Abstract
Fagraea berteroana is a tree used in traditional medicine in various islands of the South Pacific. Here, we studied its hair growth-inducing properties as suggested by one of its Marquesan ethno-uses in haircare. The ethyl acetate extract of the fruits of F. berteroana (FEAE) and four resulting fractions (FEAE-F0, FEAE-F1, FEAE-F2, and FEAE-F3) were tested on hair follicle dermal papilla cells to determine their cell proliferative activity. Furthermore, RT-qPCR analysis enabled gene modulation analysis, while immunostaining of the β-catenin protein was used to follow protein regulation. We found that the plant extracts induced a controlled, dose-dependent cell proliferation. FEAE-F0 simultaneously down-regulated Bone Morphogenetic Protein 2 (BMP2) mRNA expression and upregulated Cyclin-D1 (CCND1) gene expression, which suggests an involvement in the regulation of the Wnt and Transforming Growth Factor beta (TGFβ) pathways that control the hair cycle. FEAE-F0 exhibited a 1.34-fold increase of nuclear β-catenin protein. This is indicative of an active hair growth state. Thus, we conclude that FEAE-F0 could be an innovative candidate in hair care, which opens interesting leads to promote the Marquesan cosmetopoeia.
1. Introduction
Overall body care is an essential routine in Polynesian customs, as depicted by the confection and daily use of monoi and other natural preparations for embellishment [1,2,3]. Moreover, hair is an important physical feature in French Polynesia’s society; it is the second most cited application area of cosmetics after the skin, in the Marquesas Islands [3]. As such, the imagery of Polynesians with long, luxurious, and silky dark hair is widespread, even to this day (Figure 1, [4,5]).

Figure 1.
Left: Tahitian woman with hibiscus flower and waist-length hair. Credit: T. Theophilus, 2021. Center-left: White and yellow Fagraea berteroana flowers. Credit: J.-F. Butaud, 2005. Center-right: Unripe fruits of F. berteroana and white flower. Credit: K. Hughes, 2018. Right: Ripe F. berteroana fruit. Credit J.-F. Butaud, 2010.
An effort to study French Polynesia’s cosmetopoeia [6] led to discovering one plant with an atypical traditional application. This scarcely known ethnocosmetic use was recorded in the Marquesas Islands where, during the embalming process, the crushed fruits of Fagraea berteroana were rubbed on cadavers head to prevent hair from shedding [7].
Fagraea berteroana A.Gray ex Benth. (Gentianaceae), sometimes wrongly spelled Fagraea berteriana, is a tree of moderate height, naturally distributed throughout the South Pacific, except for Hawaii where it has been introduced. It is known under several names such as pualulu in Samoa, pua kenikeni in Hawaii, pua ‘enana or ka’upe in the Marquesas Islands [3], or simply pua in Tahiti [8]. It produces fragrant tubular white flowers that later turn yellow and seasonally yield 5 cm-long and ellipsoid, orange fruits [9] (Figure 1).
A study focused on the potential of the flowers in the fragrance industry revealed volatile compounds benzyl acetate, methyl benzoate, benzyl benzoate, myristic acid, benzyl salicylate, (E)-β-ocimene, methyl salicylate, acetoin, acetic acid, nerolidol, and heneicosane as main components of the essential oil and flower headspace [10]. The fragrant flowers are traditionally well known throughout Polynesia. In Hawaii, they are used to make ceremonial leis, while in French Polynesia they are macerated in coconut oil to obtain scented monoi [11,12]. Thus, further demonstrating the importance of F. berteroana in Polynesian cosmetopoeia.
Literature shows that little is known concerning the chemical composition of this species or its biological activities. Studies on other species of the genus Fagraea revealed diverse compound families such as alkaloids, secoiridoid glycosides, coumarins, flavanols, other phenols, and lignans in the stem bark, roots, and leaves [13,14,15,16,17,18,19,20,21]. The only chemistry-related study on the fruits is of F. fragrans Roxb. (now named Cyrtophyllum fragrans (Roxb.) DC.) and focused on the amidation of ursolic and oleanolic acids, two components naturally present in this plant part [22].
The peculiar ethnobotanical use specific to the Marquesan Islands and the lack of phytochemistry studies on this plant made it an interesting candidate for research into its hair growth activity through modulation of the hair cycle. The latter consists of three periodic phases, the anagen phase during which the hair elongates, the catagen phase when the epithelial cells of the hair undergo apoptosis, and telogen phase when the hair rests then sheds [23,24]. Several pathways have been reported to intervene during the hair cycle, namely Wnt/β-catenin, Sonic hedgehog (Shh), Transforming Growth Factor beta (TGFB), Phosphoinositide 3-kinase PI3K/AKT, Bone Morphogenetic Protein (BMP), and Fibroblast Growth Factor (FGF) [24,25,26,27,28]. Among these, Wnt, TGFB, and BMP are most discussed during the telogen-to-anagen and anagen-to-catagen transitions, for their role in signaling interactions between mesenchymal and epithelial cells [24]. Activation of the canonical Wnt pathway is essential for transitioning to and maintaining the anagen phase [26]. BMPs [29] and TGFβs [25] are paracrine effectors of the dermal papilla cells that target epithelial cells and promote the telogen and catagen phases, respectively.
In this study, we focused on Cyclin-D1 (CCND1) a downstream gene target of the Wnt pathway and its crucial regulatory protein, β-catenin [30]. We also assessed Bone Morphogenetic Protein 2 (BMP2) and Transforming growth factor beta 1 (TGFB1) levels.
We hypothesized that the extract and fractions would stimulate hair dermal papilla cell induction via regulation of the studied factors of the Wnt, TGFB, and BMP pathways. This would suppose a potential promotion of the anagen phase. To do so, cell proliferation activity of the ethyl acetate extract of the fruits of F. berteroana (FEAE) including its four resulting fractions FEAE-F0, FEAE-F1, FEAE-F2, FEAE-F3 of differing polarity obtained after open column fractioning was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay on hair follicle dermal papilla cells. RT-qPCR was performed to select the most active fraction according to its regulation of the three gene targets of the hair cycle. FEAE-F0 was thus selected and subjected to immunostaining to follow its regulation of β-catenin protein. Finally, UHPLC-ESI-MS/MS analysis of FEAE-F0 was performed to tentatively assess its chemical composition. These results are the first cellular assays focused on F. berteroana fractions and extracts.
2. Materials and Methods
2.1. Plant Material Collection, Extraction, and Fractionation
The fruits of F. berteroana A. Gray ex Benth. were collected in April 2018 in Tahiti (17°34.646′ S 149°36.363′ W), French Polynesia. The tree was identified by the botanist Jean-François Butaud. A voucher specimen (K HUGHES 4) was deposited at the herbarium of French Polynesia (PAP). The fruits were oven-dried at 40 °C for 2 days then ground to a 2 mm powder. The powder was macerated in ethyl acetate during 12 h, under agitation. The liquid ethyl acetate extract was evaporated until a viscous extract was obtained. The ethyl acetate F. berteroana extract (FEAE) was further fractioned (10.89 g) using open column chromatography with silica gel 60 Å. The solvents chosen were cyclohexane, ethyl acetate (EtOAc), and methanol in a gradually polar system. The elution started with two column volumes of cyclohexane/EtOAc (80/20) followed by one column volume of cyclohexane/EtOAc (60/40) which led to the first fraction FEAE-F0. The second fraction FEAE-F1 was obtained by recovering the 2nd volume of cyclohexane/EtOAc (60/40) and the first volume of cyclohexane/EtOAc (70/30). FEAE-F2 is composed of the 2nd volume of cyclohexane/EtOAc (70/30) and 100% EtOAc, and finally, FEAE-F3 is the fraction eluted with 100% methanol. All fractions were evaporated till dry. A fraction of the dried fractions was later dissolved in dimethylsufoxide (DMSO) to obtain a 1% solution for the cellular assays.
2.2. Cell Viability via the MTT Assay
Human hair follicle dermal papilla cells (HDPCs) were purchased from Promocell (Heidelberg, Germany) and grown in follicle dermal papilla cell growth medium (Promocell, Sickingenstr, Heidelberg, Germany) supplemented with 100 U·mL−1 penicillin and 100 μg·mL−1 streptomycin (Gibco). The cells were cultured in a humidified atmosphere at 37 °C and 5% CO2. The Cell Proliferation kit I MTT assay (Roche, Manheim, Germany) was performed to assess the influence of the extracts on the viability of HDPCs. Cells were seeded (104 and 8 × 103 for 24- and 48-h experiments, respectively) into 96-well plates. They were treated with 200 μL of increasing concentrations of the extracts, ranging from 0.1 μg·mL−1 to 50 μg·mL−1. The control group consisted of DMSO diluted in medium to concentrations ranging from 0.001% to 0.5%, similarly to those of the extracts. The plates were incubated at 37 °C and 5% CO2 for 24 h and 48 h independently. The supernatant was then discarded and 100 μL of 10% MTT in fresh medium was added to the wells and incubated for 4 h before adding 100 μL of solubilization solution. The plates were incubated overnight, and the absorbance was read on a Multiskan GO spectrophotometer (Thermoscientific, Inc., Vantaa, Finland) at 570 and 690 nm. All tests were done at least in triplicate. The absorbance of each extract concentration was normalized to its corresponding control. Statistical analyses were performed on results as described in Section 2.5 Statistical analysis.
2.3. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Cells were seeded in 6-well plates and treated with DMSO 0.5% in medium or the extracts diluted in medium. Total RNA was extracted from cells using TRIzol® reagent (Thermo Fisher Scientific, Inc., Carlsbad, CA, USA). After quantification on a Nanodrop 1000 spectrophotometer (Thermo Fisher Scientific, Inc., Wilmington, DE, USA), 750 ng of RNA were reversed transcribed to cDNA with Superscript IV (ThermoFisher Scientific, Inc., Vilnius, Lithuania). The qRT-PCR was performed to detect the expression of genes of interest using SYBR-Green PCR Master Mix (Roche, Mannheim, Germany) with a Lightcycler 480 II Roche system (Roche, Manheim, Germany). The thermocycling conditions were as follows: initial denaturation at 95 °C for 2 min, then denaturation at 95 °C for 15 s, annealing at 56 °C for 15 s and extension at 68 °C for 20 s, for a total of 40 cycles. The gene expression was normalized to Calmodulin 2 (CALM2), Glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and Pumilio homolog 1 (PUM1) expressions. The expression level of the genes of interest was calculated using the 2−ΔΔCq method. Primers were designed and obtained from Eurogentec (Table 1). The expression level obtained after the 2−ΔΔCq of each treatment for a given gene was finally normalized to the replicate mean of its corresponding DMSO control. Statistical analyses were performed on gene expression values as described in Section 2.5 Statistical analysis.

Table 1.
List of designed primer sequences used for qRT-PCR analysis.
2.4. Direct Immunofluorescence Staining
The cells were seeded on 24-well plates (Corning, Durham, NC, USA) and treated with either medium alone or FEAE-F0 for 72 h. The HDPCs were then fixed with 4% paraformaldehyde (Thermoscientific, Rockford, IL, USA) during 15 min at room temperature, then permeabilized with Triton 0.1X and saturated for 30 min in 10% fetal bovine serum (FBS). The cells were then directly stained with mouse β-catenin antibody (1:25) conjugated with AlexaFluor 488 (Santa Cruz, Dallas, TX, USA) in 1% FBS overnight at 4 °C.
The nuclei were counterstained with Hoechst 33342 Solution (Thermoscientific, Illinois, USA) the following day. The images were obtained immediately after counterstaining on the microscope AxioObserver 7 (Zeiss, Jena, Germany) and analyzed with the software Fiji coupled to ImageJ version 2.0. According to the protocol set by The University of Queensland [31] with slight modifications.
The analysis of the images obtained from the microscope was carried out as follows.
The surface of each cell nuclei was selected via a macro command on the image obtained for the β-catenin green fluorescence. The selected regions were measured for the area and integrated density (ID). All selections with an area below 640 were discarded as these did not correspond to cells but specks of fluorescence or other artefacts. A mean background value (MBGV) was also measured by selecting a dark region with no cells. The corrected total cell fluorescence (CTCF) was obtained using Equation (1).
CTCF = ID of specific cell − (Area of cell × MBGV)
The same steps were also done in blue fluorescence for the same cells selected in the green channel. A normalized CTCF value was obtained by dividing the CTCF obtained for the green channel of each cell to the corresponding CTCF in the blue channel. The results obtained were averaged to obtain a mean normalized CTCF equated to nuclear β-catenin expression levels.
2.5. Statistical Analysis
Data on cell-based tests were expressed as mean ± SE (standard error) of at least three independent experiments. The statistical tests were performed on Stata14. A F-test was performed to determine equality of the variances between the control group mean and each treatment group mean, followed by an unpaired Student’s t-test. Welch’s t-test was performed if variances were unequal. A p-value ≤ 0.05 was considered significant.
2.6. UHPLC-MS/MS Analysis of F. berteroana Fraction FEAE-F0
The chemical analyses were performed on a UHPLC system (Dionex Ultimate 3000, Thermo Scientific® equipped with a photodiode array detector: 254, 280, 340, and 450 nm) coupled to a high-resolution mass spectrometer (HRMS QqToF Impact II equipped with an electrospray ionization source, Bruker Daltonics, Germany) in positive and negative ionization modes (20 eV and 40 eV). The extracts were prepared by solubilizing 1 mg of dry extract in 1 mL of methanol then filtered with a 0.2 µm syringe filter. The separations were carried out on an Acclaim RSLC C18 column (2.1 mm × 150 mm, 2.2 µm, DIONEX Thermo Scientific®, Sunnyvale, CA, USA) at 40 °C by injecting 2 µL of the prepared solution.
The chromatographic analytical method was developed to obtain the best chromatographic separation, with H2O + 0.1% formic acid (solvent A) and acetonitrile + 0.1% formic acid (solvent B). The program consisted of 2 min at 5% B followed by a linear gradient up to 100% B for 8 min, then 100% B for 3 min and ended by a 3 min re-equilibration at 5% B (same flow rate). The injection of a formate acetate solution in basic media forming clusters on the studied mass range was used for mass calibration before each analysis.
Mass spectra were acquired ranging from 50 to 1200 m/z at 2Hz. The nebulizer pressure was set at 50.8 psi, the capillary voltage at 3000 V, the dry gas flow rate at 12 L·min−1, and dry temperature at 200 °C.
The MS/MS spectral data obtained were compared to entries in repositories such as MassBank of North America [32] and MassBank [33], the METlin library, and literature data when available (Table S1).
3. Results and Discussion
3.1. Hair Follicle Dermal Papilla Cells (HDPCs) Proliferation Dynamic after Treatment with Extracts and Target Genes Regulation
The HDPCs were treated with the extracts during both 24 and 48 h to study cell viability and proliferation dynamics as seen in Figure 2A–E.
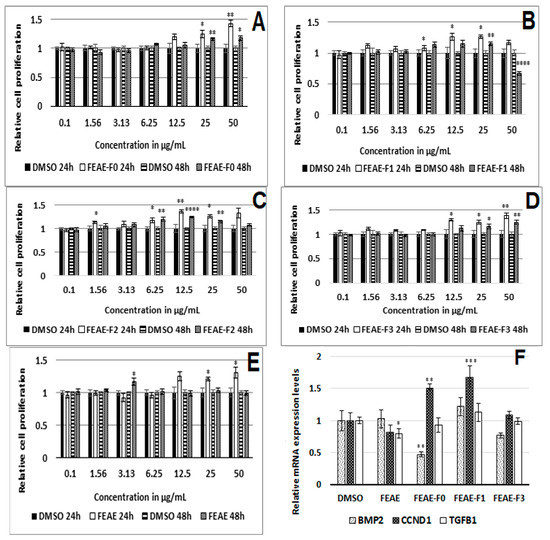
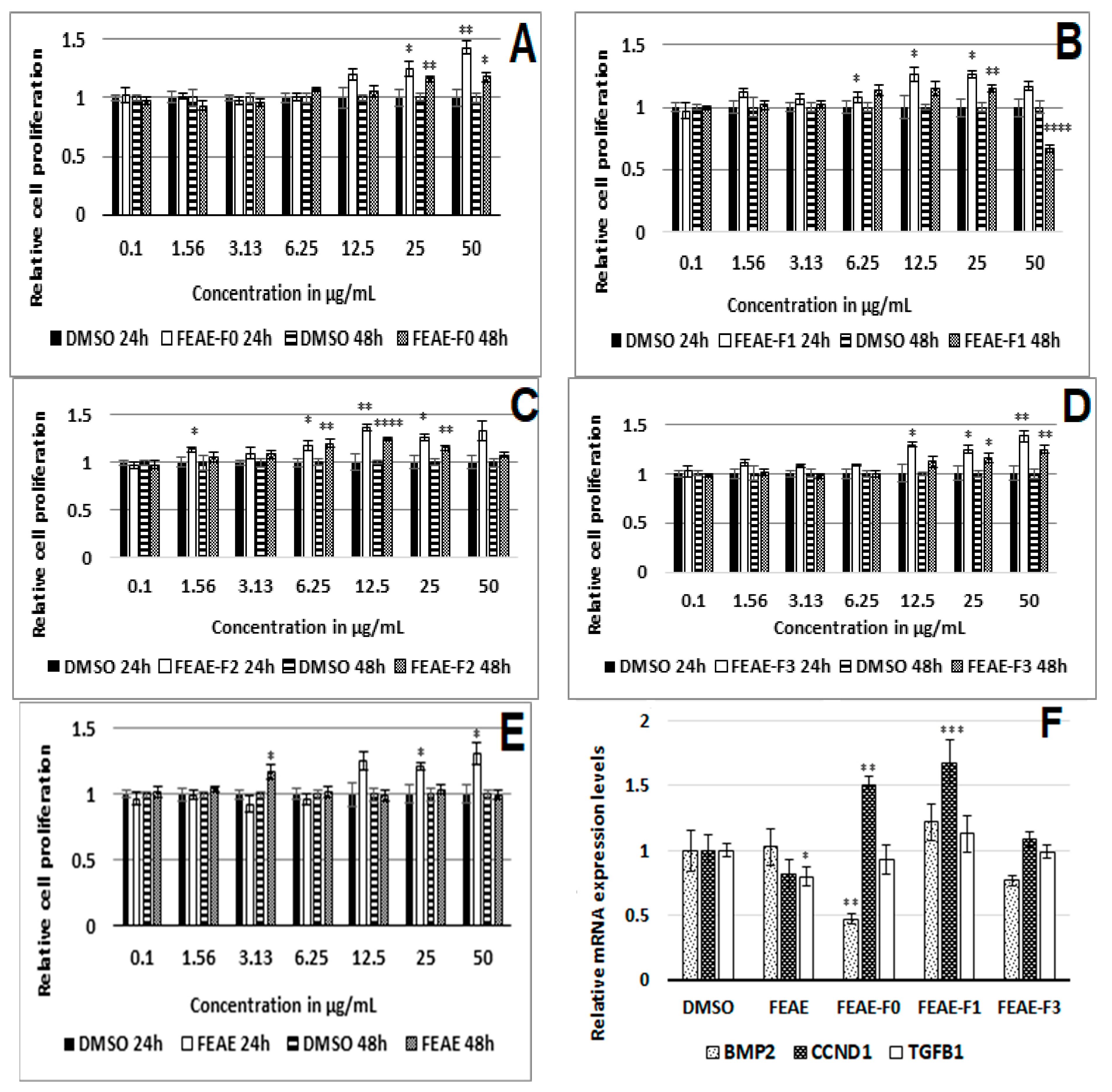
Figure 2.
Relative cell proliferation activity after 24 h and 48 h treatment with FEAE-F0 (A), FEAE-F1 (B), FEAE-F2 (C), FEAE-F3 (D), FEAE (E) and the extracts mRNA regulation of bone morphogenetic protein 2 (BMP2), cyclin-D1 (CCND1) and transforming growth factor beta (TGFB1) ± S.E. (F). Significance is determined by ‡ p < 0.05, ‡‡ p < 0.01, ‡‡‡ p < 0.001 and ‡‡‡‡ p < 0.0001.
After 24 h of treatment, FEAE and FEAE-F0 induced a dose-dependent proliferation of HDPCs (Figure 2A,E). It resulted in the highest cell proliferation after 24 h of treatment at 50 μg·mL−1 amongst all the other fractions, with a 1.43-fold increase compared to the control. FEAE-F1 and FEAE-F2, also stimulated cell proliferation compared to the control after 24 h of treatment between 6.25 and 25 μg·mL−1, although not dose-dependent. FEAE-F3 also promoted cell proliferation in cells between 12.5 and 50 μg·mL−1. Furthermore, after 48 h of treatment, FEAE-F0 also induced a dose-dependent proliferation of HDPCs, along with FEAE-F3. FEAE-F3 induced a 1.24-fold increase in cell proliferation compared to the control. On the other hand, FEAE showed no significant effect on cell proliferation after 48 h of treatment.
The RNA experiments were conducted by treating the cells with FEAE, FEAE-F0, and FEAE-F3 at 50 μg·mL−1, FEAE-F1 at 25 μg·mL−1 and the 0.5% DMSO control during 48 h, according to the results of the cell proliferation assay. Further testing on FEAE-F2 at 25 μg·mL−1 was excluded because we observed a persistent adverse effect on the physiological state of the cells when seeded in 6-well plates. Three gene targets were studied, Cyclin D1 (CCND1), Transforming growth factor beta 1 (TGFB1), and Bone Morphogenetic Protein 2 (BMP2) (Figure 2F). BMP2 is an inhibitor of the anagen, thus follicle growth, phase. Its levels are highest during the refractory telogen phase and rapidly decrease to allow entry into a new anagen phase [22]. Similarly, TGFB1 is a paracrine effector that facilitates catagen entry while Wnt activation decreases [25,34]. The extract FEAE significantly decreased TGFB1 expression 1.22-fold (p = 0.04) when compared to the control using a Student t-test. This modulation of the TGFB1 gene expression was not found in any of the fractions. Nonetheless, FEAE-F0 induced a significant 2-fold (p = 0.005) decrease of BMP2 while simultaneously increasing CCND1 1.5-fold (p = 0.002). FEAE-F1 also enabled a significant 1.7-fold (p = 0.007) increase in CCND1 but had no significant effect on the expression levels of BMP2. FEAE-F3 showed no significant regulation of any of the studied genes.
Reported studies have shown crosstalk between Wnt and TGFβ signaling [35,36]. Inhibitors of the Wnt pathway show a subsequent increase in TGFβ signaling in non-balding cells, as well as an increased TGFβ1 expression [36]. Likewise, BMP signaling prohibits activation of the Wnt pathway during the telogen phase. As the signaling environment of the cells changes, BMP levels lower to allow an upregulation of Wnt signaling and thus entry into anagen phase [24]. FEAE-F0 stood out as the most active fraction by displaying a similar regulation. Indeed, it not only increased activation of the Wnt pathway, as seen by the upregulation of CCND1, but it also downregulated BMP2. By lowering BMP signaling, FEAE-F0 facilitates transitioning to the follicle growth (anagen) phase. This can be observed by an increase in Wnt signaling [24]. CCND1 is a downstream target of the Wnt pathway and a rise in its expression levels suggested a stimulated Wnt state that we aimed to confirm by following β-catenin expression in the presence of FEAE-F0.
3.2. Modulation of β-Catenin Production by FEAE-F0
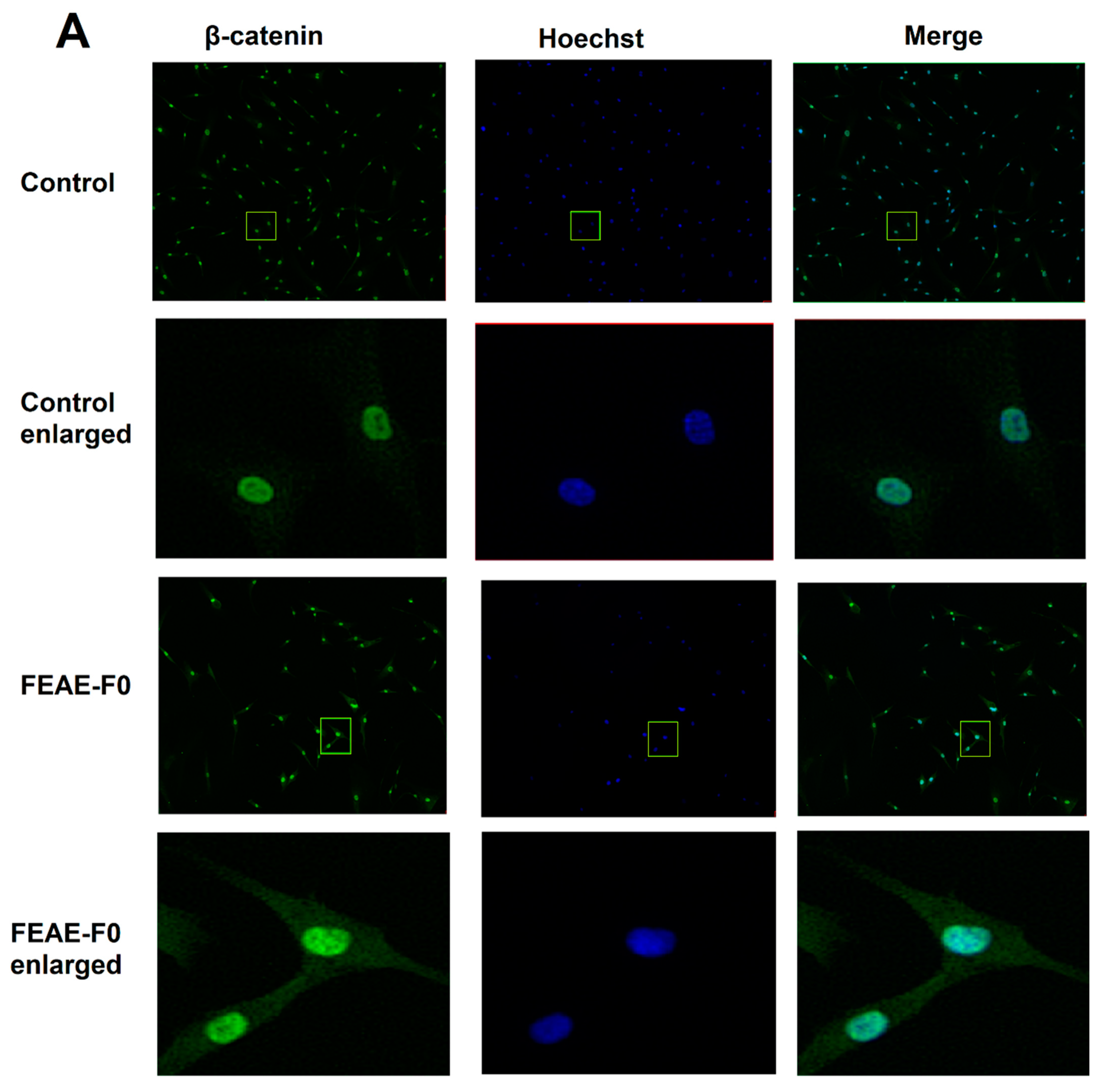
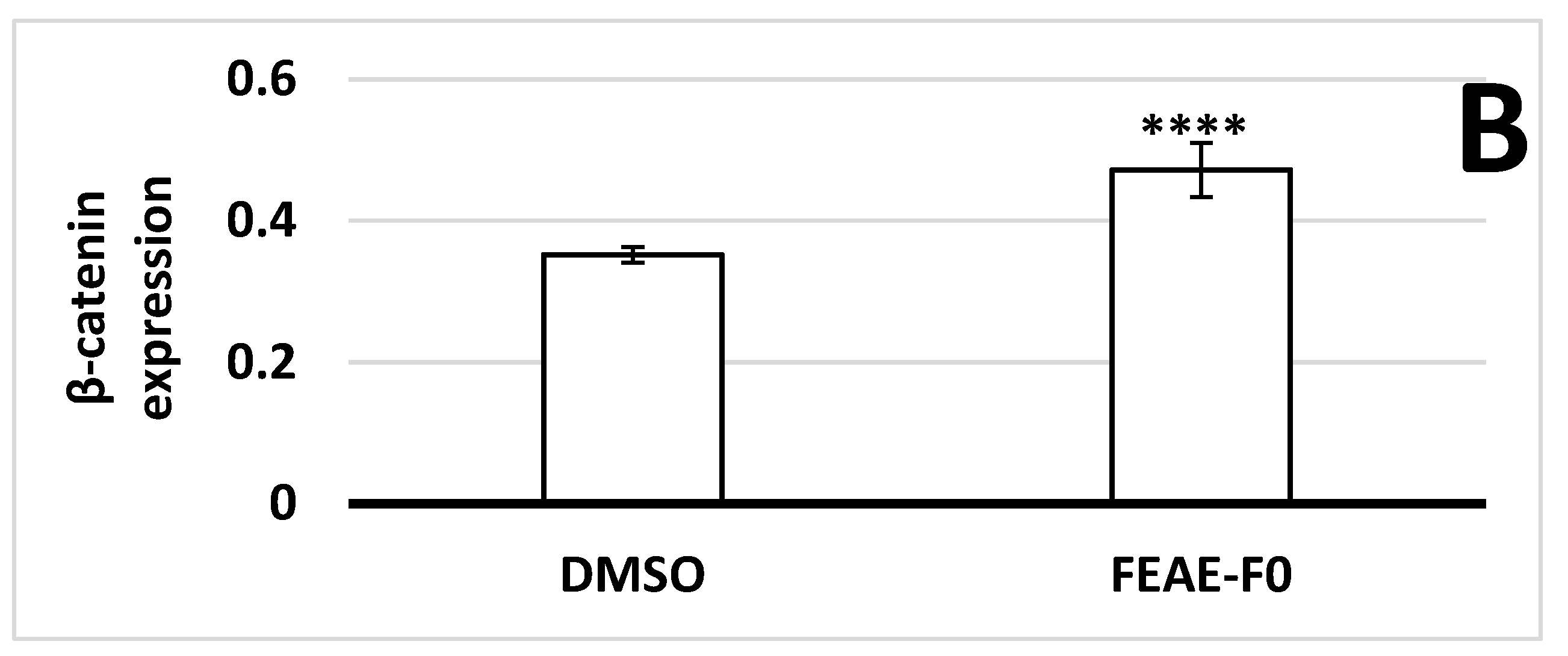
As β-catenin is a key protein of the Wnt pathway that intervenes during the growth phase of hair follicles [37], the effect of FEAE-F0 on its expression was studied (Figure 3). Indeed, when the Wnt/β-catenin pathway is inactive, β-catenin in the cytoplasm is phosphorylated by a complex composed of glycogen synthase kinase 3β, Axin, Adenomatous polyposis coli, and casein kinase 1a. The phosphorylated β-catenin is then ubiquitinated and recognized by a proteasome that degrades it before it enters the nucleus [38,39]. Conversely, when the canonical Wnt pathway is activated, the phosphorylating complex is inhibited which allows β-catenin to stabilize, accumulate in the cytoplasm, and successfully translocate to the nucleus [30]. Once in the nucleus, β-catenin serves as a co-activator of several downstream targets such as cyclin-D1 [40]. According to our experiments in Figure 3, FEAE-F0 showed a significant (p < 0.0001) 1.34-fold increase of nuclear β-catenin after treatment of HDPCs for 72 h compared to the control. Increased β-catenin levels in the nucleus are indicative of an active Wnt state allowing signaling to downstream targets [37]. Furthermore, it is important to note that this effect is done in a controlled manner. Indeed, while activation of the Wnt pathway in hair follicle cells is paramount for induction of the anagen phase and in fine hair growth, overexpression of the Wnt pathway could lead to developing cancerous cells [41]. FEAE-F0 showed a controlled proliferation and upregulation of the Wnt pathway which further crystallizes its efficiency in promoting hair growth.
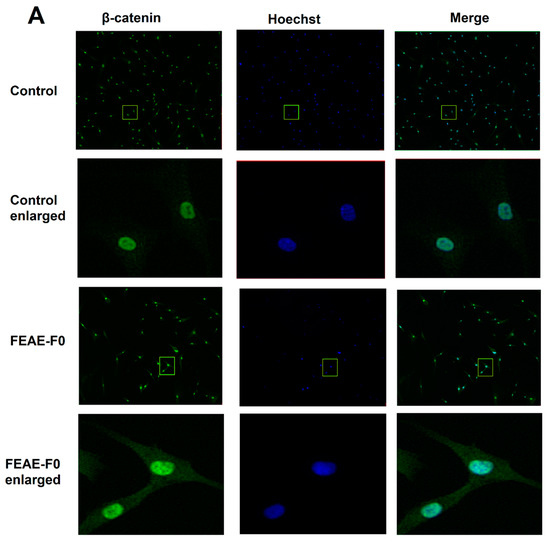
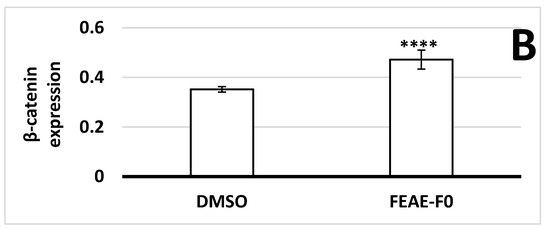
Figure 3.
(A) β-catenin expression in dermal papilla cells after 72 h of treatment in control medium or supplemented in 50 μg·mL−1 FEAE-F0 and nuclei counterstained with Hoechst followed by immunofluorescence. Objective ×10 and enlarged images ×4. Green rectangles correspond to zoomed area. (B) Relative nuclear β-catenin expression levels in immunocytochemistry (ICC) after 72 h growth in medium with 0.5% DMSO or FEAE-F0 at a concentration of 50 μg·mL−1. Significance is determined by **** p < 0.0001.
3.3. Chemical Composition of F. berteroana FEAE-F0
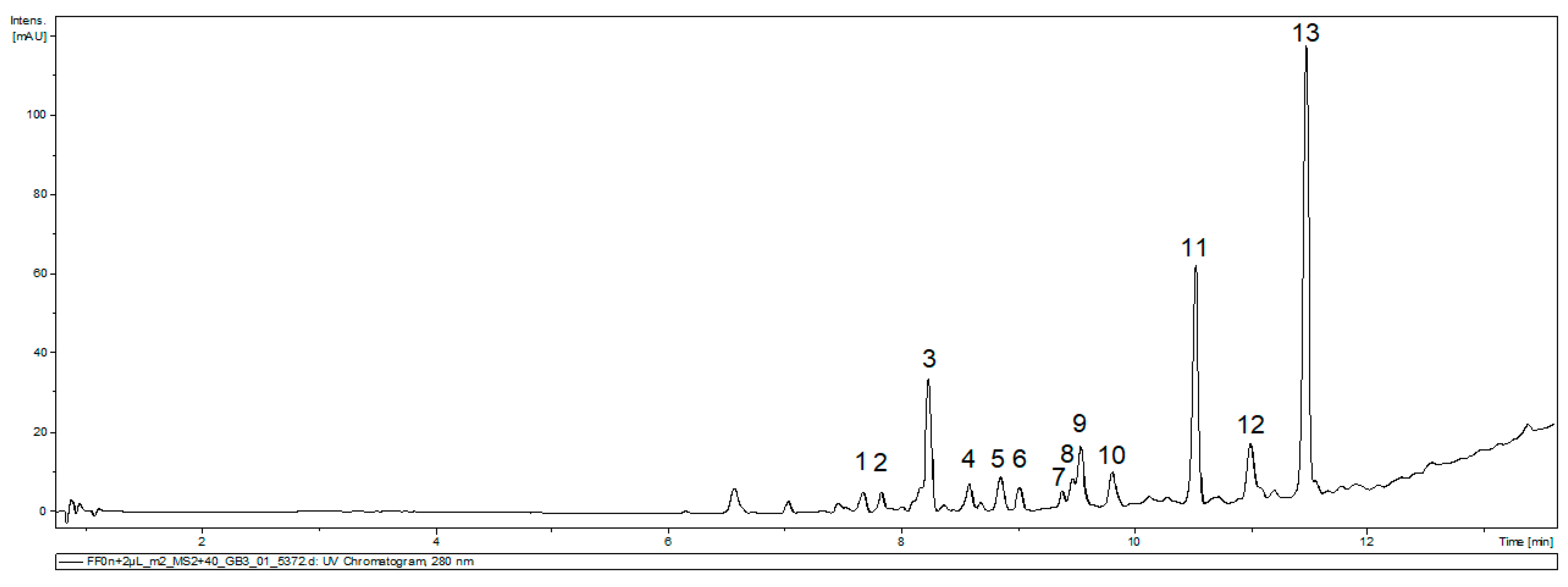
An identification of the main compounds present in FEAE-F0 was attempted by UHPLC-MS/MS analysis. The MS/MS data analyzed was that of 20 eV and 40 eV collision energy in positive and negative ionization mode. Thirteen main peaks were identified on the UV chromatogram in Figure 4. The compilation and tentative identification of the peaks by MS2 correspondence were reported in Table 2.
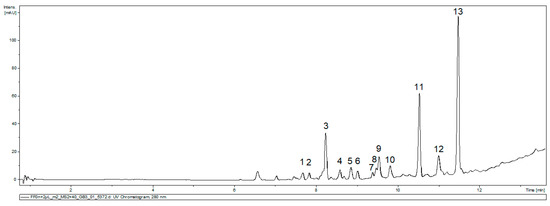
Figure 4.
UV chromatogram of FEAE-F0 at 280 nm for the 40 eV positive ionization mode.

Table 2.
Corresponding MS data compilation of peaks present in FEAE-F0 chromatogram.
Our previous work on FEAE revealed the presence of loganic and coumaric acids [42]. The present paper mainly focused on the minor bioactive fraction FEAE-F0, derived from FEAE the crude extract previously studied [42]. An exhaustive search of all compounds found in the literature for the genus Fagraea and their available MS2 was compiled in Table S1 (Supplementary Material). We compared the obtained data for the thirteen peaks to that of Table S1. Only one iridoid was successfully annotated, boonein. Indeed, boonein and several other iridoids such as (±)-gentiolactone, naucledal, angelone, swermacrolactone C amongst others (Table S1) were found in the stem bark of F. fragrans (syn. of Cyrtophyllum fragrans) [17,19,20,43]. No biological activities linked to hair care have been specified for boonein in literature. Nevertheless, iridoids are known for their anti-inflammatory and cardiovascular activity [44,45]. This could suggest an implication in hair growth by enabling proper vascularization and thus maintaining nutrient support for the efficient proliferation of dermal papilla cells, notably during the anagen phase [46]. Indeed, minoxidil, one of the only FDA-approved treatments to promote hair growth, is known to mediate its effects via different mechanisms such as promoting vascularization around HDPCs [47]. Moreover, minoxidil has been reported to prolong anagen in hair follicles [26,48].
Overall, the remaining compounds that have not yet been identified, showed carbon atoms ranging between 9 and 16, suggesting medium-sized molecules. Their molecular formulas led to many structural propositions amongst known compounds in Pubchem (Table 2), thus uncovering a plethora of structural possibilities.
A few structural hypotheses could nevertheless be proposed.
According to mass and molecular formulas, FEAE-F0 included several isomers. Compounds 6 (m/z = 181.1223) and 11 (m/z = 181.1224), as well as compounds 4 (m/z = 213.1454) and 8 (m/z = 213.1484) only differed in fragmentation pattern. The MS2 experimental data at 20 eV for compound 90552 in Metlin is remarkably similar to that of compound 6; the 40 eV spectrum fragmentation did not match as closely. Entry 90552 in Metlin corresponds to dihydroactinidiolide (DHA), which raises the hypothesis of the potential presence of DHA in our fraction, or of closely related terpenoid structures. Such a proposition may be plausible as DHA is a terpene obtained by cleavage of β-carotene [49]. As previously stated, the fruits of F. berteroana are reddish-orange when ripe. This latter point could explain a putative presence of β-carotene derivatives in our fraction. However, compound 11 had less fragments that matched with compound 90552. It could potentially correspond to an isomer of DHA. The resulting fragments of compound 11 could suggest either a linear structure with the loss of oxygen or a substituted phenyl ring (m/z = 91), followed by a successive loss of carbon atoms. Such a pattern could potentially correspond to shorter chained derivatives of the diterpene phytol, or potentially terpenoids containing an aromatic ring core. Interestingly, several terpenes have been the object of patents for their efficiency in preventing hair loss and promoting hair growth [50,51]. Nevertheless, the above propositions remain hypotheses.
4. Conclusions
Our results showed that there is a positive correlation between the ethnocosmetic use of the fruits of F. berteroana and observed molecular activities. Indeed, an ethyl acetate extract of the fruits and fractions FEAE-F0 through FEAE-F3 could potentiate hair growth via stimulation of hair follicle dermal papilla cells proliferation, shown herein by in vitro induction of HDPCs. Furthermore, FEAE-F0 was selected for its potential positive effect on the anagen phase of the hair cycle by upregulating the Wnt pathway, alongside down-regulating the BMP pathway. The in vitro activity observed for FEAE-F0 can be attributed to its chemical content, comprising of iridoids and potentially terpenoids. The fraction stands out as an interesting natural active for applications in hair growth. This could further be cemented with in and ex vivo studies geared towards the prevention of hair loss due to genetic factors such as androgenetic alopecia, or environmental stresses.
Lastly, the actual natural resource is limited in low- to mid-altitude in French Polynesia, but its cultivation is not unreasonable as the tree is often grown in marae, a large and clear area devoted to traditional or communal practices in Polynesian societies, because of its spiritual symbolism or gardens. Hence, an effort to cultivate it could be successful and interesting to obtain a sustainable resource for further development of this plant material as a potential natural cosmetic ingredient.
Supplementary Materials
The following are available online at https://www.mdpi.com/2079-9284/8/1/13/s1, Table S1: MS2 data compilation of the compounds present in the Fagraea genus according to literature.
Author Contributions
P.R., J.-Y.B., R.H., E.F. and K.H. participated in the conceptualization and general methodology design of the project and article. K.H. designed and performed the experiments, ran the data analysis, and wrote the original draft of the article. E.R. supervised fractioning of plant material. C.C. and S.H. supervised experiments in cellular biology and provided expertise for data interpretation and validation of related experiments. S.G. supervised experiments in metabolomics and provided expertise for data interpretation. J.-F.B. provided expertise in ethnobotany and in plant collection and identification. P.R., R.H., and J.-Y.B. acquired funding for the project. All authors participated in the reviewing and editing process of the article. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the French Ministry of Higher Education and Research at the University of French Polynesia (ED 469) and Greentech.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
The authors wish to acknowledge Cosmetic Valley for their financial support. UHPLC-MS/MS was performed on the Mallabar platform (granted by Provence Alpes Côte d’Azur region, ANR and Total Foundation) at IMBE. Immunofluorescence was performed on the Anipath platform (granted by Cancéropôle Lyon Auvergne Rhône Alpes 2007 and CPER 2016). The authors would also like to thank Nicolas Allègre for his expertise in molecular analysis and Pierre Pouchin for his expertise in imagery analysis. The authors wish to warmly thank Marianne, Tohei and Tuihau Theophilus for their kind contribution to the photograph in Figure 1A.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data.
References
- Butaud, J.-F. Synthèse Bibliographique Portant Sur Les Plantes Utilisées Dans La Cosmétopée de Polynésie Française; Cosmetic Valley: Chartres, France, 2013. [Google Scholar]
- Ansel, J.-L.; Ly, Q.; Butaud, J.-F.; Nicolas, M.; Herbette, G.; Peno-Mazzarino, L.; Lati, E.; Raharivelomanana, P. Activité anti-âge de l’extrait de Fitchia nutans, un ingrédient cosméticeutique d’un monoï traditionnel polynésien. Comptes Rendus Chim. 2016, 19, 1049–1055. [Google Scholar] [CrossRef]
- Jost, X.; Ansel, J.-L.; Lecellier, G.; Raharivelomanana, P.; Butaud, J.-F. Ethnobotanical Survey of Cosmetic Plants Used in Marquesas Islands (French Polynesia). J. Ethnobiol. Ethnomed. 2016, 12. [Google Scholar] [CrossRef]
- Martin, A. The Enlightenment in Paradise: Bougainville, Tahiti, and the Duty of Desire. Eighteenth Century Stud. 2007, 41, 203–216. [Google Scholar] [CrossRef]
- Gauthier, L. TAHITI—1904-1921; Les éditions du Pacifique: Paris, France, 1985. [Google Scholar]
- Hughes, K.; Ho, R.; Butaud, J.-F.; Filaire, E.; Ranouille, E.; Berthon, J.-Y.; Raharivelomanana, P. A Selection of Eleven Plants Used as Traditional Polynesian Cosmetics and Their Development Potential as Anti-Aging Ingredients, Hair Growth Promoters and Whitening Products. J. Ethnopharmacol. 2019, 245, 112159. [Google Scholar] [CrossRef] [PubMed]
- Handy, E.S.C. The Native Culture in the Marquesas; Bernice P. Bishop Museum: Honolulu, HI, USA, 1923. [Google Scholar]
- Pétard, P. Plantes Utiles de Polynésie Française et Raau Tahiti; Editions Haere Po No Tahiti: Papeete, French Polynesia, 1986; ISBN 978-2-904171-06-2. [Google Scholar]
- Whistler, W.A.; Elevitch, C.R. Fagraea berteroana (Pua kenikeni), Ver. 3.2.; Volume Species Profiles for Pacific Island Agroforestry; Elevitch, C.R., Ed.; Permanent Agriculture Resources (PAR): Honolulu, HI, USA, 2006. [Google Scholar]
- Hayashi, S.; Kameoka, H.; Hashimoto, S.; Furukawa, K.; Arai, T. Volatile Compounds of Fagraea berteriana Flowers. J. Essent. Oil Res. 1995, 7, 505–510. [Google Scholar] [CrossRef]
- Whistler, W.A. Plants in Samoan Culture: The Ethnobotany of Samoa; Isle Botanica: Honolulu, HI, USA, 2000; ISBN 978-0-9645426-6-2. [Google Scholar]
- Motley, T.J. The Ethnobotany of Fagraea Thunb. (Gentianaceae): The Timber of Malesia and the Scent of Polynesia. Econ. Bot. 2004, 58, 396–409. [Google Scholar] [CrossRef]
- Cuendet, M.; Hostettmann, K.; Potterat, O.; Dyatmiko, W. Iridoid Glucosides with Free Radical Scavenging Properties from Fagraea blumei. Helv. Chim. Acta 1997, 80, 1144–1152. [Google Scholar] [CrossRef]
- Cambie, R.C.; Lal, A.R.; Rickard, C.E.F.; Tanaka, N. Chemistry of Fijian Plants. V.: Constituents of Fagraea gracilipes A. GRAY. Chem. Pharm. Bull. 1990, 38, 1857–1861. [Google Scholar] [CrossRef]
- Ferdinal, N.; Alfajri, R.; Arifin, B. Isolation and Characterization of Scopoletin from The Bark of Fagraea ceilanica Thumb and Antioxidants Tests. Int. J. Adv. Sci. Eng. Inf. Technol. 2015, 5, 126. [Google Scholar] [CrossRef][Green Version]
- Bangprapai, A.; Thongphasuk, P.; Songsak, T. Determination of Swertiamarin Content by Tlc-Densitometer in Fagraea fragrans Roxb. Leaves. Bull. Health Sci. Technol. 2016, 14, 13–18. [Google Scholar]
- Jonville, M.-C.; Capel, M.; Frédérich, M.; Angenot, L.; Dive, G.; Faure, R.; Azas, N.; Ollivier, E. Fagraldehyde, a Secoiridoid Isolated from Fagraea fragrans. J. Nat. Prod. 2008, 71, 2038–2040. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, E.; Suzumura, K.; Yamazaki, M. Pharmacologically Active Components of Todopon Puok (Fagraea racemosa), a Medicinal Plant from Borneo. Chem. Pharm. Bull. 1995, 43, 2200–2204. [Google Scholar] [CrossRef]
- Madmanang, S.; Cheyeng, N.; Heembenmad, S.; Mahabusarakam, W.; Saising, J.; Seeger, M.; Chusri, S.; Chakthong, S. Constituents of Fagraea fragrans with Antimycobacterial Activity in Combination with Erythromycin. J. Nat. Prod. 2016, 79, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Rattanaburi, S.; Kaikaew, K.; Watanapokasin, R.; Phongpaichit, S.; Mahabusarakamb, W. A New Lignan from the Stem Bark of Fagraea fragrans Roxb. Nat. Prod. Res. 2020, 1–6. [Google Scholar] [CrossRef]
- Wan, A.S.C.; Chow, Y.L. Alkaloids of Fagraea fragrans Roxb. J. Pharm. Pharmacol. 1964, 16, 484–486. [Google Scholar] [CrossRef] [PubMed]
- Basir, D.; Hanafi, M.; Saputra, A.; Wati, T. Free Solvent Amidation of Ursolic and Oleanolic Acids of Fagraea fragrans Fruits: Their P-388 Antitumor Activity. J. Phys. Conf. Ser. 2018, 1095, 012006. [Google Scholar] [CrossRef]
- Tamura, Y.; Takata, K.; Eguchi, A.; Kataoka, Y. In Vivo Monitoring of Hair Cycle Stages via Bioluminescence Imaging of Hair Follicle NG2 Cells. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Plikus, M.V. New Activators and Inhibitors in the Hair Cycle Clock: Targeting Stem Cells’ State of Competence. J. Investig. Dermatol. 2012, 132, 1321–1324. [Google Scholar] [CrossRef] [PubMed]
- Inui, S.; Itami, S. Molecular Basis of Androgenetic Alopecia: From Androgen to Paracrine Mediators through Dermal Papilla. J. Dermatol. Sci. 2011, 61, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kwack, M.H.; Kang, B.M.; Kim, M.K.; Kim, J.C.; Sung, Y.K. Minoxidil Activates β-catenin Pathway in Human Dermal Papilla Cells: A Possible Explanation for Its Anagen Prolongation Effect. J. Dermatol. Sci. 2011, 62, 154–159. [Google Scholar] [CrossRef]
- Zhang, H.; Su, Y.; Wang, J.; Gao, Y.; Yang, F.; Li, G.; Shi, Q. Ginsenoside Rb1 Promotes the Growth of Mink Hair Follicle via PI3K/AKT/GSK-3β Signaling Pathway. Life Sci. 2019, 229, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Dastan, M.; Najafzadeh, N.; Abedelahi, A.; Sarvi, M.; Niapour, A. Human Platelet Lysate versus Minoxidil Stimulates Hair Growth by Activating Anagen Promoting Signaling Pathways. Biomed. Pharmacother. 2016, 84, 979–986. [Google Scholar] [CrossRef]
- Oshimori, N.; Fuchs, E. Paracrine TGF-β Signaling Counterbalances BMP-Mediated Repression in Hair Follicle Stem Cell Activation. Cell Stem Cell 2012, 10, 63–75. [Google Scholar] [CrossRef]
- Veltri, A.; Lang, C.; Lien, W.-H. Concise Review: Wnt Signaling Pathways in Skin Development and Epidermal Stem Cells: Wnt Signaling in Skin Development and Stem Cells. Stem Cells 2018, 36, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Hammond, L. QBI, The University of Queensland Australia Measuring Cell Fluorescence Using ImageJ—The Open Lab Book v1.0. Available online: https://theolb.readthedocs.io/en/latest/imaging/measuring-cell-fluorescence-using-imagej.html (accessed on 15 October 2020).
- MassBank of North America (MoNA)—PubChem Data Source. Available online: https://pubchem.ncbi.nlm.nih.gov/source/22043 (accessed on 20 August 2020).
- Horai, H.; Arita, M.; Kanaya, S.; Nihei, Y.; Ikeda, T.; Suwa, K.; Ojima, Y.; Tanaka, K.; Tanaka, S.; Aoshima, K.; et al. MassBank: A Public Repository for Sharing Mass Spectral Data for Life Sciences. J. Mass Spectrom. 2010, 45, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Inui, S.; Itami, S. Androgen Actions on the Human Hair Follicle: Perspectives. Exp. Dermatol. 2013, 22, 168–171. [Google Scholar] [CrossRef]
- Warner, D.R.; Greene, R.M.; Pisano, M.M. Cross-Talk between the TGFβ and Wnt Signaling Pathways in Murine Embryonic Maxillary Mesenchymal Cells. FEBS Lett. 2005, 579, 3539–3546. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.-Q.; Wu, Z.-B.; Chu, X.-Y.; Bi, Z.-G.; Fan, W.-X. An Investigation of Crosstalk between Wnt/β-catenin and Transforming Growth Factor-β Signaling in Androgenetic Alopecia. Medicine 2016, 95, e4297. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.Y. Targeting Wnt/β-catenin Pathway for Developing Therapies for Hair Loss. Int. J. Mol. Sci. 2020, 21, 4915. [Google Scholar] [CrossRef] [PubMed]
- Kimelman, D.; Xu, W. β -catenin Destruction Complex: Insights and Questions from a Structural Perspective. Oncogene 2006, 25, 7482–7491. [Google Scholar] [CrossRef] [PubMed]
- Stamos, J.L.; Weis, W.I. The β-catenin Destruction Complex. Cold Spring Harb. Perspect. Biol. 2013, 5. [Google Scholar] [CrossRef]
- Shtutman, M.; Zhurinsky, J.; Simcha, I.; Albanese, C.; D’Amico, M.; Pestell, R.; Ben-Ze’ev, A. The Cyclin D1 Gene Is a Target of the β-catenin/LEF-1 Pathway. Proc. Natl. Acad. Sci. USA 1999, 96, 5522–5527. [Google Scholar] [CrossRef]
- Schneider, J.A.; Logan, S.K. Revisiting the Role of Wnt/β-catenin Signaling in Prostate Cancer. Mol. Cell. Endocrinol. 2018, 462, 3–8. [Google Scholar] [CrossRef]
- Hughes, K.; Ho, R.; Greff, S.; Filaire, E.; Ranouille, E.; Chazaud, C.; Herbette, G.; Butaud, J.-F.; Berthon, J.-Y.; Raharivelomanana, P. Hair Growth Activity of Three Plants of the Polynesian Cosmetopoeia and Their Regulatory Effect on Dermal Papilla Cells. Molecules 2020, 25, 4360. [Google Scholar] [CrossRef] [PubMed]
- Suciati, S.; Lambert, L.K.; Ross, B.P.; Deseo, M.A.; Garson, M.J. Phytochemical Study of Fagraea Spp. Uncovers a New Terpene Alkaloid with Anti-Inflammatory Properties. Aust. J. Chem. 2011, 64, 489. [Google Scholar] [CrossRef]
- Villasenor, I. Bioactivities of Iridoids. AIAAMC 2007, 6, 307–314. [Google Scholar] [CrossRef]
- Viljoen, A.; Mncwangi, N.; Vermaak, I. Anti-Inflammatory Iridoids of Botanical Origin. CMC 2012, 19, 2104–2127. [Google Scholar] [CrossRef]
- Li, W.; Man, X.-Y.; Li, C.-M.; Chen, J.-Q.; Zhou, J.; Cai, S.-Q.; Lu, Z.-F.; Zheng, M. VEGF Induces Proliferation of Human Hair Follicle Dermal Papilla Cells through VEGFR-2-Mediated Activation of ERK. Exp. Cell Res. 2012, 318, 1633–1640. [Google Scholar] [CrossRef] [PubMed]
- Lachgar; Charveron; Gall; Bonafe Minoxidil Upregulates the Expression of Vascular Endothelial Growth Factor in Human Hair Dermal Papilla Cells. Br. J. Dermatol. 1998, 138, 407–411. [CrossRef]
- Messenger, A.G.; Rundegren, J. Minoxidil: Mechanisms of Action on Hair Growth. Br. J. Dermatol. 2004, 150, 186–194. [Google Scholar] [CrossRef]
- Shumbe, L.; Bott, R.; Havaux, M. Dihydroactinidiolide, a High Light-Induced β-Carotene Derivative That Can Regulate Gene Expression and Photoacclimation in Arabidopsis. Mol. Plant 2014, 7, 1248–1251. [Google Scholar] [CrossRef] [PubMed]
- Marin, C.L.R. Terpene Extract for the Treatment of Hair Loss 2013. Available online: https://patentscope2.wipo.int/search/en/detail.jsf?docId=WO2013174854 (accessed on 31 January 2021).
- Takahashi, H.; Hara, S.c/oK.K.Y.C.K.; Matsui, R.c/oK.K.Y.C.K. Diterpenes and Flavonoids as 5-Alpha-Reductase Inhibitors 1996. Available online: https://patents.google.com/patent/EP0747048A2/en (accessed on 31 January 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).