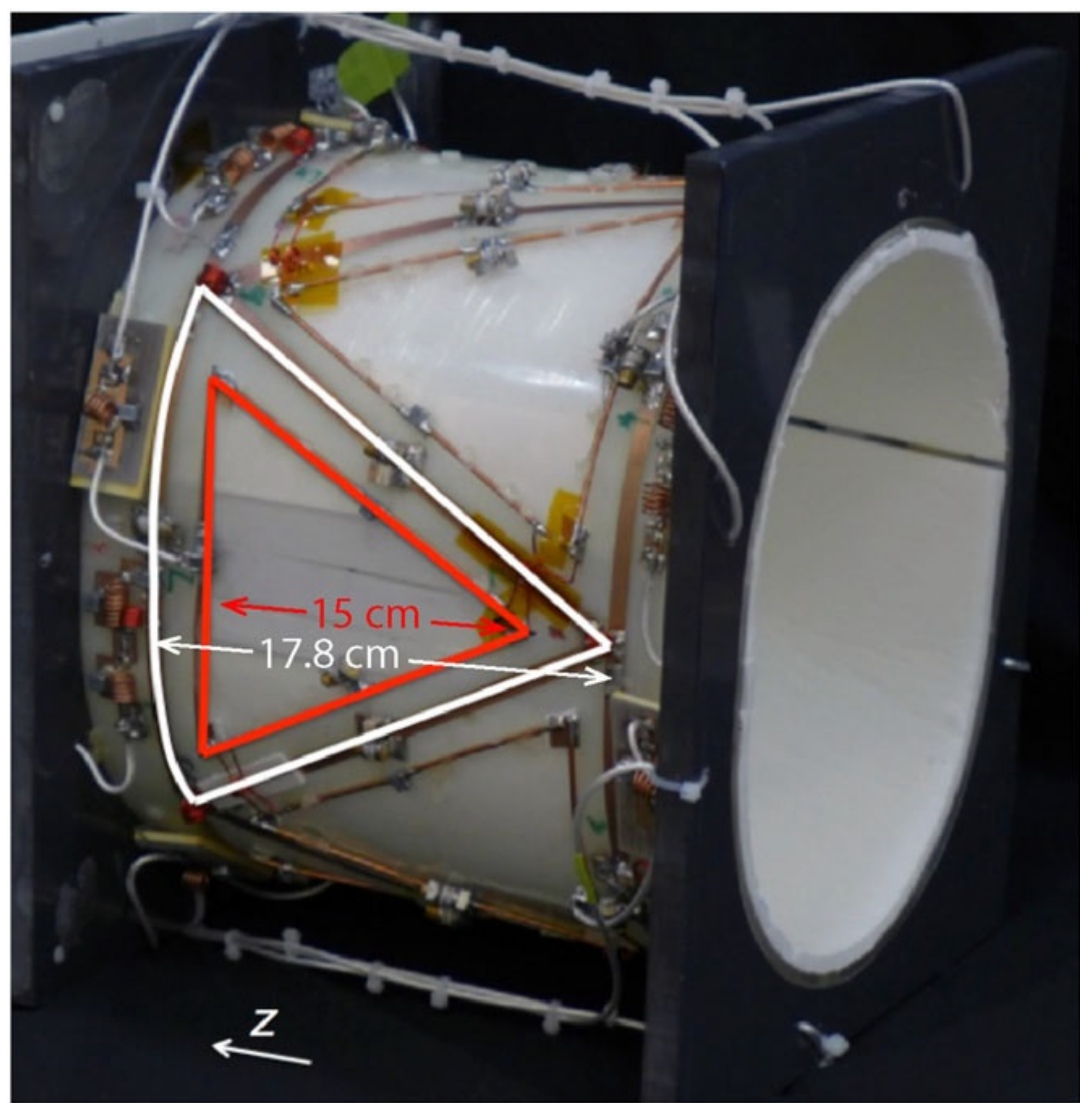
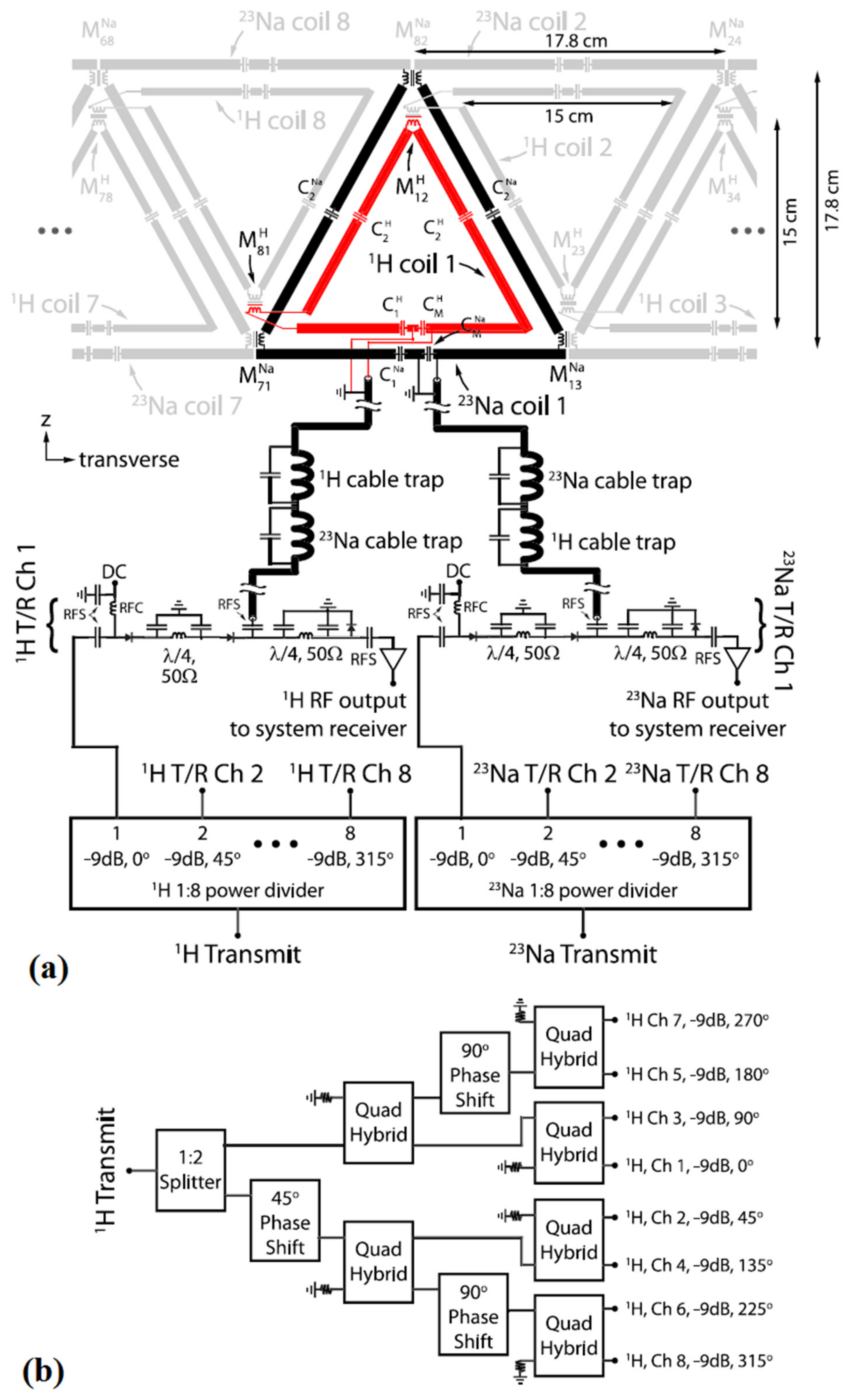
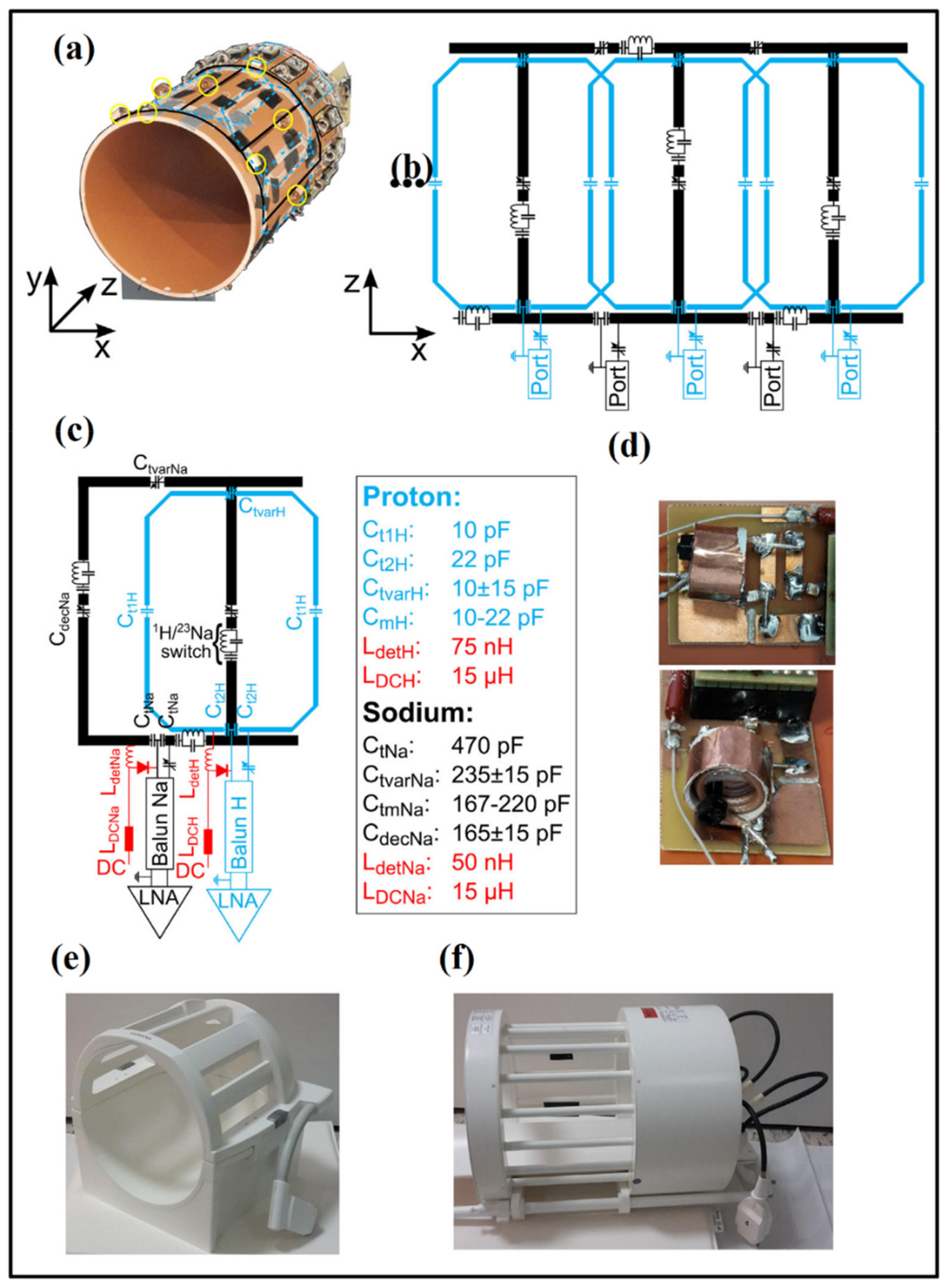
3.4. Phased-Array Dual-Tuned Sodium/Hydrogen Coils
4. Pulse Sequences and Image Reconstruction
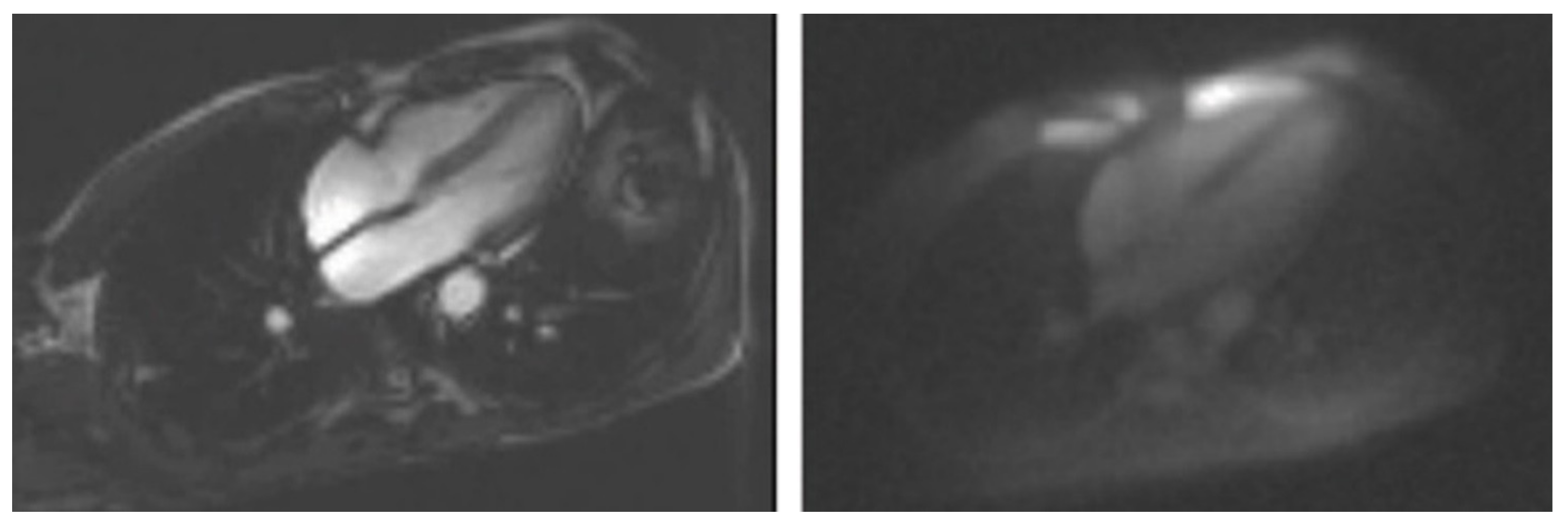
5. Clinical Applications of Sodium MR
- The tissue viability assessment. Sodium provides a marker of tissue viability in terms of the TSC that can be considered a direct biochemical marker of cellular function and viability. Thus, TSC could be considered a new parameter of tissue viability with the potential to provide additive information regarding the entity and severity of damaged tissue as well as the probability of recovery. In the brain a TSC increases at least 50% in the infarcted area in comparison to non-infarcted one. This means that TSC is around 70 mmol/L in comparison the concentration of around 45 mmol/L in normal tissue [53]. The TSC increase in continuous in the early phases of stroke and this can reduce the efficacy of reperfusion [4]. Interestingly, in an animal study of acute stroke, a TSC less than 55 mmol/L was associated a higher probability of recovery [53]. Regarding skeletal muscle, there are evidences showing that TSC can be assessed in patients with diseased muscle. Interestingly, TSC accumulation has been also observed in subjects after exercise [54]. This may be due to a physiological inhibition of the Na/K adenosine triphosphatase as a consequence of energy exhaustion, or to a change in sodium interactions with other molecules accumulating in the skeletal muscles after exercise [55].
- The increase in TSC has been also observed in an animal model of myocardial infarction. The peak of this increase occurs within the first day after induced myocardial infarction and reduces progressively in the following days due to the healing process [56]. Similarly, an increase in myocardial TSC has been documented in patients with chronic myocardial infarction [57,58]. There was no correlation between myocardial TSC and infarct size, left ventricular function and also with the occurrence of ventricular arrhythmia [59]. However, these are initial results shown in a small patient population and, thus, need to be confirmed.
- The applications in therapy. The sodium has the potential to assess the effectiveness of the therapeutical approaches to minimize or reverse organ’s lesion. The increase in TSC has been also documented in tumors. This is due to cell proliferation and also to the higher extent of extracellular matrix. In the brain tumors, extracellular matrix is increased whereas cell volume fraction is reduced in comparison to normal brain tissue. The effectiveness of the different cycles of oncogenic therapy could be assessed through the measurement of the cell volume fraction [58].
- The role of sodium MRI in the prevention policies. Initial studies were performed on the role of sodium content in skin and skeletal muscle in hypertension. In this context, TSC measurement could provide new information on the relationship between sodium and dietary salt consumption and cardiovascular risk, as well as the effects of antihypertensive treatment. Kopp et al. [60] measured TSC in skin and skeletal muscle in subjects with primary aldosteronism and found a 29% increase in muscle sodium content whereas skin Na sodium was basically but not significantly higher. Interestingly TSC in the muscle decreased after spironolactone treatment. In another study, Kopp C. et al. [61] measured water and sodium content both in skin and muscle showing water free sodium storage in muscle, and a paralleled increase in water and sodium in skin. Interestingly, sodium content increased with age in skin and muscle both in women and men, but skin increase was less pronounced in women. Moreover, in the same study, the authors showed that refractory hypertension was associated with sodium accumulation that reduced with spironolactone treatment. Moreover, myocardial TSC accumulation has also been recently documented in patients with primary hyperaldosteronism [62]. This accumulation reduced significantly after treatment. Tissue sodium accumulation has been documented also in patients with diabetes [63]. Recently, sodium glucose cotransporter 2 (SGLT-2) inhibitors, a new antidiabetic drug, that decreases reabsorption of glucose in the renal tubular system, parallel blocking sodium reabsorption, reduced sodium skin content in diabetic patients [64]. A recent study showed high reliability and agreement of sodium content in skin and skeletal muscle in healthy subjects, a key result to apply this new technique in the clinical practice [65].
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Thulborn, K.R. Quantitative Sodium MR Imaging: A Review of its Evolving Role in Medicine. Neuroimage 2018, 168, 250–268. [Google Scholar] [CrossRef] [PubMed]
- Ouwerkerk, R.; Jacobs, M.A.; Macura, K.J.; Wolff, A.C.; Stearns, V.; Mezban, S.D.; Khouri, N.F.; Bluemke, D.A.; Bottomley, P.A. Elevated tissue sodium concentration in malignant breast lesions detected with non-invasive 23Na MRI. Breast Cancer Res. Treat. 2007, 106, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Poku, L.O.; Phil, M.; Cheng, Y.; Wang, K.; Sun, X. 23Na-MRI as a Noninvasive Biomarker for Cancer Diagnosis and Prognosis. J. Magn. Reson. Imaging 2021, 53, 995–1014. [Google Scholar] [CrossRef]
- Hussain, M.S.; Stobbe, R.W.; Bhagat, Y.A.; Emery, D.; Butcher, K.S.; Manawadu, D.; Rizvi, N.; Maheshwari, P.; Scozzafava, J.; Shuaib, A.; et al. Sodium imaging intensity increases with time after human ischemic stroke. Ann. Neurol. 2009, 66, 55–62. [Google Scholar] [CrossRef]
- Boada, F.E.; Qian, Y.; Nemoto, E.; Jovin, T.; Jungreis, C.; Jones, S.C.; Weimer, J.; Lee, V. Sodium MRI and the Assessment of Irreversible Tissue Damage During Hyper-Acute Stroke. Transl. Stroke Res. 2012, 3, 236–245. [Google Scholar] [CrossRef]
- Petracca, M.; Fleysher, L.; Oesingmann, N.; Inglese, M. Sodium MRI of multiple sclerosis. NMR Biomed. 2016, 29, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.E.; Nagel, K.; Ebert, A.; Roßmanith, C.; Paschke, N.; Adlung, A.; Platten, M.; Schad, L.R.; Gass, A.; Eisele, P. Diffusely appearing white matter in multiple sclerosis: Insights from sodium (23Na) MRI. Mult. Scler. Relat. Disord. 2021, 49, 102752. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Herrmann, K.; Adlung, A.; Paschke, N.; Hausner, L.; Frölich, L.; Schad, L.; Groden, C.; Kerl, H.U. Evaluation of Sodium (23Na) MR-imaging as a Biomarker and Predictor for Neurodegenerative Changes in Patients With Alzheimer’s Disease. In Vivo 2021, 35, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Madelin, G.; Regatte, R.R. Biomedical Applications of Sodium MRI In Vivo. J. Magn. Reson. Imaging 2013, 38, 511–529. [Google Scholar] [CrossRef] [PubMed]
- Zaric, O.; Juras, V.; Szomolanyi, P.; Schreiner, M.; Raudner, M.; Giraudo, C.; Trattnig, S. Frontiers of Sodium MRI Revisited: From Cartilage to Brain Imaging. J. Magn. Reson. Imaging 2021, 54, 58–75. [Google Scholar] [CrossRef]
- Madelin, G.; Kline, R.; Walvick, R.; Regatte, R.R. A method for estimating intracellular sodium concentration and extracellular volume fraction in brain in vivo using sodium magnetic resonance imaging. Sci. Rep. 2014, 4, 4763. [Google Scholar] [CrossRef] [PubMed]
- Fleysher, L.; Oesingmann, N.; Brown, R.; Sodickson, D.K.; Wiggins, G.C.; Inglese, M. Non–invasive Quantification of Intracellular Sodium in Human Brain using Ultra–High–Field MRI. NMR Biomed. 2013, 26, 9–19. [Google Scholar] [CrossRef]
- Ianniello, C.; Moy, L.; Fogarty, J.; Schnabel, F.; Adams, S.; Axelrod, D.; Axel, L.; Brown, R.; Madelin, G. Multinuclear MRI to disentangle intracellular sodium concentration and extracellular volume fraction in breast cancer. Sci. Rep. 2021, 11, 5156. [Google Scholar] [CrossRef]
- Bottomley, P.A. Sodium MRI in human heart: A review. NMR Biomed. 2016, 29, 187–196. [Google Scholar] [CrossRef]
- Wiggins, G.C.; Brown, R.; Lakshmanan, K. High-performance radiofrequency coils for 23Na MRI: Brain and musculoskeletal applications. NMR Biomed. 2016, 29, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Jin, J. Electromagnetic Analysis and Design in Magnetic Resonance Imaging; Routledge & CRC Press: Abingdon, UK, 1999. [Google Scholar]
- Vaughan, J.T.; Griffiths, J.R. RF Coils for MRI; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar]
- Roemer, P.B.; Edelstein, W.A.; Hayes, C.E.; Souza, S.P.; Mueller, O.M. The NMR phased array. Magn. Reson. Med. 1990, 16, 192–225. [Google Scholar] [CrossRef] [PubMed]
- Gruber, B.; Froeling, M.; Leiner, T.; Klomp, D.W.J. RF coils: A practical guide for nonphysicist. J. Magn. Reson. Imaging 2018, 48, 590–604. [Google Scholar] [CrossRef]
- Chen, C.N.; Hoult, D.I. Biomedical Magnetic Resonance Technology; Adam Hilger: Bristol, UK, 1989. [Google Scholar]
- Giovannetti, G.; Viti, V.; Positano, V.; Santarelli, M.F.; Landini, L.; Benassi, A. Magnetostatic simulation for accurate design of low field MRI phased-array coils. Concepts Magn. Reson. Part. B Magn. Reson. Eng. 2007, 31, 140–146. [Google Scholar] [CrossRef]
- Giovannetti, G.; Hartwig, V.; Positano, V.; Vanello, N. Radiofrequency coils for magnetic resonance applications: Theory, design, and evaluation. Crit. Rev. Biomed. Eng. 2014, 42, 109–135. [Google Scholar] [CrossRef]
- Anisimov, N.V.; Sadykhov, E.G.; Pavlova, O.S.; Fomina, D.V.; Tarasova, A.A.; Pirogov, Y.A. Whole Body Sodium MRI at 0.5 Tesla Using Surface Coil and Long Echo Time Sequence. Appl. Magn. Reson. 2019, 50, 1149–1161. [Google Scholar] [CrossRef]
- Giovannetti, G.; Pingitore, A.; Positano, V.; De Marchi, D.; Valvano, G.; Gibiino, F.; Aquaro, G.D.; Lombardi, M.; Landini, L.; Santarelli, M.F. Improving sodium Magnetic Resonance in humans by design of a dedicated 23Na surface coil. Measurement 2014, 50, 285–292. [Google Scholar] [CrossRef]
- Maril, N.; Rosen, Y.; Reynolds, G.H.; Ivanishev, A.; Ngo, L.; Lenkinski, R.E. Sodium MRI of the Human Kidney at 3 Tesla. Magn. Reson. Med. 2006, 56, 1229–1234. [Google Scholar] [CrossRef]
- Maggiorelli, F.; Buonincontri, G.; Retico, A.; Kaggie, J.D.; Graves, M.J.; Biagi, L.; Tiberi, G.; Tosetti, M. Sodium imaging of the human knee cartilage with Magnetic Resonance at Ultra High Field: Development of a double frequency (1H/23Na) RF coil. In Proceedings of the International Applied Computational Electromagnetics Society Symposium Italy (ACES) 2017, Firenze, Italy, 26–30 March 2017; pp. 1–2. [Google Scholar]
- Wetterling, F.; Corteville, D.M.; Kalayciyan, R.; Rennings, A.; Konstandin, S.; Nagel, A.M.; Stark, H.; Schad, L.R. Whole body sodium MRI at 3T using an asymmetric birdcage resonator and short echo time sequence: First images of a male volunteer. Phys. Med. Biol. 2012, 57, 4555–4567. [Google Scholar] [CrossRef]
- Wetterling, F.; Rennings, A.; Kalayciyan, R.; Corteville, D.M.; Konstandin, S.; Schad, L.R. A dual resonator system for whole-body sodium-MRI at 3T. Proc. Intl. Soc. Mag. Reson. Med. 2011, 19, 471. [Google Scholar]
- Giovannetti, G.; Valvano, G.; Virgili, G.; Giannoni, M.; Flori, A.; Frijia, F.; De Marchi, D.; Hartwig, V.; Landini, L.; Aquaro, G.D.; et al. Design and simulation of a dual-tuned 1H/23Na birdcage coil for MRS studies in human calf. Appl. Magn. Reson. 2015, 46, 1221–1238. [Google Scholar] [CrossRef]
- Qian, Y.; Zhao, T.; Wiggins, G.C.; Wald, L.L.; Zheng, H.; Weimer, J.; Boada, F.E. Sodium Imaging of Human Brain at 7T with 15-Channel Array Coil. Magn. Reson. Med. 2012, 68, 1808–1814. [Google Scholar] [CrossRef]
- Robson, M.D.; Titus, L.; Neubauer, S. Cardiac sodium imaging with phased arrays at 3 Tesla using a 3D Ultra-short TE (UTE) approach. J. Cardiov. Magn. Reson. 2008, 10 (Suppl. 1), A109. [Google Scholar] [CrossRef]
- Brown, R.; Madelin, G.; Lattanzi, R.; Chang, G.; Regatte, R.R.; Sodickson, D.K.; Wiggins, G.C. Design of a Nested Eight-Channel Sodium and Four-Channel Proton Coil for 7T Knee Imaging. Magn. Reson. Med. 2013, 70, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Graessl, A.; Ruehl, A.; Waiczies, H.; Resetar, A.; Hoffmann, S.H.; Rieger, J.; Wetterling, F.; Winter, L.; Nagel, A.M.; Niendorf, T. Sodium MRI of the human heart at 7.0 T: Preliminary results. NMR Biomed. 2015, 28, 967–975. [Google Scholar] [CrossRef] [PubMed]
- James, J.R.; Lin, C.; Stark, H.; Dale, B.M.; Bansal, N. Optimization and Characterization of Sodium MRI Using 8-channel 23Na and 2-channel 1H RX/TX. In Proceedings of the 13th International Conference on Biomedical Engineering 2009 IFMBE Proceedings, Singapore, 3–6 December 2009; Lim, C.T., Goh, J.C.H., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; Volume 23. [Google Scholar]
- James, J.R.; Panda, A.; Lin, C.; Dydak, U.; Dale, B.M.; Bansa, N. In vivo sodium MR imaging of the abdomen at 3T. Abdom. Imaging 2015, 40, 2272–2280. [Google Scholar] [CrossRef]
- Kim, J.-H.; Moon, C.M.; Park, B.-W.; Furlan, A.; Zhao, T.; Bae, K.T. Multichannel transceiver dual-tuned RF coil for proton/sodium MR imaging of knee cartilage at 3 T. Magn. Reson. Imaging 2012, 30, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Lakshmanan, K.; Brown, R.; Madelin, G.; Qian, Y.; Boada, F.; Wiggins, G.C. An eight-channel sodium/proton coil for brain MRI at 3T. NMR Biomed. 2018, 31, e3867. [Google Scholar] [CrossRef]
- Malzacher, M.; Chacon-Caldera, J.; Paschke, N.; Schad, L.R. Feasibility study of a double resonant 8-channel 1H/ 8-channel 23Na receive-only head coil at 3 Tesla. Magn. Reson. Imaging 2019, 59, 97–104. [Google Scholar] [CrossRef]
- Woessner, D.E. NMR relaxation of spin-3/2 nuclei: Effects of structure, order, and dynamics in aqueous heterogeneous systems. Conc. Magn. Reson. 2001, 13, 294–325. [Google Scholar] [CrossRef]
- Boada, F.E.; Gillen, J.S.; Shen, G.X.; Chang, S.Y.; Thulborn, K.R. Fast three dimensional sodium imaging. Magn. Reson. Med. 1997, 37, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, M.A.; King, K.F.; Zhou, X.J. Handbook of MRI Pulse Sequences; Elsevier: San Diego, CA, USA, 2004. [Google Scholar]
- Riemer, F.; Solanky, B.S.; Stehning, C.; Clemence, M.; Wheeler-Kingshott, C.A.; Golay, X. Sodium (23Na) ultra-short echo time imaging in the human brain using a 3D-Cones trajectory. Magn. Reson. Mat. Phys. Biol. Med. 2014, 27, 35–46. [Google Scholar] [CrossRef]
- Gai, N.D.; Rochitte, C.; Nacif, M.S.; Bluemke, D.A. Optimized three-dimensional sodium imaging of the human heart on a clinical 3T scanner. Magn. Reson. Med. 2015, 73, 623–632. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ouwerkerk, R.; Weiss, R.G.; Bottomley, P.A. Measuring human cardiac tissue sodium concentrations using surface coils, adiabatic excitation, and twisted projection imaging with minimal T2 losses. J. Magn. Reson. Imaging 2005, 21, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Lott, J.; Platt, T.; Niesporek, S.C.; Paech, D.; Behl, N.G.R.; Niendorf, T.; Bachert, P.; Ladd, M.E.; Nagel, A.M. Corrections of myocardial tissue sodium concentration measurements in human cardiac 23 Na MRI at 7 Tesla. Magn. Reson. Med. 2019, 82, 159–173. [Google Scholar] [CrossRef]
- Ragab, M.; Omer, O.A.; Abdel-Nasser, M. Compressive sensing MRI reconstruction using empirical wavelet transform and grey wolf optimizer. Neural Comput. Appl. 2020, 32, 2705–2724. [Google Scholar] [CrossRef]
- Berendsen, H.J.; Edzes, H.T. The observation and general interpretation of sodium magnetic resonance in biological material. Ann. N. Y. Acad. Sci. 1973, 204, 459–485. [Google Scholar] [CrossRef] [PubMed]
- Madelin, G.; Lee, J.-S.; Regatte, R.R.; Jerschow, A. Sodium MRI: Methods and applications. Prog. Nucl. Magn. Reson. Spectrosc. 2014, 79, 14–47. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, P.S. Nonexponential nuclear magnetic relaxation by quadrupole interactions. J. Chem. Phys. 1970, 53, 985–987. [Google Scholar] [CrossRef]
- Blunck, Y.; Josan, S.; Taqdees, S.W.; Moffat, B.A.; Ordidge, R.J.; Cleary, J.O.; Johnston, L.A. 3D-multi-echo radial imaging of 23Na (3D-MERINA) for time-efficient multi-parameter tissue compartment mapping. Magn. Reson. Med. 2018, 79, 1950–1961. [Google Scholar] [CrossRef]
- Riemer, F.; Solanky, B.; Clemence, M.; Wheeler-Kingshott, C.A.; Golay, X. Bi-exponential 23Na T2* components analysis in the human brain. In Proceedings of the 20th Annual Meeting ISMRM, Melbourne, Australia, 5–11 May 2012; Volume 31, p. e3899. [Google Scholar]
- Syeda, W.; Blunck, Y.; Kolbe, S.; Cleary, J.O.; Johnston, L.A. A continuum of components: Flexible fast fraction mapping in sodium MRI. Magn. Reson. Med. 2019, 81, 3854–3864. [Google Scholar] [CrossRef] [PubMed]
- Thulborn, K.R.; Gindin, T.S.; Davis, D.; Erb, P. Comprehensive MRI protocol for stroke management: Tissue sodium concentration as a measure of tissue viability in a non-human primate model and clinical studies. Radiology 1999, 139, 26–34. [Google Scholar]
- Constantinides, C.D.; Gillen, J.S.; Boada, F.E.; Pomper, M.G.; Bottomley, P.A. Human skeletal muscle: Sodium MR imaging and quantification potential applications in exercise and disease. Radiology 2000, 216, 559–568. [Google Scholar] [CrossRef]
- Bansal, N.; Szczepaniak, L.; Ternullo, D.; Fleckenstein, J.L.; Malloy, C.R. Effect of exercise on 23Na MRI and relaxation characteristics of the human calf muscle. J. Magn. Reson. Imaging 2000, 11, 532–538. [Google Scholar] [CrossRef]
- Hillenbrand, H.B.; Becker, L.C.; Kharrazian, R.; Hu, K.; Rochitte, C.E.; Kim, R.J.; Chen, E.L.; Ertl, G.; Hruban, R.H.; Lima, J.A.C. 23Na MRI combined with contrast-enhanced 1H MRI provides in vivo characterization of infarct healing. Magn. Reson. Med. 2005, 53, 843–850. [Google Scholar] [CrossRef]
- Horn, M.; Weidensteiner, C.; Scheffer, H.; Meininger, M.; de Groot, M.; Remkes, H.; Dienesch, C.; Przyklenk, K.; von Kienlin, M.; Neubauer, S. Detection of myocardial viability based on measurement of sodium content: A Na-23-NMR study. Magn. Reson. Med. 2001, 45, 756–764. [Google Scholar] [CrossRef]
- Thulborn, K.R.; Lu, A.; Atkinson, I.C.; Damen, F.; Villano, J.L. Quantitative sodium MR imaging and sodium bioscales for the management of brain tumors. Neuroimaging Clin. N. Am. 2009, 19, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Ouwerkerk, R.; Bottomley, P.A.; Solaiyappan, M.; Spooner, A.E.; Tomaselli, G.F.; Wu, K.C.; Weiss, R.G. Tissue sodium concentration in myocardial infarction in humans: A quantitative 23Na MR imaging study. Radiology 2008, 248, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Kopp, C.; Linz, P.; Wachsmuth, L.; Dahlmann, A.; Horbach, T.; Schöfl, C.; Renz, W.; Santoro, D.; Niendorf, T.; Müller, D.N.; et al. 23Na magnetic resonance imaging of tissue sodium. Hypertension 2012, 59, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Kopp, C.; Linz, P.; Dahlmann, A.; Hammon, M.; Jantsch, J.; Muller, D.N.; Schmieder, R.E.; Cavallaro, A.; Eckardt, K.; Uder, M.; et al. 23Na magnetic resonance imaging determined tissue sodium in healthy subjects and hyoertensive patients. Hypertension 2013, 61, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Christa, M.; Weng, A.M.; Geier, B.; Wörmann, C.; Scheffler, A.; Lehmann, L.; Oberberger, J.; Kraus, B.J.; Hahner, S.; Störk, S.; et al. Increased myocardial sodium signal intensity in Conn’s syndrome detected by 23Na magnetic resonance imaging. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 263–270. [Google Scholar] [CrossRef]
- Kannenkeril, D.; Karg, M.V.; Bosch, A.; Ott, C.; Linz, P.; Nagel, A.M.; Uder, M.; Schmieder, R.E. Tissue sodium content is increased in type 2 diabetes. Diabetologia 2017, 60, 1–608. [Google Scholar]
- Karg, M.V.; Bosch, A.; Kannenkeril, D.; Striepe, K.; Ott, C.; Schneider, M.P.; Boemke-Zelch, F.; Linz, P.; Nagel, A.M.; Titze, J.; et al. SGLT-2 inhibition with dapagliflozin reduces tissue sodium content a randomised controlled trial. Cardiovasc. Diabetol. 2018, 17, 5. [Google Scholar] [CrossRef]
- Dyke, J.P.; Meyring-Wösten, A.; Zhao, Y.; Linz, P.; Thijssen, S.; Kotanko, P. Reliability and agreement of sodium (23Na) MRI in calf muscle and skin of healthy subjects from the US. Clin. Imaging 2018, 52, 100–105. [Google Scholar] [CrossRef]
- Sekino, S.Y.; Sekino, M.; Tatsuoka, H.; Ohsaki, H.; Ueno, S.; Abe, Y. Discriminative Detection of Extracellular and Intracellular Sodium in Nerve Fibers by Magnetic Resonance Spectroscopy. IEEE Trans. Magn. 2008, 44, 4500–4502. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giovannetti, G.; Flori, A.; Martini, N.; Francischello, R.; Aquaro, G.D.; Pingitore, A.; Frijia, F. Sodium Radiofrequency Coils for Magnetic Resonance: From Design to Applications. Electronics 2021, 10, 1788. https://doi.org/10.3390/electronics10151788
Giovannetti G, Flori A, Martini N, Francischello R, Aquaro GD, Pingitore A, Frijia F. Sodium Radiofrequency Coils for Magnetic Resonance: From Design to Applications. Electronics. 2021; 10(15):1788. https://doi.org/10.3390/electronics10151788
Chicago/Turabian StyleGiovannetti, Giulio, Alessandra Flori, Nicola Martini, Roberto Francischello, Giovanni Donato Aquaro, Alessandro Pingitore, and Francesca Frijia. 2021. "Sodium Radiofrequency Coils for Magnetic Resonance: From Design to Applications" Electronics 10, no. 15: 1788. https://doi.org/10.3390/electronics10151788
APA StyleGiovannetti, G., Flori, A., Martini, N., Francischello, R., Aquaro, G. D., Pingitore, A., & Frijia, F. (2021). Sodium Radiofrequency Coils for Magnetic Resonance: From Design to Applications. Electronics, 10(15), 1788. https://doi.org/10.3390/electronics10151788






