26-SNP Panel Aids Guiding Androgenetic Alopecia Therapy and Provides Insight into Mechanisms of Action
Abstract
1. Introduction
2. Materials and Methods
2.1. Database and Ethics Compliance
2.2. Data Structure and SNPs
2.3. Bioinformatics and Statistical Treatment
2.3.1. SNP Association Analysis
2.3.2. SNP Interaction Analysis
2.3.3. Odds Ratio and Chi-Squared
2.3.4. Enrichment Analysis
3. Results
3.1. Overall Descriptive Statistics
3.2. Single SNP Association Analysis
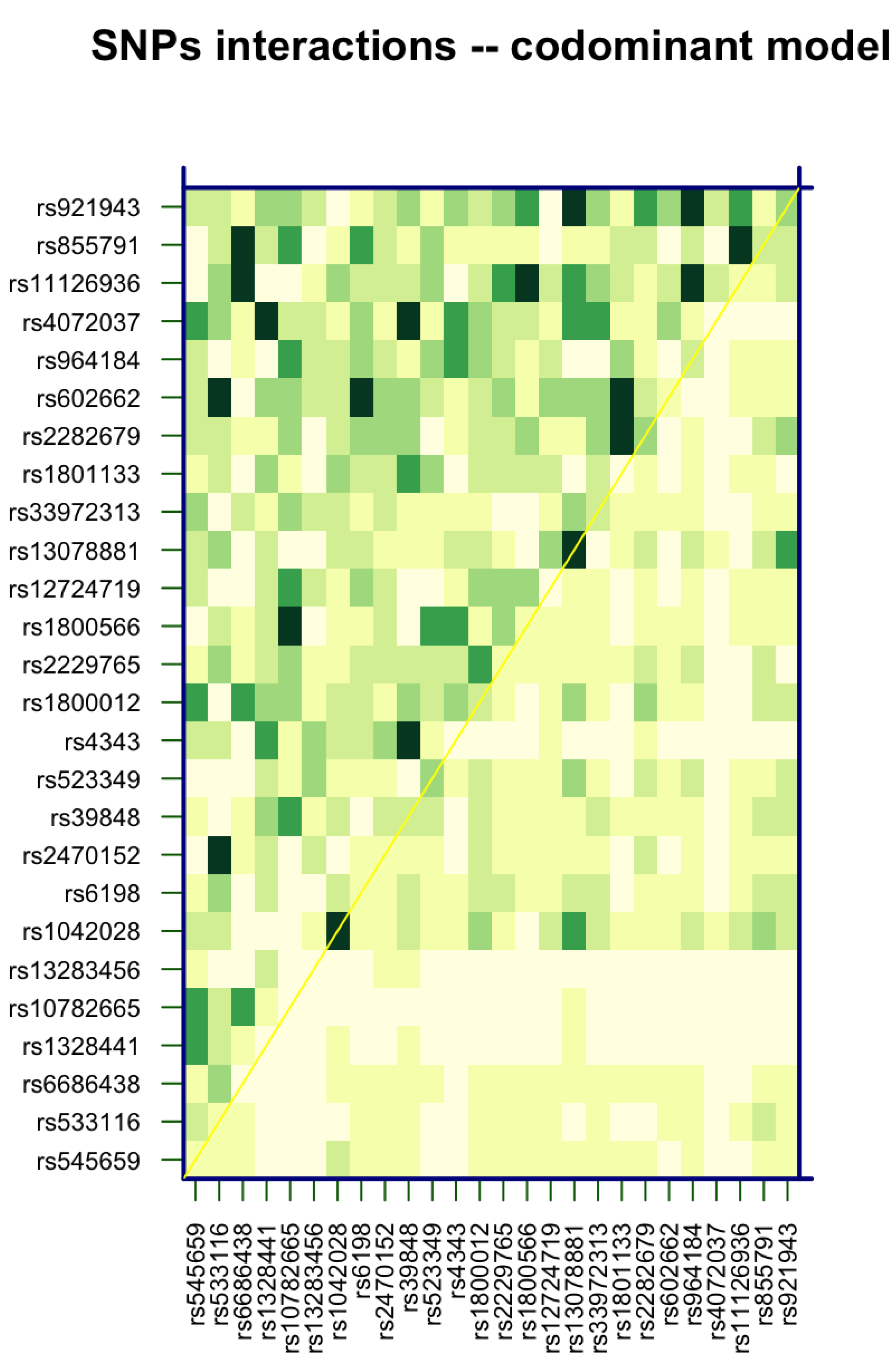
3.3. SNP–SNP Interaction Analysis
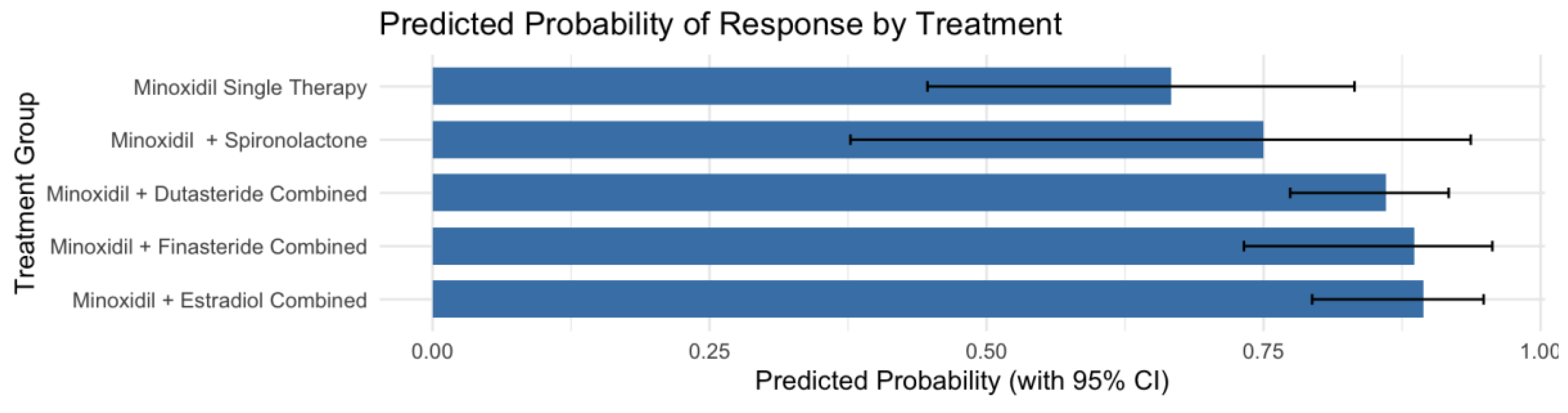
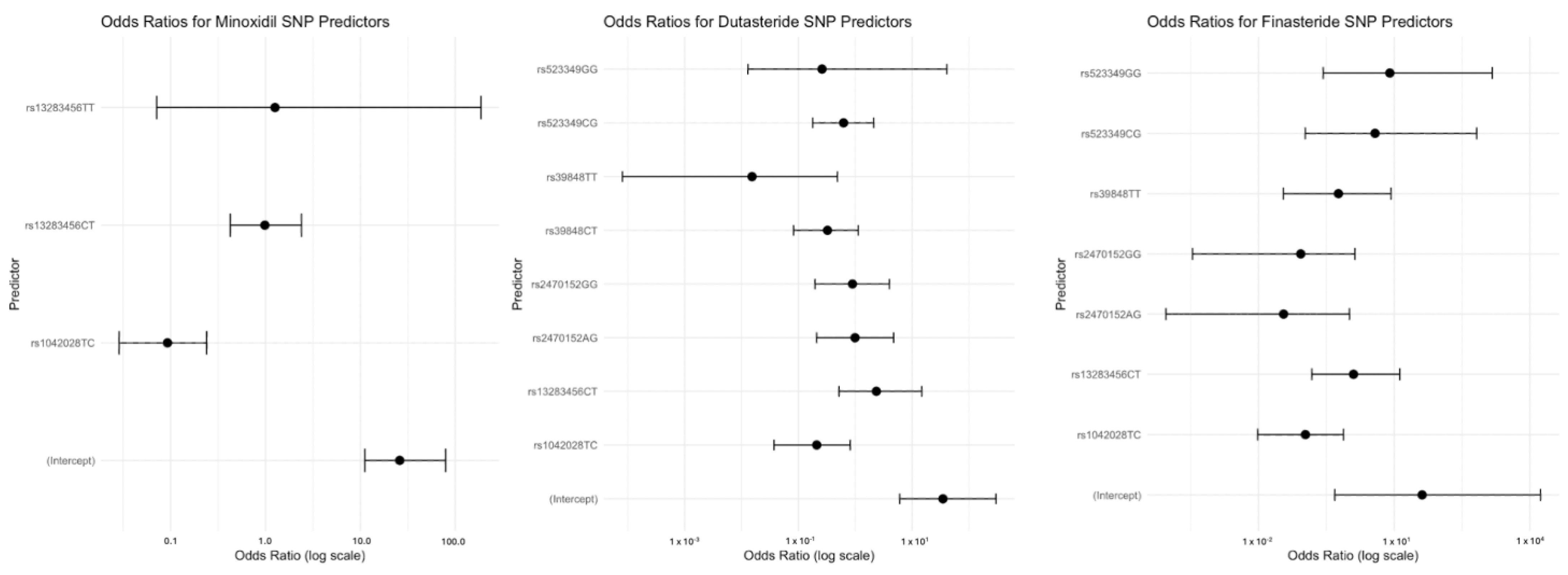
3.4. Pharmacogenetic Association by Genotype
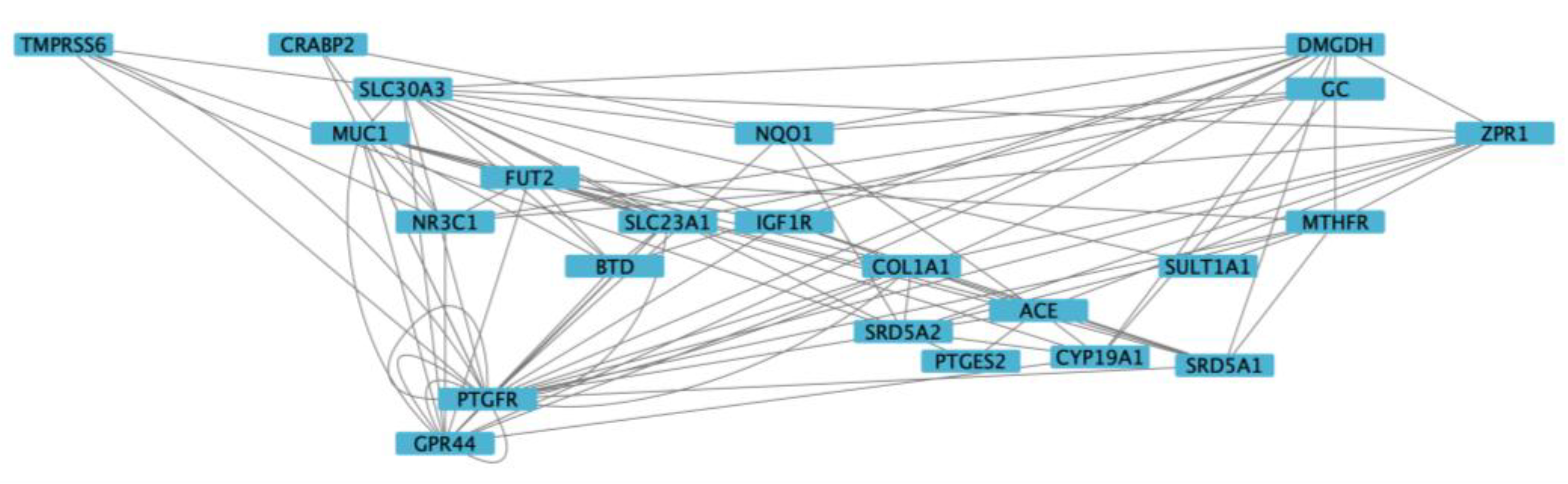
3.5. Functional Enrichment and Network Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morris, S.A.; Alsaidi, A.T.; Verbyla, A.; Cruz, A.; Macfarlane, C.; Bauer, J.; Patel, J.N. Cost Effectiveness of Pharmacogenetic Testing for Drugs with Clinical Pharmacogenetics Implementation Consortium (CPIC) Guidelines: A Systematic Review. Clin. Pharmacol. Ther. 2022, 112, 1318–1328. [Google Scholar] [CrossRef] [PubMed]
- Amaro-Álvarez, L.; Cordero-Ramos, J.; Calleja-Hernández, M.Á. Exploring the Impact of Pharmacogenetics on Personalized Medicine: A Systematic Review. Farm. Hosp. 2024, 48, 299–309. [Google Scholar] [CrossRef]
- Kirchheiner, J.; Tsahuridu, M.; Jabrane, W.; Roots, I.; Brockmöller, J. The CYP2C9 Polymorphism: From Enzyme Kinetics to Clinical Dose Recommendations. Pers. Med. 2004, 1, 63–84. [Google Scholar] [CrossRef]
- Daly, A.K. Pharmacogenetics: A General Review on Progress to Date. Br. Med. Bull. 2017, 124, 65–79. [Google Scholar] [CrossRef]
- Mosch, R.; van der Lee, M.; Guchelaar, H.J.; Swen, J.J. Pharmacogenetic Panel Testing: A Review of Current Practice and Potential for Clinical Implementation. Annu. Rev. Pharmacol. Toxicol. 2025, 65, 91–109. [Google Scholar] [CrossRef] [PubMed]
- Pirmohamed, M. Pharmacogenomics: Current Status and Future Perspectives. Nat. Rev. Genet. 2023, 24, 350–362. [Google Scholar] [CrossRef] [PubMed]
- Kabbani, D.; Akika, R.; Wahid, A.; Daly, A.K.; Cascorbi, I.; Zgheib, N.K. Pharmacogenomics in Practice: A Review and Implementation Guide. Front. Pharmacol. 2023, 14, 1189976. [Google Scholar] [CrossRef]
- Gong, L.; Klein, C.J.; Caudle, K.E.; Moyer, A.M.; Scott, S.A.; Whirl-Carrillo, M.; Klein, T.E.; Aminkeng, F.; Amr, S.; Ashcraft, K.; et al. Integrating Pharmacogenomics into the Broader Construct of Genomic Medicine: Efforts by the ClinGen Pharmacogenomics Working Group (PGxWG). Clin. Chem. 2025, 71, 36–44. [Google Scholar] [CrossRef]
- Duarte, J.D.; Thomas, C.D.; Lee, C.R.; Huddart, R.; Agundez, J.A.G.; Baye, J.F.; Gaedigk, A.; Klein, T.E.; Lanfear, D.E.; Monte, A.A.; et al. Clinical Pharmacogenetics Implementation Consortium Guideline (CPIC) for CYP2D6, ADRB1, ADRB2, ADRA2C, GRK4, and GRK5 Genotypes and Beta-Blocker Therapy. Clin. Pharmacol. Ther. 2024, 116, 939–947. [Google Scholar] [CrossRef]
- Robinson, K.M.; Eum, S.; Desta, Z.; Tyndale, R.F.; Gaedigk, A.; Crist, R.C.; Haidar, C.E.; Myers, A.L.; Samer, C.F.; Somogyi, A.A.; et al. Clinical Pharmacogenetics Implementation Consortium Guideline for CYP2B6 Genotype and Methadone Therapy. Clin. Pharmacol. Ther. 2024, 116, 932–938. [Google Scholar] [CrossRef]
- Pratt, V.M.; Cavallari, L.H.; Fulmer, M.L.; Gaedigk, A.; Hachad, H.; Ji, Y.; Kalman, L.V.; Ly, R.C.; Moyer, A.M.; Scott, S.A.; et al. DPYD Genotyping Recommendations. J. Mol. Diagn. 2024, 26, 851–863. [Google Scholar] [CrossRef]
- Zubiaur, P.; Rodríguez-Antona, C.; Boone, E.C.; Daly, A.K.; Tsermpini, E.E.; Khasawneh, L.Q.; Sangkuhl, K.; Duconge, J.; Botton, M.R.; Savieo, J.; et al. PharmVar GeneFocus: CYP4F2. Clin. Pharmacol. Ther. 2024, 116, 963–975. [Google Scholar] [CrossRef]
- Šitum, M.; Franceschi, D.; Franceschi, N. Challenges and Strategies in Dermatologic Therapy—Personalized Medicine, Patient Safety, and Pharmacoeconomics. Dermatol. Ther. 2019, 32, e13011. [Google Scholar] [CrossRef]
- Verbelen, M.; Weale, M.E.; Lewis, C.M. Cost-Effectiveness of Pharmacogenetic-Guided Treatment: Are We There Yet? Pharmacogenom. J. 2017, 17, 395–402. [Google Scholar] [CrossRef]
- Manson, L.E.N.; Swen, J.J.; Guchelaar, H.-J. Diagnostic Test Criteria for HLA Genotyping to Prevent Drug Hypersensitivity Reactions: A Systematic Review of Actionable HLA Recommendations in CPIC and DPWG Guidelines. Front. Pharmacol. 2020, 11, 567048. [Google Scholar] [CrossRef]
- Masmoudi, H.C.; Afify, N.; Alnaqbi, H.; Alhalwachi, Z.; Tay, G.K.; Alsafar, H. HLA Pharmacogenetic Markers of Drug Hypersensitivity from the Perspective of the Populations of the Greater Middle East. Pharmacogenomics 2022, 23, 695–708. [Google Scholar] [CrossRef]
- Nakkam, N.; Konyoung, P.; Kanjanawart, S.; Saksit, N.; Kongpan, T.; Khaeso, K.; Khunarkornsiri, U.; Dornsena, A.; Tassaneeyakul, W.; Tassaneeyakul, W. HLA Pharmacogenetic Markers of Drug Hypersensitivity in a Thai Population. Front. Genet. 2018, 9, 277. [Google Scholar] [CrossRef] [PubMed]
- Phillips, E.J.; Sukasem, C.; Whirl-Carrillo, M.; Müller, D.J.; Dunnenberger, H.M.; Chantratita, W.; Goldspiel, B.; Chen, Y.; Carleton, B.C.; George, A.L.; et al. Clinical Pharmacogenetics Implementation Consortium Guideline for HLA Genotype and Use of Carbamazepine and Oxcarbazepine: 2017 Update. Clin. Pharmacol. Ther. 2018, 103, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Karnes, J.H.; Rettie, A.E.; Somogyi, A.A.; Huddart, R.; Fohner, A.E.; Formea, C.M.; Ta Michael Lee, M.; Llerena, A.; Whirl-Carrillo, M.; Klein, T.E.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2C9 and HLA-B Genotypes and Phenytoin Dosing: 2020 Update. Clin. Pharmacol. Ther. 2021, 109, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.M.; Zhu, Y.; Rojas, R.L.; St. Sauver, J.L.; Bielinski, S.J.; Jacobsen, D.J.; Visscher, S.L.; Wang, L.; Weinshilboum, R.; Borah, B.J. Comparing Outcomes and Costs among Warfarin-Sensitive Patients versus Warfarin-Insensitive Patients Using The Right Drug, Right Dose, Right Time: Using Genomic Data to Individualize Treatment (RIGHT) 10K Warfarin Cohort. PLoS ONE 2020, 15, e0233316. [Google Scholar] [CrossRef]
- Weinshilboum, R.M.; Wang, L. Pharmacogenomics: Precision Medicine and Drug Response. Mayo Clin. Proc. 2017, 92, 1711–1722. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Scherer, S.E.; Bielinski, S.J.; Muzny, D.M.; Jones, L.A.; Black, J.L.; Moyer, A.M.; Giri, J.; Sharp, R.R.; Matey, E.T.; et al. Implementation of Preemptive DNA Sequence–Based Pharmacogenomics Testing across a Large Academic Medical Center: The Mayo-Baylor RIGHT 10K Study. Genet. Med. 2022, 24, 1062–1072. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Vnencak-Jones, C.L.; Roland, B.P.; Gatto, C.L.; Mathe, J.L.; Just, S.L.; Peterson, J.F.; Van Driest, S.L.; Weitkamp, A.O. A Tutorial for Pharmacogenomics Implementation Through End-to-End Clinical Decision Support Based on Ten Years of Experience from PREDICT. Clin. Pharmacol. Ther. 2021, 109, 101–115. [Google Scholar] [CrossRef]
- Pulley, J.M.; Denny, J.C.; Peterson, J.F.; Bernard, G.R.; Vnencak-Jones, C.L.; Ramirez, A.H.; Delaney, J.T.; Bowton, E.; Brothers, K.; Johnson, K.; et al. Operational Implementation of Prospective Genotyping for Personalized Medicine: The Design of the Vanderbilt PREDICT Project. Clin. Pharmacol. Ther. 2012, 92, 87–95. [Google Scholar] [CrossRef]
- Chenchula, S.; Atal, S.; Uppugunduri, C.R.S. A Review of Real-World Evidence on Preemptive Pharmacogenomic Testing for Preventing Adverse Drug Reactions: A Reality for Future Health Care. Pharmacogenom. J. 2024, 24, 9. [Google Scholar] [CrossRef]
- Wei, C.-Y.; Wen, M.-S.; Cheng, C.-K.; Sheen, Y.-J.; Yao, T.-C.; Lee, S.-L.; Wu, J.-Y.; Tsai, M.-F.; Li, L.-H.; Chen, C.; et al. Clinical Impact of Pharmacogenetic Risk Variants in a Large Chinese Cohort. Nat. Commun. 2025, 16, 6344. [Google Scholar] [CrossRef]
- Wang, X.; Wang, C.; Zhang, Y.; An, Z. Effect of Pharmacogenomics Testing Guiding on Clinical Outcomes in Major Depressive Disorder: A Systematic Review and Meta-Analysis of RCT. BMC Psychiatry 2023, 23, 334. [Google Scholar] [CrossRef]
- Magavern, E.F.; Megase, M.; Thompson, J.; Marengo, G.; Jacobsen, J.; Smedley, D.; Caulfield, M.J. Pharmacogenetics and Adverse Drug Reports: Insights from a United Kingdom National Pharmacovigilance Database. PLoS Med. 2025, 22, e1004565. [Google Scholar] [CrossRef]
- Rundegren, J. A One-Year Observational Study with Minoxidil 5% Solution in Germany: Results of Independent Efficacy Evaluation by Physicians and Patients. J. Am. Acad. Dermatol. 2004, 50, P91. [Google Scholar] [CrossRef]
- Nestor, M.S.; Ablon, G.; Gade, A.; Han, H.; Fischer, D.L. Treatment Options for Androgenetic Alopecia: Efficacy, Side Effects, Compliance, Financial Considerations, and Ethics. J. Cosmet. Dermatol. 2021, 20, 3759–3781. [Google Scholar] [CrossRef]
- Liu, C.; Liu, X.; Shi, T.; Wang, Y.; Sui, C.; Zhang, W.; Wang, B. Efficacy and Safety of Oral Minoxidil in the Treatment of Alopecia: A Single-Arm Rate Meta-Analysis and Systematic Review. Front. Pharmacol. 2025, 16, 1556705. [Google Scholar] [CrossRef]
- Johnson, H.; Huang, D.; Clift, A.K.; Bersch-Ferreira, Â.; Guimarães, G.A. Effectiveness of Combined Oral Minoxidil and Finasteride in Male Androgenetic Alopecia: A Retrospective Service Evaluation. Cureus 2025, 17, e77549. [Google Scholar] [CrossRef]
- Dominguez-Santas, M.; Diaz-Guimaraens, B.; Saceda-Corralo, D.; Hermosa-Gelbard, A.; Muñoz-Moreno Arrones, O.; Pindado-Ortega, C.; Fernandez-Nieto, D.; Jimenez-Cauhe, J.; Ortega-Quijano, D.; Suarez-Valle, A.; et al. The State-of-the-art in the Management of Androgenetic Alopecia: A Review of New Therapies and Treatment Algorithms. JEADV Clin. Pract. 2022, 1, 176–185. [Google Scholar] [CrossRef]
- Gupta, A.K.; Talukder, M.; Venkataraman, M.; Bamimore, M.A. Minoxidil: A Comprehensive Review. J. Dermatol. Treat. 2022, 33, 1896–1906. [Google Scholar] [CrossRef]
- Chen, X.; Liu, B.; Li, Y.; Han, L.; Tang, X.; Deng, W.; Lai, W.; Wan, M. Dihydrotestosterone Regulates Hair Growth Through the Wnt/β-Catenin Pathway in C57BL/6 Mice and In Vitro Organ Culture. Front. Pharmacol. 2020, 10, 1528. [Google Scholar] [CrossRef]
- Swerdloff, R.S.; Dudley, R.E.; Page, S.T.; Wang, C.; Salameh, W.A. Dihydrotestosterone: Biochemistry, Physiology, and Clinical Implications of Elevated Blood Levels. Endocr. Rev. 2017, 38, 220–254. [Google Scholar] [CrossRef]
- Kaufman, K.D.; Olsen, E.A.; Whiting, D.; Savin, R.; DeVillez, R.; Bergfeld, W.; Price, V.H.; Van Neste, D.; Roberts, J.L.; Hordinsky, M.; et al. Finasteride in the Treatment of Men with Androgenetic Alopecia. J. Am. Acad. Dermatol. 1998, 39, 578–589. [Google Scholar] [CrossRef]
- Mella, J.M.; Perret, M.C.; Manzotti, M.; Catalano, H.N.; Guyatt, G. Efficacy and Safety of Finasteride Therapy for Androgenetic Alopecia. Arch. Dermatol. 2010, 146, 1141–1150. [Google Scholar] [CrossRef] [PubMed]
- Shanshanwal, S.J.; Dhurat, R.S. Superiority of Dutasteride over Finasteride in Hair Regrowth and Reversal of Miniaturization in Men with Androgenetic Alopecia: A Randomized Controlled Open-Label, Evaluator-Blinded Study. Indian J. Dermatol. Venereol. Leprol. 2017, 83, 47. [Google Scholar] [CrossRef] [PubMed]
- Gubelin Harcha, W.; Barboza Martínez, J.; Tsai, T.-F.; Katsuoka, K.; Kawashima, M.; Tsuboi, R.; Barnes, A.; Ferron-Brady, G.; Chetty, D. A Randomized, Active- and Placebo-Controlled Study of the Efficacy and Safety of Different Doses of Dutasteride versus Placebo and Finasteride in the Treatment of Male Subjects with Androgenetic Alopecia. J. Am. Acad. Dermatol. 2014, 70, 489–498.e3. [Google Scholar] [CrossRef] [PubMed]
- Brough, K.R.; Torgerson, R.R. Hormonal Therapy in Female Pattern Hair Loss. Int. J. Womens Dermatol. 2017, 3, 53–57. [Google Scholar] [CrossRef]
- Conic, R.R.; Khetarpal, S.; Bergfeld, W. Treatment of Female Pattern Hair Loss with Combination Therapy. Semin. Cutan. Med. Surg. 2018, 37, 247–253. [Google Scholar] [CrossRef]
- Rossi, A.; Magri, F.; D’Arino, A.; Pigliacelli, F.; Muscianese, M.; Leoncini, P.; Caro, G.; Federico, A.; Fortuna, M.C.; Carlesimo, M. Efficacy of Topical Finasteride 0.5% vs 17α-Estradiol 0.05% in the Treatment of Postmenopausal Female Pattern Hair Loss: A Retrospective, Single-Blind Study of 119 Patients. Dermatol. Pract. Concept. 2020, 10, e2020039. [Google Scholar] [CrossRef]
- Burns, L.J.; De Souza, B.; Flynn, E.; Hagigeorges, D.; Senna, M.M. Spironolactone for Treatment of Female Pattern Hair Loss. J. Am. Acad. Dermatol. 2020, 83, 276–278. [Google Scholar] [CrossRef]
- Wang, C.; Du, Y.; Bi, L.; Lin, X.; Zhao, M.; Fan, W. The Efficacy and Safety of Oral and Topical Spironolactone in Androgenetic Alopecia Treatment: A Systematic Review. Clin. Cosmet. Investig. Dermatol. 2023, 16, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Georgala, S.; Katoulis, A.C.; Georgala, C.; Moussatou, V.; Bozi, E.; Stavrianeas, N.G. Topical Estrogen Therapy for Androgenetic Alopecia in Menopausal Females. Dermatology 2004, 208, 178–179. [Google Scholar] [CrossRef]
- Dhurat, R.; Daruwalla, S.; Pai, S.; Kovacevic, M.; McCoy, J.; Shapiro, J.; Sinclair, R.; Vano-Galvan, S.; Goren, A. SULT1A1 (Minoxidil Sulfotransferase) Enzyme Booster Significantly Improves Response to Topical Minoxidil for Hair Regrowth. J. Cosmet. Dermatol. 2022, 21, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Pietrauszka, K.; Bergler-Czop, B. Sulfotransferase SULT1A1 Activity in Hair Follicle, a Prognostic Marker of Response to the Minoxidil Treatment in Patients with Androgenetic Alopecia: A Review. Adv. Dermatol. Allergol. 2022, 39, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Yang, L.; Sun, Z.; Zhan, X. Personalized Drug Therapy: Innovative Concept Guided with Proteoformics. Mol. Cell. Proteom. 2024, 23, 100737. [Google Scholar] [CrossRef]
- Schaeuffele, C.; Zagorscak, P.; Langerwisch, V.; Wilke, J.; Medvedeva, Y.; Knaevelsrud, C. A Systematic Review on Personalization of Treatment Components in IBIs for Mental Disorders. Internet Interv. 2025, 41, 100840. [Google Scholar] [CrossRef]
- Zhou, Y.; Peng, S.; Wang, H.; Cai, X.; Wang, Q. Review of Personalized Medicine and Pharmacogenomics of Anti-Cancer Compounds and Natural Products. Genes 2024, 15, 468. [Google Scholar] [CrossRef]
- Brownstone, N.; Wu, J.J.; Strober, B.E.; Dickerson, T.J. Getting Personal about Skin: Realizing Precision Medicine in Dermatology. Dermatol. Rev. 2021, 2, 289–295. [Google Scholar] [CrossRef]
- Messenger, A.G.; Rundegren, J. Minoxidil: Mechanisms of Action on Hair Growth. Br. J. Dermatol. 2004, 150, 186–194. [Google Scholar] [CrossRef]
- Choi, N.; Shin, S.; Song, S.; Sung, J.-H. Minoxidil Promotes Hair Growth through Stimulation of Growth Factor Release from Adipose-Derived Stem Cells. Int. J. Mol. Sci. 2018, 19, 691. [Google Scholar] [CrossRef]
- Estill, M.C.; Ford, A.; Omeira, R.; Rodman, M. Finasteride and Dutasteride for the Treatment of Male Androgenetic Alopecia: A Review of Efficacy and Reproductive Adverse Effects. Georget. Med. Rev. 2023, 7. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, C.; Bi, L.; Du, Y.; Lu, C.; Zhao, M.; Fan, W. Dutasteride for the Treatment of Androgenetic Alopecia: An Updated Review. Dermatology 2024, 240, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Olsen, E.A.; Dunlap, F.E.; Funicella, T.; Koperski, J.A.; Swinehart, J.M.; Tschen, E.H.; Trancik, R.J. A Randomized Clinical Trial of 5% Topical Minoxidil versus 2% Topical Minoxidil and Placebo in the Treatment of Androgenetic Alopecia in Men. J. Am. Acad. Dermatol. 2002, 47, 377–385. [Google Scholar] [CrossRef]
- Rossi, A.; Caro, G. Efficacy of the Association of Topical Minoxidil and Topical Finasteride Compared to Their Use in Monotherapy in Men with Androgenetic Alopecia: A Prospective, Randomized, Controlled, Assessor Blinded, 3-arm, Pilot Trial. J. Cosmet. Dermatol. 2024, 23, 502–509. [Google Scholar] [CrossRef]
- Francès, M.P.; Vila-Vecilla, L.; Russo, V.; Caetano Polonini, H.; de Souza, G.T. Utilising SNP Association Analysis as a Prospective Approach for Personalising Androgenetic Alopecia Treatment. Dermatol. Ther. 2024, 14, 971–981. [Google Scholar] [CrossRef] [PubMed]
- González, J.R.; Armengol, L.; Solé, X.; Guinó, E.; Mercader, J.M.; Estivill, X.; Moreno, V. SNPassoc: An R Package to Perform Whole Genome Association Studies. Bioinformatics 2007, 23, 654–655. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING V11: Protein–Protein Association Networks with Increased Coverage, Supporting Functional Discovery in Genome-Wide Experimental Datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]
- Arranz, M.J.; Gonzalez-Rodriguez, A.; Perez-Blanco, J.; Penadés, R.; Gutierrez, B.; Ibañez, L.; Arias, B.; Brunet, M.; Cervilla, J.; Salazar, J.; et al. A Pharmacogenetic Intervention for the Improvement of the Safety Profile of Antipsychotic Treatments. Transl. Psychiatry 2019, 9, 177. [Google Scholar] [CrossRef]
- Qahwaji, R.; Ashankyty, I.; Sannan, N.S.; Hazzazi, M.S.; Basabrain, A.A.; Mobashir, M. Pharmacogenomics: A Genetic Approach to Drug Development and Therapy. Pharmaceuticals 2024, 17, 940. [Google Scholar] [CrossRef] [PubMed]
- McLeod, H.L.; Nguyen, D.G. Pharmacogenomics in Oncology—Running Out of Excuses for Slow Adoption. JAMA Netw. Open 2024, 7, e2449453. [Google Scholar] [CrossRef] [PubMed]
- Franczyk, B.; Rysz, J.; Gluba-Brzózka, A. Pharmacogenetics of Drugs Used in the Treatment of Cancers. Genes 2022, 13, 311. [Google Scholar] [CrossRef] [PubMed]
- Spratlin, J.; Sawyer, M.B. Pharmacogenetics of Paclitaxel Metabolism. Crit. Rev. Oncol. Hematol. 2007, 61, 222–229. [Google Scholar] [CrossRef]
- Duarte, J.D.; Cavallari, L.H. Pharmacogenetics to Guide Cardiovascular Drug Therapy. Nat. Rev. Cardiol. 2021, 18, 649–665. [Google Scholar] [CrossRef]
- Chang, V.Y.; Wang, J.J. Pharmacogenetics of Chemotherapy-Induced Cardiotoxicity. Curr. Oncol. Rep. 2018, 20, 52. [Google Scholar] [CrossRef]
- Sato, A.; Takeda, A. Evaluation of Efficacy and Safety of Finasteride 1 Mg in 3177 Japanese Men with Androgenetic Alopecia. J. Dermatol. 2012, 39, 27–32. [Google Scholar] [CrossRef]
- Choi, G.-S.; Sim, W.-Y.; Kang, H.; Huh, C.H.; Lee, Y.W.; Shantakumar, S.; Ho, Y.-F.; Oh, E.-J.; Duh, M.S.; Cheng, W.Y.; et al. Long-Term Effectiveness and Safety of Dutasteride versus Finasteride in Patients with Male Androgenic Alopecia in South Korea: A Multicentre Chart Review Study. Ann. Dermatol. 2022, 34, 349. [Google Scholar] [CrossRef]
- Dhurat, R.; Sharma, A.; Rudnicka, L.; Kroumpouzos, G.; Kassir, M.; Galadari, H.; Wollina, U.; Lotti, T.; Golubovic, M.; Binic, I.; et al. 5-Alpha Reductase Inhibitors in Androgenetic Alopecia: Shifting Paradigms, Current Concepts, Comparative Efficacy, and Safety. Dermatol. Ther. 2020, 33, e13379. [Google Scholar] [CrossRef] [PubMed]
- Graupp, M.; Wehr, E.; Schweighofer, N.; Pieber, T.R.; Obermayer-Pietsch, B. Association of Genetic Variants in the Two Isoforms of 5α-Reductase, SRD5A1 and SRD5A2, in Lean Patients with Polycystic Ovary Syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 157, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huang, Y.; Fu, X.; Chen, C.; Zhang, D.; Yan, L.; Xie, Y.; Mao, Y.; Li, Y. Meta-Analysis of Three Polymorphisms in the Steroid-5-Alpha-Reductase, Alpha Polypeptide 2 Gene (SRD5A2) and Risk of Prostate Cancer. Mutagenesis 2011, 26, 371–383. [Google Scholar] [CrossRef]
- Ha, S.-J.; Kim, J.-S.; Myung, J.-W.; Lee, H.-J.; Kim, J.-W. Analysis of Genetic Polymorphisms of Steroid 5α-Reductase Type 1 and 2 Genes in Korean Men with Androgenetic Alopecia. J. Dermatol. Sci. 2003, 31, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Michelet, J.F.; Commo, S.; Billoni, N.; Mahe, Y.F.; Bernard, B.A. Activation of Cytoprotective Prostaglandin Synthase-1 by Minoxidil as a Possible Explanation for Its Hair Growth-Stimulating Effect. J. Investig. Dermatol. 1997, 108, 205–209. [Google Scholar] [CrossRef]
- Goren, A.; Castano, J.A.; Mccoy, J.; Bermudez, F.; Lotti, T. Therapeutic Hotline Novel Enzymatic Assay Predicts Minoxidil Response in the Treatment of Androgenetic Alopecia. Dermatol. Ther. 2013, 27, 171–173. [Google Scholar] [CrossRef]
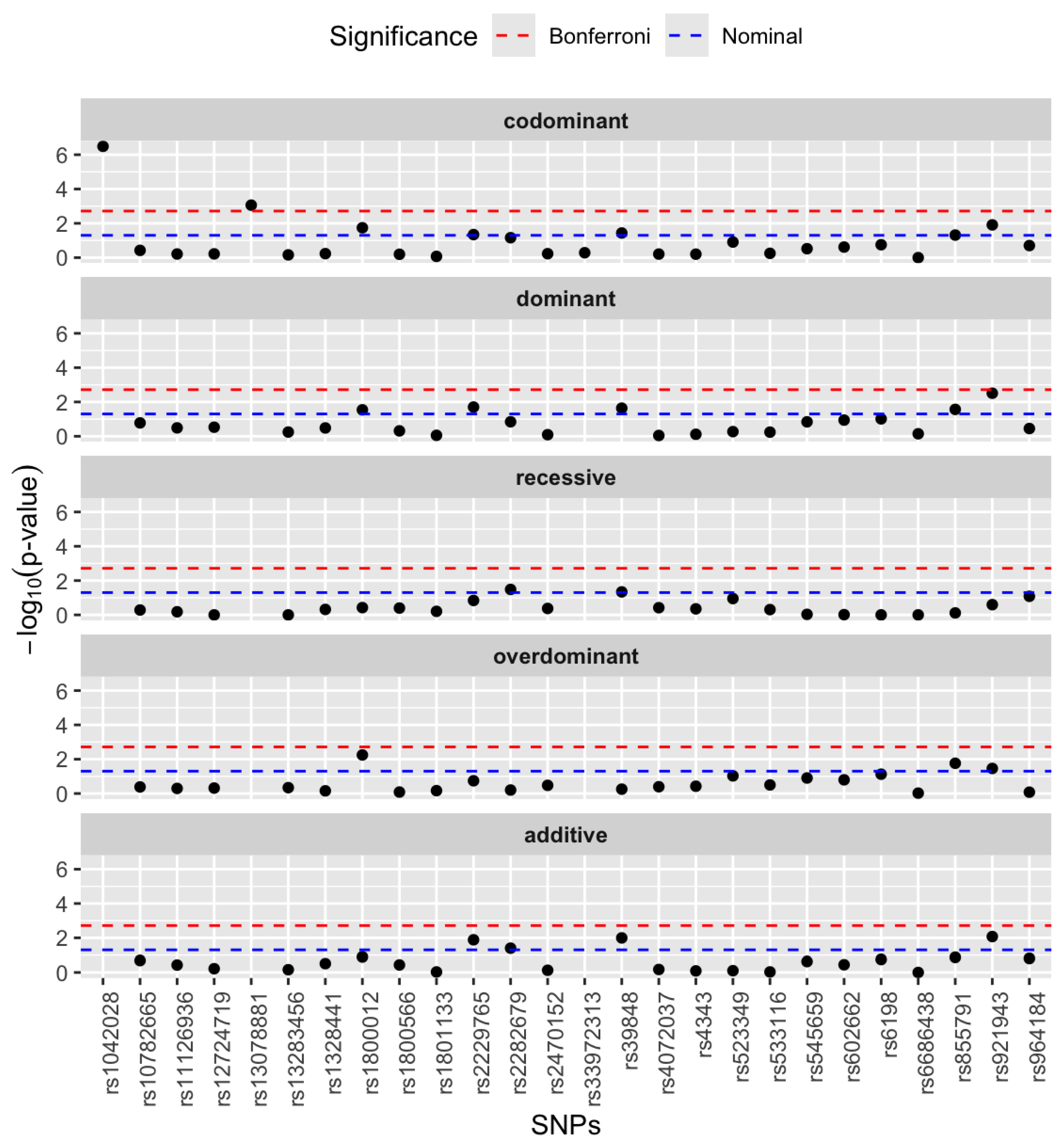
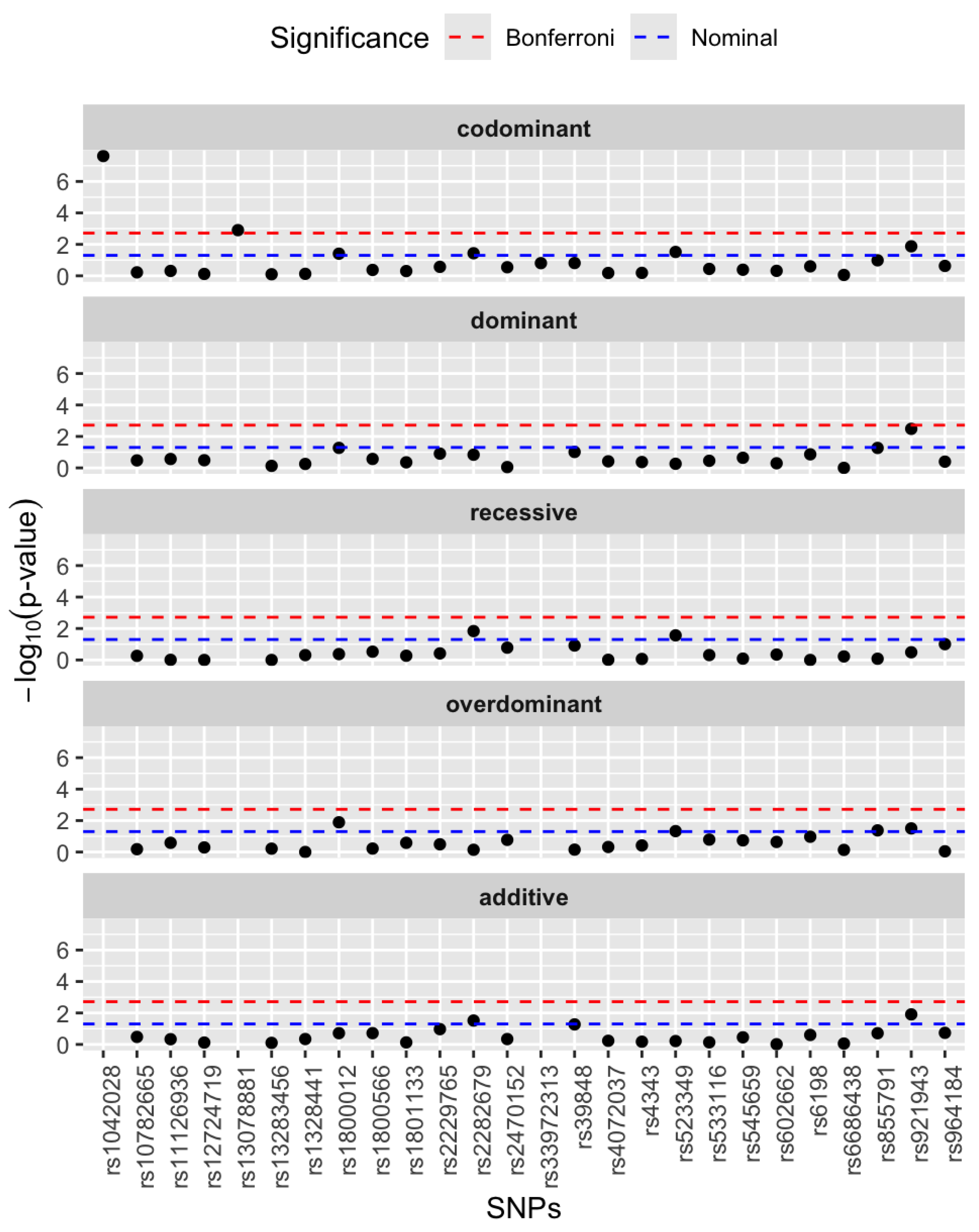
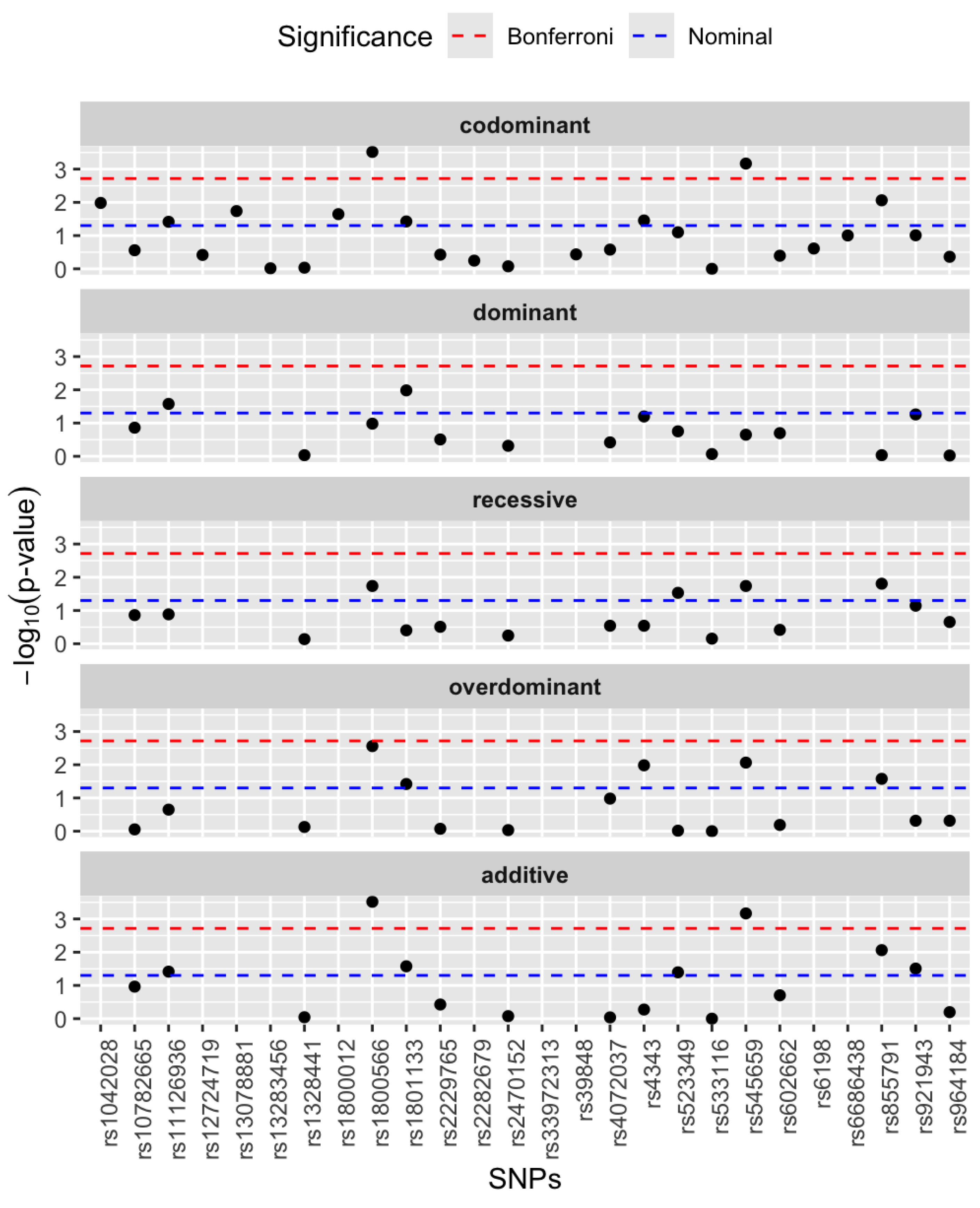
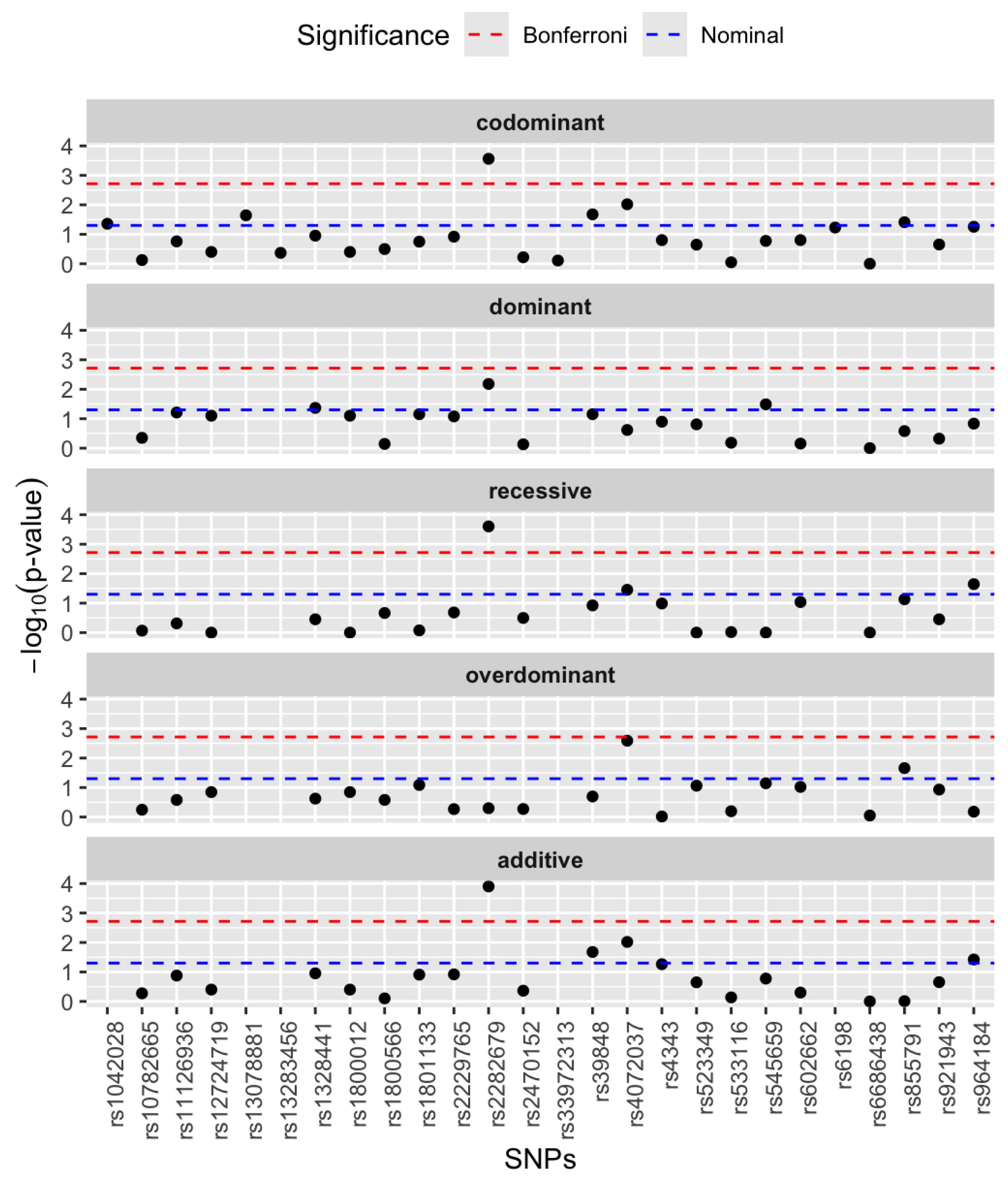
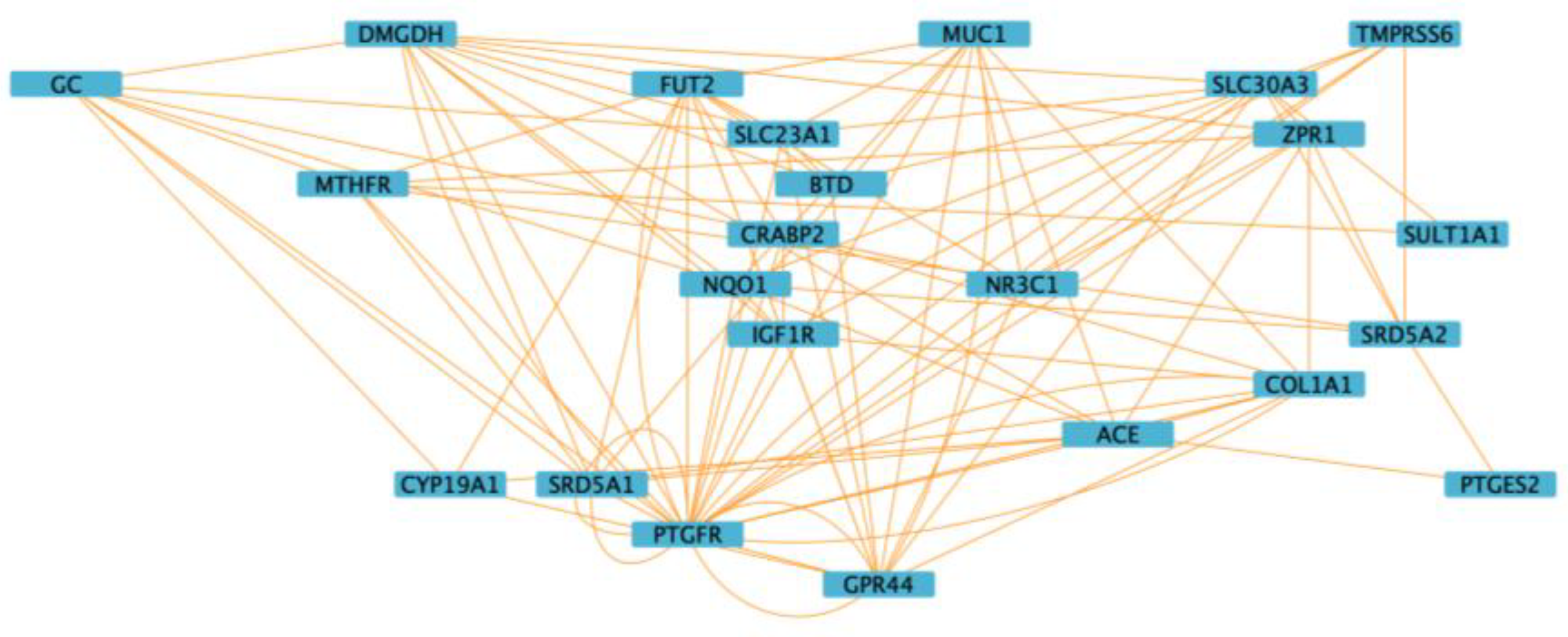
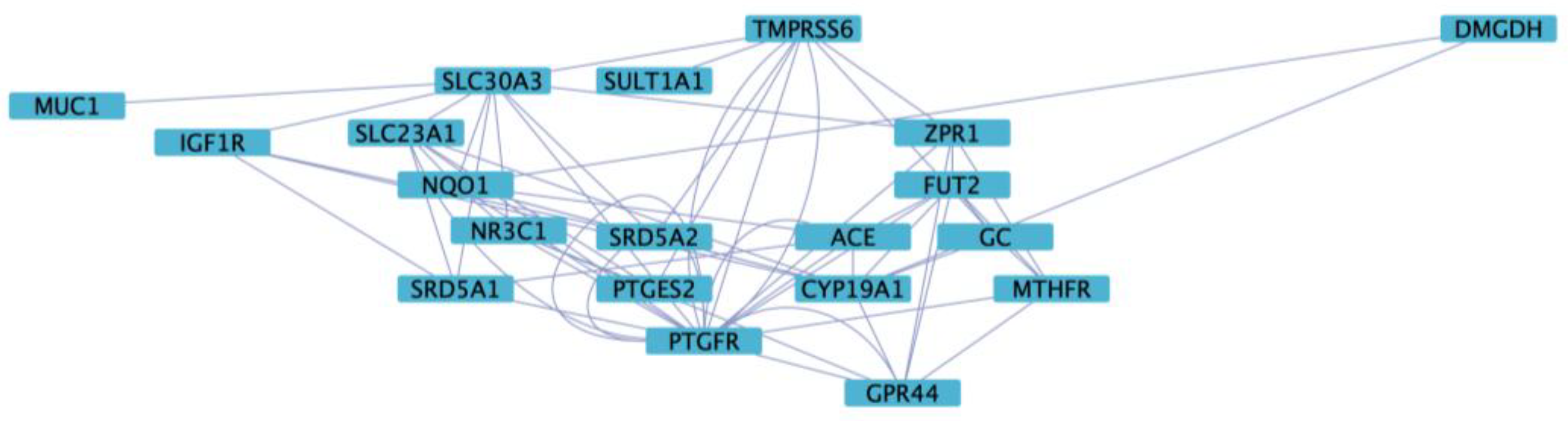
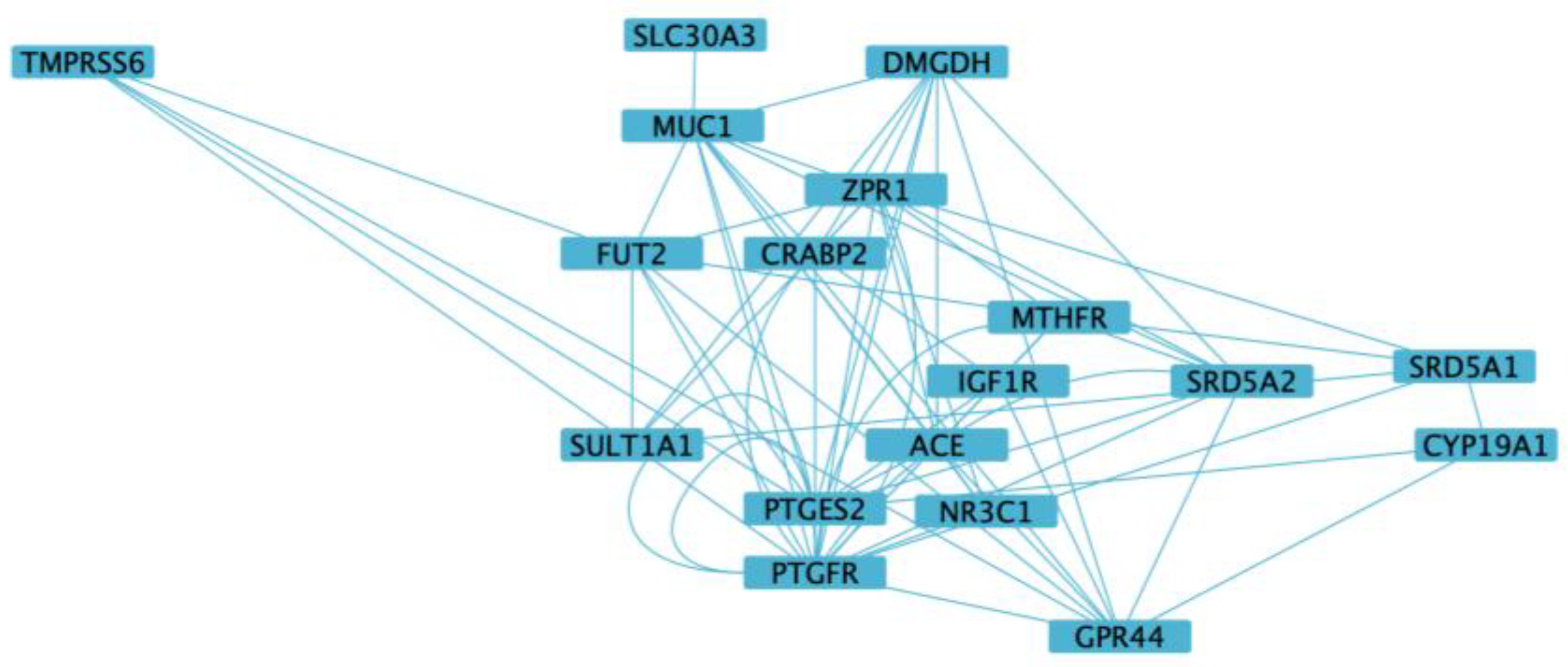
| Genes | rsIDs | Major Allele Frequency | Minor Allele Frequency |
|---|---|---|---|
| GPR44 | rs545659 | T | C |
| GPR44 | rs533116 | C | T |
| PTGFR | rs6686438 | G | T |
| PTGFR | rs1328441 | C | T |
| PTGFR | rs10782665 | G | T |
| PTGES2 | rs13283456 | C | T |
| SULT1A1 | rs1042028 | C | T |
| GR-alpha | rs6198 | T | C |
| CYP19A1 | rs2470152 | G | A |
| SRD5A1 | rs39848 | T | C |
| SRD5A2 | rs523349 | C | G |
| ACE | rs4343 | A | G |
| COL1A1 | rs1800012 | C | A |
| IGF1R | rs2229765 | G | A |
| NQO1 | rs1800566 | G | A |
| CRABP2 | rs12724719 | G | A |
| BTD | rs13078881 | G | C |
| SLC23A1 | rs33972313 | C | T |
| MTHFR | rs1801133 | G | A |
| GC | rs2282679 | T | G |
| FUT2 | rs602662 | G | A |
| ZPR1 | rs964184 | C | G |
| MUC1 | rs4072037 | T | C |
| SLC30A3 | rs11126936 | G | T |
| TMPRSS6 | rs855791 | G | A |
| DMGDH | rs921943 | C | T |
| rsIDs | Gene | All Patients | Dutasteride | Estradiol + Spironolactone | Finasteride | Finasteride + Dutasteride | Minoxidil |
|---|---|---|---|---|---|---|---|
| rs1042028 | SULT1A1 | 0.00566 | 0.0466 | 0.0226 | 0.00290 | 0.0128 | |
| rs11126936 | SLC30A3 | 0.000304 | 0.00457 | ||||
| rs13078881 | BTD | 0.0104 | |||||
| rs1328441 | PTGFR | 0.01299 | 0.0276 | 0.0306 | |||
| rs1800012 | COL1A1 | 0.0328 | 0.000125 | 0.000398 | 0.0146 | ||
| rs1800566 | NQO1 | 0.0101 | 0.0210 | ||||
| rs1801133 | MTHFR | 0.00257 | |||||
| rs2229765 | IGF1R | 0.0104 | |||||
| rs2282679 | GC | 0.0294 | 0.0273 | ||||
| rs39848 | SRD5A1 | 0.0325 | 0.000681 | 0.00196 | |||
| rs4072037 | MUC1 | 0.0134 | |||||
| rs4343 | ACE | 0.0179 | 0.0254 | ||||
| rs523349 | SRD5A2 | 0.0172 | 0.0219 | 0.00867 | 0.000883 | 0.0419 | |
| rs545659 | GPR44 | 0.00310 | 0.000576 | 0.0311 | 0.00329 | ||
| rs602662 | FUT2 | 0.0227 | 0.0104 | ||||
| rs6198 | GR-alpha | 0.00566 | 0.0466 | 0.0226 | 0.00290 | 0.0128 | |
| rs855791 | TMPRSS6 | 0.000304 | 0.00457 | ||||
| rs921943 | DMGDH | 0.0104 | |||||
| rs964184 | ZPR1 | 0.01299 | 0.0276 | 0.0306 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaboardi, H.; Russo, V.; Vila-Vecilla, L.; Patel, V.; De Souza, G.T. 26-SNP Panel Aids Guiding Androgenetic Alopecia Therapy and Provides Insight into Mechanisms of Action. Cosmetics 2025, 12, 190. https://doi.org/10.3390/cosmetics12050190
Gaboardi H, Russo V, Vila-Vecilla L, Patel V, De Souza GT. 26-SNP Panel Aids Guiding Androgenetic Alopecia Therapy and Provides Insight into Mechanisms of Action. Cosmetics. 2025; 12(5):190. https://doi.org/10.3390/cosmetics12050190
Chicago/Turabian StyleGaboardi, Hannah, Valentina Russo, Laura Vila-Vecilla, Vishal Patel, and Gustavo Torres De Souza. 2025. "26-SNP Panel Aids Guiding Androgenetic Alopecia Therapy and Provides Insight into Mechanisms of Action" Cosmetics 12, no. 5: 190. https://doi.org/10.3390/cosmetics12050190
APA StyleGaboardi, H., Russo, V., Vila-Vecilla, L., Patel, V., & De Souza, G. T. (2025). 26-SNP Panel Aids Guiding Androgenetic Alopecia Therapy and Provides Insight into Mechanisms of Action. Cosmetics, 12(5), 190. https://doi.org/10.3390/cosmetics12050190





