Abstract
The potent sensitizer PPD is considered a key sensitizer in hair dye contact allergy. Modification of its molecular structure to 2-methoxymethyl-p-phenylenediamine (ME-PPD) reduces its skin sensitizing potency. We investigated the usage, behavior, and tolerance profile of ME-PPD-containing professional hair color products in a specifically tailored proactive market surveillance program in hairdresser salons across 5 countries. Hairdressers completed record cards for their clients, which were evaluated at the end of the program. 497 individuals received in total 2461 hair color treatments with ME-PPD-containing hair color. Feedback on compatibility was provided for 194 individuals: 6 individuals reported intolerance reactions, which were assessed as likely allergic contact dermatitis (2), likely irritation (2), or were unassessable (2); none of these reactions were severe or serious. Mild discomfort was reported by 46 individuals, while 142 individuals explicitly reported good tolerance to the ME-PPD-containing hair color. A total of 27 individuals applied ME-PPD-containing hair color more than 15 times (long-term tolerability). The study confirms good tolerability of ME-PPD-containing hair color. This is consistent with the primary prevention benefit of ME-PPD in terms of significantly reduced risk of skin sensitization induction and the reduced severity of elicitation reactions for all hair dye users.
1. Introduction
Hair dyeing is a common cosmetic procedure widely used to change natural hair color or cover gray hair, either at home or in a salon (professional use). These formulations contain a mixture of hair dye precursors, which combine chemically in the presence of hydrogen peroxide to form the color pigment inside the hair shaft. Several of these hair dye precursors, in particular the primary intermediates p-phenylenediamine (PPD) and p-toluenediamine (PTD), provide excellent hair coloring performance, but are also considered to be major contact allergens [1]. As a result, allergic contact dermatitis related to the use of hair dye products is an important health concern for a subgroup of consumers.
In this context, substitution of these dyes with alternatives that lower the probability of skin sensitization, and therefore the onset of contact dermatitis, is desirable [2]. The newly developed precursor 2-methoxymethyl-p-phenylenediamine [3] (ME-PPD, see Figure 1) provides excellent hair coloring performance [4] with significantly reduced skin sensitization properties compared to PPD or the structurally related compound PTD [3]. By comparison, the maximal on-head exposure to ME-PPD from hair dye use is more than 100-fold lower than the contact allergy induction threshold (8.8 versus 1075 µg/cm2), whereas the maximal on-head exposures to PPD and PTD are much closer to the induction threshold (i.e., 16.1 versus 27.5 and 22.7 versus 41.3 µg/cm2, respectively) [3]. Consequently, the risk of contact allergy induction through ME-PPD use is considered unlikely, whereas that through PPD and PTD use is well known [3].
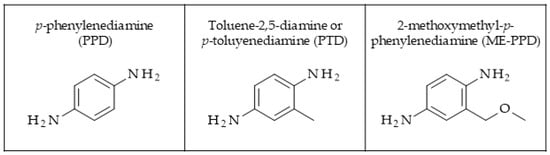
Figure 1.
Chemical structures of the hair dye primary intermediates p-phenylenediamine (PPD), p-toluenediamine (PTD), and 2-methoxymethyl-p-phenylenediamine (ME-PPD).
For oxidative hair dyes, cross-reactions between PPD, PTD, and other para-substituted benzene components have been frequently reported [1,5,6,7]. Consequently, dermatologists generally recommend discontinuing the use of oxidative hair colors for individuals with contact allergy to PPD or PTD to avoid elicitation and cross-reaction with other para-substituted benzene compounds. Nevertheless, many consumers consider hair dyeing as an important personal-care need [8], in particular when related to gray hair coverage, and continue to use hair dyes even though they have been advised to discontinue using respective hair colorants [5]. However, there is no standard predictive approach for the identification of safe exposure levels to avoid elicitation in already allergic individuals [5]. Furthermore, elicitation thresholds cannot be predicted from the relative induction potency of skin sensitization [9].
In a prior study in the Netherlands (n = 30), our group investigated whether individuals with patch test-proven PPD contact allergy from hair dye use respond to ME-PPD-containing hair dye test products. Exposure to ME-PPD under conditions simulating hair dyeing resulted in cross-elicitation to ME-PPD only in 9/30 individuals [10]. In a similar study of an ethnically diverse population of PPD-allergic individuals in the US (n = 20), cross-elicitation to the ME-PPD-containing hair dye test product occurred only in 6/20 individuals [11].
In another cross-elicitation study of PPD-allergic individuals (n = 25) in the Netherlands, 21 reacted to open-use testing with a hair dye containing 2.0% PPD, but only 12 reacted to open-use testing with a hair dye containing 2.0% ME-PPD. After patch testing with increasing ME-PPD concentrations, 13 individuals cross-reacted at 0.1% and 21 at 2.0%, indicating decreased sensitivity when compared with the published PPD elicitation dose–response data [12].
In a further study in Germany (n = 43), 29 of 43 PPD allergic individuals tolerated continued hair dyeing with ME-PPD-containing hair color products with an average of nine treatments per year, if they had no prior reaction to the 45 min forearm exposure [13]. These findings demonstrate a quantitative reduction in cross-reactivity to ME-PPD as compared to PPD and a potential toleration of continuous use of ME-PPD provided negative pre-testing.
ME-PPD dye technology was first commercially introduced in professional hair dyes in 2014 by the Wella Company. ME-PPD, without PPD or PTD, was formulated into 26 shades of a sub-brand (Koleston Perfect Innosense) of a large, already existing professional hair color brand (Koleston Perfect). The new sub-brand was advertised as the first permanent hair color using the ME-PPD, yielding excellent color performance and a reduced sensitization risk.
To increase our knowledge about the user behavior and experience in the context of advertising the reduced sensitization risk of ME-PPD, we developed a market surveillance program specifically tailored to monitor contact allergy related to ME-PPD shades at hairdressing salons. For the participating hairdressers, the study was positioned as a learning program about the new hair dye technology. Hairdressers were asked to document their personal experiences and observations without any specific focus on scalp comfort to avoid potential bias and thus over-reporting.
For this purpose, the record cards normally used in the hairdresser business to document the client’s history were slightly adapted to include an open comment field and handed out to the participating hairdressers. The hairdressers were informed that the adapted record cards do not replace Wella Company’s market surveillance and that any relevant observations had to also be communicated to the company through the usual channels. In this study, we reviewed the record cards with specific attention to the frequency of tolerated ME-PPD hair colorations per consumer, the specific details of the applied shades, and the number of incompatibility reactions to ME-PPD color products. Based on these data, we estimated the tolerance of consumers towards marketed ME-PPD hair colors over an extended period of time.
2. Materials and Methods
2.1. Cases
The study duration was almost 2 years (May 2014 until April 2016), with individual hairdresser salons participating between 11 and 23 months. Individuals (529 salon clients, ID 1-529) who were regular clients of the respective hairdresser salon, and whose hair was to be dyed with Koleston Perfect Innosense as part of everyday hairdresser routine, were recruited in 5 countries (France, Germany, Russia, the UK, and the US) by the 22 participating hairdresser salons. This brand, formulated without PPD and PTD with ME-PPD dye technology, had been introduced to the hairdressers as a vibrant permanent color designed to reduce the risk of developing an allergy. The 26 available shades of this brand were recommended for clients who were ingredient-conscious and interested in the latest innovations. It was emphasized during the hairdresser education that the color was not suitable for allergic consumers due to its potential risk of cross-reactivity, particularly with PPD and PTD, which can lead to severe reactions. Correspondingly, an Allergy Alert Test must be performed 48 h before each coloration as indicated in the use instructions and safety warnings.
2.2. Hair Color Treatments with ME-PPD-Containing Hair Dye Products
The hairdressers performed the hair color applications as usual. In addition, they were asked to complete record cards to document their personal experiences and observations under comments (see Figure 2). In order to avoid bias and potential overreporting, the study was positioned to learn about a hair color product with new hair dye technology (“interest of participation”, “loyalty driver”), rather than to learn about skin reactions. Accordingly, no specifications were made regarding the entries in the comment field (open text field).
Figure 2.
Record card.
At the end of the program, these color record cards were collected and evaluated. Only 21 of the 26 shades in the product range (very light to dark, see Table 1) were considered for the evaluation, as 5 clear or intense red tones did not contain ME-PPD. In addition, one or more of the following additional hair dye precursors were also present depending on the applied shade(s): m-aminophenol, resorcinol, 2-methylresorcinol, hydroxyethyl-3,4-methylene-dioxyanline HCL, 1-hydroxyethyl-4,5-diamino pyrazole sulfate, 2,4-diaminophenoxyethanol HCL, 4-amino-2-hydroxytoluene, 2-methyl-5-hydroxyethylaminophenol, and 2-amino-4-hydroxyethylaminoanisole sulfate.

Table 1.
Grouping of ME-PPD-containing hair dye shades in different tonal shade levels with corresponding concentration ranges of ME-PPD.
The varied and partly non-specific comments on the record cards regarding product compatibility were grouped. As frontline professionals with frequent client interaction and practical experience, hairdressers contribute to the early detection of scalp conditions with their documentation providing a reliable basis for dermatological assessment. In cases where an intolerance reaction was noted by the hairdresser, a dermatologist subsequently assessed the case for causality and seriousness, thereby using a standardized algorithm methodology or seriousness criteria according to the Serious Undesirable Effects (SUE) Reporting Guidelines [14]. Intolerance reactions were also assessed in terms of the severity of the reaction. For our initial assessments, in cases where hairdressers mixed several shades to obtain the desired color result, the shade with the lowest ME-PPD concentration was considered for the evaluation, representing a worst-case exposure. For subsequent evaluation of country-specific shade preferences when several ME-PPD shades were used in a single treatment, analyses were based on the darkest shade used, enabling a clearer picture of country-specific coloration habits.
3. Results
3.1. Composition of Study and Shade Selection
The study population consisted of 529 individuals who received one or more professional hair color treatments with their Koleston Perfect Innosense shade(s) of choice from the 26 available ones at a participating hairdresser salon. A total of 497 selected ME-PPD-containing shades, while 32 selected only non-ME-PPD-containing ones. In total, 2461 full hair color treatments with ME-PPD-containing hair colors were recorded (see Table 2). Thereof, 187 individuals received a single application, and 310 individuals received multiple treatments. Multiple treatments ranged from 2 applications to 23 applications, with an average of 7 applications per individual.

Table 2.
Participation pattern of individuals, hairdresser salons, and number of total hair color treatments per country within the 23-month observation period.
The 21 ME-PPD-containing shades ranging from dark brown to lightest blond were grouped into three tonal shade levels (dark to medium dark, medium dark to light, and light to very light) with ME-PPD on-head concentration ranges of 1.0 ± 0.2%, 0.5 ± 0.2%, and 0.15 ± 0.2%, respectively (see Table 1). Country-specific shade preferences were noted (Table 3). Most individuals were from Russia (209), Germany (131), and France (129); other individuals were from the US (19) and the UK (9). Color shades of the dark to medium dark range were preferred in Russia (92), France (72), and the US (9). Shades of the medium dark to light range were preferred in Germany (62) and in the UK (5).

Table 3.
Distribution of individual preferences for different shade tone levels per country.
3.2. Hair Color Treatment and Compatibility Evaluation
Based on the client record cards, the hairdressers provided their experiences and observations, mainly referring to technical aspects like color result, gray coverage, etc. Comments on dermal compatibility were made for 194 (39%) of the 497 individuals (see Table 4). For evaluation purposes these comments were standardized and grouped into the categories good tolerance, skin discomfort, or intolerance reaction. Under the term skin discomfort, mild transient symptoms were summarized. These were mostly sensorial perturbations such as tingling and/or burning generally not preventing the hairdresser from further hair color applications. Intolerance reactions included symptoms consistent with contact dermatitis (irritation or allergic contact dermatitis), leading to discontinuation of further hair coloring treatments. If noted, reasons why no further hair color treatments took place and comments on historical hair color compatibility (allergies, etc.) were included in the compatibility evaluation.

Table 4.
Grouping of skin compatibility comments from individual record cards per country.
Out of 497 individuals using ME-PPD shades, 6 (ID: 57, 87, 88, 156, 225, and 521) reported an intolerance reaction (see Table 5). Four reported a history of intolerance reactions during or following previous hair colorations.

Table 5.
Characteristics of individuals with intolerance reactions to ME-PPD-containing hair color.
These six intolerances were assessed by a dermatologist for causality, seriousness, and severity according to national vigilance standards.
For the causality assessment, based on the hairdressers’ comments in the record cards, a standardized algorithm methodology was used, which was developed within the framework of the SUE Reporting Guidelines [14]. According to this algorithm, for individuals 57 and 521, an allergic reaction was likely as increasing skin symptoms and itching occurred after the 7th application, which led them to discontinue the program. Epicutaneous patch testing to confirm this suspicion could not be performed. Based on the hairdressers’ comments, an irritant contact dermatitis was most likely for individuals 87 and 88, as symptoms had already appeared during hair color application. For individuals 156 and 225, the reactions were found to be unassessable because the information captured in the comments was insufficient. Thus, no differentiation between irritation and allergic contact dermatitis was possible.
None of the six intolerance reactions was considered serious taking the seriousness criteria into account, which are listed in the European Commission SUE Reporting Guidelines [14]. The guideline defines serious undesirable effects as those that result in temporary or permanent functional incapacity, disability, hospitalization, congenital anomalies, or an immediate vital risk or death.
Based on the reported skin symptoms that did not require treatment, the severity degree of all six intolerance reactions was considered to be mild.
An additional 46 of the 497 ME-PPD users reported some skin discomfort, whereas for 303 participants, no comments related to skin compatibility were provided (Table 4).
By contrast, 142 individuals (29%) reported explicitly good tolerance to the ME-PPD-containing shades. Out of these 142 individuals, 24 had mentioned issues with hair colorations in the past reported as allergy (2); reactions like burning, itching, redness, or irritation (4); or reactions not further specified (18). Another 6 reported a sensitive scalp, while 112 did not comment further.
Next, we evaluated the number of applications per individual for the 491 individuals who did not report an intolerance reaction. The number of applications was 1–5 times for 323 individuals, 5–15 times for 141 individuals, and more than 15 times for 27 individuals.
Finally, we assessed the application frequency per shade of the 21 ME-PPD-containing shades. We found that the distribution of shade tone levels (dark to light) was independent of the number of applications per individual (see Figure 3).
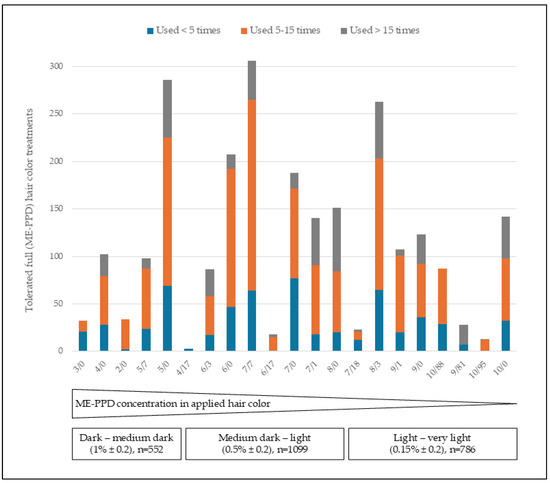
Figure 3.
Number of tolerated full hair color treatments related to 2-methoxymethyl-para-phenlenediamine (ME-PPD) on-head concentration range per shade tone level in percent and use frequency within the 23-month observation period; n = number of tolerated full hair color treatments.
4. Discussion
With the introduction of ME-PPD into commercial hair dye products, a PPD/PTD alternative has become available that is considered to avoid de novo generation of hair dye contact allergy for hair dye users, in line with the principles of primary prevention of skin sensitization and of the onset of contact dermatitis [3]. Although ME-PPD appears to be tolerated in some PPD/PTD-allergic individuals [10,11,12,13] the advice to these individuals is not to dye with a ME-PPD-containing hair dye product. However, despite warnings to not use ME-PPD hair colorants in case of a known hair dye allergy, some individuals with existing allergy to PPD and/or PTD do consider the use of ME-PPD-containing hair dye products as an alternative, especially in cases when patch testing results in only mild skin reactivity to PPD or PTD.
In the present study, we developed an ME-PPD-tailored market surveillance program to monitor skin reactions or contact dermatitis related to ME-PPD-containing hair color, and thereby assess the introduction and marketing of hair colors in the context of the “reduced allergy induction risk” concept. We especially wanted to gain more insights into usage behavior and tolerance profiles of this hair color. This program has been carried out in addition to, and independently from, regulatory requirements. These demands for monitoring of spontaneous reports and collection, as well as analysis of post-marketing adverse event data, are an invaluable tool for identifying potential safety issues associated with a marketed product. But there are limitations in the use and interpretation of spontaneous post-marketing adverse event data, as the adverse event reports from usage of marketed products are made on a voluntary basis by consumers. As such, the rate at which cases are reported is dependent on many factors, including the time since product launch, cultural consumer habits, media attention, and public health concerns. Consequently, not all occurrences of an adverse event may be reported. By carrying out a proactive program, to our knowledge the first of its kind, we wanted to overcome these limitations and obtain complete usage and behavior data of consumers using a new hair brand of ME-PPD-containing hair dyes over a certain time period, resulting in a more complete picture of the in-market situation.
As this new hair dye technology was initially marketed exclusively for professional use, we cooperated with hairdressers and asked them to document usages of the newly launched hair dye over an extended time period, thereby capturing real-world data under typical salon conditions.
The participating hairdressers had to perform the hair color applications as usual and complete record cards (Figure 2). Thus, we managed to capture data from a close follow-up of more than 500 clients and almost 2500 hair color applications.
Among the 497 individuals receiving full hair color treatments with ME-PPD-containing hair colors, only 6 individuals reported that they had any intolerance (Table 5).
These six intolerance reactions were assessed by a dermatologist based on the entries in the individual record cards. The reactions were evaluated for causality, seriousness, and severity according to national vigilance standards.
For the causality assessment, a standardized algorithm methodology was used, which was developed within the framework of the SUE Reporting Guidelines [14]. For two (ID: 156, 225) of the six individuals, the reaction was not assessable, while for two individuals (ID: 87, 88), an irritant contact dermatitis would be most likely. For two individuals (ID: 57, 521) an allergic reaction would be most likely (considering the symptoms and chronology of the reaction). This corresponds to a prevalence rate of a possible allergic reaction of 4/1000 (2/497) to 8/1000 (4/497). This rate is comparable to the general risk profile in the population and is in line with others [15] who found a prevalence rate of sensitization to PPD of 8/1000 in the general population.
All six cases were assessed as non-serious, being of mild severity.
In total, 187 out of 497 individuals received a single treatment. From there, 3 individuals used the hair color product only once due to an intolerance reaction (ID: 88, 156, and 225; see Table 5), while 21 individuals where not satisfied with the performance of the color (gray coverage, color result, shade availability), 1 individual had moved, and 32 individuals had to stop as they only started to participate when the program ended before the next coloration appointment. For 130 individuals, the reason is not known. Not knowing the reason is a limitation of the study, which we had to accept in order not to bias the hairdressers and the clients.
Out of 497 individuals, 310 individuals received multiple hair color treatments, which ranged from 2 to 23 applications with an average of 7 applications per individual. For 46 individuals some skin discomfort was reported, whereas for 303 individuals no comment on compatibility had been given, which we interpreted as good skin tolerance. Further, 142 out of 497 individuals reported explicitly good tolerance to ME-PPD-containing hair color (see Table 4). Out of these 142 individuals, 24 had mentioned having had scalp issues following hair coloring in the past. These data are based solely on individuals’ self-reports, which were documented by the hairdressers. While this represents a minor limitation, it reflects real-world conditions and is unlikely to have significantly influenced the main findings, especially since this aspect was not the primary focus of the study.
During the observation period, out of 491 individuals, 323 individuals had used the hair color 1–5 times, 141 individuals 5–15 times, and 27 individuals had used the ME-PPD-containing hair color more than 15 times. A comparison with the frequency of use of other alternative hair dye products cannot be made because corresponding data are not available. When analyzing the frequency of usage in more detail, we found that individuals with ME-PPD-containing hair color applications more often than 15 times were not only among users of shades with light tone levels, but also among users of shades with dark tone levels, which contain higher concentrations of ME-PPD than light shades. These data also indicate a good long-term tolerability of hair color shades with higher ME-PPD concentrations (Figure 3).
Our study for the first time proactively collected data of individuals using ME-PPD-containing hair color products. Other data of this kind are not yet available, likely because such studies are complex and difficult to implement. In order not to bias the hairdressers regarding the indication of skin intolerance, only a comment field was offered in the provided record cards. This, in turn, made the evaluation difficult because the partially incomplete answers had to be standardized first. Another difficulty was that the hairdressers only agreed to participate if there was no further follow-up. Nevertheless, we decided on this design and considered the data obtained to be very valuable. The strength of this study lies precisely in these unbiased data. This study enabled a comprehensive analysis of the market situation when introducing a new hair dye in the context of the “reduced allergy induction risk” concept. In contrast to a study based on spontaneous reporting, this study is not limited by influencing factors like cultural consumer habits, media attention, public health concerns, or others.
As an alternative study design, it might also have been possible to collect these data directly from the clients through an agency. However, our study design had the advantage that we were able to carry out the observation over almost 2 years, and it was guaranteed that only salons using specifically the new Wella line versus other “scalp comfort” colors were picked. In addition, this study design had the advantage that the individual hairdresser was able to provide precise information, particularly about the hair color shades used.
The study confirms that introducing a hair color in the context of the “reduced allergy induction risk” advertising message did not lead to any relevant concerns related to allergy, e.g., increased usage of the hair color by people allergic to PPD. There is not a single comment in the record cards indicating that this new hair color was used by a study participant with a known hair dye allergy due to its reduced allergy risk. In addition to risk management measures such as detailed warnings on product labels and ingredient listing, good communication was critical in achieving this. Complementary education of hairdresser professionals is essential when introducing a hair color in the context of a reduced allergy induction risk.
This is also confirmed by the data from our regular market surveillance, where the seriousness of adverse events is assessed according to the seriousness criteria of the EU Cosmetics Regulation Article 21. (p) [16] or similar national regulatory requirements. ME-PPD dye technology was broadly introduced by the Wella Company into the global professional market between April 2018 and December 2018 as part of a relaunch of a large, existing professional hair color brand (Koleston Perfect). It was shown that the percentage of serious adverse events, compared to all the reported adverse events related to professional hair colors during and after the market launch of ME-PPD-containing hair colors, decreased from 3.4% (2017) pre-market to 2.6% (2018) and 2.5% (2019), respectively. These regular market surveillance data confirm, despite the limitations of regular market surveillance described above, a slight decrease in serious adverse reactions in line with the concept of primary prevention of skin sensitization and of the onset of contact dermatitis. Accordingly, it can be concluded that there is no trend indicating that PPD/PTD allergy sufferers were increasingly using ME-PPD-containing hair color products (and reacting to) or are not heeding the warnings.
The data of the study confirm a good tolerability of the ME-PPD-containing hair color. To our knowledge this is the first proactive study assessing tolerability of a hair dye product line through hairdresser comments. The proactively obtained results are supported by the regular market surveillance data, also indicating good tolerability of hair dyes containing ME-PPD. The data are also consistent with previous application data of ME-PPD-containing hair dyes to PPD/PTD-allergic individuals: after negative allergy alert pre-testing of 38, 29 tolerated continuous hair dyeing during a one-year observation period without any severe allergic reaction [13]. This is consistent with the primary prevention benefit of ME-PPD in terms of significantly reduced risk of skin sensitization induction as well as the reduced severity of elicitation reactions for all hair dye users [3].
Due to the limitations of regular market surveillance described above and the lack of further data from proactive data collection, the data situation on compatibility of ME-PPD-containing hair colors is in its early stages of development. Further collection of cosmetovigilance data is important to substantiate the positive results reported here.
Author Contributions
Conceptualization, M.K., C.G., and M.S.; methodology, M.K., C.G., L.M., and M.S., validation, M.K. and L.M., formal analysis, M.K., L.M., and A.M.; investigation, M.K. and L.M.; resources, M.S.; data curation, M.K. and A.M.; writing—original draft preparation, M.K.; writing—review and editing, C.G., M.S., A.A.G., and B.B.; visualization, M.K.; supervision, C.G. and B.B.; project administration, M.S. and L.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable. The study was based on observations in everyday life and secondary analysis of fully anonymized, non-sensitive data. No personal data was processed, and no direct contact with participants occurred. Ethical review was not required.
Informed Consent Statement
Participants’ consent was waived due to the observational nature of the study in a real-life setting and the use of anonymized data. As all data were fully anonymized prior to analysis and no identifiable personal information was accessible to the investigators, obtaining informed consent was not required.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The authors thank Karma Fussell for critical review of the manuscript.
Conflicts of Interest
The authors Carsten Goebel, Monika Kock, Lidia Mihailescu, and Maike Seib are employees of the Wella Company. The hair dye ingredient studied in this paper is currently used in commercial products marketed by the Wella Company. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors alone are responsible for the content and writing of the paper.
Abbreviations
The following abbreviations are used in this manuscript:
| PPD | p-phenylenediamine |
| PTD | p-toluenediamine |
| ME-PPD | 2-methoxymethyl-p-phenylenediamine |
| SUE | Serious Undesirable Effect |
References
- Søsted, H.; Rustemeyer, T.; Gonçalo, M.; Bruze, M.; Goossens, A.; Giménez-Arnau, A.M.; Le Coz, C.J.; White, I.R.; Diepgen, D.L.; Andersen, K.E.; et al. Contact allergy to common ingredients in hair dyes. Contact Dermat. 2013, 69, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Kimber, I.; Dearman, R.J. Contact hypersensitivity: Immunological mechanisms. In Toxicology of Contact Hypersensitivity, 1st ed.; Kimber, I., Maurer, T., Eds.; Taylor and Francis: London, UK, 1996; pp. 14–25. [Google Scholar] [CrossRef]
- Goebel, C.; Troutman, J.; Hennen, J.; Rothe, H.; Schlatter, H.; Gerberick, G.F.; Blömeke, B. Introduction of a methoxymethyl side chain into p-phenylenediamine attenuates its sensitizing potency and reduces the risk of allergy induction. Toxicol. Appl. Pharmacol. 2014, 274, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Mohr-Hautavoine, C.V.; Herrlein, M.; Guthrie, M.T. In-situ hair coloration-chromophore development from selected aromatic amines—A high throughput delivery perspective. Dye. Pigment 2015, 117, 157–162. [Google Scholar] [CrossRef]
- McFadden, J.P.; Yeo, L.; White, J.L. Clinical and experimental aspects of allergic contact dermatitis to para-phenylenediamine. Clin. Dermatol. 2011, 29, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Uter, W.; Lessmann, H.; Geier, J.; Becker, D.; Fuchs, T.; Richter, G. The spectrum of allergic (cross-)sensitivity in clinical patch testing with ‘para amino’ compounds. Allergy 2002, 57, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Koopmans, A.K.; Bruynzeel, D.P. Is PPD a useful screening agent? Contact Dermat. 2003, 48, 89–92. [Google Scholar] [CrossRef] [PubMed]
- White, I.R. Risk assessment in practice—Clinical problems in ACD. Consumer perspectives. Contact Dermat. 2004, 50, 122–123. [Google Scholar] [CrossRef]
- Basketter, D.A.; Andersen, K.E.; Liden, C.; Van Loveren, H.; Bomann, A.; Kimber, I.; Alanko, K.; Berggren, E. Evaluation of the skin sensitizing potency of chemicals using existing methods and considerations of relevance for elicitation. Contact Dermat. 2005, 52, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Blömeke, B.; Pot, L.M.; Coenraads, P.J.; Hennen, J.; Kock, M.; Goebel, C. Cross-elicitation responses to 2-methoxymethyl-p-phenylenediamine under hair dye use conditions in p-phenylenediamine-allergic individuals. Br. J. Dermatol. 2015, 172, 976–980. [Google Scholar] [CrossRef] [PubMed]
- Zahir, A.; Chesahna, K.; Blömeke, B.; Goebel, C.; Gaspari, A.A. Tolerance to a hair dye product containing 2-methoxymethyl-p-phenylenediamine in an ethnically diverse population of p-phenylenediamine-allergic individuals. Dermatitis 2016, 27, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Schuttelaar, M.L.; Dittmar, D.; Burgerhof, J.G.M.; Blömeke, B.; Goebel, C. Cross-elicitation responses to 2-methoxymethyl-p-phenylenediamine in p-phenylenediamine-allergic individuals: Results from open use testing and diagnostic patch testing. Contact Dermat. 2018, 79, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Kock, M.; Coenraads, P.J.; Blömeke, B.; Goebel, C. Continuous usage of a hair dye product containing 2-methoxymethyl-para-phenylenediamine by hair-dye-allergic individuals. Br. J. Dermatol. 2016, 174, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- SUE Reporting Guidelines. European Commission, Directorate General for Internal Market, Industry, Entrepreneurship and SMEs. 2013. Available online: https://single-market-economy.ec.europa.eu/sectors/cosmetics/market-surveillance_en (accessed on 27 May 2025).
- Diepgen, T.L.; Naldi, L.; Bruze, M.; Cazzaniga, S.; Schuttelaar, M.L.; Elsner, P.; Goncalo, M.; Ofenloch, R.; Svensson, Å. Prevalence of contact allergy to p-phenylenediamine in the European general population. J. Investig. Dermatol. 2016, 136, 409–415. [Google Scholar] [CrossRef] [PubMed]
- No 1223/2009; Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products. EC: Brussels, Belgium, 2009. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32009R1223 (accessed on 27 May 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).