Monfortinho Thermal Water-Based Creams: Effects on Skin Hydration, Psoriasis, and Eczema in Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Physicochemical Analysis of Thermal Water
2.2. Processing and Physicochemical Characterization of the Creams
2.3. Skin Biometrics Evaluation
2.4. Clinical Study
2.5. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Analysis of Thermal Water
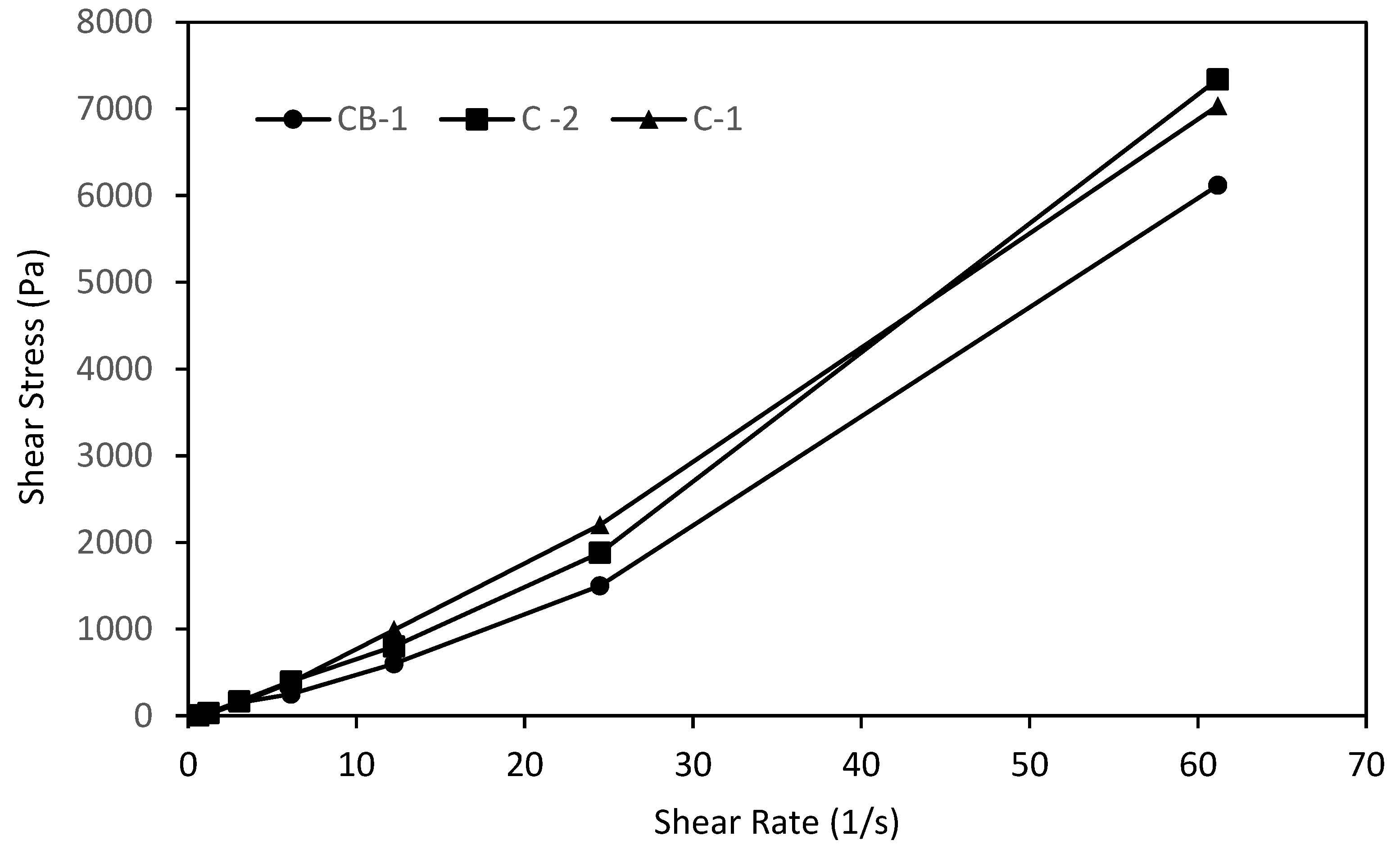
3.2. Physicochemical Characterization of the Creams
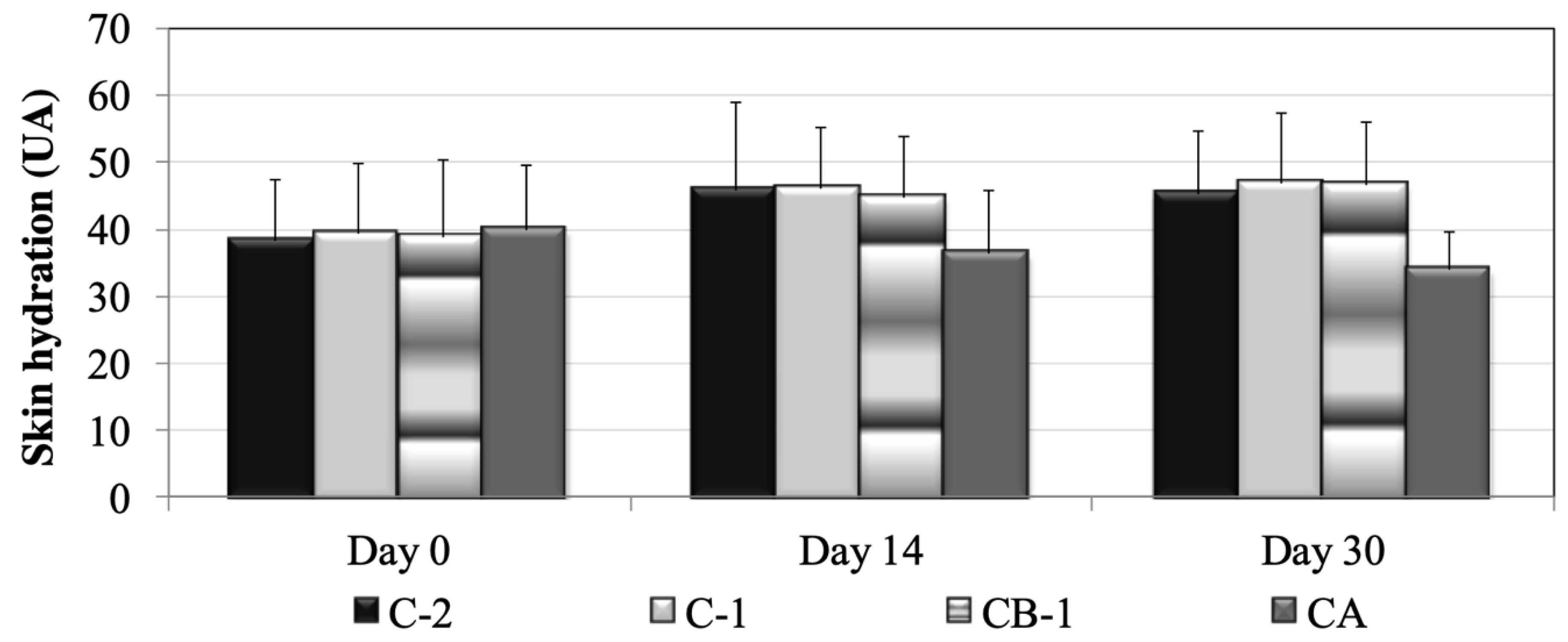
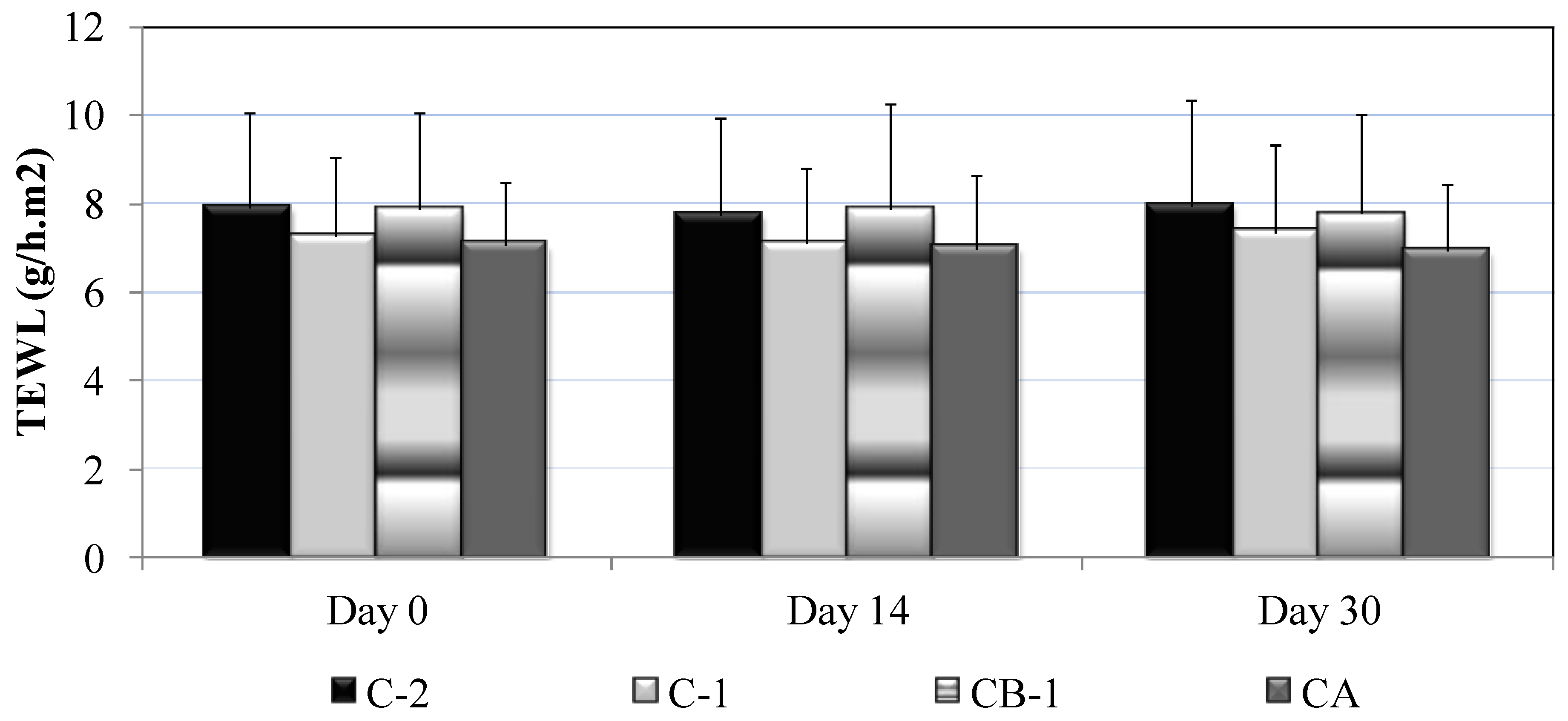
3.3. Skin Biometrics Evaluation
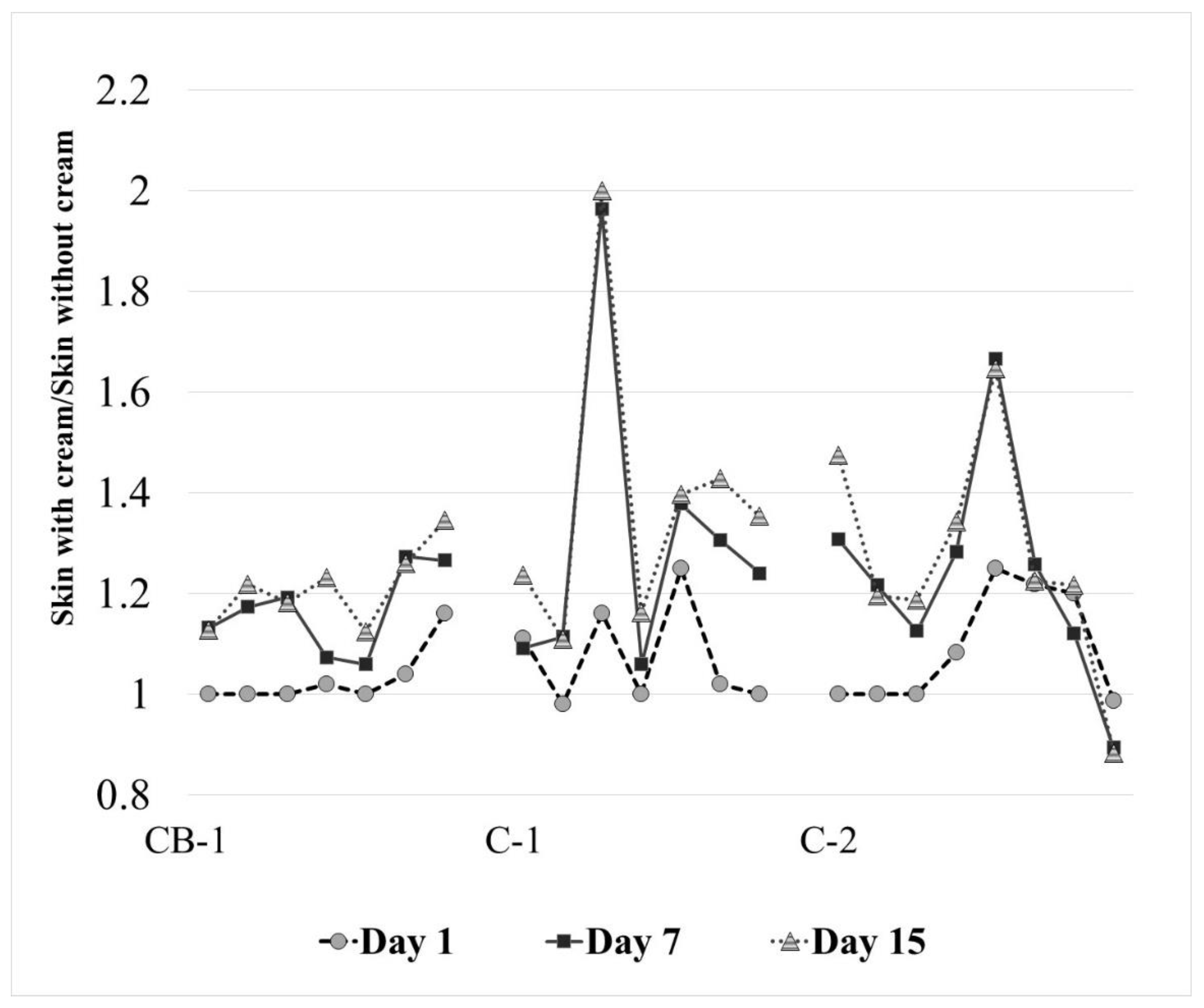
3.4. Clinical Study
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Picoto, A. Mineral water and spas in Portugal. Clin. Dermatol. 1996, 14, 637–639. [Google Scholar] [CrossRef]
- Association TdP. Termas de Monfortinho. Available online: http://www.termasdeportugal.pt/estanciastermais/Termas-de-Monfortinho (accessed on 27 April 2016).
- Ghersetich, I.; Freedman, D.; Lotti, T. Balneology today. J. Eur. Acad. Dermatol. 2000, 14, 346–348. [Google Scholar] [CrossRef]
- Seite, S. Thermal waters as cosmeceuticals: La Roche-Posay thermal spring water example. Clin. Cosmet. Investig. Dermatol. 2013, 6, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.O.; Costa, P.C.; Bahia, M.F. Effect of São Pedro do Sul thermal water on skin irritation. Int. J. Cosmet. Sci. 2010, 32, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Gattu, S.; Maibach, H. Enhanced Absorption through Damaged Skin: An Overview of the in vitro Human Model. Ski. Pharmacol. Physiol. 2010, 23, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Gattu, S.; Maibach, H. Modest but Increased Penetration through Damaged Skin: An Overview of the in vivo Human Model. Skin Pharmacol. Phys. 2011, 24, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Meges, S.; Lauze, C.; Dupuy, P.; Bacle, I.; MacLeod, P. Sensory analysis of four medical spa spring waters containing various mineral concentrations. Int. J. Dermatol. 1999, 38, 784–786. [Google Scholar]
- Fabre, P. Avène 2016. Available online: http://www.eau-thermale-avene.fr/eau-thermale/apaisement-anti-irritation/eau-thermale-avene (accessed on 10 April 2018).
- Uriage. Thermal Center: l’eau de la Peau 2016. Available online: http://www.uriage.com/AA/en/the-thermal-center (accessed on 10 April 2018).
- Clesceri, L.; Greenberg, A.; Eaton, A. Standard Methods for the Examination of Water and Wastewater, 21th ed.; American Public Association: Washington, DC, USA, 2005. [Google Scholar]
- International Organization for Standardization I. Water Quality—Determination of Permanganate Index; ISO 8467:1993; Internation Organization for Standardization: Geneve, Switzerland, 1993. [Google Scholar]
- Neves, A.; Marto, J.; Duarte, A.; Gonçalves, L.M.; Pinto, P.; Figueiredo, A.C.; Ribeiro, H.M. Characterization of Portuguese Thymbra capitata, Thymus caespititius and Myrtus communis essential oils in topical formulations. Flavour Fragr. J. 2017, 32, 392–402. [Google Scholar] [CrossRef]
- Sengupta, P. Potential Health Impacts of Hard Water. Int. J. Prev. Med. 2013, 4, 866–875. [Google Scholar] [PubMed]
- Ertel, K.; Warner, R.; Boissy, W. Effect of moisturizers on the structure of lipids in the outer stratum corneum of humans. In Dry Skin and Moisturizers, Chemistry and Function, 2nd ed.; Lodén, M., Maibach, H., Eds.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Fluhr, J.B.; Berardesca, A.; Glycerol, E. Just a moisturizer? biological and biophysical effects. In Dry Skin and Moisturizers, Chemistry and Function, 2nd ed.; Lodén, M., Maibach, H., Eds.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Lodén, M. Treatments Improving Skin Barrier Function. Curr. Probl. Dermatol. 2016, 49, 112–122. [Google Scholar] [PubMed]
- Lodén, M. Effect of moisturizers on epidermal barrier function. Clin. Dermatol. 2012, 30, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, H.; Marto, J.; Raposo, S.; Agapito, M.; Isaac, V.; Chiari, B.G.; Lisboa, P.F.; Paiva, A.; Barreiros, S.; Simões, P. From coffee industry waste materials to skin-friendly products with improved skin fat levels. Eur. J. Lipid Sci. Technol. 2013, 115, 330–336. [Google Scholar] [CrossRef]
- Barkat, A.K.; Naveed, A.; Abder, M.; Farid, M. A Novel Cassia fistula (L.)-Based Emulsion Elicits Skin Anti-Aging Benefits in Humans. Cosmetics 2015, 2, 368–383. [Google Scholar]
- Tsoureli-Nikita, E.; Menchini, G.; Ghersetich, I.; Hercogova, J.; Tsoureli-Nikita, E. Alternative treatment of psoriasis with balneotherapy using Leopoldine spa water. J. Eur. Acad. Dermatol. Venereol. 2002, 16, 260–262. [Google Scholar] [CrossRef] [PubMed]
- Goldman, M.P.; Merial-Kieny, C.; Nocera, T.; Méry, S. Comparative benefit of two thermal spring waters after photodynamic therapy procedure. J. Cosmet. Dermatol. 2007, 6, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Lodén, M. Role of topical emollients and moisturizers in the treatment of dry skin barrier disorders. Am. J. Clin. Dermatol. 2003, 4, 771–788. [Google Scholar] [CrossRef] [PubMed]
| Ingredients (Chemical Name) | Qualitative and Quantitative Composition % (w/w) | ||
|---|---|---|---|
| C-Bl | C-1 | C-2 | |
| Ceteareth-12 (and) Ceteareth-20 | 3.0 | 3.0 | 3.0 |
| Cetyl alcohol | 5.0 | 5.0 | 5.0 |
| Decyl oleate | 4.5 | 4.5 | 4.5 |
| Glycerin | 5.0 | 5.0 | 5.0 |
| Phenoxyethanol | 0.2 | 0.2 | 0.2 |
| Ultra-pure laboratory water | q.s. 100% | - | - |
| Thermal water | - | q.s. 100% | - |
| Concentrated thermal water (2:1) | - | - | q.s. 100% |
| Parameter | Units | Results |
|---|---|---|
| Inorganic and Physical Factors | ||
| Alkalinity | mg/L CaCO3 | 2.5 |
| Ammonia | mg/L NH4 | <0.05 * |
| Boron | mg/L B | <0.17 * |
| Chloride | mg/L Cl | 3.7 |
| Conductivity (20 °C) | μS/cm | 38 |
| Dissolved carbon dioxide | mg/L CO2 | 27 |
| Dissolved oxygen | mg/L O2 | 7.5 |
| % Saturation | 87 | |
| Fluoride | mg/L F | 0.05 |
| Hardness | mg/L CaCO3 | 17 |
| Nitrate | mg/L NO3 | <5.0 * |
| Nitrite | mg/L NO2 | <0.01 * |
| Ortho-phosphate | mg/L PO4 | <0.05 * |
| Permanganate index | mg/L O2 | 0.67 |
| pH (25 °C) | pH scale | 5.7 |
| Silicon | mg/L SiO2 | 16 |
| Sulfide | mg/L S | <0.17 * |
| Sulphate | mg/L SO4 | <5.0 * |
| Total dissolved solids (TDS) | mg/L | 53 |
| True color | mg/L Pt/Co | <0.50 * |
| Turbidity | UNT | 0.17 |
| Parameter | Units | Results |
|---|---|---|
| Metals | ||
| Aluminum | μg/L Al | <30 * |
| Antimony | μg/L Sb | <2.0 * |
| Arsenic | μg/L As | <1.0 * |
| Barium | μg/L Ba | <60 * |
| Beryllium | μg/L Be | <2.0 * |
| Cadmium | μg/L Cd | <1.0 * |
| Calcium | mg/L Ca | 1.6 |
| Chromium | μg/L Cr | <10 * |
| Cobalt | μg/L Co | <4.0 * |
| Copper | μg/L Cu | <10 * |
| Iron | μg/L Fe | <20 * |
| Lead | μg/L Pb | <6.0 * |
| Magnesium | mg/L Mg | 2.7 |
| Manganese | μg/L Mn | <15 * |
| Mercury | μg/L Hg | <0.30 * |
| Nickel | μg/L Ni | <6.0 * |
| Potassium | mg/L K | 0.90 |
| Selenium | μg/L Se | <1.0 * |
| Sodium | mg/L Na | 3.3 |
| Vanadium | μg/L V | <5.0 * |
| Zinc | mg/L Zn | <0.20 * |
| Case Code | Age | Skin Disorder | Location | Erythema | Itching | Flaking | |
|---|---|---|---|---|---|---|---|
| CB-1, n = 7 | 29 | 60 | Psoriasis | Elbow | 0 | 0 | 0 |
| 26 | 77 | Psoriasis | Left arm | 1 | 1 | 1 | |
| 19 | 73 | Psoriasis | Forearm | 1 | 0 | 1 | |
| 17 | 46 | Psoriasis | Left elbow | 1 | 1 | 1 | |
| 9 | 73 | Psoriasis | Leg | 1 | 1 | 0 | |
| 24 | 65 | Psoriasis | Elbow | 1 | 1 | 0 | |
| 4 | 38 | Psoriasis | Left shoulder | 1 | 1 | 1 | |
| C-1, n = 7 | 23 | 82 | Dermatitis | Leg | 0 | 0 | 0 |
| 20 | 27 | Psoriasis | Arm | 0 | 1 | 1 | |
| 11 | 12 | Psoriasis | Chest | 1 | 1 | 1 | |
| 3 | 53 | Psoriasis | Forearm | 1 | 1 | 1 | |
| 27 | 35 | Psoriasis | Left foot | 1 | 1 | 1 | |
| 28 | 83 | Psoriasis | Knee | 1 | 1 | 1 | |
| 26 | 61 | Psoriasis | Elbow | 1 | 1 | 1 | |
| C-2, n = 8 | 7 | 55 | Psoriasis | Arms | 1 | 1 | 1 |
| 30 | 63 | Psoriasis | Elbows | 0 | 0 | 1 | |
| 21 | 66 | Psoriasis | Right hand | 0 | 0 | 0 | |
| 25 | 52 | Psoriasis | Left arm | 1 | 0 | 0 | |
| 22 | 35 | Psoriasis | Forearm | 0 | 1 | 1 | |
| 15 | 18 | Atopic eczema | Arm | 1 | 1 | 1 | |
| 1 | 55 | Atopic eczema | Right elbow | 0 | 1 | 1 | |
| 2 | 66 | Psoriasis | Right elbow | 1 | 1 | 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida, C.; Madeira, A.; Marto, J.; Graça, A.; Pinto, P.; Ribeiro, H. Monfortinho Thermal Water-Based Creams: Effects on Skin Hydration, Psoriasis, and Eczema in Adults. Cosmetics 2019, 6, 56. https://doi.org/10.3390/cosmetics6030056
Almeida C, Madeira A, Marto J, Graça A, Pinto P, Ribeiro H. Monfortinho Thermal Water-Based Creams: Effects on Skin Hydration, Psoriasis, and Eczema in Adults. Cosmetics. 2019; 6(3):56. https://doi.org/10.3390/cosmetics6030056
Chicago/Turabian StyleAlmeida, Cristina, Ana Madeira, Joana Marto, Angélica Graça, Pedro Pinto, and Helena Ribeiro. 2019. "Monfortinho Thermal Water-Based Creams: Effects on Skin Hydration, Psoriasis, and Eczema in Adults" Cosmetics 6, no. 3: 56. https://doi.org/10.3390/cosmetics6030056
APA StyleAlmeida, C., Madeira, A., Marto, J., Graça, A., Pinto, P., & Ribeiro, H. (2019). Monfortinho Thermal Water-Based Creams: Effects on Skin Hydration, Psoriasis, and Eczema in Adults. Cosmetics, 6(3), 56. https://doi.org/10.3390/cosmetics6030056








