Surface Activity of Surfactant–Polyelectrolyte Mixtures through Nanoplasmonic Sensing Technology
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.2.1. Effect of the Cationic Polymer Fraction
2.2.2. Effect of the Electrolyte Fraction
2.3. Methods
2.3.1. Deposition
2.3.2. Wet Combing Force
3. Results and Discussion
3.1. Deposition
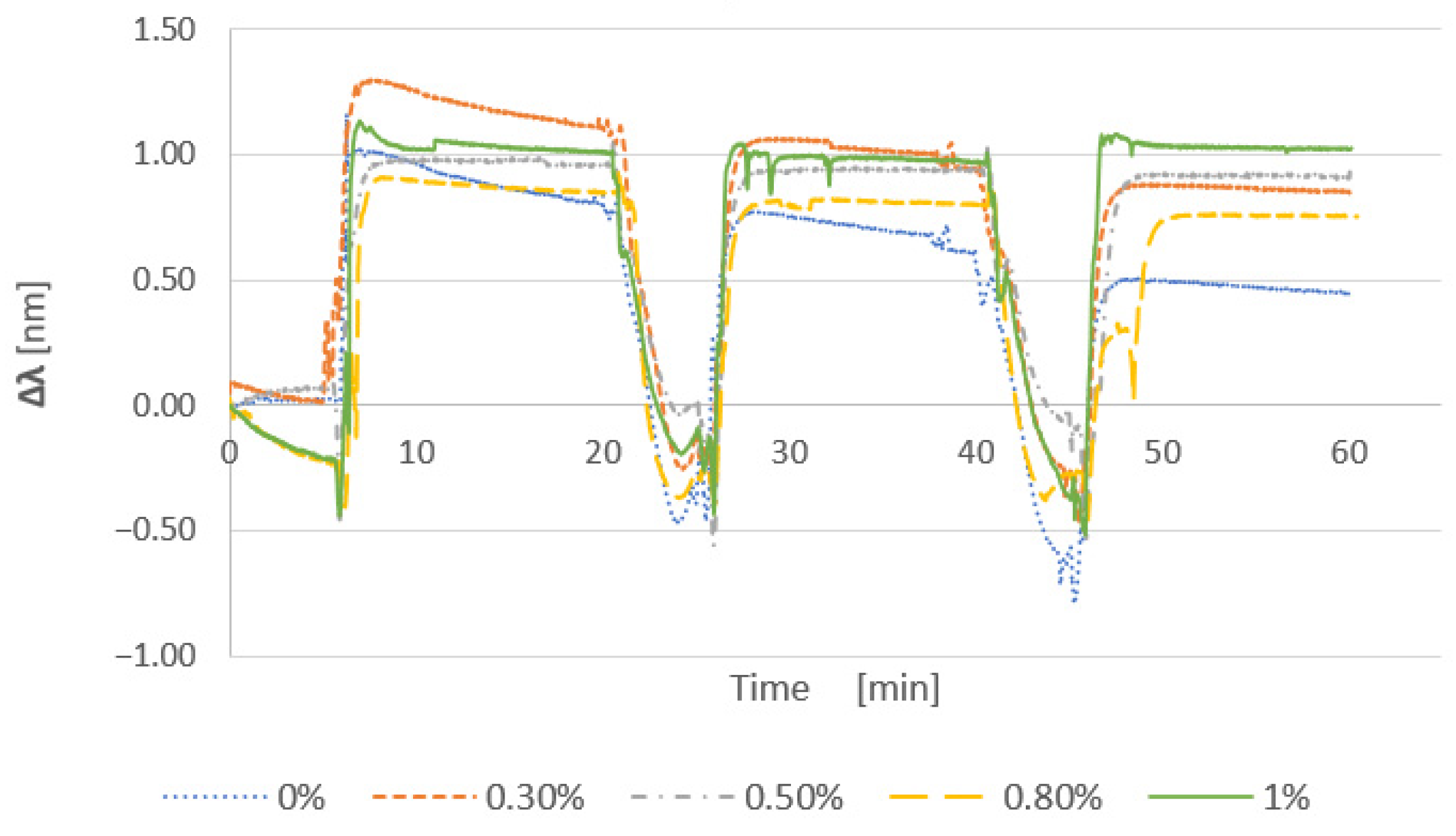
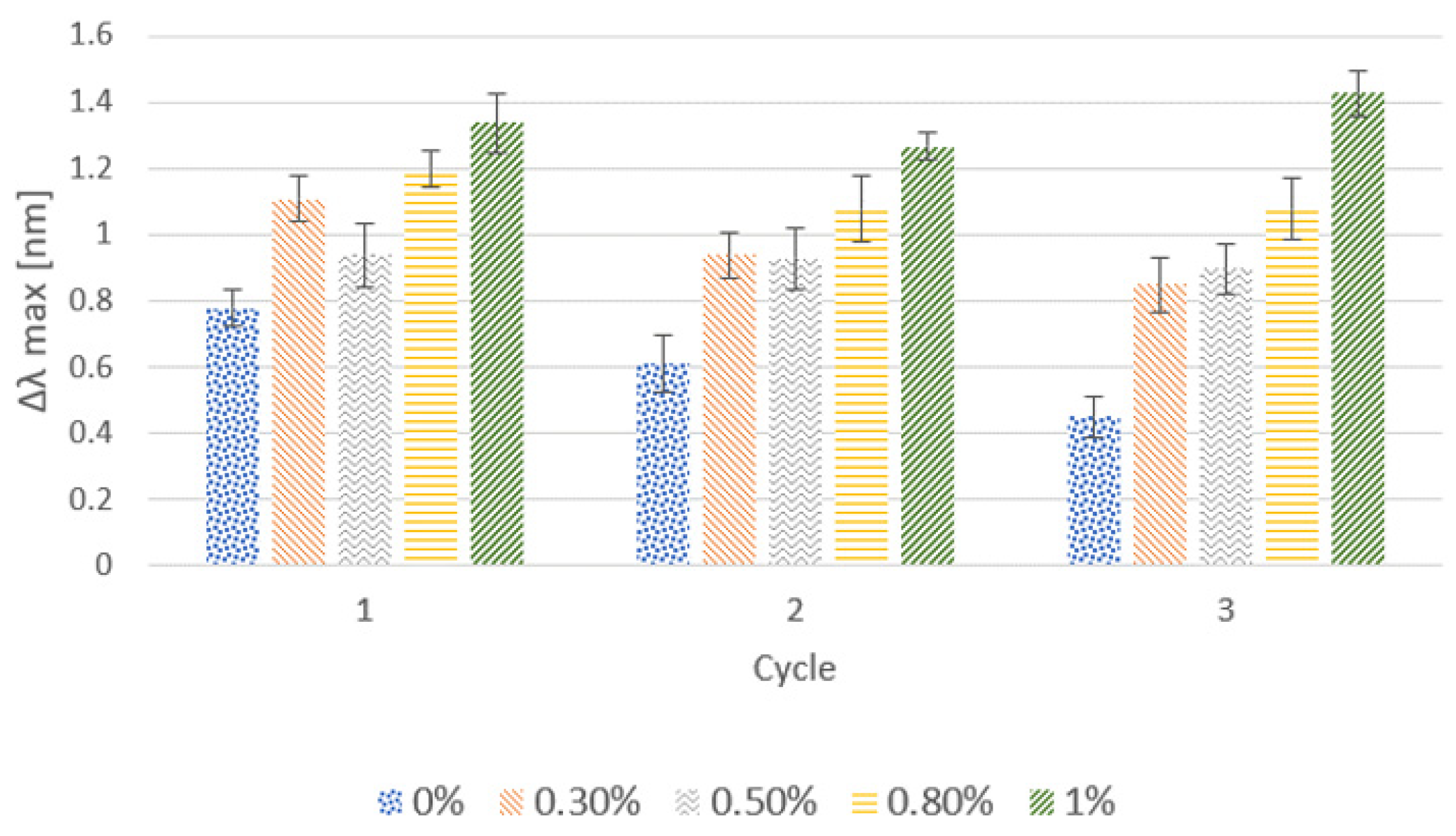
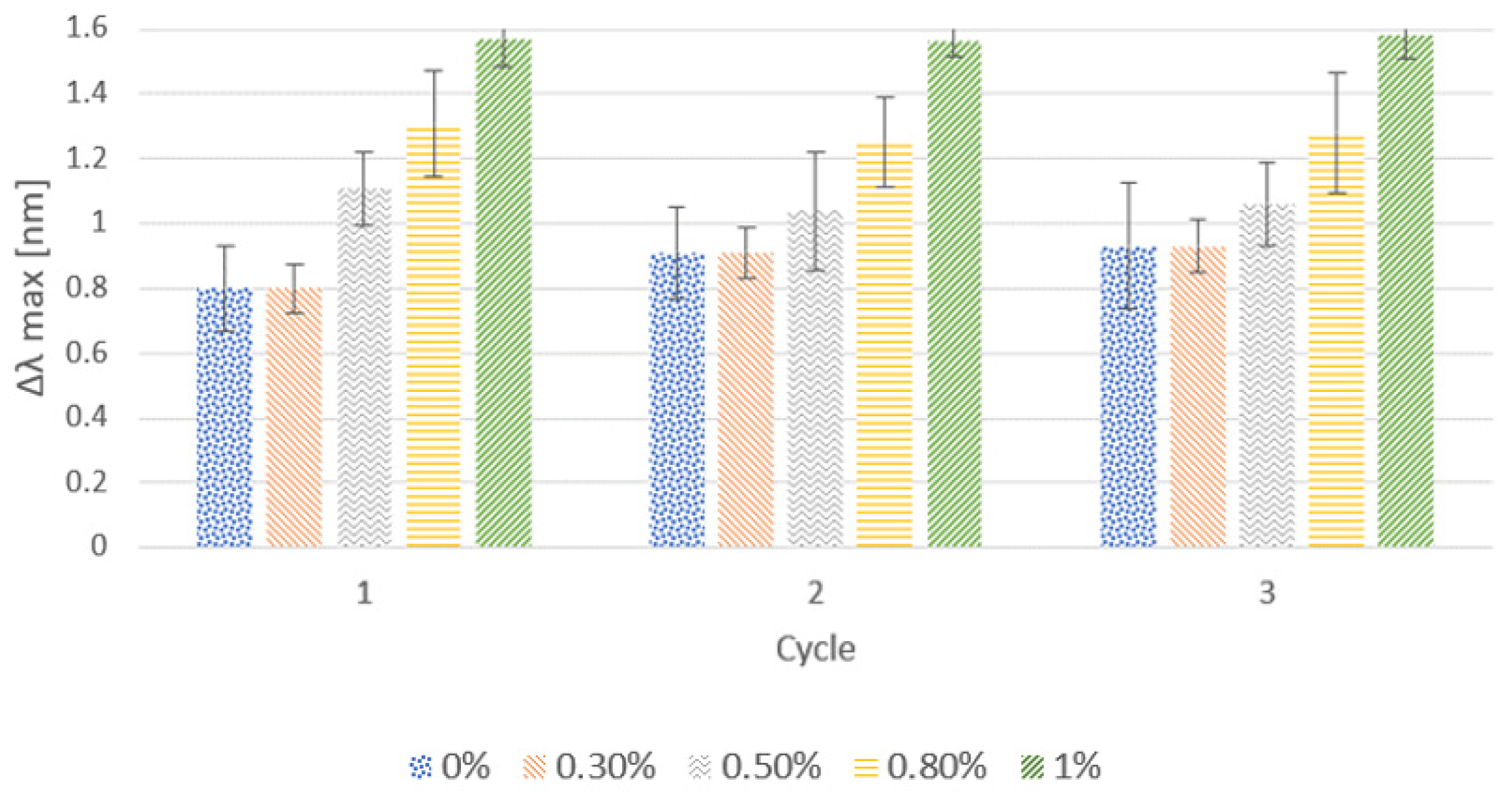
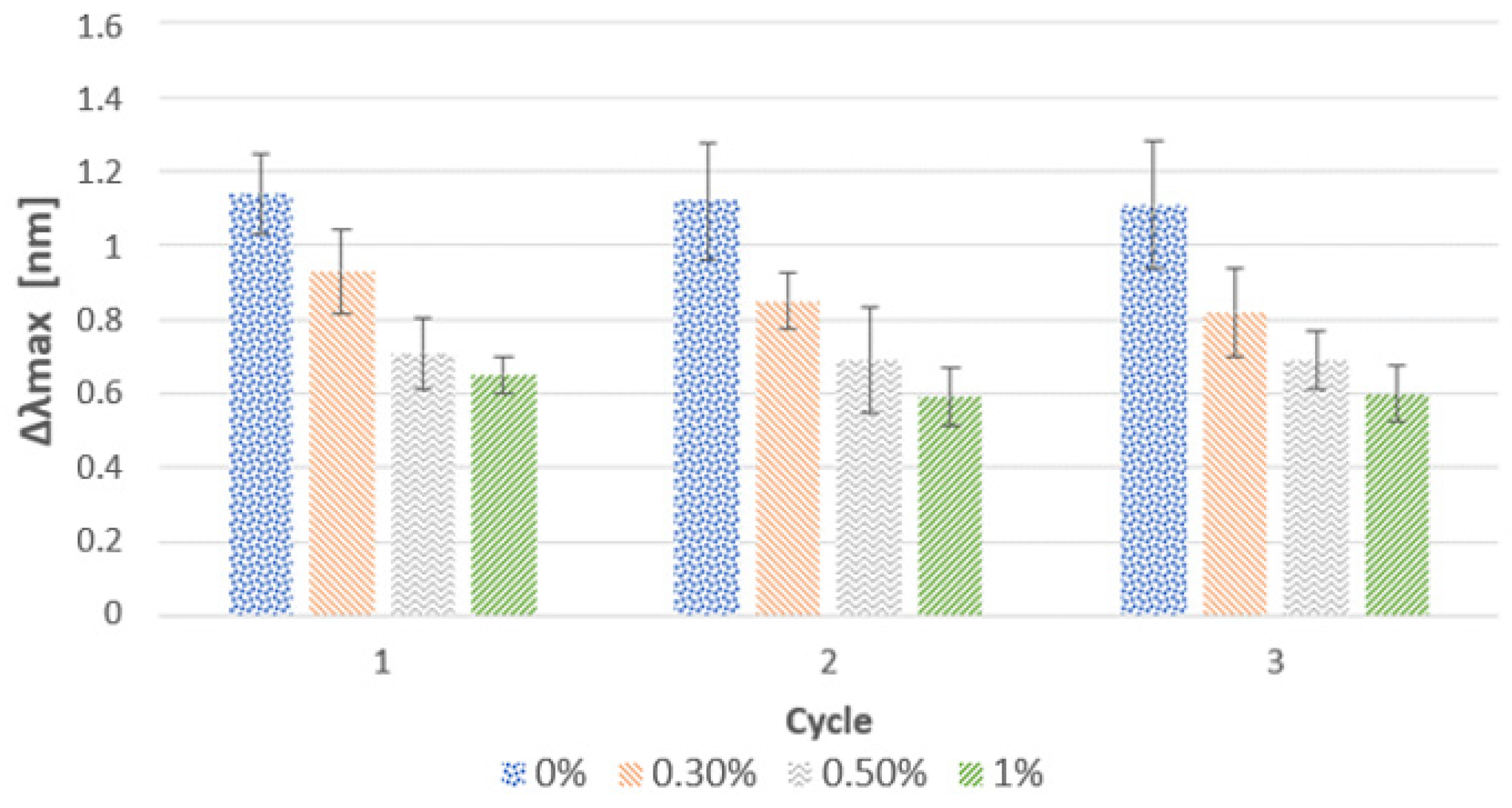
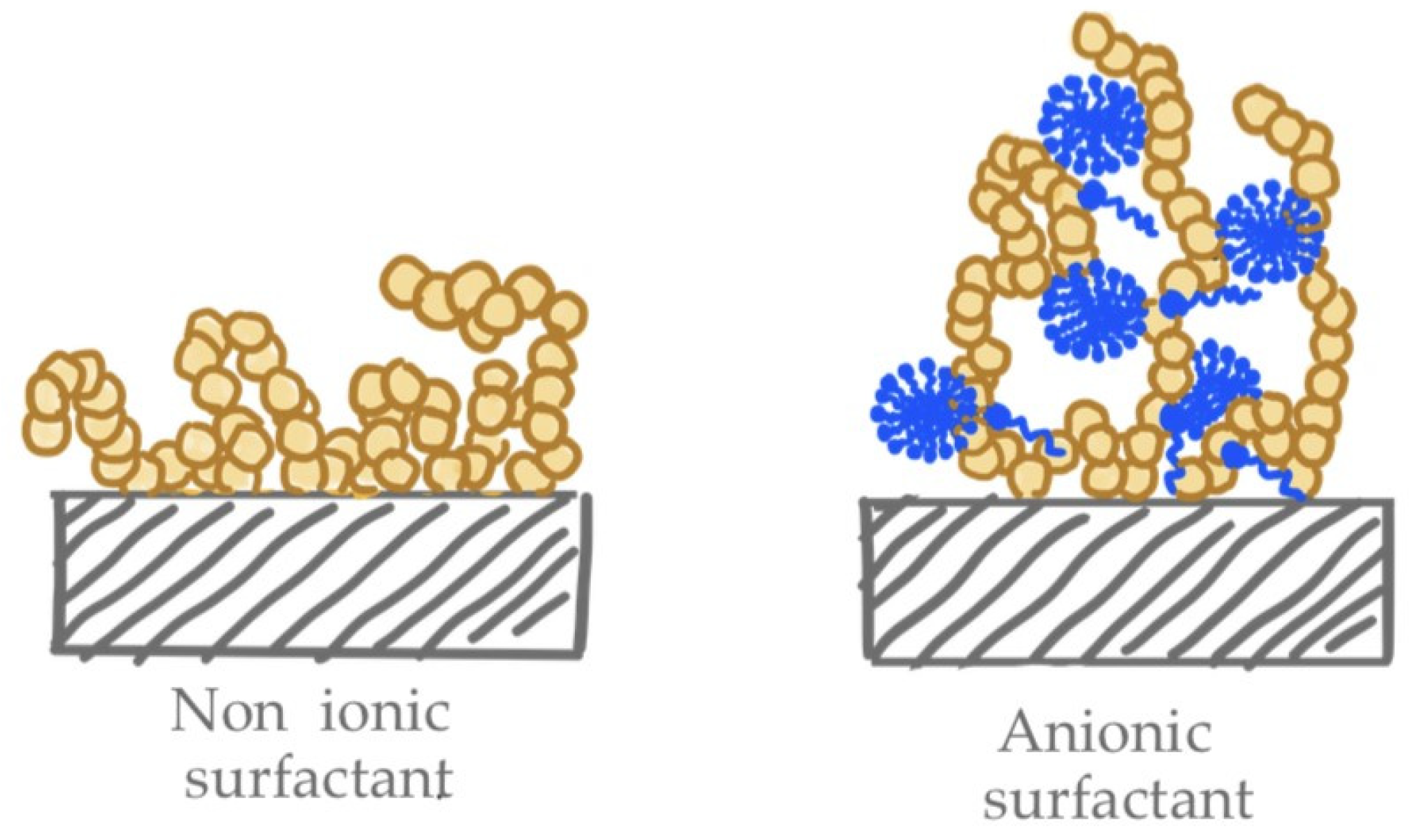
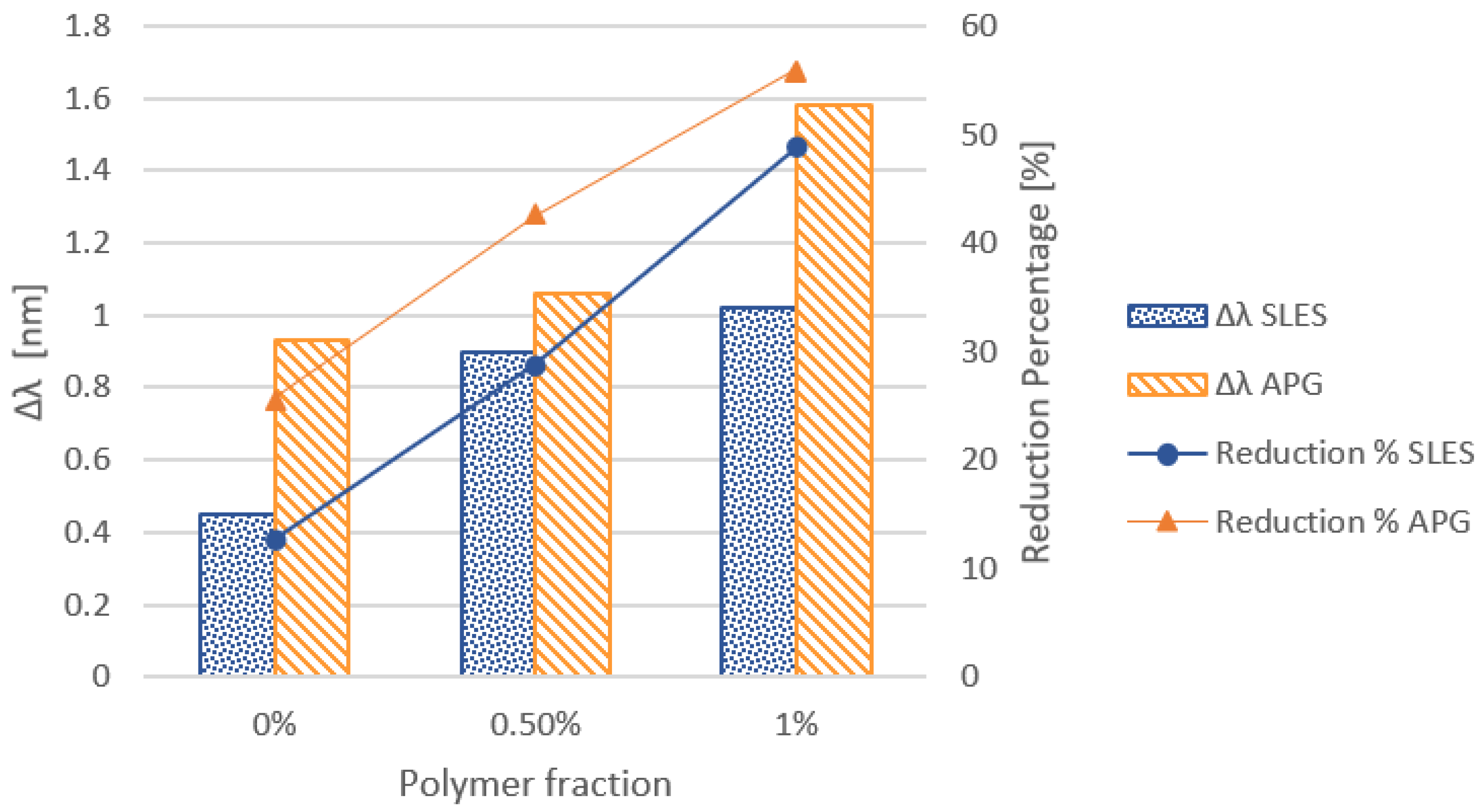
3.1.1. Effect of the Cationic Polymer
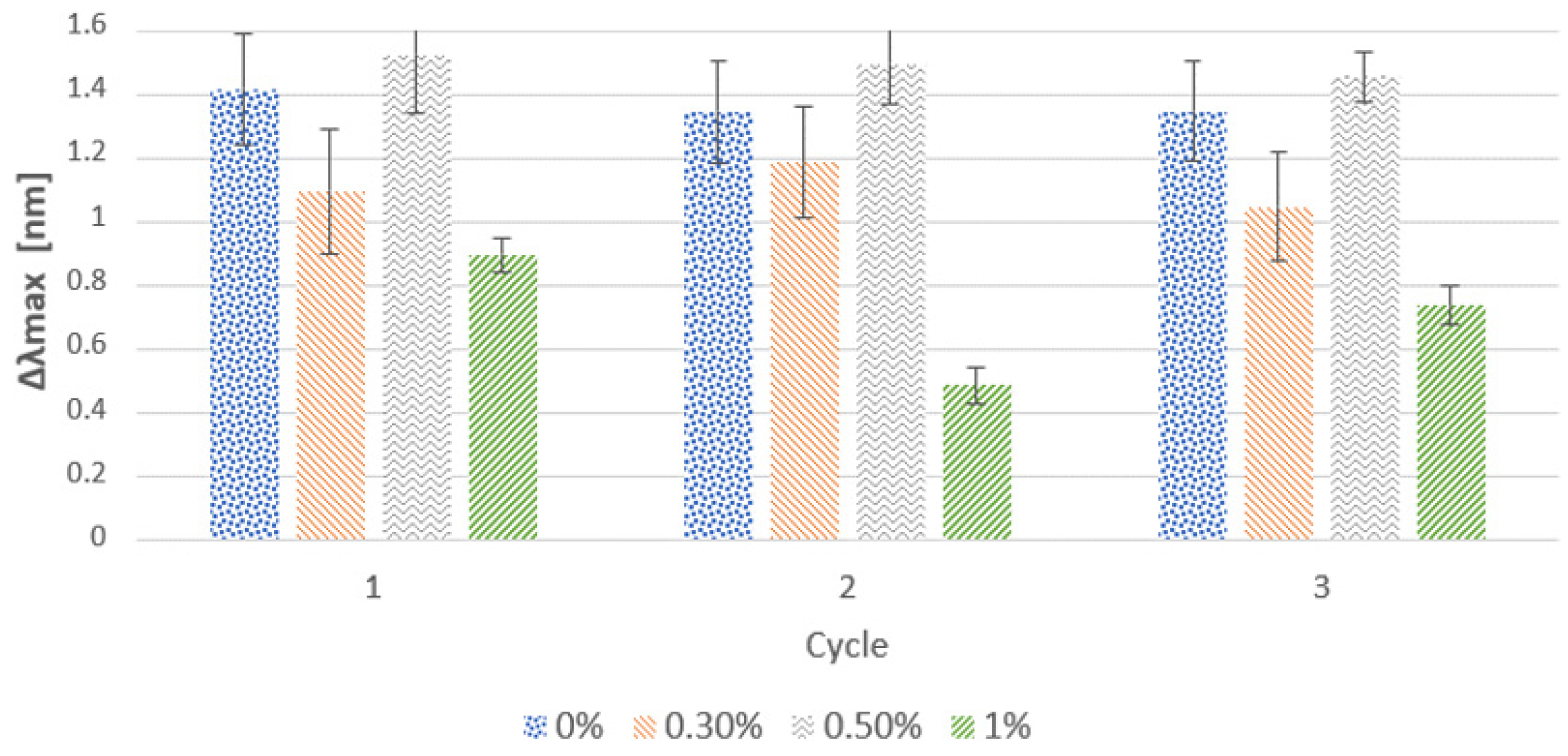
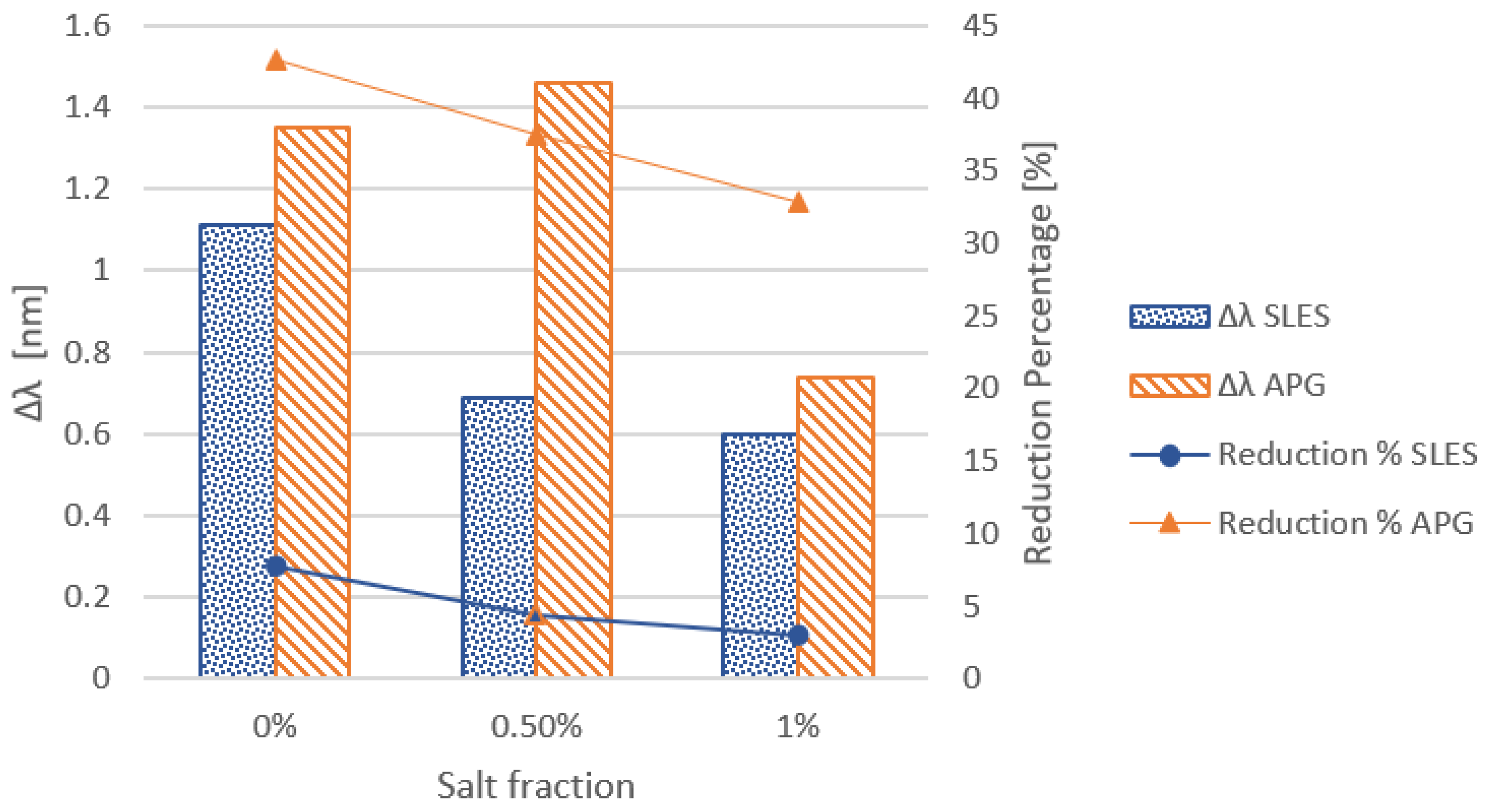
3.1.2. Effect of Salt Addition
3.2. Wet Combing Force
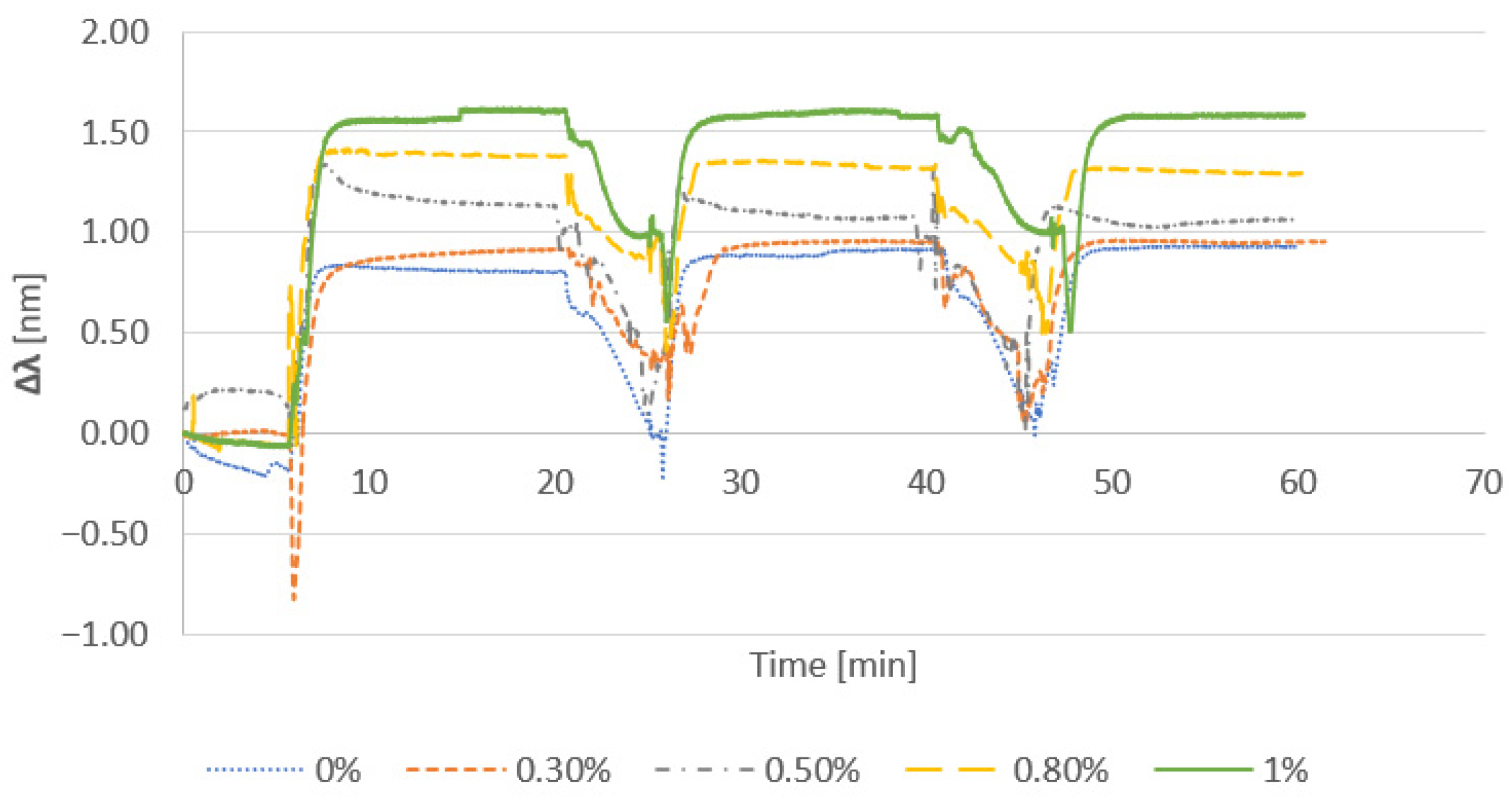
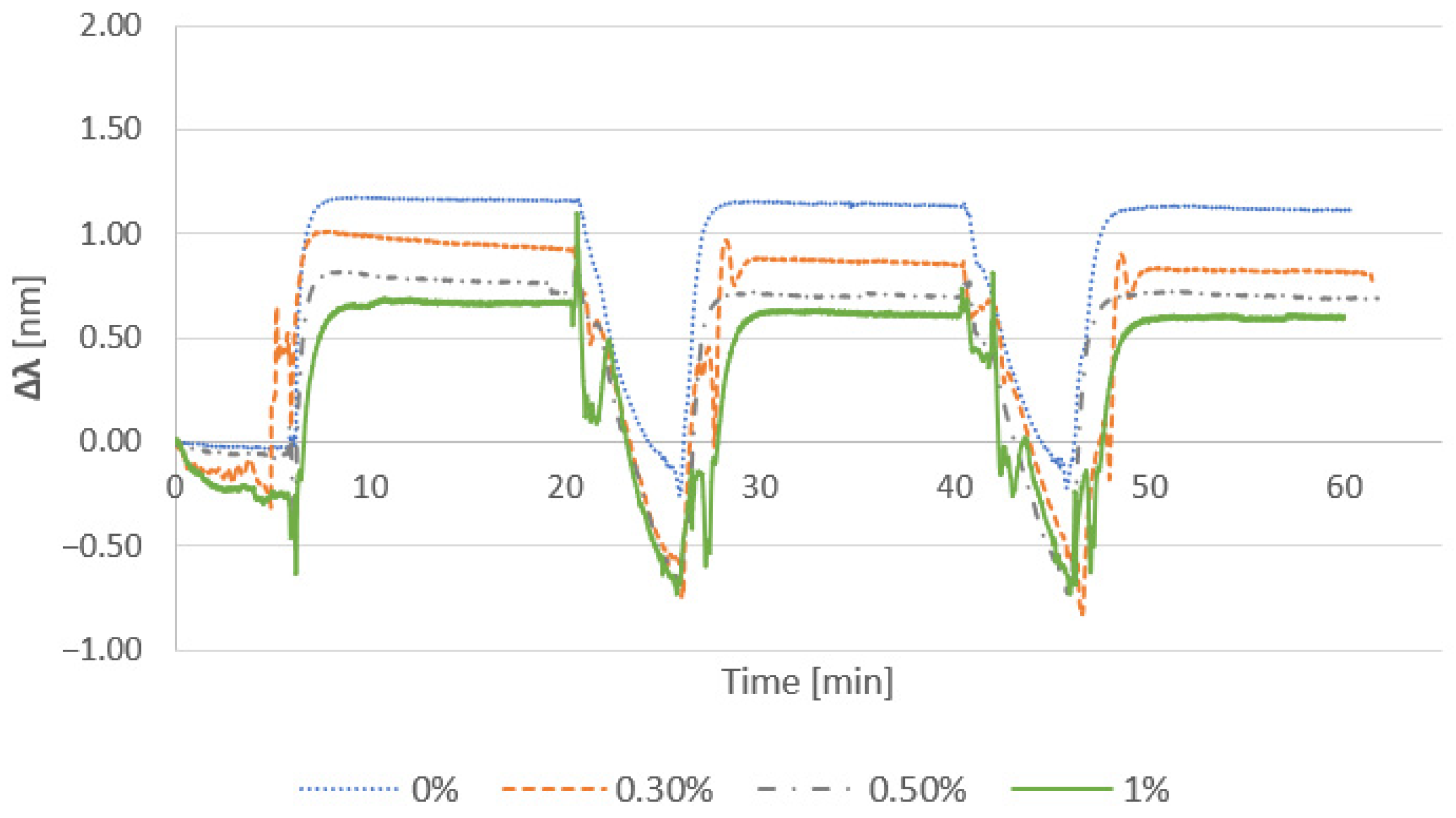
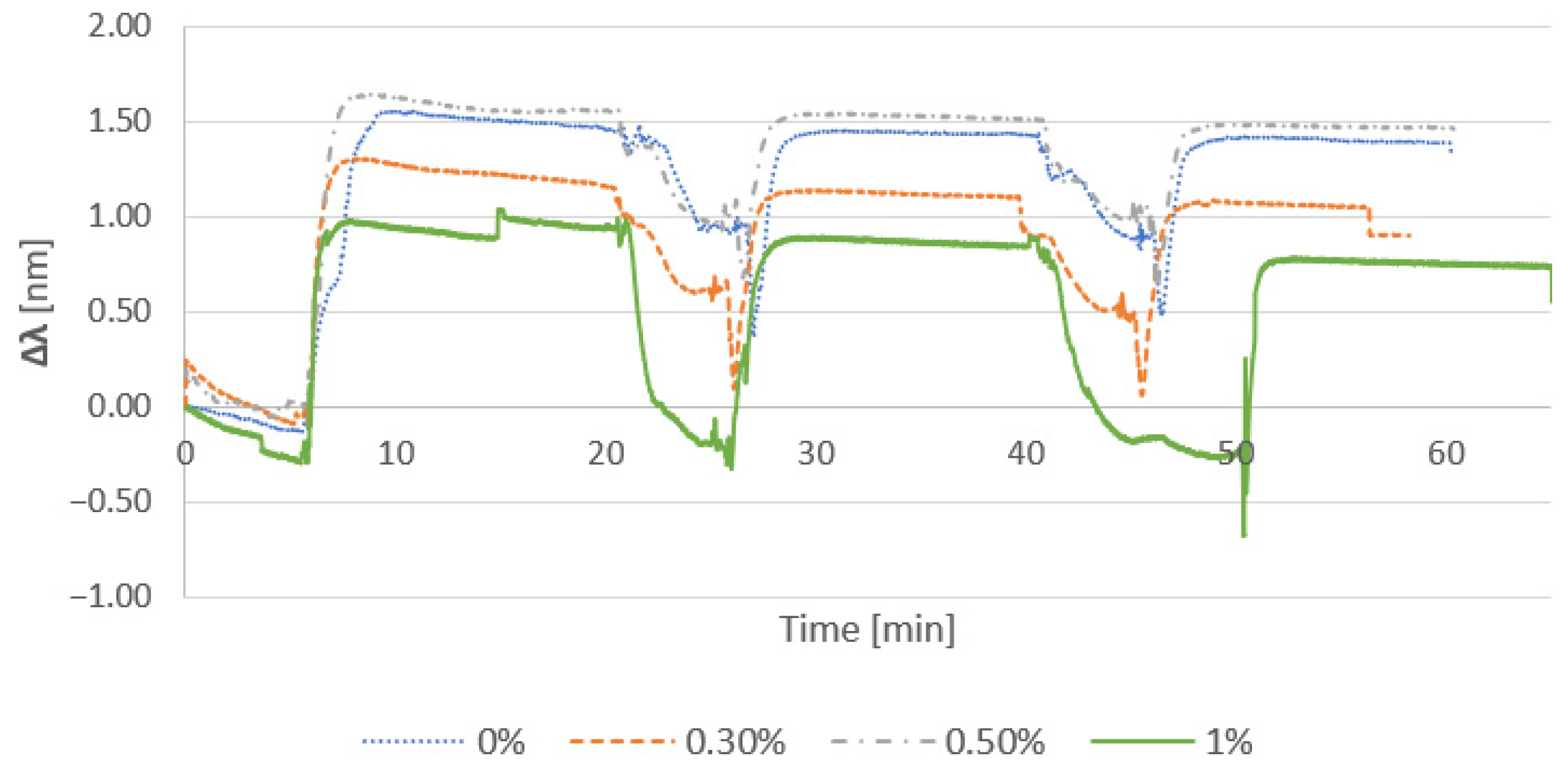
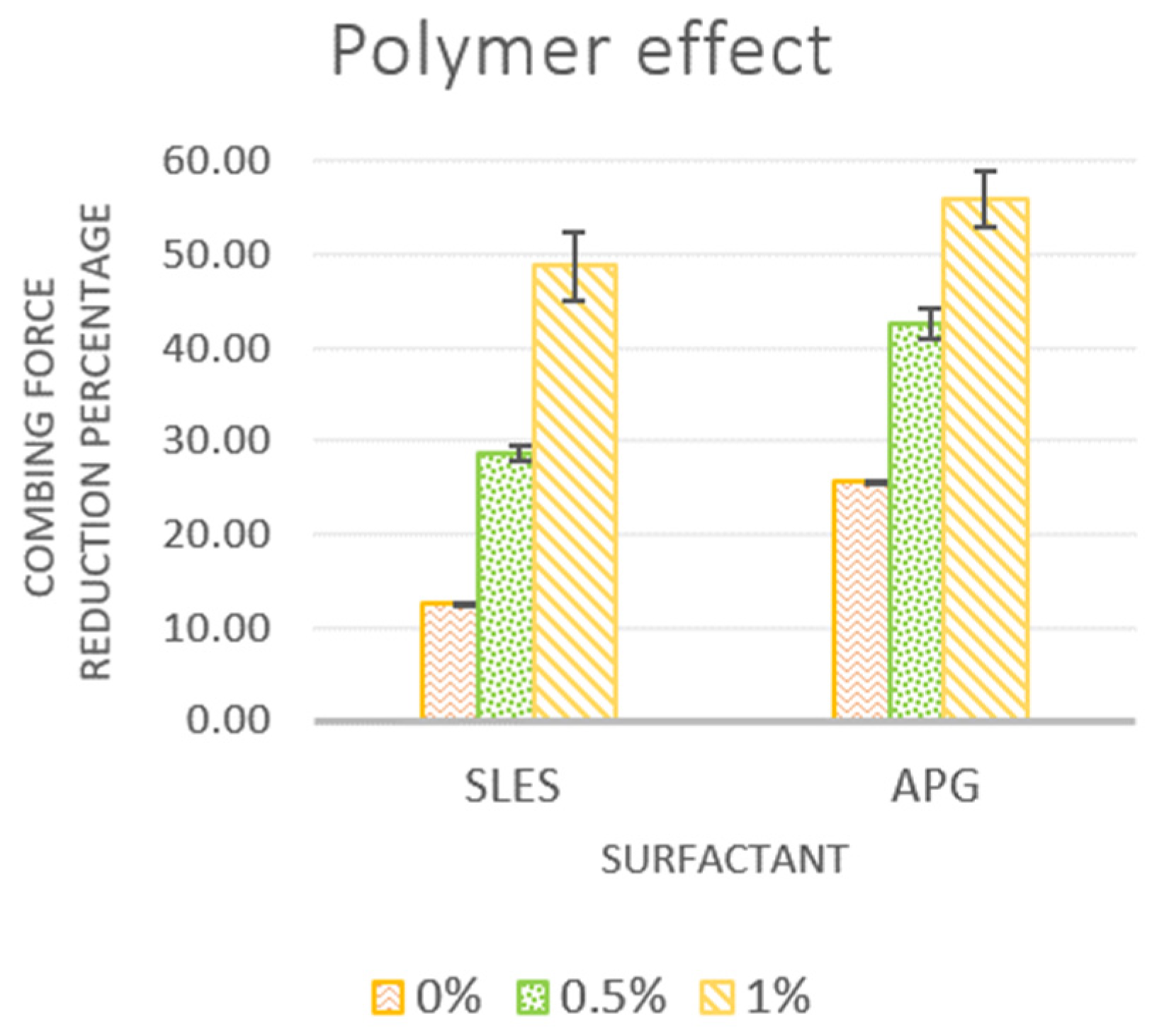
3.2.1. Effect of Cationic Polymer
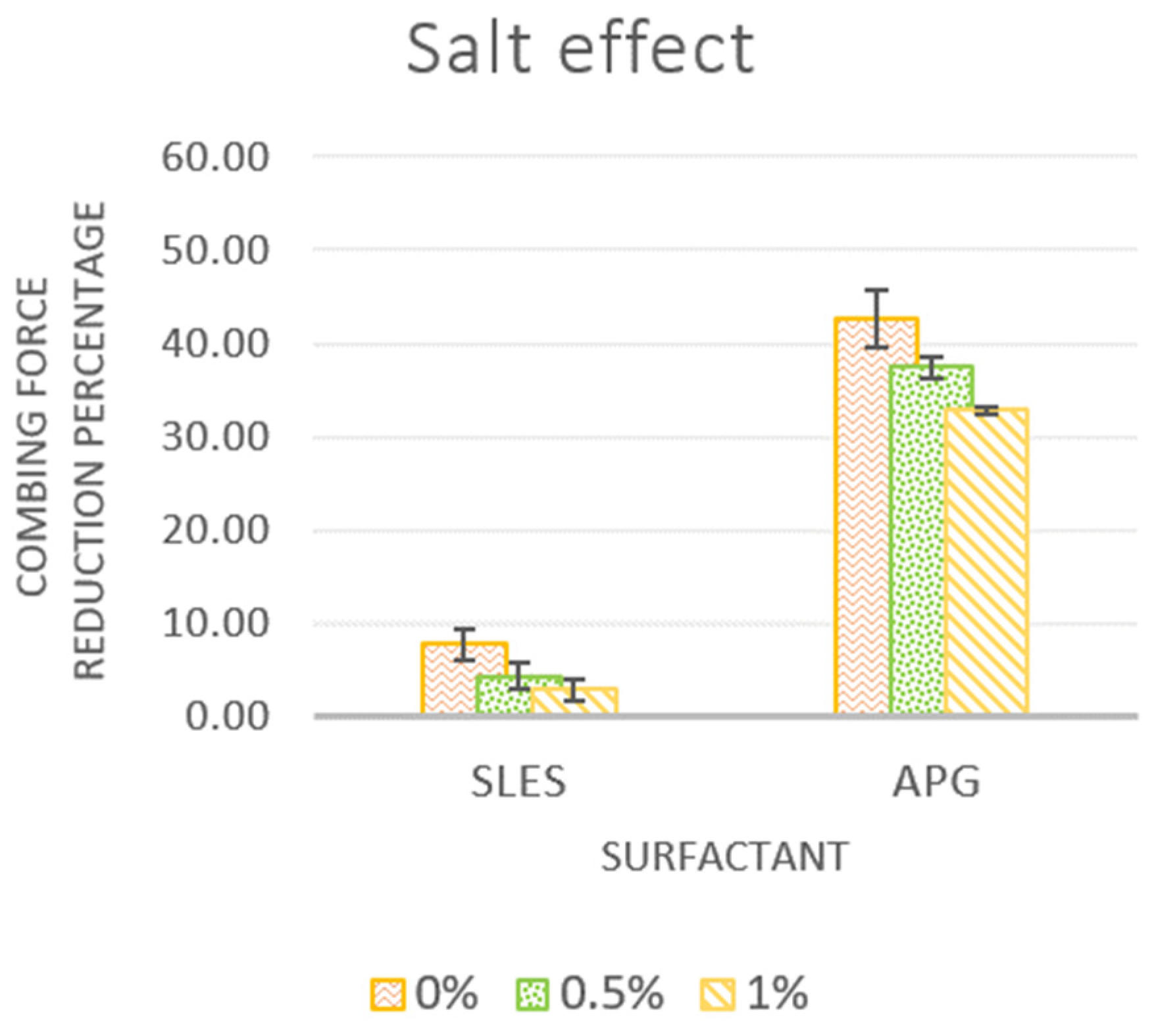
3.2.2. Effect of Salt Addition
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robbins, C.R. Chemical and Physical Behavior of Human Hair, 4th ed.; Springer: New York, NY, USA, 2013. [Google Scholar]
- Dawber, R. Hair: Its structure and response to cosmetic preparations. Clin. Dermatol. 1996, 14, 105–112. [Google Scholar] [CrossRef]
- Fernández-Peña, L.; Guzmán, E. Physicochemical Aspects of the Performance of Hair-Conditioning Formulations. Cosmetics 2020, 7, 26. [Google Scholar] [CrossRef]
- Dias, M.F.R.G. Hair Cosmetics: An Overview. Int. J. Trichology 2015, 7, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Draelos, Z.D. Hair Care. An Illustrated Dermatologic Handbook; Taylor & Francis: London, UK, 2004. [Google Scholar]
- Lee, W.; Idson, B. Update on hair conditioner ingredients. Cosmet. Toilet. 1983, 98, 42–46. [Google Scholar]
- O’Lenick, T. Anionic/cationic complexes in hair care. J. Cosmet. Sci. 2011, 62, 209–228. [Google Scholar] [PubMed]
- Harusawa, F.; Nakama, Y.; Muneo, T. Anionic-cationic ion-pairs: As conditioning agents in shampoos. Cosmet. Toilet. 1991, 106, 35–39. [Google Scholar]
- Hunting, A.L. Can there be cleaning and conditioning in the same product? Cosmet. Toilet. 1988, 103, 73–82. [Google Scholar]
- Andreas, P.; Peter, H.; Sabine, V.; Ralf, S.; Wolfgang, S. The interaction of cationic polymers with human hair. Macromol. Symp. 1997, 126, 241–252. [Google Scholar]
- Bain, C.D.; Claesson, P.M.; Langevin, D.; Meszaros, R.; Nylander, T.; Stubenrauch, C.; Titmuss, S.; Klitzin, R. Complexes of surfactants with oppositely charged polymers at surfaces and in bulk. Adv. Colloid Interface Sci. 2010, 155, 32–49. [Google Scholar] [CrossRef] [PubMed]
- Clauzel, M.; Johnson, E.S.; Nylander, T.; Panandiker, R.K.; Sivik, M.R.; Piculell, L. Surface Deposition and Phase Behavior of Oppositely Charged Polyion–Surfactant Ion Complexes. Delivery of Silicone Oil Emulsions to Hydrophobic and Hydrophilic Surfaces. ACS Appl. Mater. Interfaces 2011, 3, 2451–2462. [Google Scholar] [CrossRef]
- Somasundaran, P.; Chakraborty, S.; Qiang, Q.; Deo, P.; Wang, J.; Zhang, R.; Chakraborty, S. Surfactants, polymers and their nanoparticles for personal care applications. J. Cosmet. Sci. 2004, 55, 1–17. [Google Scholar]
- Carole, L.; Mullay, J.; Kyer, C.; McCalister, P.; Clifford, T. Use of statistical modeling to predict the effect silicone deposition, and conditioning sensory performance of cationic cassia polymers. J. Cosmet. Sci. 2011, 62, 161–177. [Google Scholar]
- Stanimirova, R.D.; Kralchevsky, P.A.; Danov, K.D.; Xu, H.; Ung, Y.W.; Petkov, J.T. Oil drop deposition on solid surfaces in mixed polymer-surfactant solutions in relation to hair- and skin-care applications. Colloids Surf. A Physicochem. Eng. Asp. 2019, 577, 53–61. [Google Scholar] [CrossRef]
- Hössel, P.; Dieing, R.; Nörenberg, R.; Pfau, A.; Sander, R. Conditioning polymers in today’s shampoo formulations—Efficacy, mechanism and test methods. Int. J. Cosmet. Sci. 2000, 22, 1–10. [Google Scholar] [CrossRef]
- Llamas, S.; Guzmán, E.; Ortega, F.; Bagdhalli, N.; Cazeneuve, C.; Rubio, R.; Luengo, G. Adsorption of polyelectrolytes and polyelectrolytes-surfactant mixtures at surfaces: A physico-chemical approach to a cosmetic challenge. Adv. Colloid Interface Sci. 2015, 222, 461–487. [Google Scholar] [CrossRef]
- Banerjee, S.; Cazeneuve, C.; Baghdadli, N.; Ringeissen, S.; Léonforte, F.; Leermakers, F.A.M.; Luengo, G.S. Modeling of Polyelectrolyte Adsorption from Micellar Solutions onto Biomimetic Substrates. J. Phys. Chem. 2017, 121, 8368–8651. [Google Scholar] [CrossRef]
- Guzmán, E.; Llamas, S.; Fernández-Peña, L.; Léonforte, F.; Baghdadli, N.; Cazeneuve, C.; Ortega, F.; Rubioa, R.G.; Luengo, G.S. Effect of a natural amphoteric surfactant in the bulk and adsorption behavior of polyelectrolyte-surfactant mixtures. Colloids Surf. A 2019, 585, 124178. [Google Scholar] [CrossRef]
- Llamas, S.; Guzmán, E.; Baghdadli, N.; Ortega, F.; Cazeneuve, C.; Rubio, R.; Luengo, G. Adsorption of poly(diallyldimethylammonium chloride)—Sodium methyl-cocoyl-taurate complexes onto solid surfaces. Colloids Surf. A Physicochem. Eng. Asp. 2016, 505, 150–157. [Google Scholar] [CrossRef]
- Svensson, A.V.; Huang, L.; Johnson, E.S.; Nylander, T.; Piculell, L. Surface Deposition and Phase Behavior of Oppositely Charged Polyion/Surfactant Ion Complexes. 1. Cationic Guar versus Cationic Hydroxyethylcellulose in Mixtures with Anionic Surfactants. Appl. Mater. Interfaces 2009, 1, 2431–2442. [Google Scholar] [CrossRef]
- Guzmán, E.; Fernández-Peña, L.; SLuengo, G.; Rubio, A.M.; Rey, A.; Léonforte, F. Self-Consistent Mean Field Calculations of Polyelectrolyte-Surfactant Mixtures in Solution and upon Adsorption onto Negatively Charged Surfaces. Polymers 2020, 3, 624. [Google Scholar] [CrossRef]
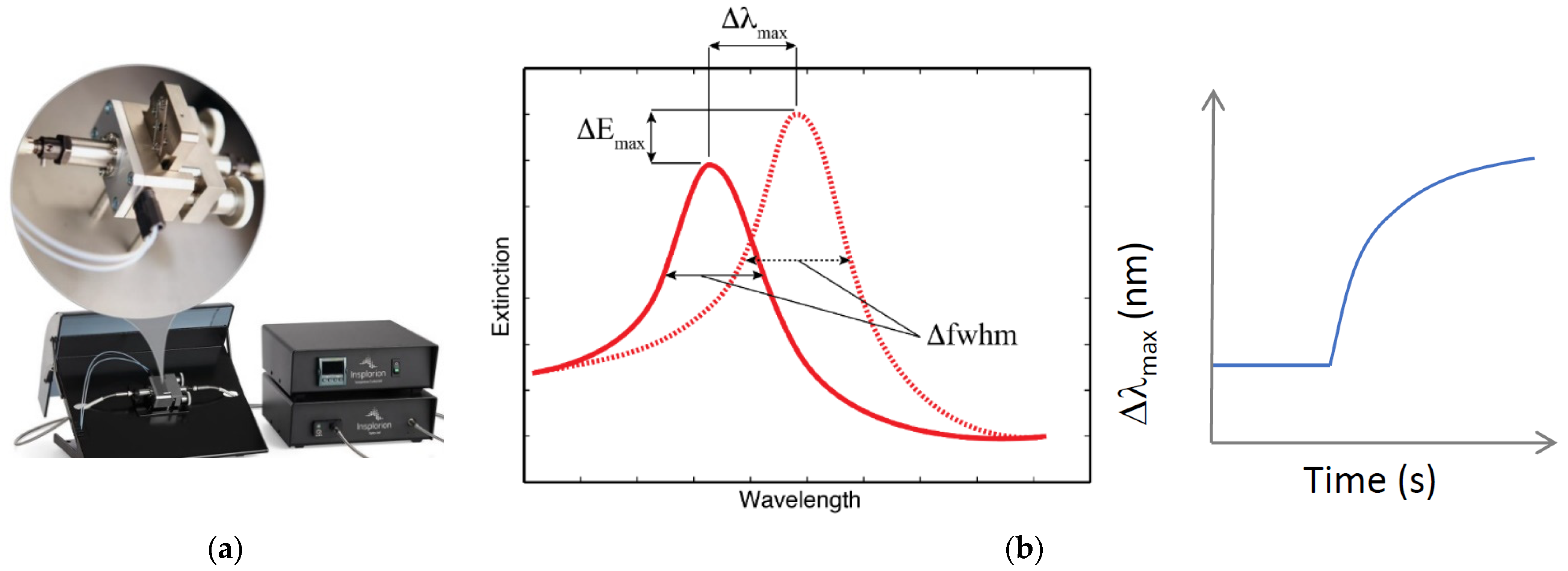
- Insplorium. NanoPlasmonic Sensing. s.f. [Online]. Available online: https://www.insplorion.com/app/uploads/NPS-Technology-1.pdf (accessed on 3 July 2022).
- Savic, S.; Lukic, M.; Jaksic, I.; Reichl, S.; Tamburic, S.; Müller-Goymann, C. An alkyl polyglucoside-mixed emulsifier as stabilizer of emulsion systems: The influence of colloidal structure on emulsions skin hydration potential. J. Colloid Interface Sci. 2011, 358, 182–191. [Google Scholar] [CrossRef]
- Cornwell, P.A. A review of shampoo surfactant technology: Consumer benefits, raw materials and recent developments. Int. J. Cosmet. Sci. 2017, 40, 16–30. [Google Scholar] [CrossRef]
- Ríos, F.; Fernández-Arteaga, A.; Lechuga, M.; Jurado, E.; Fernández-Serrano, M. Kinetic study of the anaerobic biodegradation of alkyl polyglucosides and the influence of their structural parameters. Environ. Sci. Pollut. Res. 2016, 23, 8286–8293. [Google Scholar] [CrossRef]
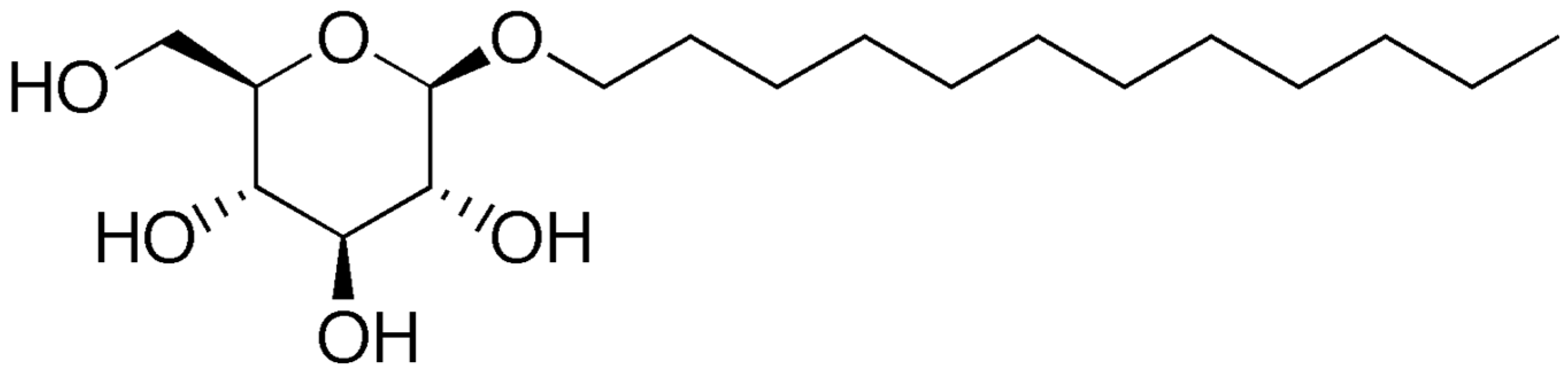
- Wikipedia. Available online: https://en.wikipedia.org/wiki/Lauryl_glucoside#/media/File:Lauryl_glucoside.png (accessed on 25 September 2022).
- Insplorium. Sensor technology for cleaner air, accelerated transition to fossil-free energy and advancing research in life science and clean tech. 2022; (Application notes obtained from Insplorion company). [Google Scholar]
- Insplorion. Quantitative interpretation of the NPS signal. 2017; (Application notes obtained from Insplorion company). [Google Scholar]
- McMullen, R.L.; Laura, D.; Zhang, G.; Kroon, B. Investigation of the interactions of cationic guar with human hair by electrokinetic analysis. Int. J. Cosmet. Sci. 2021, 43, 375–390. [Google Scholar] [CrossRef]
- Shokri, J.; Shamseddini Lori, M.; Monajjemzadeh, F. Examining polyquaternium polymers deposition on human excised hair fibers. J. Cosmet. Dermatol. 2017, 17, 1225–1232. [Google Scholar] [CrossRef]
- Luengo, G.S.; Guzman, E.; Fernández-Peña, L.; Leonforte, F.; Ortega, F.; Rubio, R.G. Interaction of polyelectrolytes and surfactants on hair surfaces. Desposits and their characterization. In Surface Science and Adhesion in Cosmetics; Wiley: Hoboken, NJ, USA, 2021. [Google Scholar]
- Sigal, G.B.; Mrksich, M.; Whitesides, G.M. Using Surface Plasmon Resonance Spectroscopy To Measure the Association of Detergents with Self-Assembled Monolayers of Hexadecanethiolate on Gold. Langmuir 1997, 13, 2749–2755. [Google Scholar] [CrossRef]
- Petkova, R.; Tcholakova, S.; Denkov, N.D. Foaming and Foam Stability for Mixed Polymer−Surfactant Solutions: Effects of Surfactant Type and Polymer Charge. Langmuir 2012, 28, 4996–5009. [Google Scholar] [CrossRef]
- Lochhead, R.Y.; Huisinga, L.R. A Brief Review of Polymer/Surfactant Interaction. Cosmet. Toilet. 2004, 119, 37–45. [Google Scholar]
- Faucher, J.A.; Goddard, E.D.; Hannan, R.B. Sorption and Desorption of a Cationic Polymer by Human Hair: Effects of salt solutions. Text. Res. J. 2015, 47, 616–620. [Google Scholar] [CrossRef]
- Goddard, E.D. Polymer-surfactant interaction. Part II: Polymer and surfactant of opposite charge. In Interactions of Surfactants with Polymers and Proteins; Taylor & Francis Group: Abingdon, UK, 1993. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perea Cubides, T.A.; Amin, S. Surface Activity of Surfactant–Polyelectrolyte Mixtures through Nanoplasmonic Sensing Technology. Cosmetics 2022, 9, 105. https://doi.org/10.3390/cosmetics9050105
Perea Cubides TA, Amin S. Surface Activity of Surfactant–Polyelectrolyte Mixtures through Nanoplasmonic Sensing Technology. Cosmetics. 2022; 9(5):105. https://doi.org/10.3390/cosmetics9050105
Chicago/Turabian StylePerea Cubides, Tatiana Andrea, and Samiul Amin. 2022. "Surface Activity of Surfactant–Polyelectrolyte Mixtures through Nanoplasmonic Sensing Technology" Cosmetics 9, no. 5: 105. https://doi.org/10.3390/cosmetics9050105
APA StylePerea Cubides, T. A., & Amin, S. (2022). Surface Activity of Surfactant–Polyelectrolyte Mixtures through Nanoplasmonic Sensing Technology. Cosmetics, 9(5), 105. https://doi.org/10.3390/cosmetics9050105






