Cosmeceutical Properties of Two Cultivars of Red Raspberry Grown under Different Conditions
Abstract
:1. Introduction
2. Material and Methods
2.1. Plant Materials
2.2. Physicochemical Properties of Fruit Samples
2.3. Determination of Soluble Sugars and Ascorbic Acid
2.4. Determination of Metabolites
2.5. Total Antioxidant Capacity Assays
2.6. Enzyme Inhibition Assays
2.7. Assay of Protein Concentration
2.8. Topical Skin Care Cream Formulation and Preliminary Stability Analysis
2.9. Statistical Analysis
3. Results and Discussion
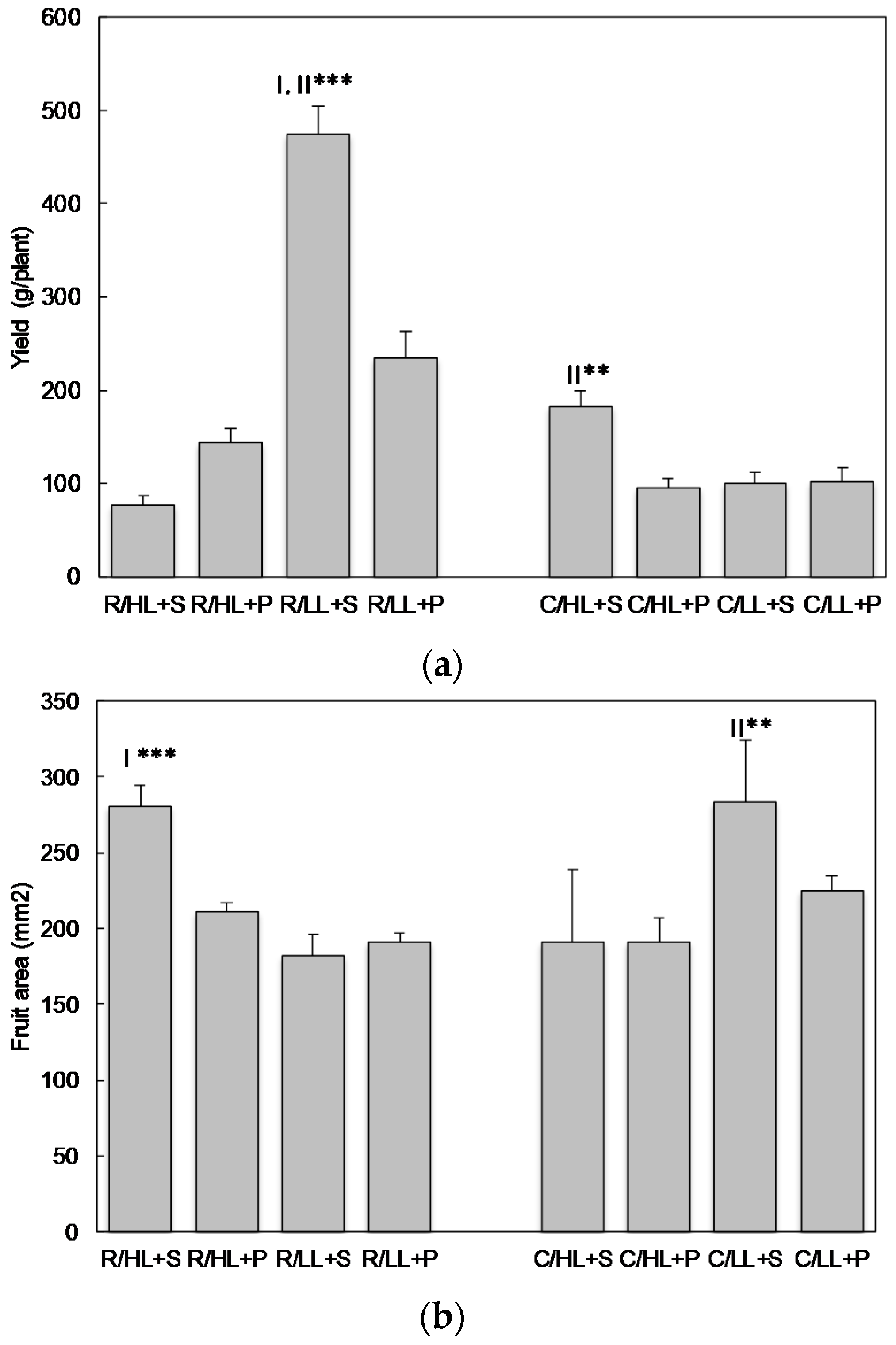
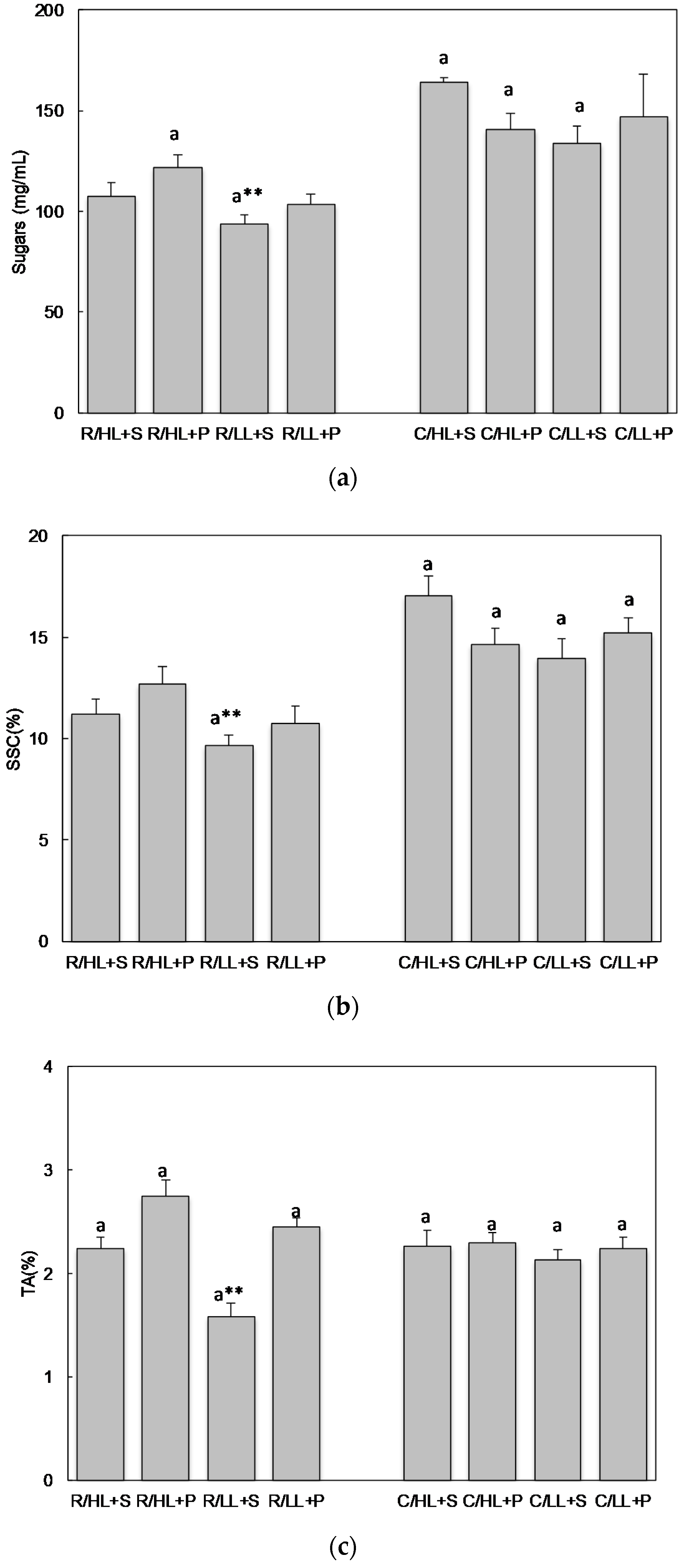
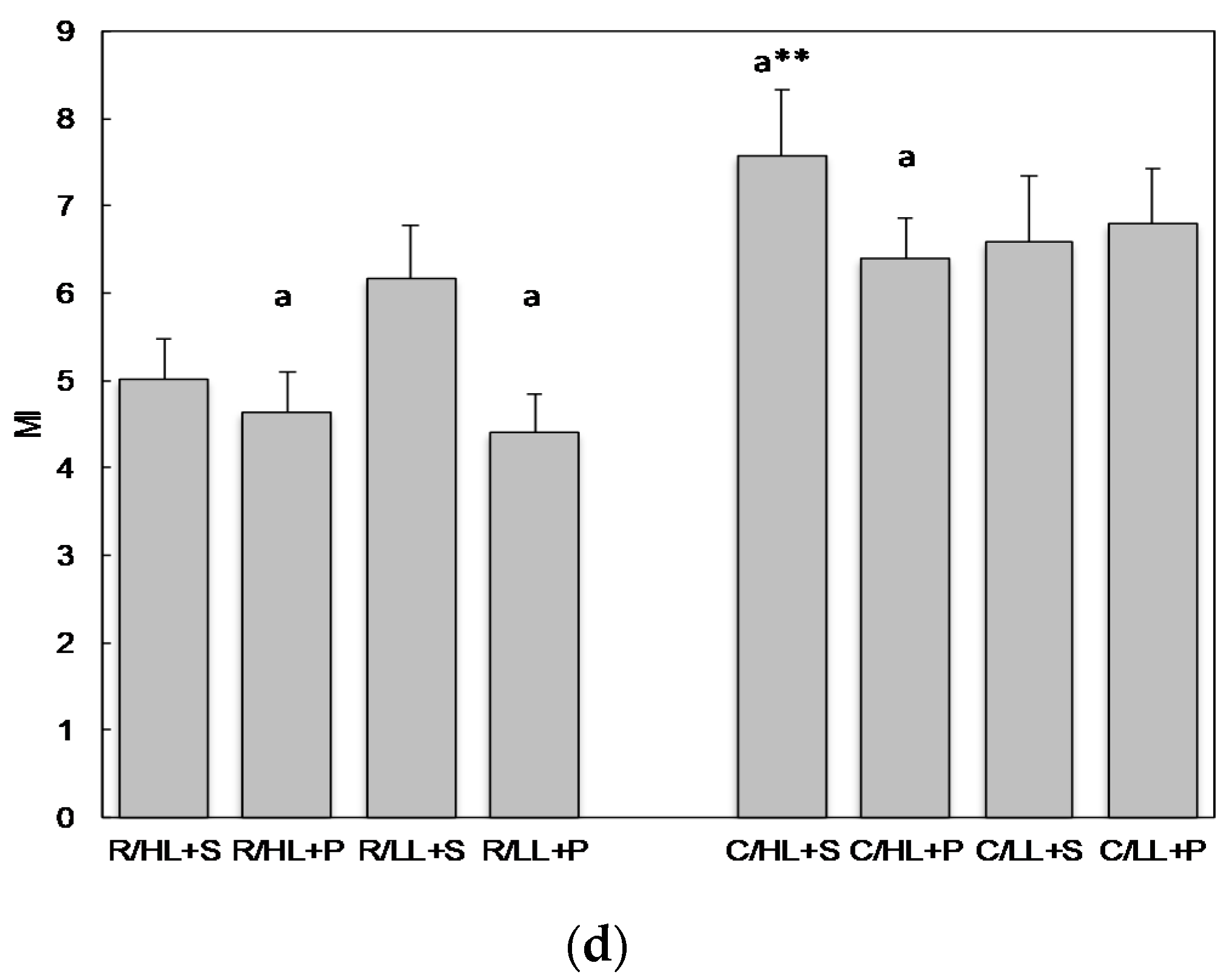
3.1. The Effect of Cultivars, Light Intensity and Substrate on Selected Agronomic Parameters
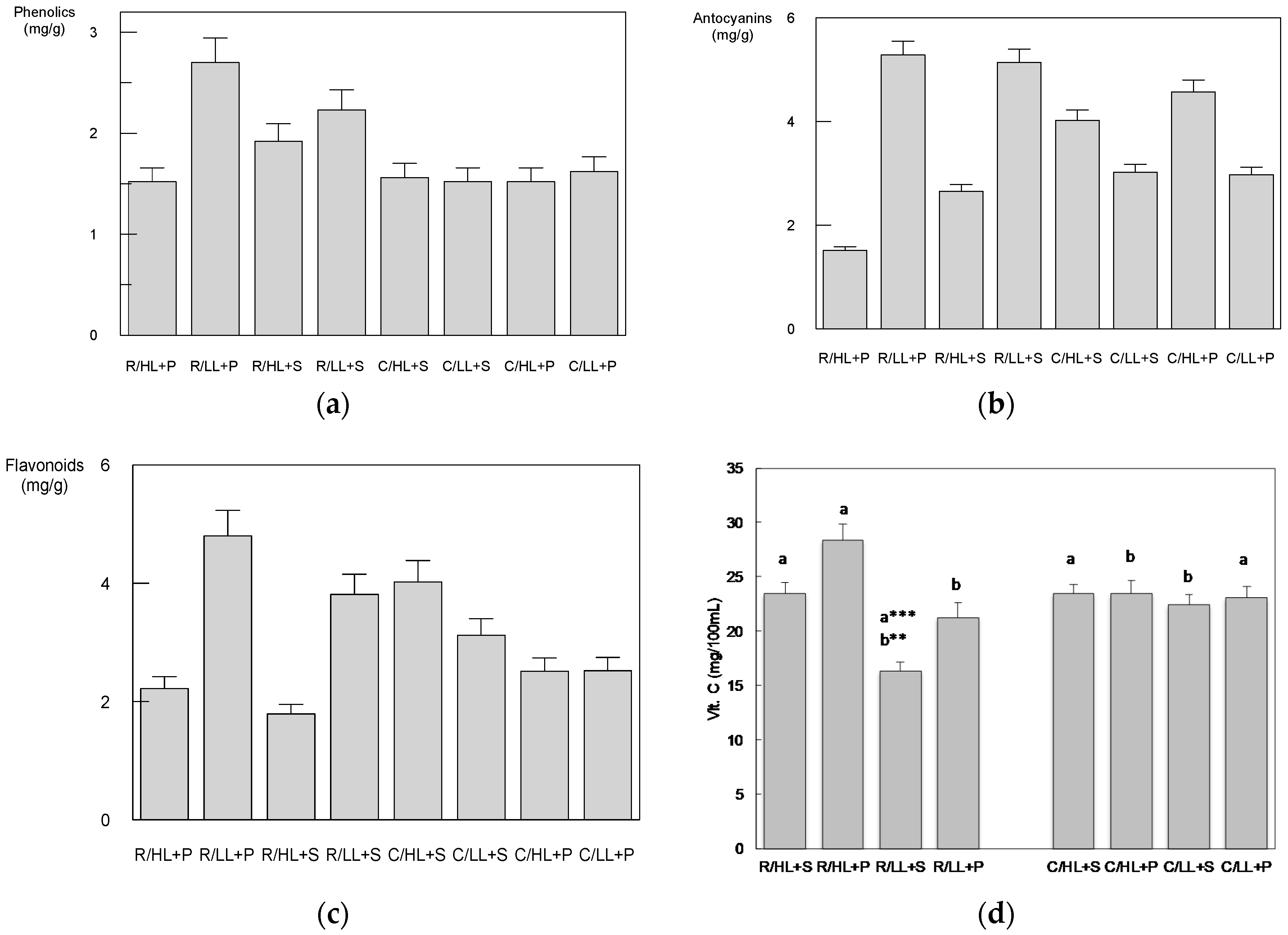
3.2. The Effect of Cultivars, Light Intensity and Substrate Compositions on Selected Secondary Metabolites
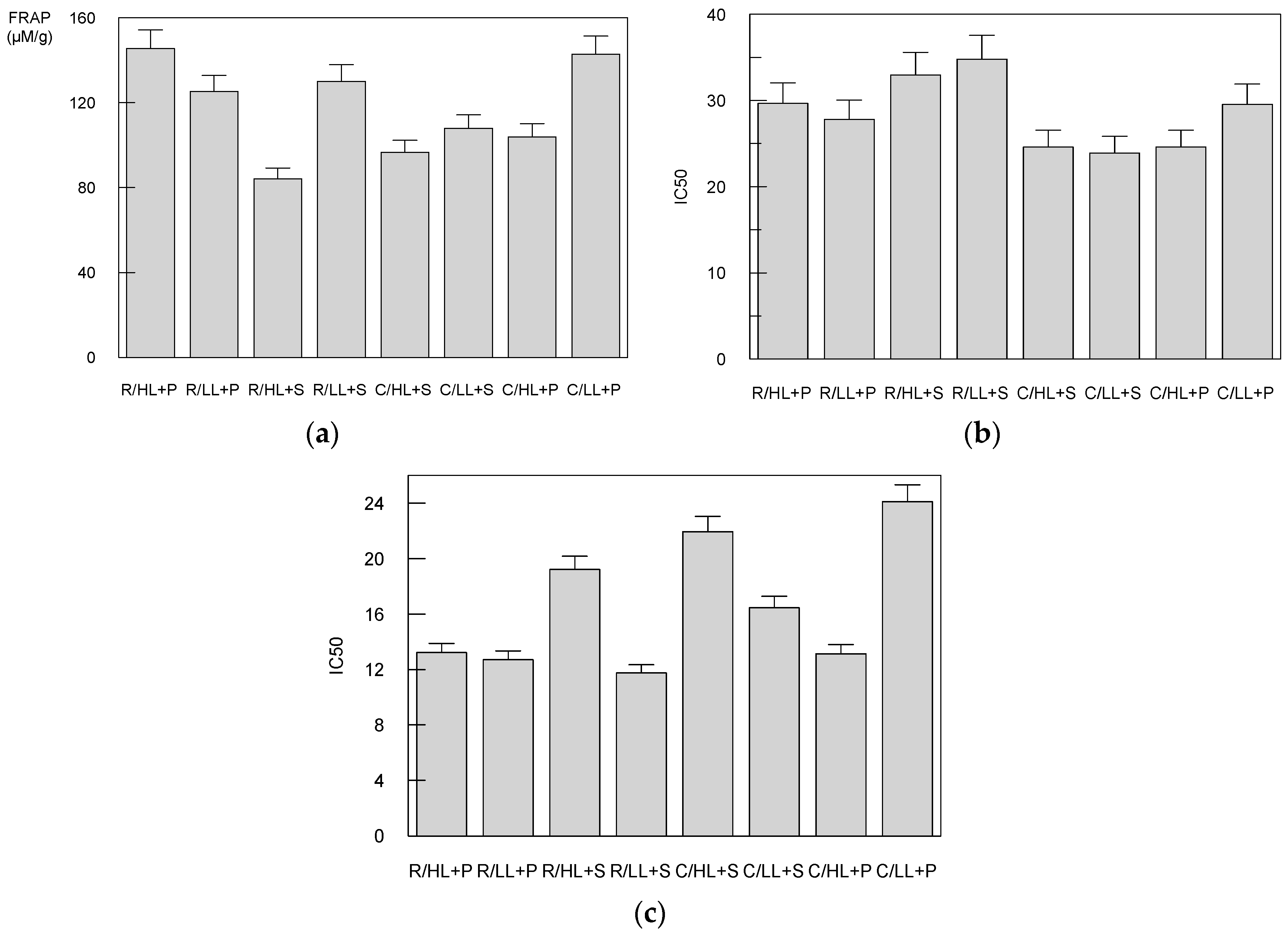
3.3. The Effect of Cultivars, Light Intensity and Substrate Compositions on Antioxidant Capacity
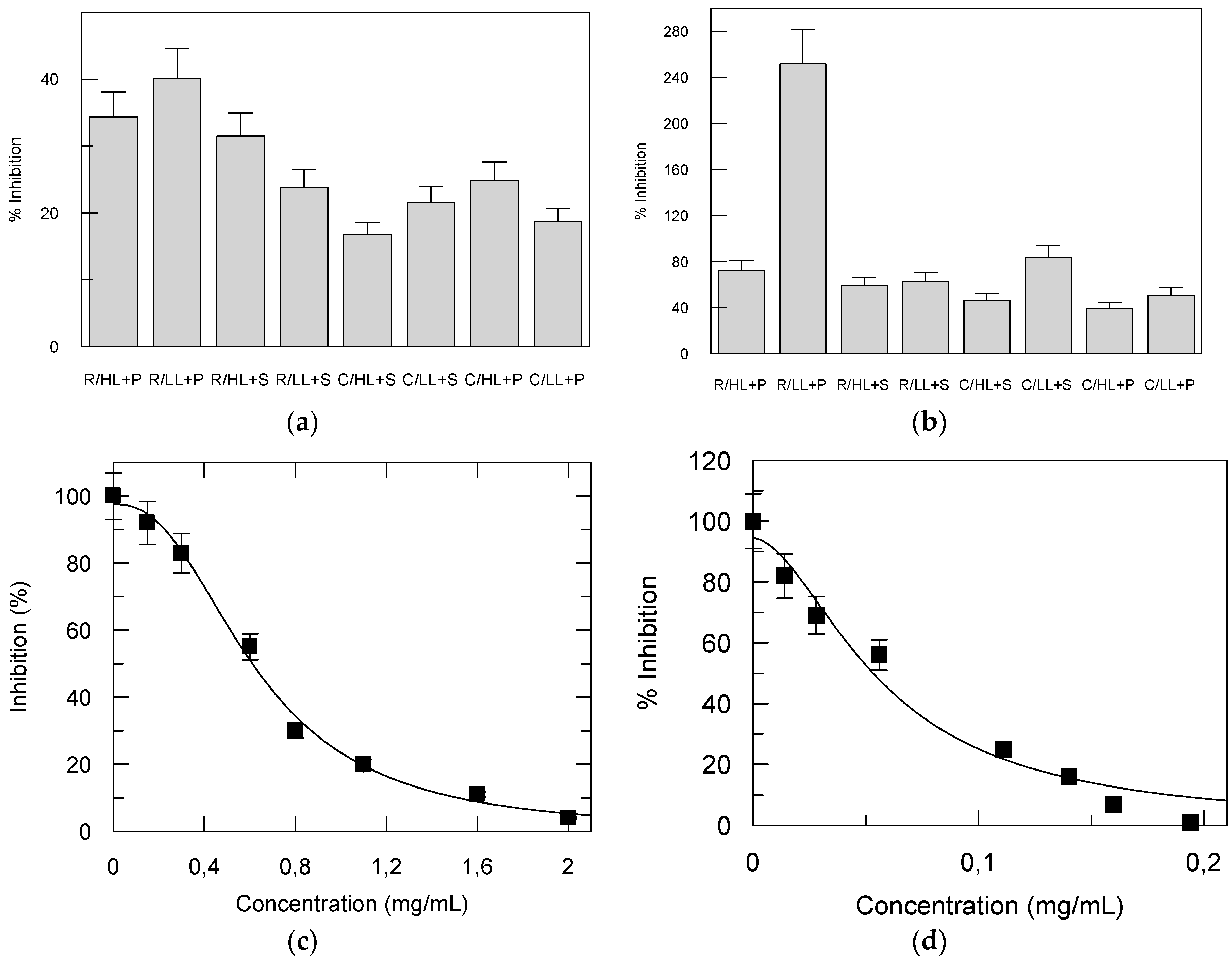
3.4. The Effect of Cultivars, Light Intensity and Substrate Compositions on Elastase, Tyrosinase and Acetylcholinesterase Inhibition
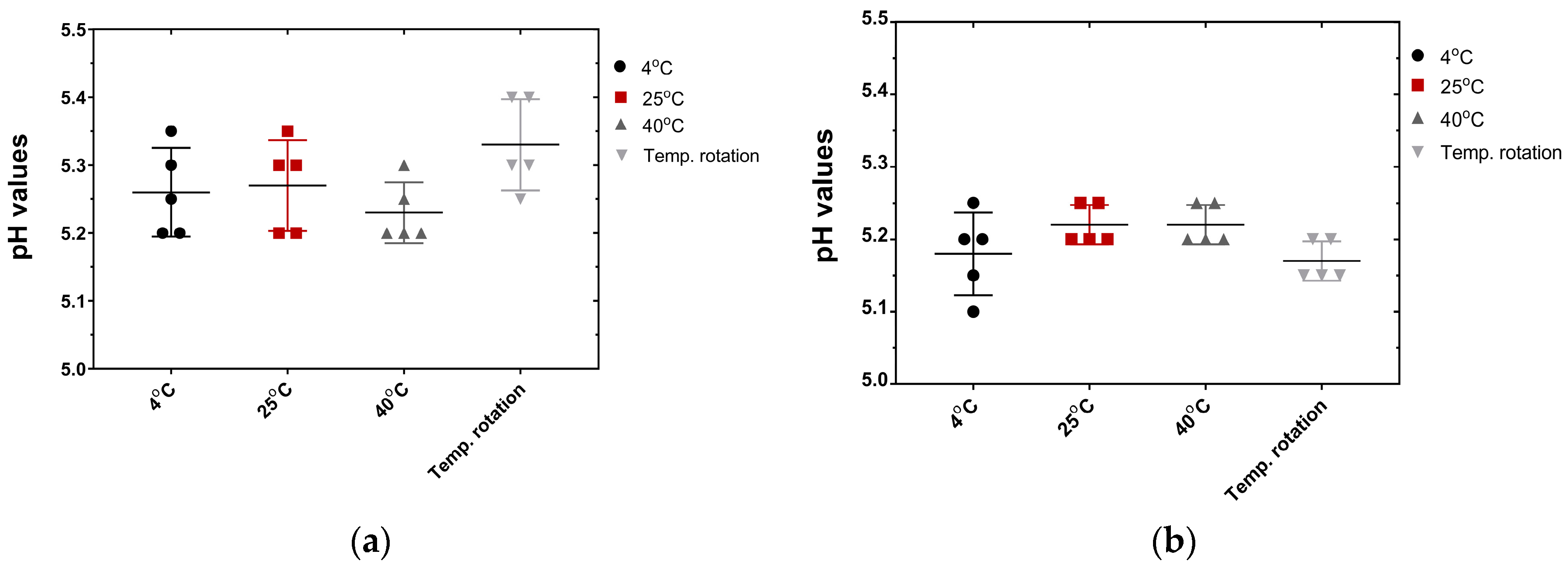
3.5. Formulation of a Topical Skin Care Cream and Preliminary Stability Evaluation
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ACC | Anthocyanins content |
| ABTS | 2,2′-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid free radical scavenging method |
| ACNS | Anthocyanins |
| DPPH | α,α-diphenyl-β-picrylhydrazyl free radical scavenging method |
| DW | Dry weight |
| El | Elastase |
| FRAP | Ferric reducing ability of plasma |
| GAE | Gallic acid equivalents |
| HL | High light |
| % I | % Inhibition |
| LL | Low light |
| MI | Maturity index (ratio of total soluble solids/titratable acidity, SSC/TA) |
| S | Soil |
| P | Soil/peat mixture |
| SSC | Soluble solids content |
| TA | Titratable acidity |
| TAC | Total antioxidant capacity |
| TP | Total phenolics |
| TF | Total flavonoids |
| TYR | Tyrosinase |
References
- Kapsak, W.R.; Rahavi, E.B.; Childs, N.M.; White, C. Functional foods: Consumer attitudes, perceptions, and behaviors in a growing market. J. Am. Diet. Assoc. 2011, 111, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Burton-Freeman, B.M.; Sandhu, A.K.; Edirisinghe, I. Red Raspberries and Their Bioactive Polyphenols: Cardiometabolic and Neuronal Health Links. Adv. Nutr. 2016, 7, 44–65. [Google Scholar] [CrossRef] [PubMed]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive Compounds and Antioxidant Activity in Different Types of Berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [PubMed]
- Manganaris, G.A.; Goulas, V.; Vicente, A.R.; Terry, L.A. Berry antioxidants: Small fruits providing large benefits. J. Sci. Food Agric. 2014, 94, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Antonopoulou, I.; Varriale, S.; Topakas, E.; Rova, U.; Christakopoulos, P.; Faraco, V. Enzymatic synthesis of bioactive compounds with high potential for cosmeceutical application. Appl. Microbiol. Biotechnol. 2016, 100, 6519–6543. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.M. Fifty years of research and development of cosmeceuticals: A contemporary review. J. Cosmet. Dermatol. 2016, 5, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Valls, J.; Millán, S.; Martí, M.P.; Borràs, E.; Arola, L. Advanced separation methods of food anthocyanins, isoflavones and flavanols. J. Chromatogr. A 2009, 1216, 7143–7172. [Google Scholar] [CrossRef] [PubMed]
- Beattie, J.; Crozier, A.; Duthie, G.G. Potential health benefits of berries. Curr. Nutr. Food Sci. 2005, 1, 77–86. [Google Scholar] [CrossRef]
- Rao, A.V.; Snyder, D.M. Raspberries and human health: A review. J. Agric. Food Chem. 2010, 58, 3871–3883. [Google Scholar] [CrossRef] [PubMed]
- Kafkas, E.; Özgen, M.; Özoğul, Y.; Türemiş, N. Phytochemical and fatty acid profile of selected red raspberry cultivars: A comparative study. J. Fruit Qual. 2008, 31, 67–78. [Google Scholar] [CrossRef]
- Kahkonen, M.P.; Hopia, A.I.; Heinonen, M. Berry phenolics and their antioxidant activity. J. Agric. Food Chem. 2001, 49, 4076–4082. [Google Scholar] [CrossRef] [PubMed]
- Juranic, Z.; Zizak, Z. Biological activities of berries: From antioxidant capacity to anti-cancer effects. Biofactors 2005, 23, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Zafra-Stone, S.; Yasmin, T.; Bagchi, M.; Chatterjee, A.; Vinson, J.A.; Bagchi, D. Berry anthocyanins as novel antioxidants in human health and disease prevention. Mol. Nutr. Food Res. 2007, 51, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Maro, L.A.C.; Pio, R.; Guedes, M.N.S.; Patto de Abreu, C.M.; Curi, P.N. Bioactive compounds, antioxidant activity and mineral composition of fruits of raspberry cultivars grown in subtropical areas in Brazil. Fruits 2013, 68, 209–217. [Google Scholar] [CrossRef]
- Corradini, E.; Foglia, P.; Giansanti, P.; Gubbiotti, R.; Samperi, R.; Lagana, A. Flavonoids: Chemical properties and analytical methodologies of identification and quantitation in foods and plants. Nat. Prod. Res. 2011, 25, 469–495. [Google Scholar] [CrossRef] [PubMed]
- Puupponen-Pimia, R.; Nohynek, L.; Meier, C.; Kähkönen, M.; Heinonen, M.; Hopia, A.; Oksman-Caldentey, K.M. Antimicrobial properties of phenolic compounds from berries. J. Appl. Microbiol. 2001, 90, 494–507. [Google Scholar] [CrossRef] [PubMed]
- Zia-Ul-Haq, M.; Riaz, M.; De Feo, V.; Jaafar, H.Z.; Moga, M. Rubus fruticosus L.: Constituents, biological activities and health related uses. Molecules 2014, 19, 10998–11029. [Google Scholar] [CrossRef] [PubMed]
- Anttonen, M.J.; Karjalainen, R.O. Environmental and genetic variation of phenolic compounds in red raspberry. J. Food Compos. Anal. 2005, 18, 759–769. [Google Scholar] [CrossRef]
- Connor, A.M.; Stephens, M.J.; Hall, H.K.; Alspach, P.A. Variation and heritabilities of antioxidant activity and total phenolic content estimated from a red raspberry factorial experiment. J. Am. Soc. Hortc. Sci. 2005, 130, 403–411. [Google Scholar]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Huda-Faujan, N.; Noriham, A.; Norrakiah, A.S.; Babji, A.S. Antioxidant activity of plants methanolic extracts containing phenolic compounds. Afr. J. Biotechnol. 2009, 8, 484–489. [Google Scholar]
- Sønste, A.; Opstada, N.; Myrheim, U.; Heideb, O.M. Interaction of short day and timing of nitrogen fertilization on growth and flowering of ‘Korona’ strawberry (Fragaria × ananassa Duch.). Sci. Hortic. 2009, 123, 204–209. [Google Scholar] [CrossRef]
- Garner, D.; Crisosto, C.H.; Wiley, P.; Crisosto, G.M. Measurement of Soluble Solids Content. Measurement of pH and Titratable Acidity. Cent. Valley Postharvest Newslett. 2008, 17, 2–4. [Google Scholar]
- Sadasivam, S.; Manickam, A. Vitamins. In Biochemical Methods, 2nd ed.; Sadasivam, S., Manickam, A., Eds.; New Age International (P) Ltd.: New Delhi, India, 1997; pp. 185–186. [Google Scholar]
- Papaioanou, M.; Chronopoulou, E.; Ciobotari, G.; Efrose, R.C.; Sfichi-Duke, L.; Chatzikonstantinou, M.; Ganopoulos, I.; Madesis, P.; Nianiou-Obeidat, I.; Labrou, N.E. Evaluation of the nutraceutical and cosmeceutical potential of two cultivars of Rubus fruticosus L. under different cultivation conditions. Curr. Pharm. Biotechnol. 2017, 18, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Huo, L.; Su, W.; Lu, R.; Deng, C.; Liu, L.; Deng, Y.; Guo, N.; Lu, C.; He, C. Free radical-scavenging capacity, antioxidant activity and phenolic content of Pouzolzia zeylanica. J. Serb. Chem. Soc. 2011, 76, 709–717. [Google Scholar] [CrossRef]
- Pellegrini, N.; Serafini, M.; Colombi, B.; Del Rio, D.; Salvatore, S.; Bianchi, M.; Brighenti, F. Total Antioxidant Capacity of Plant Foods, Beverages and Oils Consumed in Italy Assessed by Three Different In Vitro Assays. J. Nutr. 2003, 133, 2812–2819. [Google Scholar] [CrossRef] [PubMed]
- Molyneux, P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Bieth, J.; Spiess, B.; Wermuth, C.G. The synthesis and analytical use of a highly sensitive and convenient substrate of elastase. Biochem. Med. 1974, 11, 350–357. [Google Scholar] [CrossRef]
- Duckworth, H.W.; Coleman, J.E. Physicochemical and kinetic properties of mushroom tyrosinase. J. Biol. Chem. 1970, 245, 1613–1625. [Google Scholar] [PubMed]
- Worek, F.; Eyer, P.; Thiermann, H. Determination of acetylcholinesterase activity by the Ellman assay: A versatile tool for in vitro research on medical countermeasures against organophosphate poisoning. Drug Test Anal. 2012, 4, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M. A rapid and sensitive method for the detection of microgram quantities of proteins utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Rieger, M.M. Stability testing of Macroemulsions. Cosmet. Toilet. 1991, 106, 60–69. [Google Scholar]
- Morais, J.M.; Santos, O.D.H.; Delicato, H.; Goncalves, T.; Azzini, R.; Rocha-Filcho, P.A. Physicochemical characterization of canola oil/water nanoemulsions obtained by determination of required HBL number and emusion phase inversion methods. J. Dispers. Sci. Technol. 2006, 27, 109–115. [Google Scholar] [CrossRef]
- Hussain, I.; Roberto, S.R.; Koyama, R.; Marinho de Assis, A.; Colombo, R.C.; Batista Fonseca, I.C.; Correa Antunes, L.E. Performance of ‘Tupy’ and ‘Xavante’ blackberries under subtropical conditions. Fruits 2017, 72, 166–173. [Google Scholar] [CrossRef]
- Antunes, L.E.C.; Gonçalves, E.D.; Trevisan, R. Phenology and production of blackberry cultivars in agroecological system. Ciência Rural 2010, 40, 1929–1933. [Google Scholar] [CrossRef]
- Clark, J.R.; Finn, C.E. Blackberry breeding and genetics. In Methods in Temperate Fruit Breeding. Fruit, Vegetable, and Cereal Science and Biotechnology; Flachowsky, H., Hanke, V.M., Eds.; Global Science Books Ltd.: London, UK, 2011; Volume 5, pp. 27–43. [Google Scholar]
- Wright, C.J.; Waister, P.D. Light interception and fruiting cane architecture in the red raspberry grown under annual and biennial management systems. J. Hortc. Sci. 1984, 59, 395–402. [Google Scholar] [CrossRef]
- Pritts, M.P.; Brennan, R.M.; Gordon, S.L.; Williamson, B. From plant to plate: How can we redesign Rubus production systems to meet future expectations? Acta Hortc. 2002, 585, 537–543. [Google Scholar] [CrossRef]
- Fernandez, G.E.; Ballington, J.R. Performance of primocane-fruiting experimental blackberry cultivars in the Southern Appalachian Mountains. HortTechnology 2010, 20, 996–1000. [Google Scholar]
- Lobos, G.A.; Retamales, J.B.; del Pozo, A.; Hancock, J.F.; Flore, J.A. Physiological response of Vaccinium corymbosum “Elliott” to shading nets in Michigan. Acta Hortic. 2009, 810, 465–470. [Google Scholar] [CrossRef]
- Guerrero, V.M.; Orozco, J.A.; Romo, A.; Gardea, A.A.; Molina, F.J.; Sastre, B.; Martinez, J.J. The effect of hail nets and Ethephon on color development of ‘Red Chief Delicious’ apple fruit in the highlands of Chihuahua, Mexico. J. Am. Pomol. Soc. 2002, 56, 132–135. [Google Scholar]
- Pastenes, C.; Santa-Marία, E.; Infante, R.; Franck, N. Domestication of the Chilean guava (Ugni molinae Turcz.), a forest understory shrub, must consider light intensity. Sci. Hortic. 2003, 98, 71–84. [Google Scholar] [CrossRef]
- Gent, M.P.N. Effect of Degree and Duration of Shade on Quality of Greenhouse Tomato. HortScience 2007, 42, 514–520. [Google Scholar]
- Awang, Y.B.; Atherton, J.G. Salinity and Shading Effects on Leaf Water Relations and Ionic Composition of Strawberry Plants Grown on Rockwool. J. Hortic. Sci. 1994, 69, 377–383. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Stampar, F.; Veberic, R. Composition of sugars, organic acids, and total phenolics in 25 wild or cultivated berry species. J. Food Sci. 2012, 77, C1064–C1070. [Google Scholar] [CrossRef] [PubMed]
- Mikulic-Petkovsek, M.; Rescic, J.; Schmitzer, V.; Stampar, F.; Slatnar, A.; Koron, D.; Veberic, R. Changes in fruit quality parameters of four Ribes species during ripening. Food Chem. 2015, 173, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Etienne, A.; Génard, M.; Lobit, P.; Mbeguié-A-Mbéguié, D.; Bugaud, C. What controls fleshy fruit acidity? A review of malate and citrate accumulation in fruit cells. J. Exp. Bot. 2013, 64, 1451–1469. [Google Scholar] [CrossRef] [PubMed]
- Zorenc, Z.; Veberic, R.; Koron, D.; Mikulic-Petkovsek, M. Impact of Raspberry (Rubus idaeus L.) Primocane Tipping on Fruit Yield and Quality. Not. Bot. Horti Agrobot Cluj-Napoca 2017, 45, 417–424. [Google Scholar] [CrossRef]
- Lewers, K.S.; Wang, S.Y.; Vinyard, B.T. Evaluation of blackberry cultivars and breeding selections for fruit quality traits and flowering and fruiting dates. Crop Sci. 2010, 50, 2475–2491. [Google Scholar] [CrossRef]
- Petrussa, E.; Braidot, E.; Zancani, M.; Peresson, C.; Bertolini, A.; Patui, S.; Vianello, A. Plant Flavonoids—Biosynthesis, Transport and Involvement in Stress Responses. Int. J. Mol. Sci. 2013, 14, 14950–14973. [Google Scholar] [CrossRef] [PubMed]
- Falcone Ferreyra, M.L.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci. 2012, 3, 222. [Google Scholar] [CrossRef] [PubMed]
- Bartosz, G. Non-enzymatic antioxidant capacity assays: Limitations of use in biomedicine. Free Radic. Res. 2010, 44, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Veberic, R.; Slatnar, A.; Bizjak, J.; Stampar, F.; Mikulic-Petkovsek, M. Anthocyanin composition of different wild and cultivated berry species. LWT Food Sci. Technol. 2015, 60, 509–517. [Google Scholar] [CrossRef]
- Wang, S.Y.; Chen, C.-T.; Wang, C.Y. The influence of light and maturity on fruit quality and flavonoid content of red raspberries. Food Chem. 2009, 112, 676–684. [Google Scholar] [CrossRef]
- Beretta, G.; Facino, R.M. Recent advances in the assessment of the antioxidant capacity of pharmaceutical drugs: From in vitro to in vivo evidence. Anal. Bioanal. Chem. 2010, 398, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Choochote, W.; Suklampoo, L.; Ochaikul, D. Evaluation of antioxidant capacities of green microalgae. J. Appl. Phycol. 2014, 26, 43–48. [Google Scholar] [CrossRef]
- Leong, L.P.; Shui, G. An investigation of antioxidant capacity of fruits in Singapore markets. Food Chem. 2002, 76, 69–75. [Google Scholar] [CrossRef]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Lebensm. Wiss. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Kim, Y.J.; Uyama, H. Tyrosinase inhibitors from natural and synthetic sources: Structure, inhibition mechanism and perspective for the future. Cell. Mol. Life Sci. 2005, 62, 1707–1723. [Google Scholar] [CrossRef] [PubMed]
- Kamagaju, L.; Morandini, R.; Bizuru, E.; Nyetera, P.; Nduwayezu, J.B.; Stevigny, C.; Ghanem, G.; Duez, P. Tyrosinase modulation by five Rwandese herbal medicines traditionally used for skin treatment. J. Ethnopharmacol. 2013, 146, 824–834. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.J.; Choi, J.N.; Kim, J.; Yeo, S.H.; Choi, J.H.; Lee, C.H. Metabolomics-Based Optimal Koji Fermentation for Tyrosinase Inhibition Supplemented with Astragalus Radix. Biosci. Biotechnol. Biochem. 2012, 76, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.K.; Kim, J.H.; Cho, J.J.; Choi, J.D. Inhibitory effect of 150 plant extracts on elastase activity and their anti-in ammatory effects. Int. J. Cosmet. Sci. 1999, 21, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Thring, T.S.A.; Hili, P.; Naughton, D.P. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complement. Altern. Med. 2009, 9, 27. [Google Scholar] [CrossRef]
- Čolović, M.; Krstić, D.Z.; Lazarević-Pašti, T.D.; Bondžić, A.M.; Vasić, V. Acetylcholinesterase Inhibitors: Pharmacology and Toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef] [PubMed]
- Riefenrath, W.G.; Hawkins, G.S.; Kurtz, M.S. Percutaneous penetration and skin retention of topically applied compounds: An in vitro/in vivo study. J. Pharm. Pharm. Sci. 1991, 80, 526–532. [Google Scholar] [CrossRef]

| Species | Cultivar | Light | Substrate | Abbreviation |
|---|---|---|---|---|
| Raspberry | ‘Ruvi’ | High light (HL) | Soil (S) | R/HL + S |
| Soil/peat (P) | R/HL + P | |||
| Low light (LL) | Soil (S) | R/LL + S | ||
| Soil/peat (P) | R/LL + P | |||
| ‘Cayuga’ | High light (HL) | Soil (S) | C/HL + S | |
| Soil/peat (P) | C/HL + P | |||
| Low light (LL) | Soil (S) | C/LL + S | ||
| Soil/peat (P) | C/LL + P |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papaioanou, M.; Chronopoulou, E.G.; Ciobotari, G.; Efrose, R.C.; Sfichi-Duke, L.; Chatzikonstantinou, M.; Pappa, E.; Ganopoulos, I.; Madesis, P.; Nianiou-Obeidat, I.; et al. Cosmeceutical Properties of Two Cultivars of Red Raspberry Grown under Different Conditions. Cosmetics 2018, 5, 20. https://doi.org/10.3390/cosmetics5010020
Papaioanou M, Chronopoulou EG, Ciobotari G, Efrose RC, Sfichi-Duke L, Chatzikonstantinou M, Pappa E, Ganopoulos I, Madesis P, Nianiou-Obeidat I, et al. Cosmeceutical Properties of Two Cultivars of Red Raspberry Grown under Different Conditions. Cosmetics. 2018; 5(1):20. https://doi.org/10.3390/cosmetics5010020
Chicago/Turabian StylePapaioanou, Maria, Evangelia G. Chronopoulou, Gheorghii Ciobotari, Rodica C. Efrose, Liliana Sfichi-Duke, Marianna Chatzikonstantinou, Evangelia Pappa, Ioannis Ganopoulos, Panagiotis Madesis, Irini Nianiou-Obeidat, and et al. 2018. "Cosmeceutical Properties of Two Cultivars of Red Raspberry Grown under Different Conditions" Cosmetics 5, no. 1: 20. https://doi.org/10.3390/cosmetics5010020
APA StylePapaioanou, M., Chronopoulou, E. G., Ciobotari, G., Efrose, R. C., Sfichi-Duke, L., Chatzikonstantinou, M., Pappa, E., Ganopoulos, I., Madesis, P., Nianiou-Obeidat, I., Zeng, T., & Labrou, N. E. (2018). Cosmeceutical Properties of Two Cultivars of Red Raspberry Grown under Different Conditions. Cosmetics, 5(1), 20. https://doi.org/10.3390/cosmetics5010020








