Rutin—Increased Antioxidant Activity and Skin Penetration by Nanocrystal Technology (smartCrystals)
Abstract
:1. Introduction
- The increased saturation solubility of nanosized rutin and subsequently increased concentration gradient between dermal formulation and skin, leading to increased diffusion into the skin.
- The increased dissolution velocity caused by the large surface area of the nanocrystals.
- High adhesion to skin, as all nanosized materials are adhesive.
2. Experimental Section
2.1. Materials
2.2. Production of Rutin Nanocrystal (smartCrystal) Suspension
2.3. Production of Rutin Containing Gel Formulation
2.4. Characterization of Nanocrystals
2.4.1. Photon Correlation Spectroscopy (PCS)
2.4.2. Laser Diffractometry (LD)
2.4.3. Light Microscopy (LM)
2.4.4. Zeta Potential (ZP)
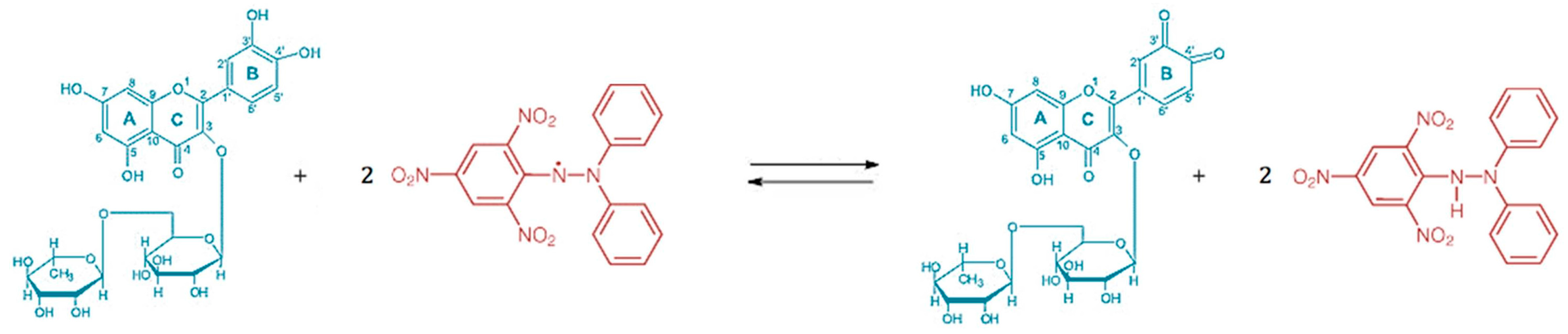
2.5. In-Vitro Antioxidant Activity
2.6. Ex-Vivo Penetration Study on Porcine Ear Skin
2.7. High-Performance Liquid Chromatography (HPLC)
3. Results and Discussion
3.1. Characterization of Rutin Nanocrystal Suspension and the Nanocrystal Gel
3.2. Safety of Rutin and Rutin Nanocrystals in Dermal Formulations
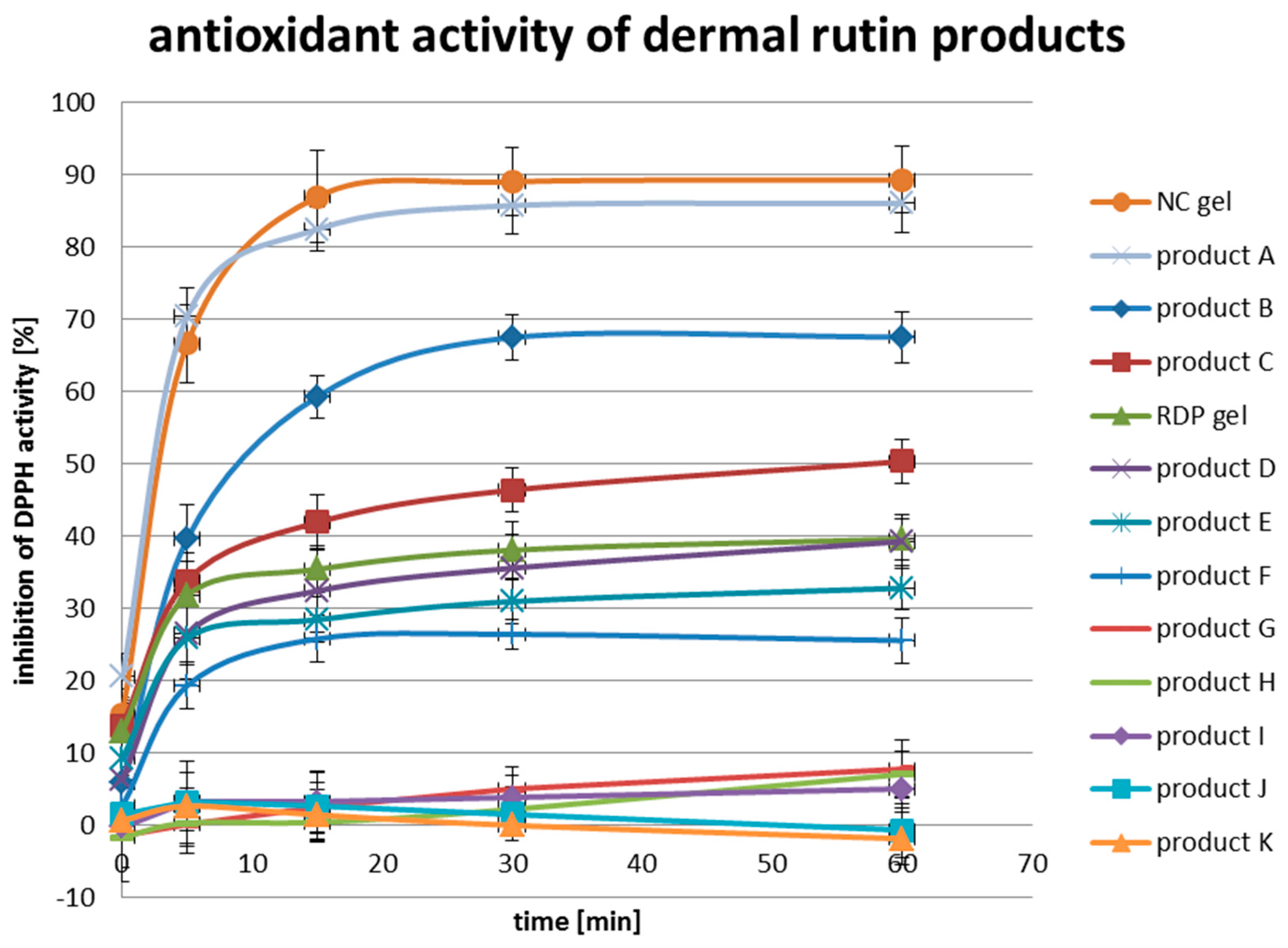
3.3. In-Vitro Antioxidant Activity
3.4. Ex-Vivo Penetration Study on Porcine Ears
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Imber, G.M.D. Antioxidants, free radicals and skin care. In The Youth Corridor: Your Guide to Timeless Beauty; KCM Publishing: Carol Stream, IL, USA, 2013. [Google Scholar]
- Howard, D. Structural Changes Associated with Aging Skin; The International Dermal Institute: Burlington, MA, USA, 2012. [Google Scholar]
- Del Rosso, J.Q.; Levin, J. The clinical relevance of maintaining the functional integrity of the stratum corneum in both healthy and disease-affected skin. J. Clin. Aesthet. Dermatol. 2011, 4, 22–42. [Google Scholar] [PubMed]
- Halliwell, B.; Gutteridge, J.M. Free Radicals in Biology and Medicine; Oxford University Press: New York, NY, USA, 1999; Volume 3, pp. 1–543. [Google Scholar]
- Yuting, C.; Rongliang, Z.; Zhongjian, J.; Yong, J. Flavonoids as superoxide scavengers and antioxidants. Free Radic. Biol. Med. 1990, 9, 19–21. [Google Scholar] [CrossRef]
- Silva, A.R.; Menezes, P.F.C.; Martinello, T.; Novakovich, G.F.L.; Praes, C.E.O.; Feferman, I.H.S. Antioxidant kinetics of plant-derived substances and extracts. Int. J. Cosmet. Sci. 2010, 32, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R. Nanocrystals for Use in Topical Cosmetic Formulations and Method of Production Thereof. U.S. Patent 12/514,621, 25 February 2010. [Google Scholar]
- Lai, F.; Schlich, M.; Pireddu, R.; Corrias, F.; Maria Fadda, A.; Sinico, C. Production of nanosuspensions as a tool to improve drug bioavailability: Focus on topical delivery. Curr. Pharm. Des. 2015, 21, 6089–6103. [Google Scholar] [CrossRef] [PubMed]
- Al Shaal, L.; Müller, R.H.; Shegokar, R. smartCrystal combination technology—Scale up from lab to pilot scale and long term stability. Die Pharm. Int. J. Pharm. Sci. 2010, 65, 877–884. [Google Scholar]
- Mauludin, R.; Müller, R.H.; Keck, C.M. Kinetic solubility and dissolution velocity of rutin nanocrystals. Eur. J. Pharm. Sci. 2009, 36, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Keck, C.M.; Kobierski, S.; Mauludin, R.; Müller, R.H. Second generation of drug nanocrystals for delivery of poorly soluble drugs: smartCrystals technology. Dosis 2008, 24, 124–128. [Google Scholar]
- Keck, C.M.; Müller, R.H. Nanotoxicological classification system (NCS)—A guide for the risk-benefit assessment of nanoparticulate drug delivery systems. Eur. J. Pharm. Biopharm. 2013, 84, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.X.D.; Mazo, L.H.; Cavalheiro, É.T. The use of a graphite-silicone rubber composite electrode in the determination of rutin in pharmaceutical formulation. J. Braz. Chem. Soc. 2008, 19, 1600–1606. [Google Scholar] [CrossRef]
- Craft, B.D.; Kerrihard, A.L.; Amarowicz, R.; Pegg, R.B. Phenol-based antioxidants and the in vitro methods used for their assessment. Compr. Rev. Food Sci. Food Saf. 2012, 11, 148–173. [Google Scholar] [CrossRef]
- Mauludin, R.; Müller, R.H. Preparation and storage stability of rutin nanosuspensions. J. Pharm. Investig. 2013, 43, 395–404. [Google Scholar] [CrossRef]
- Lindemann, U.; Wilken, K.; Schaefer, H.; Sterry, W. Quantification of the horny layer using tape stripping and microscopic techniques. J. Biomed. Opt. 2003, 8, 601–607. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pyo, S.M.; Meinke, M.; Keck, C.M.; Müller, R.H. Rutin—Increased Antioxidant Activity and Skin Penetration by Nanocrystal Technology (smartCrystals). Cosmetics 2016, 3, 9. https://doi.org/10.3390/cosmetics3010009
Pyo SM, Meinke M, Keck CM, Müller RH. Rutin—Increased Antioxidant Activity and Skin Penetration by Nanocrystal Technology (smartCrystals). Cosmetics. 2016; 3(1):9. https://doi.org/10.3390/cosmetics3010009
Chicago/Turabian StylePyo, Sung Min, Martina Meinke, Cornelia M. Keck, and Rainer H. Müller. 2016. "Rutin—Increased Antioxidant Activity and Skin Penetration by Nanocrystal Technology (smartCrystals)" Cosmetics 3, no. 1: 9. https://doi.org/10.3390/cosmetics3010009
APA StylePyo, S. M., Meinke, M., Keck, C. M., & Müller, R. H. (2016). Rutin—Increased Antioxidant Activity and Skin Penetration by Nanocrystal Technology (smartCrystals). Cosmetics, 3(1), 9. https://doi.org/10.3390/cosmetics3010009






