Non-Invasive Nanoparticle Imaging Technologies for Cosmetic and Skin Care Products
Abstract
:1. Introduction
| Technique | Advantages | Disadvantages |
|---|---|---|
| Scanning electron microscopy/Transmission electron microscopy |
|
|
| Single photon laser scanning microscopy |
|
|
| Multiphoton microscopy |
|
|
| Optical coherence tomography |
|
|
| Coherent anti-Stokes Raman Scattering |
|
|
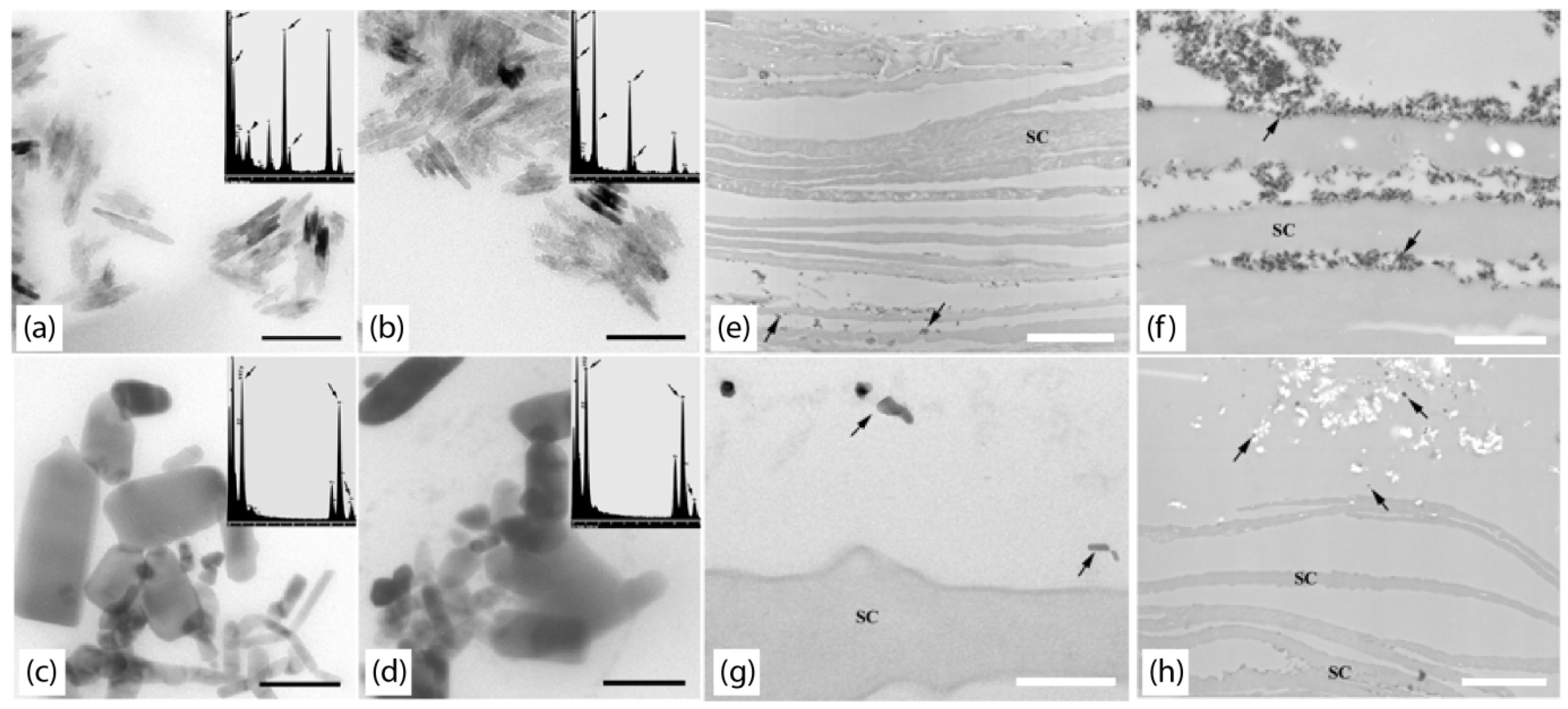
2. Non-Invasive Imaging Techniques
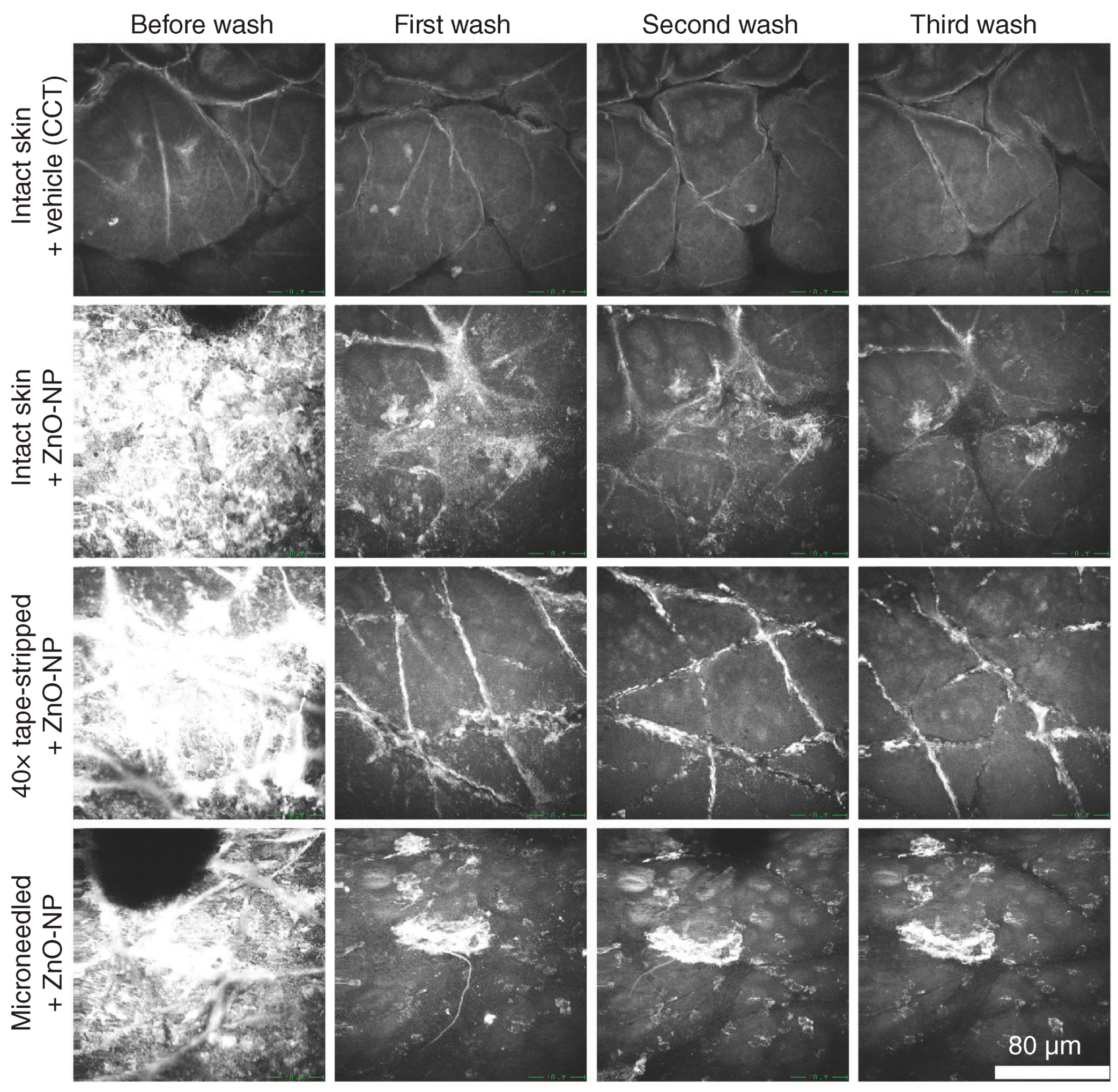
2.1. Single-Photon Confocal Microscopy

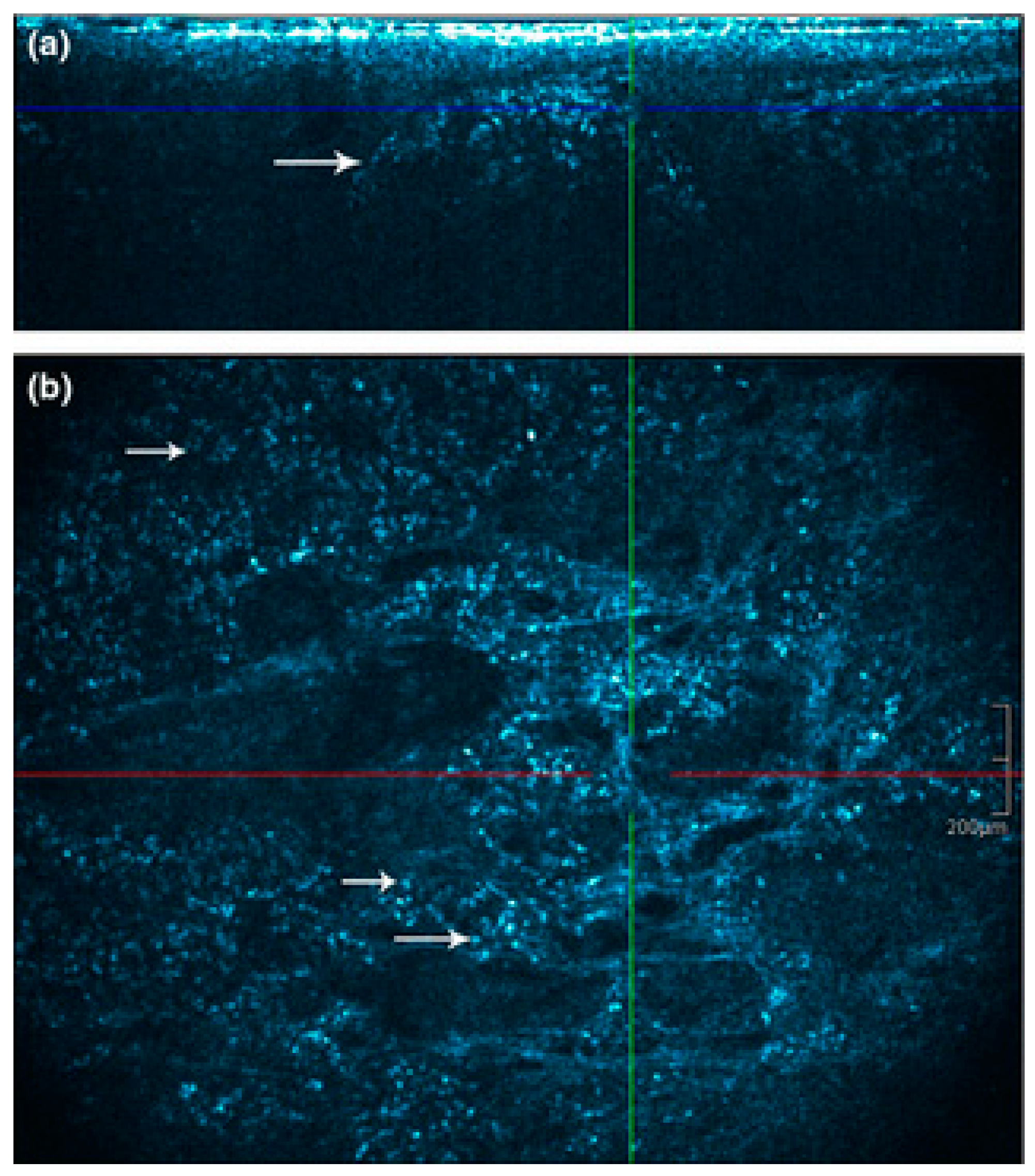
2.2. Multiphoton Microscopy and Time-Correlated Single-Photon Counting
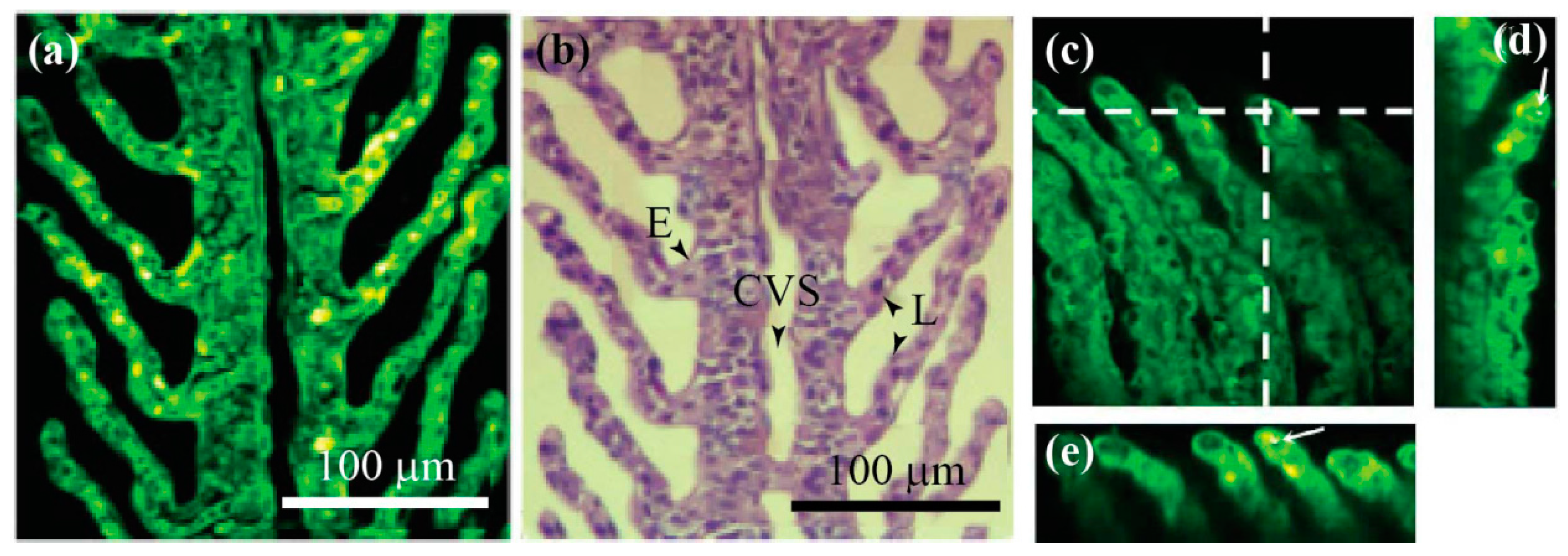
2.3. Optical Coherence Tomography
2.4. Advanced Raman Spectroscopy
3. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, S.Q.; Tooley, I.R. Photoprotection in the era of nanotechnology. Semin. Cutan. Med. Surg. 2011, 30, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Badihi, A.; Benita, S.; Karra, N.; Nasser, T. Nanoparticles for Cosmetic Applications. Patent WO2012101638 A2, 2 August 2012. [Google Scholar]
- Tsujimoto, H.; Hara, K.; Tsukada, Y.; Huang, C.; Kawashima, Y.; Arakaki, M.; Okayasu, H.; Mimura, H.; Miwa, N. Evaluation of the permeability of hair growing ingredient encapsulated PLGA nanospheres to hair follicles and their hair growing effects. Bioorgan. Med. Chem. Lett. 2007, 17, 4771–4777. [Google Scholar] [CrossRef] [PubMed]
- Morganti, P.; Palombo, M.; Tishchenko, G.; Yudin, V.; Guarneri, F.; Cardillo, M.; del Ciotto, P.; Carezzi, F.; Morganti, G.; Fabrizi, G. Chitin-hyaluronan nanoparticles: A multifunctional carrier to deliver anti-aging active ingredients through the skin. Cosmetics 2014, 1, 140–158. [Google Scholar] [CrossRef]
- Matos, B.N.; Reis, T.A.; Gratieri, T.; Gelfuso, G.M. Chitosan nanoparticles for targeting and sustaining minoxidil sulphate delivery to hair follicles. Int. J. Biol. Macromol. 2015, 75, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Zohra, R.R.; Qader, S.A.U.; Mumtaz, M. Scanning electron microscopy analysis of hair index on Karachi’s population for social and professional appearance enhancement. Int. J. Cosmet. Sci. 2015, 37, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Prow, T.W. Multiphoton microscopy applications in nanodermatology. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2012, 4, 680–690. [Google Scholar] [CrossRef] [PubMed]
- Monteiro-Riviere, N.A.; Wiench, K.; Landsiedel, R.; Schulte, S.; Inman, A.O.; Riviere, J.E. Safety evaluation of sunscreen formulations containing titanium dioxide and zinc oxide nanoparticles in UVB sunburned skin: An in vitro and in vivo study. Toxicol. Sci. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Sarder, P.; Yazdanfar, S.; Akers, W.J.; Tang, R.; Sudlow, G.P.; Egbulefu, C.; Achilefu, S. All-near-infrared multiphoton microscopy interrogates intact tissues at deeper imaging depths than conventional single- and two-photon near-infrared excitation microscopes. J. Biomed. Opt. 2013, 18, 106012. [Google Scholar] [CrossRef] [PubMed]
- Claxton, N.S.; Fellers, T.J.; Davidson, M.W. Laser Scanning Confocal Microscopy. Available online: http://www.olympusconfocal.com/theory/LSCMIntro.pdf (accessed on 24 May 2015).
- Ulrich, M.; Lange-Asschenfeldt, S.; Gonzalez, S. Clinical applicability of in vivo reflectance confocal microscopy in dermatology. G. Ital. Dermatol. Venereol. 2012, 147, 171–178. [Google Scholar] [PubMed]
- Nori, S.; Rius-Díaz, F.; Cuevas, J.; Goldgeier, M.; Jaen, P.; Torres, A.; González, S. Sensitivity and specificity of reflectance-mode confocal microscopy for in vivo diagnosis of basal cell carcinoma: A multicenter study. J. Am. Acad. Dermatol. 2004, 51, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Pellacani, G.; Guitera, P.; Longo, C.; Avramidis, M.; Seidenari, S.; Menzies, S. The impact of in vivo reflectance confocal microscopy for the diagnostic accuracy of melanoma and equivocal melanocytic lesions. J. Investig. Dermatol. 2007, 127, 2759–2765. [Google Scholar] [CrossRef] [PubMed]
- Labouta, H.I.; Liu, D.C.; Lin, L.L.; Butler, M.K.; Grice, J.E.; Raphael, A.P.; Kraus, T.; El-Khordagui, L.K.; Soyer, H.P.; Roberts, M.S. Gold nanoparticle penetration and reduced metabolism in human skin by toluene. Pharm. Res. 2011, 28, 2931–2944. [Google Scholar] [CrossRef] [PubMed]
- Prow, T.W.; Grice, J.E.; Lin, L.L.; Faye, R.; Butler, M.; Becker, W.; Wurm, E.M.; Yoong, C.; Robertson, T.A.; Soyer, H.P. Nanoparticles and microparticles for skin drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 470–491. [Google Scholar] [CrossRef] [PubMed]
- Høgsberg, T.; Löschner, K.; Löf, D.; Serup, J. Tattoo inks in general usage contain nanoparticles. Br. J. Dermatol. 2011, 165, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Tattoos & Permanent Makeup: Fact Sheet. Available online: http://www.fda.gov/Cosmetics/ProductsIngredients/Products/ucm108530.htm (accessed on 18 May 2015).
- Maier, T.; Flaig, M.; Ruzicka, T.; Berking, C.; Pavicic, T. High-definition optical coherence tomography and reflectance confocal microscopy in the in vivo visualization of a reaction to permanent make-up. J. Eur. Acad. Dermatol. Venereol. 2014, 29, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Powers, K.W.; Brown, S.C.; Krishna, V.B.; Wasdo, S.C.; Moudgil, B.M.; Roberts, S.M. Research strategies for safety evaluation of nanomaterials. Part VI. Characterization of nanoscale particles for toxicological evaluation. Toxicol. Sci. 2006, 90, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Wokovich, A.; Tyner, K.; Doub, W.; Sadrieh, N.; Buhse, L.F. Particle size determination of sunscreens formulated with various forms of titanium dioxide. Drug Dev. Ind. Pharm. 2009, 35, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Cicchi, R.; Kapsokalyvas, D.; Pavone, F.S. Clinical nonlinear laser imaging of human skin: A review. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Darvin, M.; Konig, K.; Kellner-Hoefer, M.; Breunig, H.; Werncke, W.; Meinke, M.; Patzelt, A.; Sterry, W.; Lademann, J. Safety assessment by multiphoton fluorescence/second harmonic generation/hyper-Rayleigh scattering tomography of ZnO nanoparticles used in cosmetic products. Skin Pharmacol. Physiol. 2012, 25, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.L.; Grice, J.E.; Butler, M.K.; Zvyagin, A.V.; Becker, W.; Robertson, T.A.; Soyer, H.P.; Roberts, M.S.; Prow, T.W. Time-correlated single photon counting for simultaneous monitoring of zinc oxide nanoparticles and NAD (P) H in intact and barrier-disrupted volunteer skin. Pharm. Res. 2011, 28, 2920–2930. [Google Scholar] [CrossRef] [PubMed]
- Zvyagin, A.V.; Zhao, X.; Gierden, A.; Sanchez, W.; Ross, J.A.; Roberts, M.S. Imaging of zinc oxide nanoparticle penetration in human skin in vitro and in vivo. J. Biomed. Opt. 2008, 13, 064031–064031. [Google Scholar] [CrossRef] [PubMed]
- Raphael, A.P.; Sundh, D.; Grice, J.E.; Roberts, M.S.; Soyer, H.P.; Prow, T.W. Zinc oxide nanoparticle removal from wounded human skin. Nanomedicine 2013, 8, 1751–1761. [Google Scholar] [CrossRef] [PubMed]
- Gambichler, T.; Jaedicke, V.; Terras, S. Optical coherence tomography in dermatology: Technical and clinical aspects. Arch. Dermatol. Res. 2011, 303, 457–473. [Google Scholar] [CrossRef] [PubMed]
- Arevalo, J.F.; Mendoza, A.J.; Fernandez, C.F.; Sanchez, J.G.; Reinaldo, A. Clinical applications of optical coherence tomography in macular diseases. In Retinal Angiography and Optical Coherence Tomography; Springer: New York, NY, USA, 2009; pp. 223–238. [Google Scholar]
- Han, S.H.; Yoon, C.H.; Conroy, L.; Vitkin, I.A. OCT monitoring of cosmetic creams in human skin in vivo. Proc. SPIE 2012, 8207. [Google Scholar] [CrossRef]
- Vasquez-Pinto, L.; Maldonado, E.; Raele, M.; Amaral, M.; Freitas, A. Optical coherence tomography applied to tests of skin care products in humans—A case study. Skin Res. Technol. 2015, 21, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Skoulika, S.G.; Georgiou, C.A.; Polissiou, M.G. FT-Raman spectroscopy—Analytical tool for routine analysis of diazinon pesticide formulations. Talanta 2000, 51, 599–604. [Google Scholar] [CrossRef]
- Mulvaney, P. Surface plasmon spectroscopy of nanosized metal particles. Langmuir 1996, 12, 788–800. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Stone, N.; Kendall, C.; Smith, J.; Crow, P.; Barr, H. Raman spectroscopy for identification of epithelial cancers. Faraday Discuss. 2004, 126, 141–157. [Google Scholar] [CrossRef] [PubMed]
- Shetty, G.; Kendall, C.; Shepherd, N.; Stone, N.; Barr, H. Raman spectroscopy: Elucidation of biochemical changes in carcinogenesis of oesophagus. Br. J. Cancer 2006, 94, 1460–1464. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.C.; Jin, R.; Mirkin, C.A. Nanoparticles with Raman spectroscopic fingerprints for DNA and RNA detection. Science 2002, 297, 1536–1540. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.L.; Xie, X.S. Coherent anti-Stokes Raman scattering microscopy: Chemical imaging for biology and medicine. Annu. Rev. Anal. Chem. 2008, 1, 883–909. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Shi, J.; Yang, Y.; Zhang, Y.; Engle, J.W.; Nickles, R.J.; Wang, X.; Cai, W. Cancer-targeted optical imaging with fluorescent zinc oxide nanowires. Nano Lett. 2011, 11, 3744–3750. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Wen, X.; Zheng, Z.; Kable, E.; Cox, G.; Zhu, H. Two-photon optical characteristics of zinc oxide in bulk, low-dimensional and nano-forms. Proc. SPIE 2005, 5931. [Google Scholar] [CrossRef]
- Moger, J.; Johnston, B.D.; Tyler, C.R. Imaging metal oxide nanoparticles in biological structures with CARS microscopy. Opt. Express 2008, 16, 3408–3419. [Google Scholar] [CrossRef] [PubMed]
- Johnston, B.D.; Scown, T.M.; Moger, J.; Cumberland, S.A.; Baalousha, M.; Linge, K.; van Aerle, R.; Jarvis, K.; Lead, J.R.; Tyler, C.R. Bioavailability of nanoscale metal oxides TiO2, CeO2, and ZnO to fish. Environ. Sci. Technol. 2010, 44, 1144–1151. [Google Scholar] [CrossRef] [PubMed]
- Banan, P.; Lin, L.L.; Lambie, D.; Prow, T.; Soyer, H.P. Effects of ex vivo skin microbiopsy on histopathologic diagnosis in melanocytic skin lesions. JAMA Dermatol. 2013, 149, 1107–1109. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.L.; Prow, T.W.; Raphael, A.P.; Harrold, R.L., III; Primiero, C.A.; Ansaldo, A.B.; Soyer, H.P. Microbiopsy engineered for minimally invasive and suture-free sub-millimetre skin sampling. F1000Res. 2013, 2. [Google Scholar] [CrossRef]
- McClenahan, P.; Lin, L.L.; Tan, J.M.; Flewell-Smith, R.; Schaider, H.; Jagirdar, K.; Atkinson, V.; Lambie, D.; Prow, T.W.; Sturm, R.A.; et al. BRAFV600E mutation status of involuting and stable nevi in dabrafenib therapy with or without trametinib. JAMA Dermatol. 2014, 150, 1079–1082. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.M.; Lin, L.L.; Lambie, D.; Flewell-Smith, R.; Jagirdar, K.; Schaider, H.; Sturm, R.A.; Prow, T.W.; Soyer, H.P. BRAF wild-type melanoma in situ arising in a BRAF V600E mutant dysplastic nevus. JAMA Dermatol. 2015, 151, 417–421. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, L.L.; Nufer, K.L.; Tomihara, S.; Prow, T.W. Non-Invasive Nanoparticle Imaging Technologies for Cosmetic and Skin Care Products. Cosmetics 2015, 2, 196-210. https://doi.org/10.3390/cosmetics2030196
Lin LL, Nufer KL, Tomihara S, Prow TW. Non-Invasive Nanoparticle Imaging Technologies for Cosmetic and Skin Care Products. Cosmetics. 2015; 2(3):196-210. https://doi.org/10.3390/cosmetics2030196
Chicago/Turabian StyleLin, Lynlee L., Kaitlin L. Nufer, Shoko Tomihara, and Tarl W. Prow. 2015. "Non-Invasive Nanoparticle Imaging Technologies for Cosmetic and Skin Care Products" Cosmetics 2, no. 3: 196-210. https://doi.org/10.3390/cosmetics2030196
APA StyleLin, L. L., Nufer, K. L., Tomihara, S., & Prow, T. W. (2015). Non-Invasive Nanoparticle Imaging Technologies for Cosmetic and Skin Care Products. Cosmetics, 2(3), 196-210. https://doi.org/10.3390/cosmetics2030196





