Abstract
Skin photoageing remains a topic of considerable concern. Retinol (RT) and retinyl palmitate (RP) have shown preliminary therapeutic efficacy; nevertheless, the high irritation associated with RT and the relatively modest efficacy of RP have constrained their broader application. Consequently, this study explored the effects and biosafety of RT and RP in repairing UV-induced skin ageing through a series of in vitro cell experiments, in vitro hemolysis assays, UV-irradiated mouse models, and molecular simulation techniques. The findings revealed that the interaction between RT and RP achieved complementary and enhanced therapeutic outcomes. Specifically, this combination improved the biosafety profile of retinoid formulations, accelerated cell migration rates, and facilitated the activation of the peroxisome proliferator-activated receptor α (PPARα) pathway. Moreover, the action of RT and RP further mitigated epidermal hyperplasia, mast cell infiltration, and the expression levels of interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumour necrosis factor-α (TNF-α), while stimulating the synthesis of type I collagen. Metabolomics and transcriptomics analyses indicated that RT and RP exerted complementary effects through metabolic pathways, significantly elevating the overall therapeutic efficacy. Network pharmacology and molecular docking studies unveiled that the structural similarity between RT and RP was one of the contributors to their enhancement. In conclusion, this study demonstrated that the combined application of RT and RP exhibited marked effects. Through their mutual action, they not only potentiated each other’s therapeutic effects but also achieved complementary and optimised therapeutic outcomes, thereby substantially enhancing the overall efficacy.
1. Introduction
Skin ageing is a multifaceted process influenced by intrinsic biological factors, genetic predispositions, and external impacts, among which photoageing induced by ultraviolet (UV) radiation significantly accelerates this process [1,2]. Persistent photoageing damages skin DNA and degrades collagen and elastin. It triggers the production of free radicals, leading to a series of skin-related issues such as uneven pigmentation, laxity, yellowing, wrinkles, telangiectasia, leathery skin texture, and malignant skin tumours [3,4,5]. Based on the Market Analysis Report [6], the survey indicates that driven by rising consumer demand for anti-ageing products and advances in technology that improve stability and reduce side effects, the application of retinol and its derivatives in the skincare and pharmaceutical markets is rapidly expanding.
Numerous studies have demonstrated that retinol and its derivatives can be oxidised to retinaldehyde, which is further metabolised into retinoic acid. Retinoic acid can bind to specific proteins and nuclear receptors, thereby stimulating skin regeneration and enhancing various skin physiological properties [7]. It not only promotes collagen production in the dermis [8], improves skin elasticity, and reduces wrinkles [9] but also effectively inhibits UV-induced matrix metalloproteinase (MMP) activity in the skin, preventing photoageing [10]. Quan [11] emphasised that topical retinol shows promise in enhancing skin texture, reducing fine lines, and increasing the thickness of both the epidermis and dermis. [9] also pointed out that topical retinol, at specific concentrations and exposure times, can maintain epidermal barrier function and promote skin health. However, there are some limitations to the daily use of retinol [12]. Due to its specific physicochemical properties, retinol is highly sensitive to light, temperature, oxygen, and other conditions. It is prone to losing activity, resulting in poor transdermal absorption, low utilisation, and complex storage. Additionally, retinol can be irritating, with some users experiencing mild irritation, skin dryness, flaking, and redness [7]. High concentrations (0.5–1%) of retinol may cause more frequent and intense symptoms, such as dermatitis [13,14], while low doses typically do not significantly improve photoaged skin.
Retinyl palmitate, a derivative of retinol, although requiring two-step conversion to become retinoic acid to exert its effects, exhibits relatively better stability and mildness [15]. Quickly absorbed by the skin, retinyl palmitate is converted into retinol, thereby exerting anti-ageing effects within the skin. The primary functions of retinyl palmitate include accelerating skin metabolism, promoting cell proliferation, and stimulating collagen production [16]. In a study comparing the anti-photoageing effects of five types of retinoids, retinyl palmitate and retinol both demonstrated significant anti-photoageing activity and showed the best therapeutic effects in a UVB-induced photoageing mouse model [17]. However, the single use of retinyl palmitate also presents issues of limited functionality, making it difficult to address the multiple problems caused by skin photoageing comprehensively. On the one hand, as an effective anti-wrinkle agent, RP has limited water solubility and is prone to degradation during topical application [18]. On the other hand, numerous clinical applications have demonstrated its translation (or efficacy) in practical use. The anti-ageing effects of retinyl palmitate require long-term and continuous use to become apparent, and cessation of use may lead to a resurgence of ageing signs. Therefore, further research is needed to investigate the biological photoprotective effects of topical application of high-concentration retinyl palmitate.
Another aspect is some studies have already investigated the actions of natural chemical compounds [19,20,21]. In our preliminary research, we discussed the effects and mechanisms of combined therapy using natural active ingredients such as hydroxycinnamoyl esters and retinol on skin anti-photoageing. Our studies have shown that the combined use of retinol and hydroxycinnamoyl esters can effectively soothe the skin through pathways such as protein metabolism, the tricarboxylic acid (TCA) cycle, ketone metabolism, glucose generation of pyruvate, and metabolism of aspartate and glutamate. Although there are currently no articles specifically addressing the effects of retinol and retinyl palmitate in combating skin ageing, recent research has shown that retinol and retinyl palmitate have great potential in the transdermal delivery of pharmaceutical and cosmetic active ingredients by altering the dense secondary structure of keratin, thereby reducing the skin barrier effect [22].
To overcome the limitations of using retinol and retinyl palmitate as single ingredients, this study proposes an innovative strategy: the combined use of retinol (RT) and retinyl palmitate (RP), aiming to overcome the limitations of single ingredients in anti-photoageing applications and enhance the biological safety during use. Our core objective is to systematically investigate the effects of RP, RT, and their combination (RP&RT) on the epidermal and dermal structures, as well as their potential roles in metabolic regulation under UVB radiation through a series of in vitro experiments and animal model studies. Specifically, we aim to effectively repair skin damage caused by photoageing by reducing the concentration of highly irritant RT and leveraging the effect of RP and RT in combination. Furthermore, an in-depth exploration of the impact of RP&RT combination therapy on epidermal cell structure, proliferative capacity, oxidative damage levels, and the expression of key proteins will further deepen our understanding of the changes in epidermal and dermal structures induced by UVB, as well as their associated metabolic regulation mechanisms, providing a scientific basis for the development of efficient and safe anti-photoageing strategies.
2. Materials and Methods
2.1. Materials
Retinol (C20H30O, ≥95% purity) and retinyl palmitate (C36H60O2, ≥90% purity) were provided by Shenzhen Hujia Technology Co., Ltd. (Shenzhen, China). HaCaT cells were purchased from the China Center for Type Culture Collection (Beijing, China). Dulbecco’s modified eagle medium (DMEM), phosphate-buffered saline (PBS), and Trypsin-EDTA (0.25%) were from Gibco (Grand Island, NE, USA). Fetal bovine serum (FBS) was from Vivacell (Shanghai, China). 4′,6-diamidino-2-phenylindole (DAPI) solution was purchased from Beyotime (Shanghai, China). Bovine serum albumin (BSA), Triton X-100, PPAR-α rabbit pAb, goat anti-rabbit IgG second antibody, IL-1β antibody, IL-6 antibody, TNF-α antibody, COL-1 antibody, and HRP-labeled second antibody were from Sigma-Aldrich (St. Louis, MO, USA). The chemical reagents were of analytical grade and provided by Aladdin (Shanghai, China). HaCaT cells (the cell line used) originate from the Natural Products and Big Health Team Laboratory at the School of Biomedicine, Guangdong University of Technology. Sterile, defibrinated sheep blood was purchased from Hongquan Biotech Co., Ltd. (Guangzhou, China).
2.2. Cell Migration Assay
The experiment was divided into control, UVB, RP, RT, and RP&RT groups. HaCaT cells were inoculated in a 24-well plate at a density of 50,000 cells per well and cultured in a CO2 incubator at 37 °C for 24 h. After 24 h, the plates were removed and washed twice with PBS. All groups, except for the control group, were exposed to UVB irradiation at 40 mJ/cm2. Using sterile 200 μL pipette tips, scratches were made on the cells in the wells. The wells were washed twice with PBS and imaged under a microscope to obtain 0 h images. Subsequently, PBS was replaced with DMEM containing FBS, RT (2 μg/mL), RP (1 μg/mL), or RT&RP (0.5 μg/mL) for each group, and the plates were placed back into the incubator for 24 h. After 24 h, the plates were removed from the incubator, and after washing each well twice with PBS, they were examined under a microscope and photographed to obtain 24 h images. Images were analysed using Image-Pro Plus 6.0 software, where 6 to 8 horizontal lines were randomly drawn, and the average distance between the cells was measured, with migration rate = (0 h scratch width–24 h scratch width)/0 h scratch width × 100%.
2.3. Expression of PPAR-α in HaCaT Cells
The steps for subculturing were repeated, maintaining consistency with the initial cell seeding, grouping for drug administration, and modelling procedures employed in the Cell Migration Assay. Following 24 h of modelling and drug administration, the supernatant from the cell culture plate was removed. The HaCaT cells were fixed with fixing solution for 10 min, permeabilised with 0.5% Triton X-100 for 10 min, and washed thrice with PBS. A 1% BSA solution was introduced for blocking for one hour. Post-blocking, PPAR-α Antibody (1:200) was added to the cell culture plate and left to incubate overnight at 4 °C. Subsequently, the plate was washed three times with PBS. Cy3-labeled goat anti-rabbit IgG (1:500) was added and incubated for 2 h under light exclusion. Following this, a dark incubation with 10 μg/mL of DAPI for 10 min was conducted, followed by three PBS washes. Fluorescence microscopy images were captured using an inverted microscope, and Image-Pro Plus 6.0 was utilised for light density calculations. Data analysis and visualisation were performed using GraphPad Prism 8.0.3.
2.4. In Vitro Hemolysis Assay and Skin Irritation by Hemoglobin Degeneration
Add 1 mL of sterile defibrinated sheep blood to a 15 mL centrifuge tube. Add 8 mL of 37 °C PBS and mix thoroughly. Then, centrifuge at 3800 rpm for 15 min to remove the protein layer from the supernatant. Repeat these steps 3 times. After completion, add 10 mL of 37 °C PBS and set aside. Prepare solutions of 16 mg/mL retinol (RT), 80 mg/mL retinyl palmitate (RP), and a mixture of 16 mg/mL RT and 80 mg/mL RP dissolved in 100 µL ethanol and 900 µL PBS. Mix these three sample groups and PBS in equal volumes, diluting them into four gradients for each group: RT at 2 mg/mL, 1 mg/mL, 0.5 mg/mL, and 0.25 mg/mL. Mix the red blood cell suspension with the solutions from the four gradients mentioned above in equal volumes, with a total volume of 1 mL. Incubate at 37 °C for 1 h. Stop the reaction by centrifuging at 1000 rpm for 5 min. Take the supernatant for imaging, measure the absorbance values at 410 nm, 540 nm, and 575 nm using an ELISA reader, and calculate the hemolysis rate. The positive control group for this experiment (complete hemolysis) is deionised water, and the negative control group (no hemolysis) is the PBS group.
2.5. Establishment of Animal Model
A total of 30 KM mice, 6 weeks old, male, SPF grade, weighing 20–25 g, were purchased from the Animal Ethics Committee of Guangdong University of Technology (Guangzhou, China; SCXK/20231101). Before the experiment, the mice were randomly assigned to five groups with 6 mice in each group: a control group, a model group, an RP group (0.25% RP), an RT group (0.05% RT), and a combination group (0.25% RP + 0.05% RT). After acclimatisation, the mice underwent treatment with the respective compounds.
The control group received standard feeding without any treatment. Other groups were exposed to UVB irradiation (Each group dose was 300 mJ/day), with irradiation once every other day for 28 days; subsequently, the drugs were applied daily to the back skin of mice in each group. In the model group, 200 μL of saline was used, while 200 μL of a solution containing 0.25% retinyl palmitate was applied in the RP group. The RT group received 200 μL of a solution containing 0.05% retinol, and the RP&RT group was treated with 200 μL of a combined solution containing 0.25% retinyl palmitate and 0.05% retinol. On the 29th day, photographs of the back skin of the mice were taken, and the severity of inflammation was scored. The scoring criteria were defined as follows: (1) skin was smooth with no wrinkles or erythema; (2) a small amount of erythema, fine lines, or scabs were present; (3) slight shallow wrinkles, moderate erythema, or scabbing was observed; (4) numerous shallow wrinkles and severe erythema or scabs were evident; (5) skin exhibited thickening, deep wrinkles, or erosion [23]. After the images were taken, the mice were then euthanised by cervical dislocation, and their back skin was collected, fixed in 4% paraformaldehyde, and prepared for further analysis.
2.6. Hematoxylin and Eosin Staining
The skin tissue fixed in 4% paraformaldehyde was embedded. Following standard histological procedures, the embedded tissue was sectioned into thin slices, typically 4–5 micrometres in thickness, using a microtome. These sections were then stained with a Hematoxylin and Eosin staining solution. Subsequently, dehydration and sealing were performed. Finally, the fully automated slide scanning system was used to analyse stained sections and capture high-resolution images of the tissue. Image-Pro Plus 6.0 software was used to analyse the optical density values in the images.
2.7. Toluidine Blue Staining
The skin tissue was embedded in paraffin, then immersed in xylene and a series of gradient ethanol solutions, followed by hydration in distilled water. The hydrated samples were stained with toluidine blue solution for 10 min, thoroughly rinsed with running water for 2 min and differentiated with 1% glacial acetic acid. After differentiation, the samples were washed with tap water for 5 min, dried in an oven, and dehydrated in absolute ethanol. The dehydrated sections were immersed in xylene for 2 min and mounted with neutral resin, and images were captured using a scanning system. The stained sections were analysed for mast cell infiltration using Image-Pro Plus 6.0 software.
2.8. Masson Staining
The paraffin-embedded skin tissue was sequentially immersed in xylene, gradient ethanol, and water. It was then stained with Weigert’s iron hematoxylin for 5 min, followed by washing with water. Differentiation was performed with 1% hydrochloric acid alcohol for a few seconds, and the sections were rinsed with running water. Next, the tissue was stained with Ponceau acidic fuchsin solution for 5 min, washed with water, and then stained with phosphomolybdic acid aqueous solution for another 5 min. This was followed by counterstaining with aniline blue solution for 5 min and differentiation with 1% glacial acetic acid for 1 min. The sections were then dehydrated using gradient ethanol and mounted with neutral resin. Images were captured using an automated slide scanning system, and the optical density values in the images were analysed using Image-Pro Plus 6.0 software.
2.9. Immunohistochemistry Staining
The paraffin-embedded tissue sections were dewaxed in xylene for 15 min and then sequentially immersed in 100%, 95%, and 80% ethanol solutions for 5 min each. The sections were further soaked in pure water for 5 min and washed with PBS buffer for 5 min. They were then immersed in 3% hydrogen peroxide solution for 15 min to block endogenous peroxidase activity, followed by another PBS wash for 5 min. Antigen retrieval was performed, and after a subsequent PBS wash, the sections were covered with 5% BSA solution and incubated in a 37 °C incubator for 30 min for blocking. Primary antibodies targeting COL-1, TNF-α, IL-6, and IL-1β were applied, and the sections were incubated overnight at 4 °C. After washing with PBST, HRP-labeled anti-rabbit IgG were added, and the sections were incubated at room temperature for 1 h. DAB chromogen solution was then applied to develop the colour, and the reaction was stopped using PBS buffer. The sections were rinsed with running water for 8 min, followed by dehydration using gradient ethanol, clearing in xylene, and sealing with neutral resin. Images were captured using an automated digital slide scanner, and the optical density values of the images were analysed using Image-Pro Plus 6.0 software 6.0.
2.10. Transcriptome Analysis
For transcriptome sequencing, data libraries were processed using the Illumina HiSeq 4000 platform (St. Louis, MO, USA). Low-quality data were filtered out using a stringent quality control method to ensure the integrity of the sequencing data. This process involved the removal of sequences containing adapters, sequences with more than 10% ambiguous bases (N), sequences predominantly composed of base A, and sequences with over 50% low-quality bases (Q ≤ 20). Subsequent analyses, including ribosomal RNA alignment, sequence alignment, reference genome alignment, and transcript reconstruction, were conducted using Bowtie2, HISAT2, and StringTie. Gene expression levels for all samples were quantified, followed by sample correlation analysis and differential gene expression analysis. Genes with an FDR < 0.05 and |log2(FC)| > 1 were identified as significantly differentially expressed. Differentially expressed proteins were further analysed in the GO database, and genes were categorised and functionally annotated using the GO and KEGG databases for a comprehensive analysis of biological processes and pathways.
2.11. Metabolomics Analysis
The selected metabolomics experimental method was based on earlier studies. A total of 0.1 g of skin tissue was mixed with 1 mL of chromatography-grade methanol, then homogenised by centrifugation at 13,000 rpm for 15 min at 4 °C. The supernatant was subsequently filtered using a 0.22 μm filter (NEST Biotechnology), and 100 μL of the filtrate was transferred into vials for further analysis. Target compounds were chromatographically separated using an ultra-performance liquid chromatography (UPLC) system with a liquid chromatography column. The chromatographic setup included the Agilent 1290 Infinity LC UPLC system, a hydrophilic interaction liquid chromatography (HILIC) column, a separation temperature of 25 °C, a flow rate of 0.5 mL/min, and an injection volume of 2 µL. The mobile phase consisted of solvent A, a mixture of water with 25 mmol/L ammonium acetate and 25 mmol/L ammonia solution, and solvent B, acetonitrile. The elution conditions were as follows: 0→0.5 min, 95% B; 0.5→7 min, 95%→65% B; 7→8 min, 65%→40% B; 8→9 min, 40% B; 9→9.1 min, 40%→95% B; 9.1→12 min, 95% B. Sample spectra, including both primary and secondary levels, were acquired using an AB Triple TOF 6600 mass spectrometer. The ESI source conditions included ion source gas one and gas 2 at 60 psi, curtain gas at 30 psi, and ion source voltage of ±5500 V (applicable for both positive and negative ion modes). The TOF-MS scan ranged from m/z 60 to 1000 Da, and the product ion scan ranged from m/z 25 to 1000 Da. Secondary mass spectrometry was conducted in data-dependent acquisition mode with high sensitivity. The accumulation potentials for positive and negative ions were set to ±60 V, and the collision energy was (35 ± 15) eV.
2.12. Chemical Similarity Analysis of RT and RP
Previous studies showed that the level of effect in drug combinations was significantly correlated with structural compound differences and their similarity in inducing changes in gene expression [24]. Chemical structure information in SMILES format was downloaded from PubChem, and the MACCS fingerprint for each drug was calculated using RDKit (https://rdkit.org (accessed on 2 January 2024)). A molecular fingerprint is a binary (bit string) representation of a molecule’s structure. If two drug molecules have the A and B bits set in their MACCS fragment bit strings, and C represents the bits set in both fingerprints, the Tanimoto coefficient (T) for the drug pair was defined as follows [25]: T = C/(A + B − C).
2.13. Network Pharmacology
The search for the “skin dry”, “skin allergies”, “skin ageing”, “photoageing”, and “skin soothing” keywords in the GeneCards database (www.genecards.org/ (accessed on 10 February 2024)) generated targets related to skin ageing. The small molecule substances RP (CAS: 79-81-2) and RT (CAS: 68-26-8) were input into the Pubchem website (https://pubchem.ncbi.nlm.nih.gov/ (accessed on 10 February 2024)), and the SMILES structural formulas of RP and RT were obtained. After analysing and selecting the species as “Homo sapiens” from SwisstargetPrediction (SwissTargetPrediction), potential targets were obtained after the screening with a Probability >0.1. Their intersections with senescence were determined through Venny (https://bioinfogp.cnb.csic.es/tools/venny/index.html (accessed on 10 February 2024)). The tool STRING (www.string-db.org/ (accessed on 11 February 2024)) was used to examine the direct and indirect interplays among these targets. Subsequently, a PPI network map was created, visualising the top 10 targets of the highest degree. Subsequently, a PPI network map was created, visualising the top 10 targets of the highest degree through Cytoscape software 3.9.1. DAVID (https://david.ncifcrf.gov (accessed on 12 February 2024)) served the purpose of categorising Gene Ontology (GO) and enriching pathways in the Kyoto Encyclopedia of Genes and Genomes (KEGG). Visualisation of GO terms and KEGG pathways was achieved through the online tool Weishengxin (https://www.bioinformatics.com.cn/ (accessed on 12 February 2024)).
2.14. Molecular Docking
First, the ligand structures of retinol and retinyl palmitate were prepared in SDF file format and imported into the Discovery Studio 2019 (DS) client software for preprocessing, such as structure conversion and optimisation. Then, the crystal structure PDB files of the target proteins COL-I (5N3K), IL-6 (1ALU), IL-1β (1ITB), and TNF-α (7KPA) were downloaded from the RCSB PDB website (https://www.rcsb.org/ (accessed on 20 February 2024)) and preprocessed in DS by removing “water” and “ligands” and adding “hydrogens.” After preparing the proteins, the active site prediction was conducted by clicking “Define Site from Receptor Cavity” to locate the docking pocket. Using the centre of the predicted pocket position as the origin of coordinates, a docking box was created, with the shape and size depending on the project requirements. Once these steps were completed, docking parameters were set using “Dock Ligands (CDOCKER)” with the appropriate docking accuracy selected. The structure files and parameter files were checked, and server resources were utilised to initiate the docking process according to the project requirements.
2.15. Statistical Analysis
The results were expressed as mean ± standard deviation (SD) of triple values. One-way analysis of variance (ANOVA) was utilised to compare the means across multiple groups. In contrast, pairwise comparisons between smaller groups were performed using a two-sided Student’s t-test, conducted with GraphPad Prism software 8.0.2(263), where * p < 0.05 and ** p < 0.01 were considered statistically significant.
3. Results
3.1. Effects of RT& RP on HaCaT Cells in UV-Induced Damage
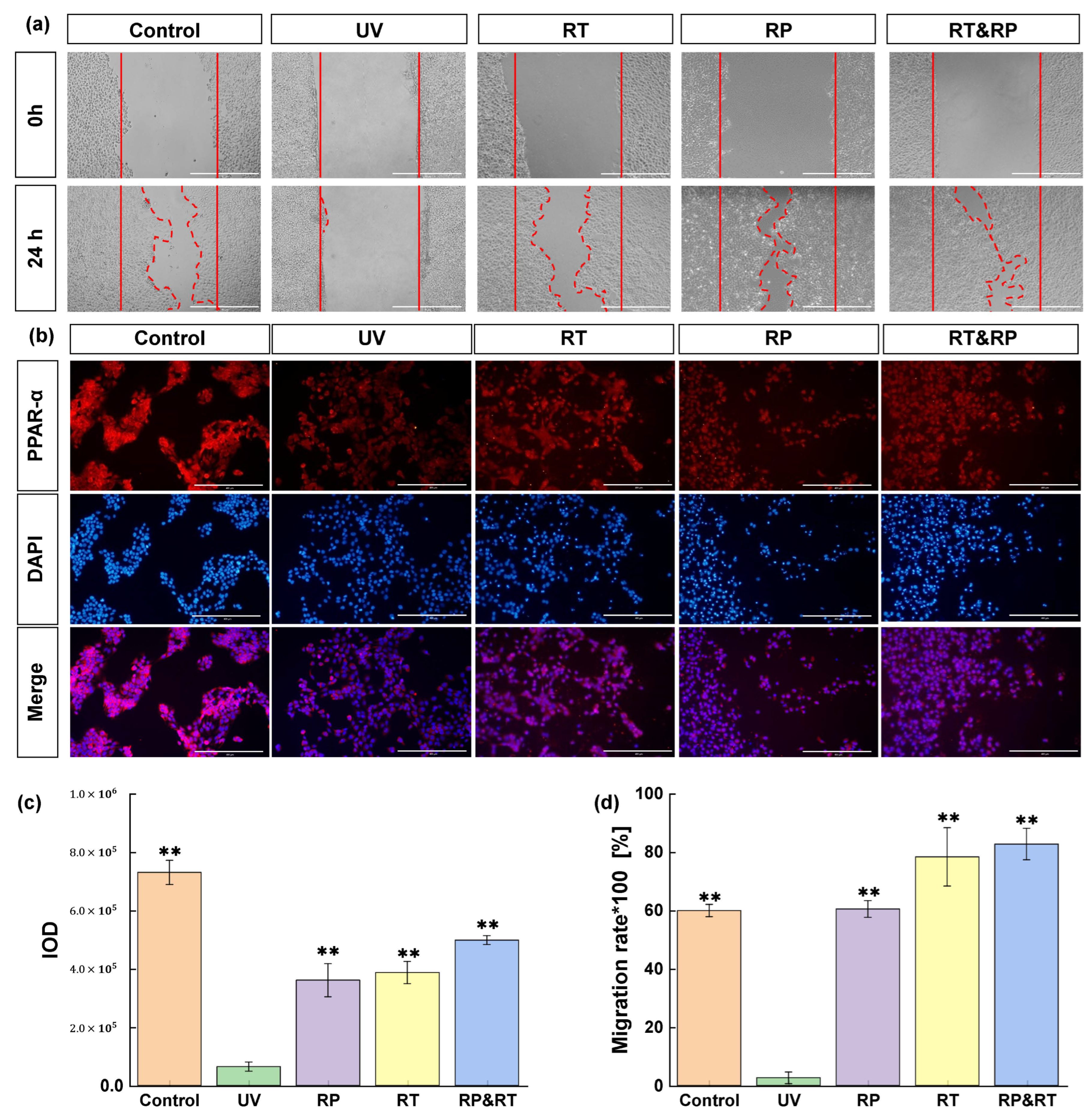
Scratch assay is often employed to assess cell migration and repair effectiveness. It entails creating a gap or scratch on a mature cell layer. Edge cells then fill this gap, resulting in scratch disappearance. This test, akin to wound repair, is frequently used in vitro to evaluate drug anti-photoageing effects on the skin. Research shows that cell migration nearly halts following UV damage. Yet, 24 h post-treatment with RT, RP, and RT&RP, the RT group’s migration rate mirrors the control group’s. Furthermore, subsequent to RP and RT&RP application, the cell migration rates are comparable, and both outperform the control group. These results suggest that RT, RP, and RT&RP exhibit significant efficacy in promoting cell migration and repairing damage (Figure 1a,d) (p < 0.01). Furthermore, the combined treatment of RT&RP demonstrates superior efficacy compared to RT treatment alone. It also indicates that the combined treatment of RT and RP exhibits effects without increasing cellular stimulation.
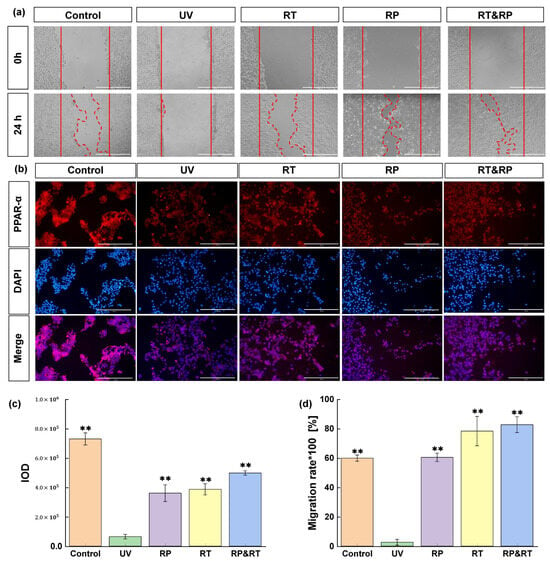
Figure 1.
Effects of RT&RP on HaCaT cells in UV-induced damage. (a,c) Results of scratch migration experiments; (b,d) Results of PPAR-α immunofluorescence assay (n = 3, ** p < 0.01).
PPARs (peroxisome proliferator-activated receptors) represent a ligand-activated nuclear hormone receptor family that governs most cellular metabolic processes. Among these, PPAR-α, a pivotal member, is crucial in regulating proliferation in keratinocytes, maintaining skin homeostasis, and modulating skin inflammation. Activation of PPAR-α enhances the expression of catalase, effectively promoting the degradation of reactive oxygen species (ROS) and reducing ROS generation induced by ultraviolet exposure. Activated PPAR-α also diminishes lymphocyte infiltration and the expression of pro-inflammatory cytokines such as TNF-α and IL-1β, thereby attenuating the inflammatory response triggered by UVB-induced stimulation. This study assessed the impact of RT, RP, and RT&RP treatments on the expression of PPAR-α in UVB-induced ageing HaCaT cells using immunofluorescence. As shown in Figure 1b,c, following UVB damage at 400 mJ/cm2, there was a significant (p < 0.01) reduction in PPAR-α levels within the cells. Subsequent treatment with RT, RP, and RT&RP led to a notable elevation in PPAR-α protein expression compared to the model group. Remarkably, RP monotherapy exhibited a more pronounced effect in upregulating PPAR-α compared to RT monotherapy, while the combined RT&RP treatment surpassed the impact of RP monotherapy. These findings indicate that RT and RP potentially modulate oxidative homeostasis and inflammatory responses in cells by regulating the PPAR-α pathway, thereby ameliorating UVB-induced cellular damage and mitigating oxidative stress and inflammation. Notably, the effects observed with the combined treatment highlight the superior efficacy of RT and RP in concert.
3.2. The Analysis of Biocompatibility
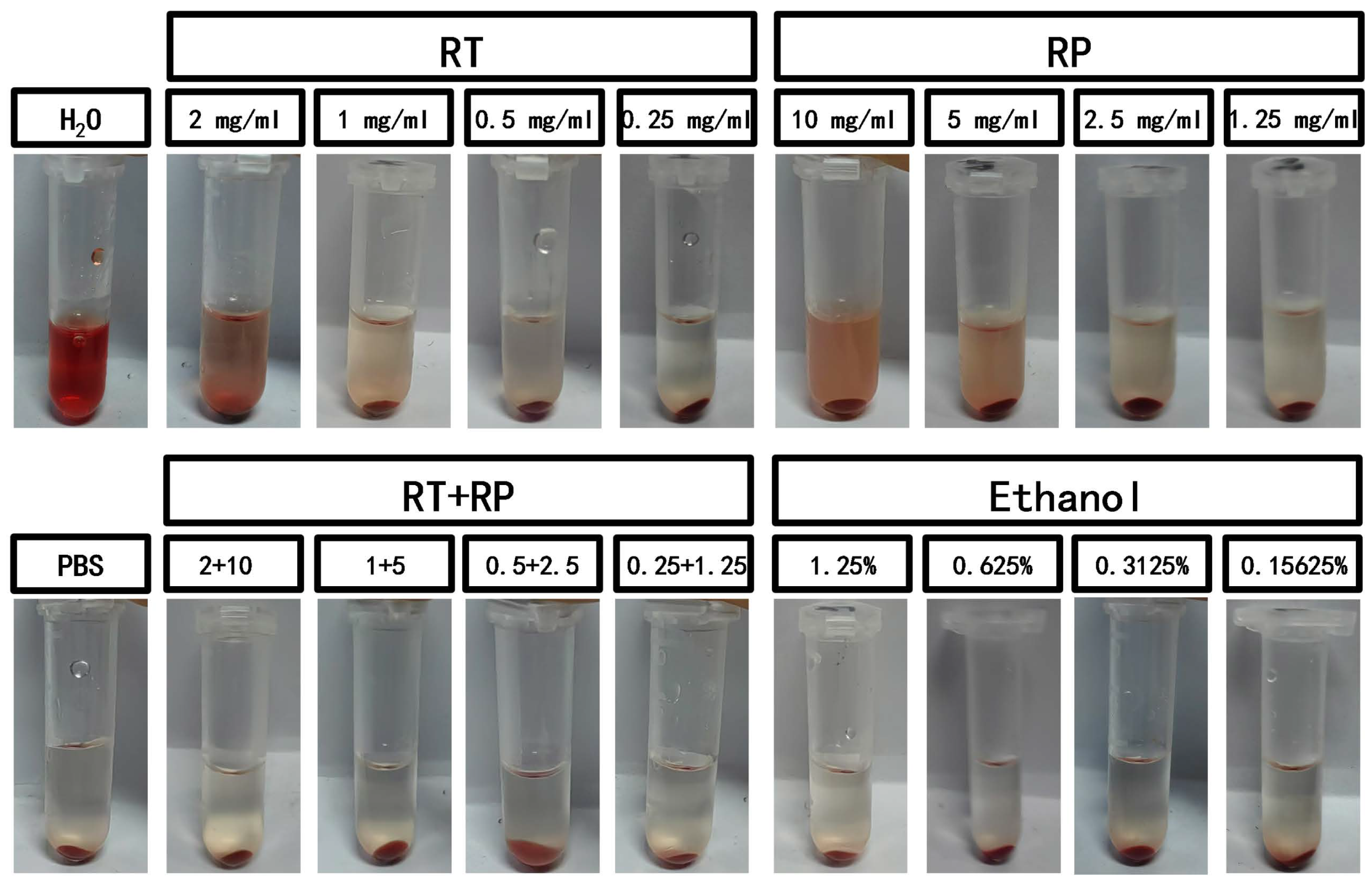
A hemolysis experiment was conducted using defibrinated sheep blood with different concentrations of retinol (RT), retinyl palmitate (RP), and RP&RT. The results in Figure 2 indicate that when retinol is combined with retinyl palmitate, the hemolysis rates for both individual drugs show a significant decrease (Table 1). According to the ECVAM cosmetic product RBC test irritation grading criteria, a material is considered non-irritating when the L/D value is >100, mildly irritating when 10 < L/D ≤ 100, and slightly irritating when 1 < L/D ≤ 10. The experimental results (Table 2) demonstrate that the RT group exhibits mild irritation at concentrations between 0.25–1 mg/mL, the RP group shows mild irritation at concentrations between 1.25–10 mg/mL, and the RP&RT group exhibits non-irritation within the specified concentration ranges. This suggests that the application of RP can reduce the biological irritability of RT at the same concentrations, enhancing the prospects of RP and RT in dermatologically applied cosmetics.

Figure 2.
The hemolysis experiment of RT&RP.

Table 1.
Comparison of hemolysis rates between individual use and combined use of RT and RP.

Table 2.
Biological safety rating of combined use of retinol and retinyl palmitate.
3.3. He Staining Analysis
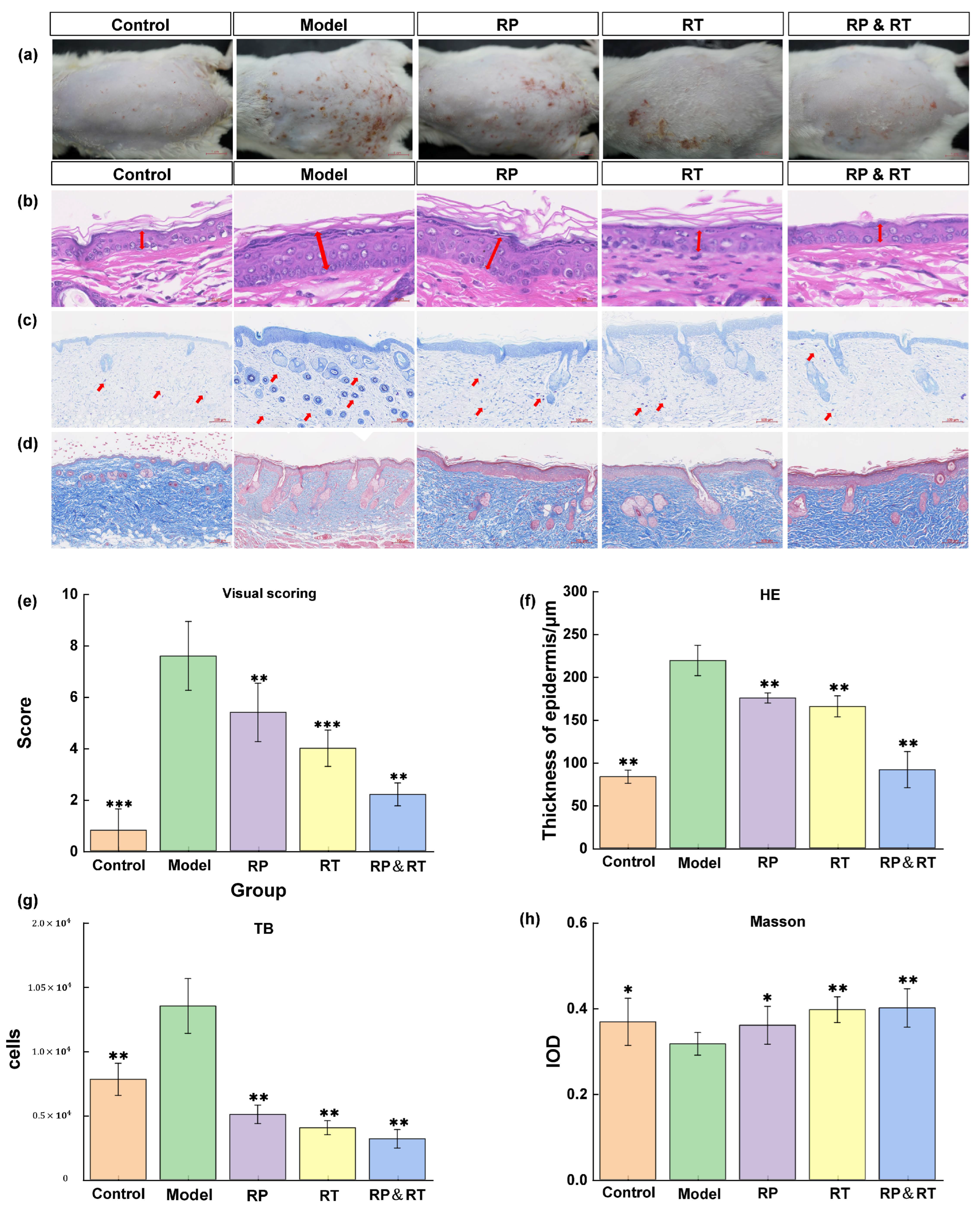
Following UVB irradiation, the model group mice exhibited a marked increase in wrinkles and erythema on their hairless backs compared to the control group. Treatments with RP, RT, and RP&RT were subsequently administered to the model animals. To assess the morphological changes in the skin, the Back Status Assessment Score (Figure 3a) and HE staining were performed (Figure 3b). The control group displayed standard epidermal thickness, while the model group showed significant stratum corneum thickening (p < 0.01). Post-treatment, the skin abnormalities in the experimental animals demonstrated noticeable improvement, with the RP&RT group showing the most pronounced effect (p < 0.01). Based on the statistical results of epidermal thickness in Figure 3f from the HE staining analysis, the RT&RP group shows a significantly lower epidermal thickness of 150 μm compared to 200 μm in the RT group and 220 μm in the RP group, approaching the epidermal thickness of the control group. Therefore, it was inferred that the combination of RT and RP in the RT&RP group acts on the skin epidermis in the UVB-induced skin ageing model, helping to maintain the epidermal thickness at a relatively normal level.
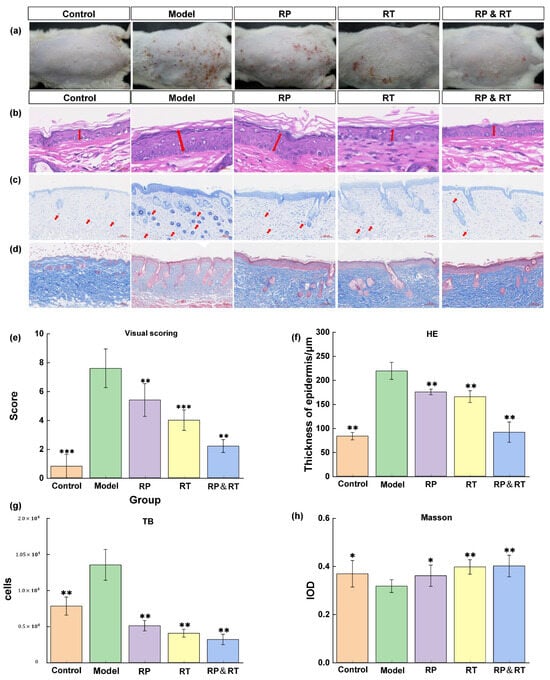
Figure 3.
Tissue staining results. (a,e) The control group displayed standard epidermal thickness back skin assessment score; (b,f) HE staining; (c,g) mast cell infiltration; (d,h) Masson staining. There were significant differences between the experimental groups (n = 3, * p < 0.05, ** p < 0.01, *** p < 0.001).
3.4. Analysis of Blue Toluidine Staining
Research has shown that inflammation plays a crucial role in accelerating skin aging. Toluidine blue staining was employed to evaluate the effects of RT, RP, and their combination (RT&RP) on mast cell infiltration. There was a significant increase in mast cell numbers within the dermis in the model group compared to both the control and model groups. However, following treatment with RT, RP, and RT&RP, the number of mast cells was markedly reduced, approaching levels observed in the control group. As illustrated in Figure 3c, these findings suggest that RT, RP, and RT&RP can effectively reduce the inflammation-induced increase in mast cell numbers, with the RT&RP treatment demonstrating the most substantial effect. Based on the statistical results in Figure 3g, the RT&RP group had approximately 2000 hypertrophic cells, fewer than the 3000 counted in the RT group and the 2500 in the RP group. It was also significantly lower than in the blank and model groups. Therefore, the combination of RT and RP in the RT&RP group demonstrates an effect in reducing hypertrophic cell proliferation.
3.5. Masson Staining Analysis
Collagen fibres are stained blue in Masson-stained sections, while muscle fibres appear red. In the control group, the collagen fibres in the dorsal skin are tightly arranged. In contrast, the model group exhibits a significant reduction and disorganisation of collagen fibres, with some fibres even appearing fragmented (Figure 3d). Compared to the model group, treatments with RP, RT, and RT&RP result in a significant increase in collagen fibres in the hairless dorsal skin, as evidenced by the stronger blue staining observed. The topical application of RP and RT effectively repairs collagen fibre damage induced by UV irradiation, thereby mitigating the effects of skin ageing. Based on the collagen tissue IOD statistics in (Figure 3h), the RT&RP group exhibits the highest IOD, approximately 0.4. Compared to the model, RT, and RP groups, the collagen content shows the most significant increase, and compared with the blue collagen fibres and red muscle fibres in the dermis layer, the messy fibres in the model group were significantly reshaped. This suggests that the components in the RT&RP group exert an effect in promoting collagen tissue formation when used in combination.
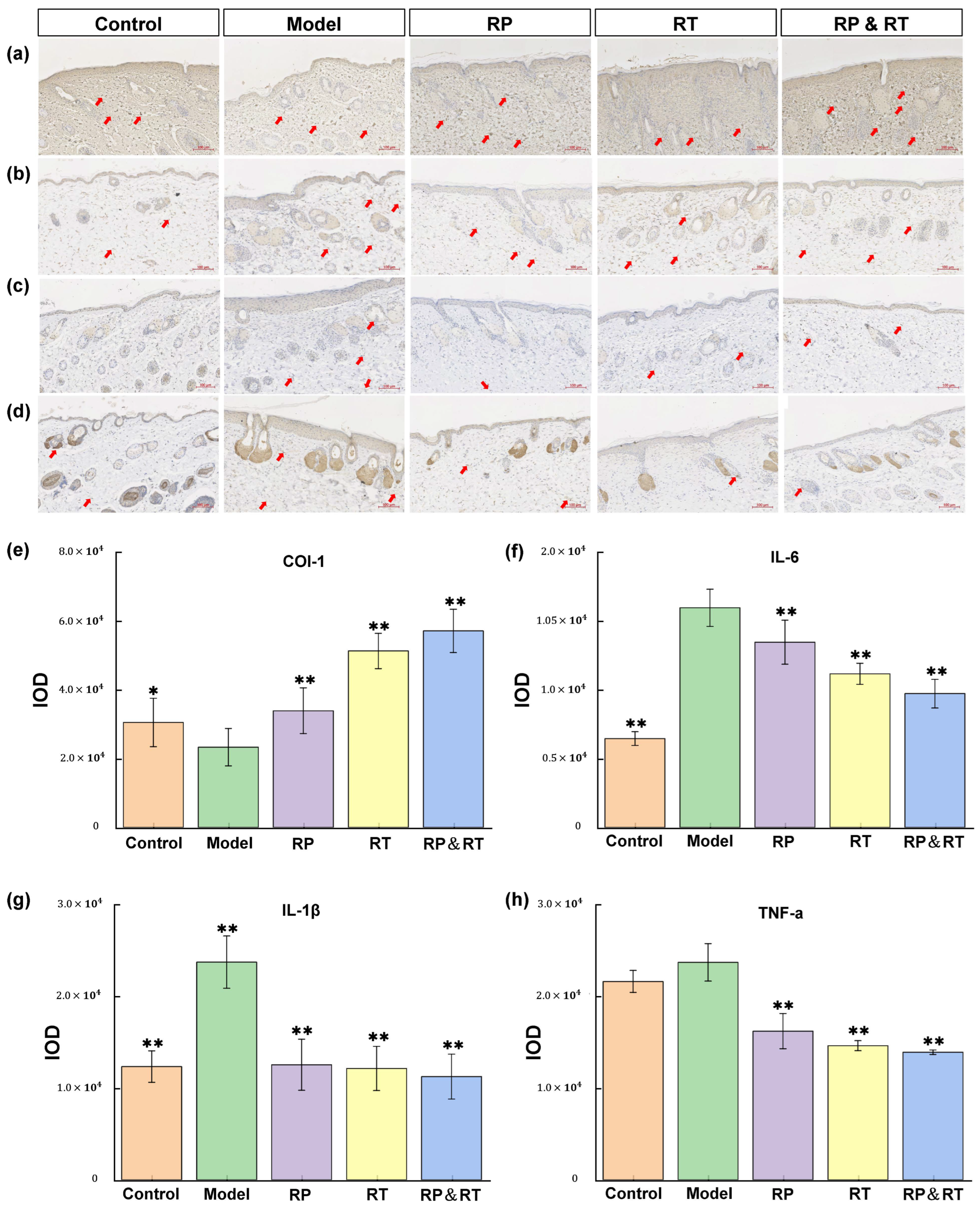
3.6. Immunohistochemical Analysis
Pro-inflammatory cytokines significantly influence the process of skin ageing. Key cytokines involved in the pathological mechanisms of skin ageing include COL-I [26,27,28,29], TNF-α [30], and IL-6 [31]. Immunohistochemical analysis reveals that in the UVB model group, the levels of IL-1β, IL-6, and TNF-α are markedly elevated compared to the control group (p < 0.05). At the same time, Col-I expression is significantly reduced (Figure 4a–d). Following treatment with RP, RT, and RP&RT, the experimental groups demonstrated a substantial decrease in IL-1β, IL-6, and TNF-α expression compared to the UVB model group. Specifically, RP&RT treatment is most effective in reducing IL-1β expression (p < 0.01), RP is particularly effective in lowering IL-6 levels (p < 0.01), and RT is incredibly potent in reducing TNF-α expression (p < 0.01). Additionally, RP&RT treatment results in higher Col-I expression levels in the experimental group compared to the UVB model group. These findings indicate that RP, RT, and RP&RT mitigate UVB-induced photoageing by downregulating IL-1β, IL-6, and TNF-α while enhancing Col-I expression. Based on the IOD statistics for the four immunohistochemical markers in Figure 4e–h, the RT&RP group shows a significantly higher IOD for the COL-I marker than the other groups. The effect surpasses that of the individual RT and RP groups. Since COL-I plays a key role in wound healing, the combination of RT and RP in the RT&RP group mitigates UVB-induced skin ageing by enhancing COL-I production. Regarding the inflammatory markers IL-1β, IL-6, and TNF-α, the IOD statistics indicate that the RT&RP group has lower optical densities than the other groups. Although the IOD for IL-6 in the RT&RP group is not lower than in the blank group, it is still significantly reduced compared to the RT and RP groups. Therefore, the combination of RT and RP in the RT&RP group demonstrates an effect by reducing the inflammatory factors IL-1β, IL-6, and TNF-α, thereby combating skin ageing.
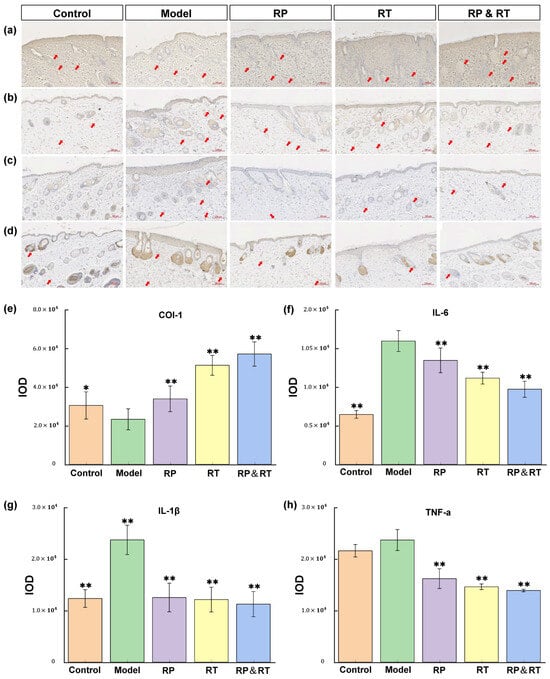
Figure 4.
Immunohistochemical test results. (a,e) Col-I, (b,g) IL-1β, (c,f) IL-6; (d,h) TNF-α. There were significant differences between the experimental groups (n = 3, * p < 0.05, ** p < 0.01).
3.7. Skin Transcriptome Analysis
3.7.1. Analysis and Reproducibility of RT, RP, and RT&RP Components
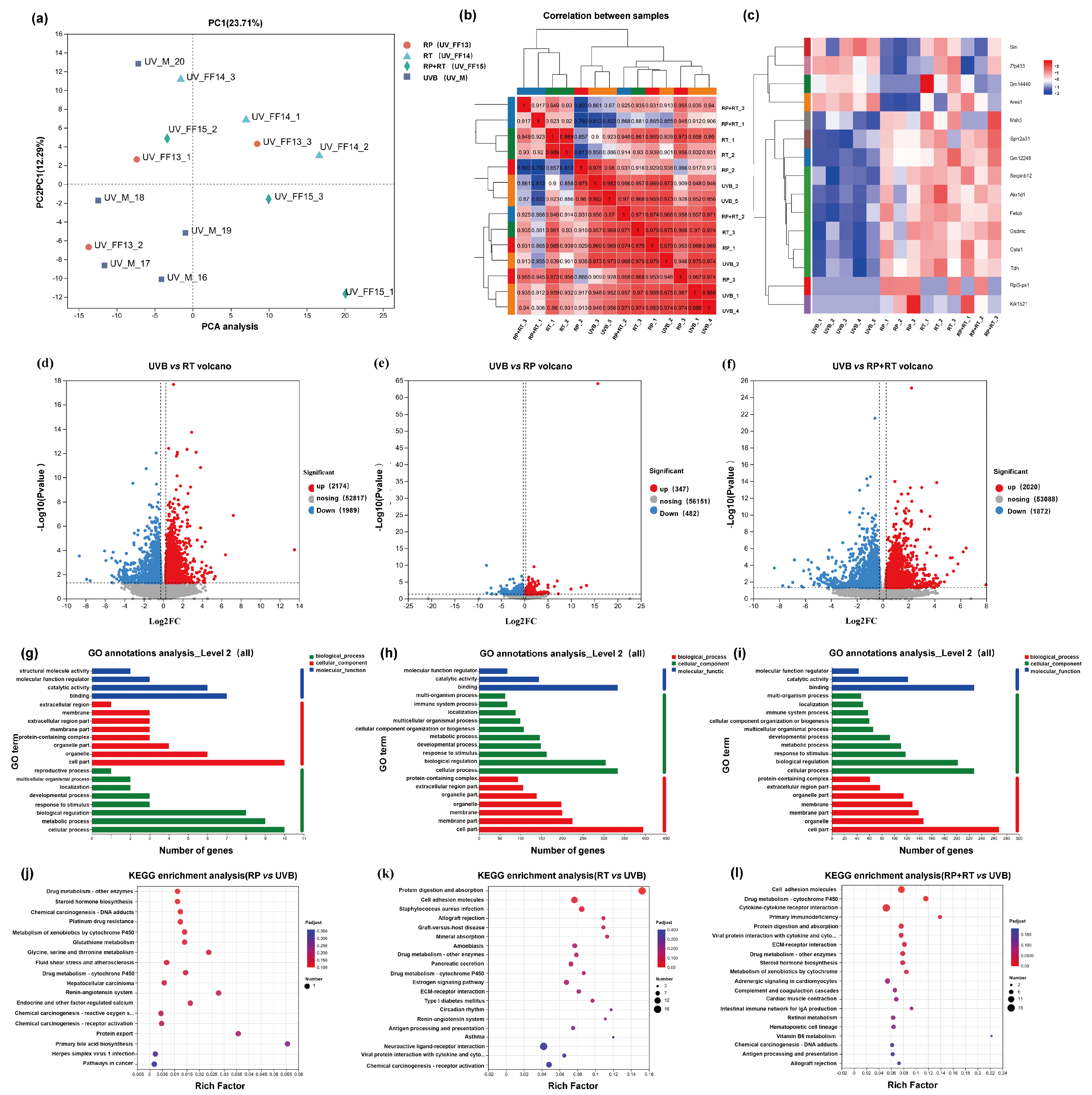
To evaluate the validity of the model, an unsupervised principal component analysis was conducted across all samples. The study revealed that the RT, RP, and RP&RT treatment groups were distinct outliers compared to the UVB model group, as illustrated in Figure 5a. In the principal component analysis plot, stronger red colouration indicates a higher reproducibility correlation, while blue represents a lower reproducibility correlation. Figure 5b highlights that the reproducibility within each group is superior to that between different groups, underscoring the consistency of intra-group data.
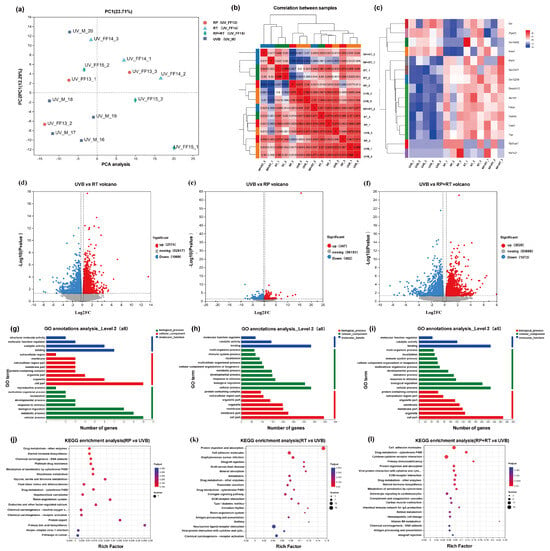
Figure 5.
(a) Principal component analysis. (b) Repetitive correlation assessment. A volcanic map showing the number of differential genes. (c) Cluster heat ma; (d) UV vs. RP; (e) UV vs. RT; (f) UV vs. RP&RT. GO classification of differentially expressed genes. (g) UV vs. RP; (h) UV vs. RT; (i) UV vs. RP&RT. KEGG cluster analysis of differentially expressed genes. (j) UV vs. RP; (k) UV vs. RT; (l) UV vs. RP&RT.
3.7.2. Differential Gene Analysis
The volcano plot illustrates the total number of genes and the significantly regulated differentially expressed genes (DEGs) across different groups. The horizontal axis represents the fold change in gene expression, while the vertical axis indicates the significance level of the genes. In this plot, red dots correspond to upregulated DEGs, blue dots to downregulated DEGs, and grey dots to non-differentially expressed genes. Specifically, the number of upregulated and downregulated genes for RP, RT, and RP&RT are 347 and 482, 2020 and 1872, and 2174 and 1989, respectively. Notably, the RP&RT treatment exhibits a substantial impact on gene regulation, as shown in Figure 5d–f.
3.7.3. Gene Function Analysis
Gene Ontology (GO) serves as an international standard system for classifying gene functions, encompassing molecular function, biological process, and cellular component categories. In Figure 5g–i, the horizontal axis represents gene expression levels. In contrast, the vertical axis corresponds to the secondary GO classification, providing insight into the functional distribution of differentially expressed genes (DEGs).
The Kyoto Encyclopedia of Genes and Genomes (KEGG) database is employed to explore the enrichment and regulatory relationships of DEGs within various pathways. In Figure 5j–l, the horizontal axis denotes the enrichment factor, with the vertical axis indicating the specific KEGG pathways. A more prominent enrichment factor is represented by a redder dot, signifying a higher degree of enrichment. The analysis reveals that RP is significantly enriched in pathways related to drug metabolism by other enzymes, steroid hormone biosynthesis, chemical carcinogen–DNA adducts, platinum drug resistance, cytochrome P450 metabolism of xenobiotics, and glutathione metabolism. On the other hand, RT is primarily enriched in pathways associated with protein digestion and absorption, cell adhesion molecules, and Staphylococcus aureus infection. The combined treatment of RP&RT is notably enriched in cell adhesion molecules, drug metabolism-cytochrome P450, and cytokine–cytokine receptor interaction pathways.
3.7.4. Cluster Analysis of Differentially Expressed Genes
The clustering heatmap illustrates the differentially expressed genes (DEGs) across samples, with the horizontal axis representing sample information and hierarchical clustering results, and the vertical axis representing DEGs and their respective hierarchical clustering outcomes. The expression levels are colour-coded, where red indicates high expression and blue indicates low expression. The analysis reveals that genes such as Msh5, Sprr2a3, Gm12248, Serpinb12, Akr1d1, Fetub, Gsdmc, Gsta1, and Tdh are upregulated in the model group following treatment with either RP, RT, or the combination of RP&RT. Notably, Serpinb12 is associated with the collagen-containing extracellular matrix, Fetub exhibits endopeptidase inhibitor activity, and Gsta1 plays a role in conjugating glutathione to electrophilic compounds. In the RP group, the expression levels of Sin, Zfp433, Gm14440, and Arxes1 are lower compared to the model group, whereas gene expression in the RT group remains unaffected. Zfp433 is potentially involved in RNA polymerase II-mediated transcription, aligning with the molecular function (MF) predicted by GO enrichment in network pharmacology. Additionally, Arxes1 and Arxes2 mRNA is upregulated during adipogenesis via C/EBPα and PPARγ/RXRα pathways (Figure 5c).
3.8. Metabolomics Analysis
3.8.1. Principal Component Analysis (PCA)
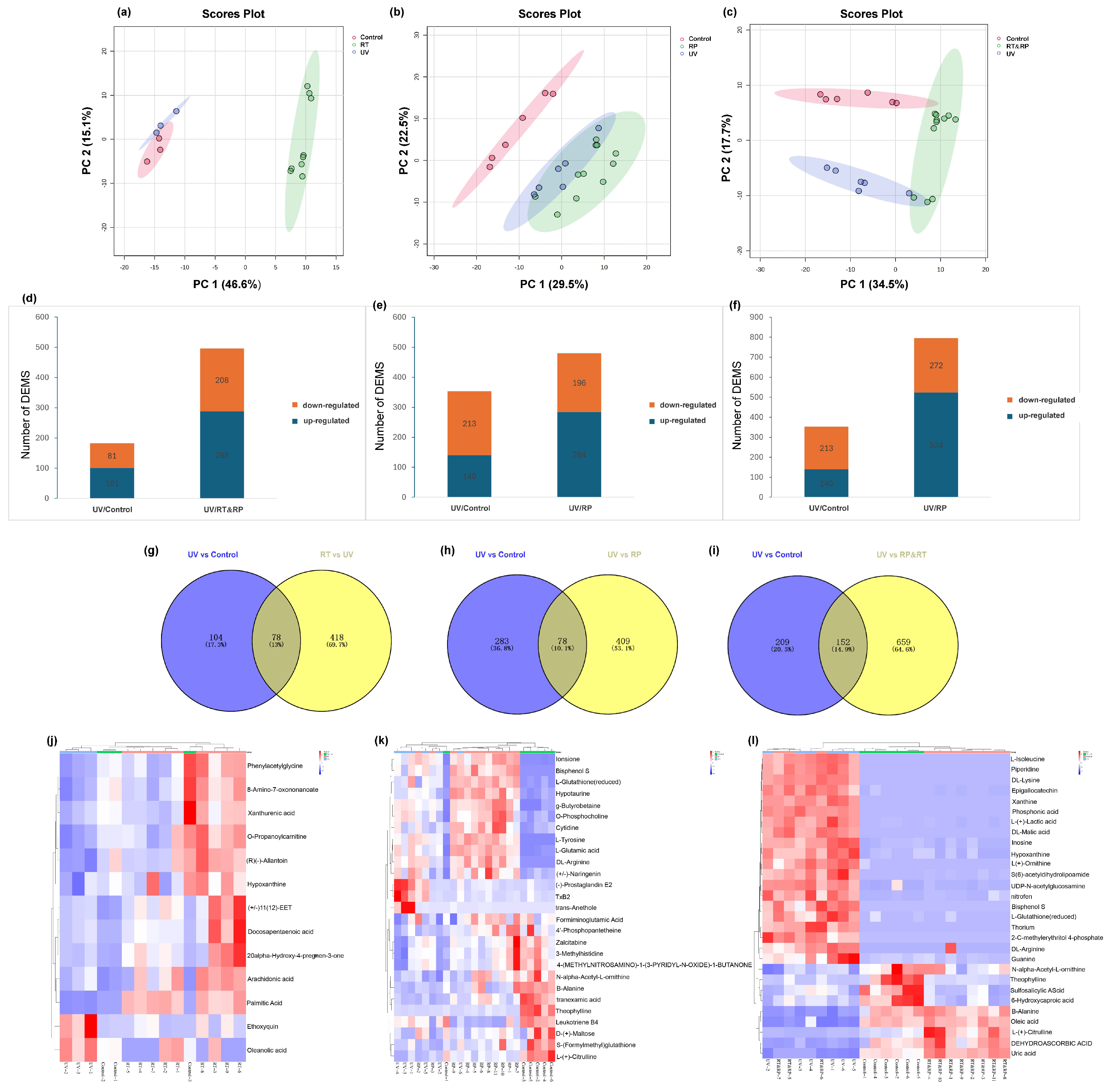
As shown in Figure 6a–c, PCA demonstrates a good separation effect between the drug-treated group (RT), the combined drug-treated and recovery-treated (RT&RP) group, the control group (Control), and the model group (UV). Each group exhibits good intra-group clustering, indicating low within-group differences and high similarity. Inter-group separation is relatively distinct, showing good distinctiveness. The drug-treated group (RP) is well separated from the control group (Control).
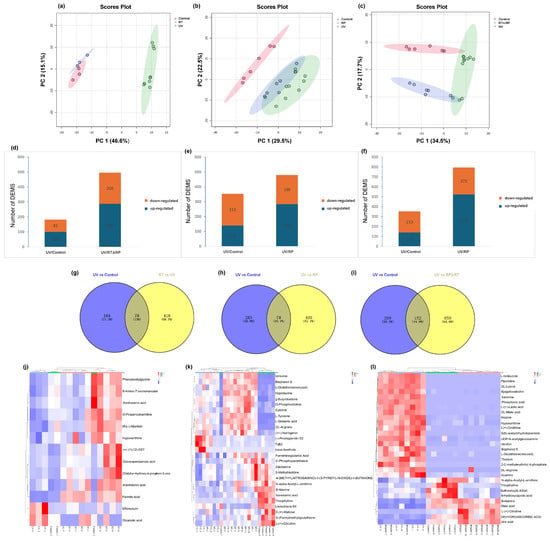
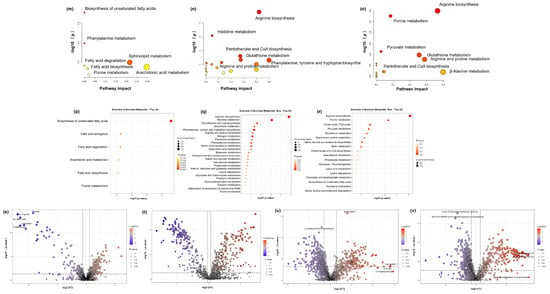
Figure 6.
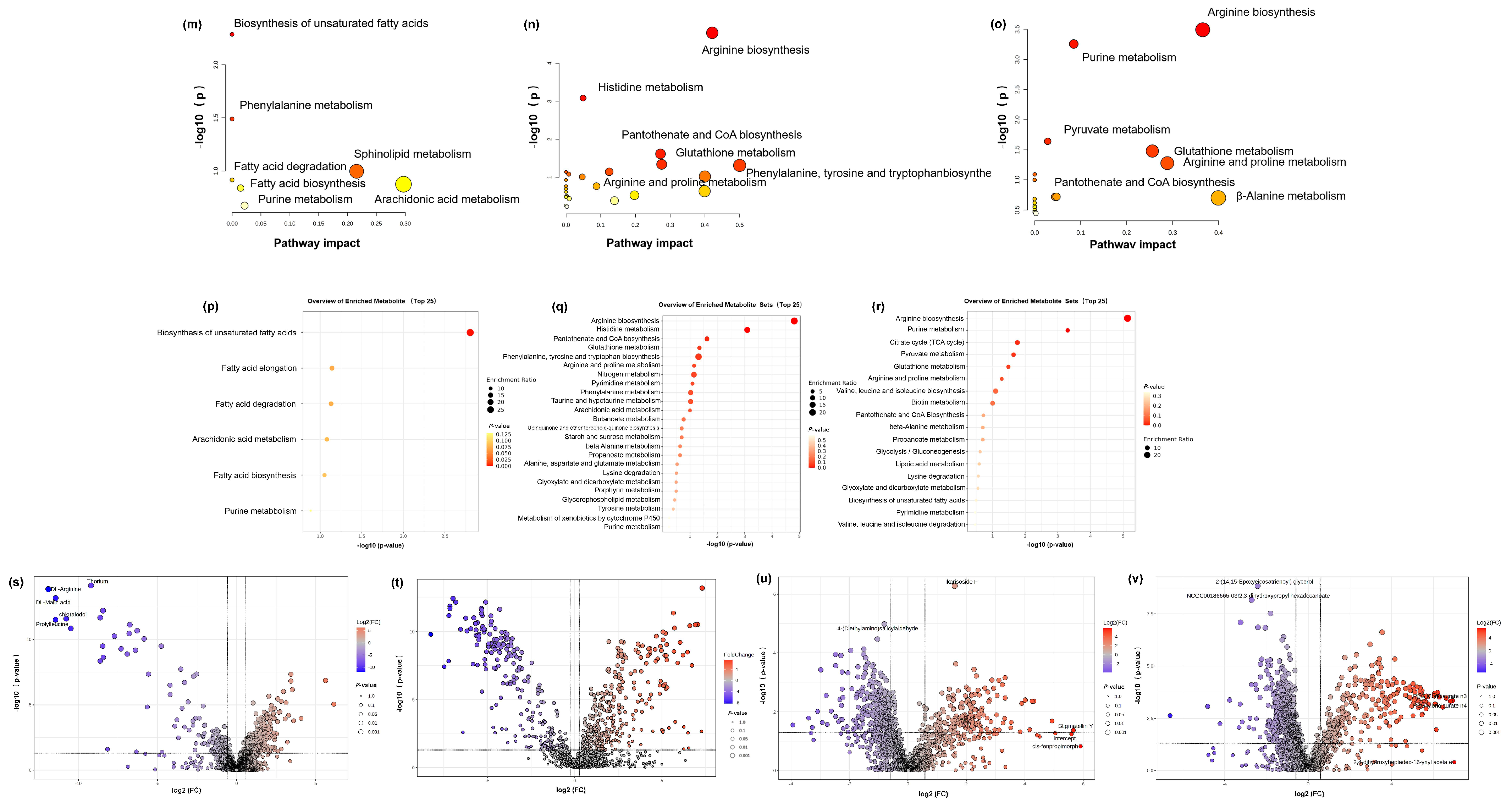
Principal component analysis (PCA). (a) RT; (b) RP; (c) RT&RP. Differences in the number of metabolites expressed in different groups. (d) RT; (e) RP; (f) RT&RP. Venn diagram of common differentially expressed metabolites. (g) RT; (h) RP; (i) RT&RP. Clustered heatmap of common differentially expressed metabolites. (j) RT; (k) RP; (l) RT&RP. Metabolic pathway diagram. (m) RT; (n) RP; (o) RT&RP. Enrichment level diagram of different metabolic pathways. (p) RT; (q) RP; (r) RT&RP. Volcano plot of significantly expressed regulated metabolites. (s) UV; (t) RT; (u) RP; (v) RT&RP.
3.8.2. Selection of DEMs
Pairwise comparisons were conducted to identify differentially expressed metabolites (DEMs) across various comparison groups. The UV group exhibited 101 upregulated DEMs and 81 downregulated DEMs compared to the Control group. The RT group (Figure 6d) showed 288 upregulated DEMs and 208 downregulated DEMs compared to the UV group. When analysing the RP group compared to the RT&RP group, the UV group had 140 upregulated DEMs and 213 downregulated DEMs compared to the Control group. The RP group (Figure 6e) had 284 upregulated DEMs and 196 downregulated DEMs compared to the UV group. In comparison, the RT&RP group (Figure 6f) had 524 upregulated DEMs and 272 downregulated DEMs compared to the UV group, which shows that the combined administration of retinol and retinyl palmitate is more effective in modulating skin inflammation by influencing metabolites than administering retinol or retinyl palmitate alone. Subsequently, the DEMs from the RT group (Figure 6g) were compared to the UV group, resulting in a total of 496 DEMs. Venn analysis was performed with these 496 DEMs and the 182 DEMs from the Control and UV groups, yielding 78 common DEMs. Similarly, the DEMs from the RP group (Figure 6h) were compared to the UV group, resulting in 487 DEMs. Venn analysis with these 487 DEMs and the 361 DEMs from the Control and UV groups yielded 78 common DEMs. Furthermore, the DEMs from the RT&RP group (Figure 6i) were compared to the UV group, resulting in 811 DEMs. Venn analysis with these 811 DEMs and the 361 DEMs from the Control and UV groups yielded 152 common DEMs. Subsequently, using MetaboAnalysis, 13, 27, and 29 endogenous metabolites were selected. Figure 6j–l presents these results in clustered heatmaps, displaying the expression levels of common DEMs. In the heatmap, red indicates higher expression levels of the metabolite in the sample, while blue indicates lower expression levels. The metabolite expression levels after combined treatment with retinol and retinyl palmitate more prominently reverted to the levels of the control group.
3.8.3. Metabolic Pathway Analysis of Anti-Ageing Effects
Subsequently, we uploaded the common differentially expressed metabolites of the three groups to MetaboAnalysis to analyse the metabolic pathways involved in alleviating skin photoageing by RT, RP, and RT&RP. The metabolic pathways for the RT group (Figure 6m) include biosynthesis of unsaturated fatty acids, phenylalanine metabolism, sphingolipid metabolism, arachidonic acid metabolism, fatty acid degradation, purine metabolism, and steroid hormone biosynthesis. For the RP group (Figure 6n), the metabolic pathways include arginine biosynthesis, histidine metabolism, pantothenate and CoA biosynthesis, glutathione metabolism, arginine and proline metabolism, and phenylalanine, tyrosine, and tryptophan biosynthesis. The metabolic pathways for the RT&RP group (Figure 6o) comprise arginine biosynthesis, purine metabolism, pyruvate metabolism, glutathione metabolism, arginine and proline metabolism, pantothenate and CoA biosynthesis, and β-Alanine metabolism. When administered in combination with retinyl palmitate compared to retinol alone, the regulatory impact on purine metabolism is retained, indicating a closeness in pathway regulation to that of retinyl palmitate administered alone. Arginine serves as a crucial substrate for the synthesis of prolyl hydroxylase and lysyl oxidase, two enzymes involved in the cross-linking formation of collagen. By enhancing the activity of these enzymes, arginine can facilitate the synthesis of collagen. Additionally, arginine stimulates the production of growth factors, such as epidermal growth factor and fibroblast growth factor, which play a role in promoting skin repair and regeneration [32]. Proline is one of the primary amino acids involved in collagen synthesis. It is converted into hydroxylysine and hydroxyproline to assist in the formation of collagen. Hydroxyproline, a unique amino acid in collagen, is derived from the hydroxylation of proline by prolyl hydroxylase. Proline constitutes approximately 15% of the amino acids in collagen, alongside glycine and hydroxyproline, collectively forming the main amino acid components of collagen [33]. Glutathione metabolism regulates cellular redox balance, which is essential for cellular health and survival. Arginine and proline metabolism encompasses the metabolic pathways of a rginine and proline, involving their synthesis, conversion, and degradation processes within the body. Arginine serves various physiological functions, such as protein synthesis, nitrogen metabolism, and immune regulation, while proline plays a key role in protein, DNA, and amino acid synthesis. These amino acids are pivotal in cellular metabolism and physiological processes, highlighting their essential roles. This observation suggests that retinyl palmitate may play a more significant role in combination therapy. The enrichment levels of different metabolic pathways shown in Figure 6p–r indicate that the redder the colour, the higher the enrichment level, reflecting more significant changes.
3.8.4. Differential Metabolite Analysis of Anti-Ageing Effects
Finally, based on PCA, differential metabolite screening was conducted utilising a volcano plot to visualise VIP values, p-values, and FC values, as depicted in Figure 6s–v. In the volcano plot, each point represents a metabolite, with the point size proportional to the VIP value. The x-axis represents FC (log2-based), while the y-axis represents p-values (negative logarithm base 10). Different metabolites are colour-coded in the volcano plot to indicate the results of the differential metabolite screening (criteria being VIP > 1 and p < 0.05). Significantly upregulated metabolites are denoted by red dots, significantly downregulated metabolites by blue dots, and non-significant differential metabolites by grey dots.
3.9. Chemical Similarity Analysis of Retinol and Retinyl Palmitate
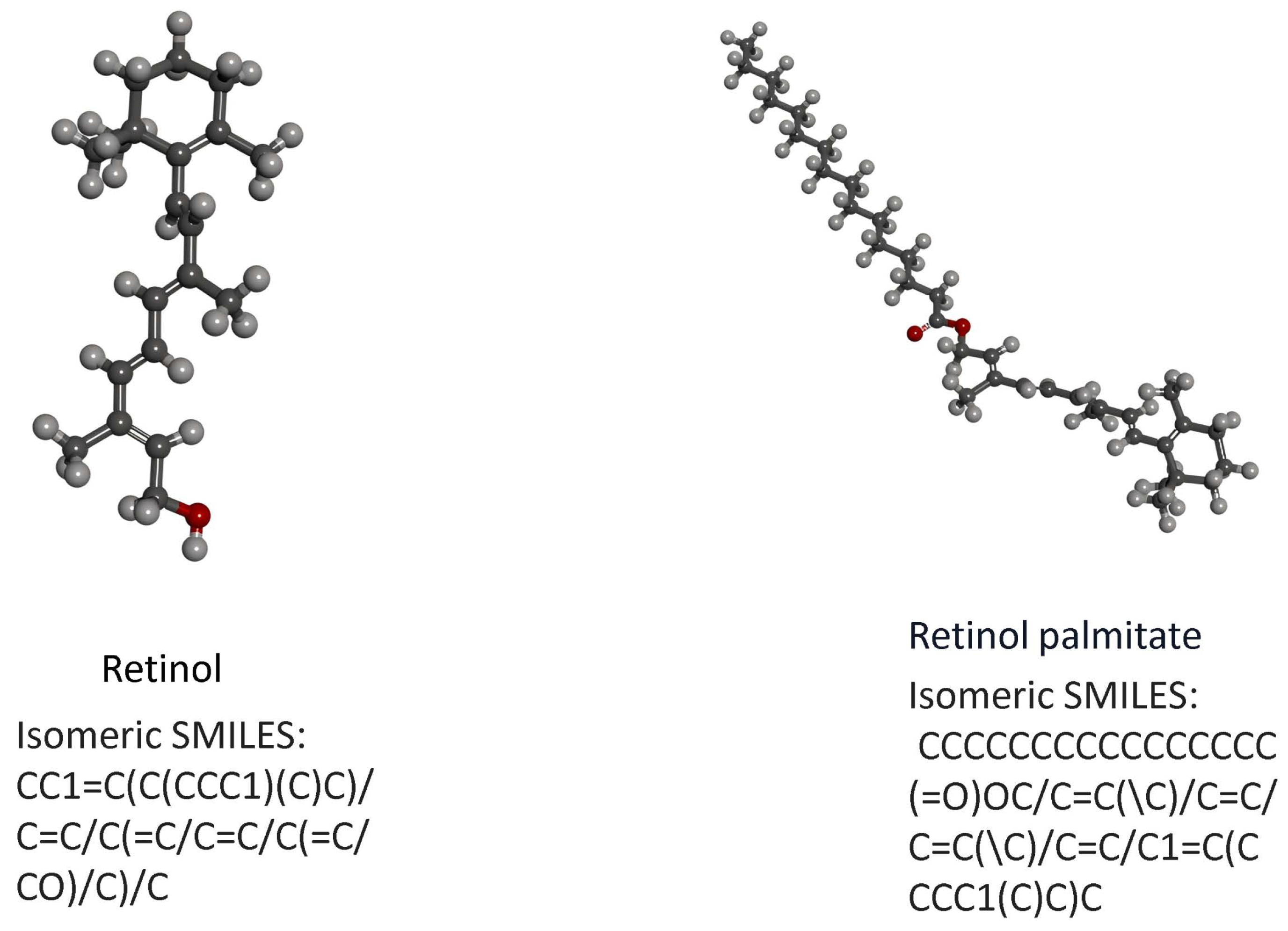
The effect of retinol and retinyl palmitate can be predicted based on their structural similarity and impact on the transcriptome. Research has confirmed that when nodes in a network have structural similarities, they are more likely to form groups or functional substructures, which may promote effects. Because nodes with similar structures are more likely to cooperate or participate in certain functions, they concluded that highly correlated independent drug responses could explain most combined clinical trials. In contrast, low-correlated independent drug responses make the independent action of drugs the dominant mechanism in the clinical population (additivity). The results found that the molecular structural similarity between retinol and retinyl palmitate was 0.46428, indicating that there was no significant difference in structure between retinol and retinyl palmitate. Still, both were below the threshold (0.68), suggesting that retinol and retinyl palmitate may exert a potential effect on anti-photoageing by interacting with different proteins or performing different functions (Figure 7). To verify this effect, we subsequently conducted network pharmacology and molecular docking analysis.
Figure 7.
(a) Structural formula of retinol. (b) Structural formula of retinyl palmitate.
3.10. Network Pharmacological Analysis
3.10.1. Targets Related to Skin Ageing Components
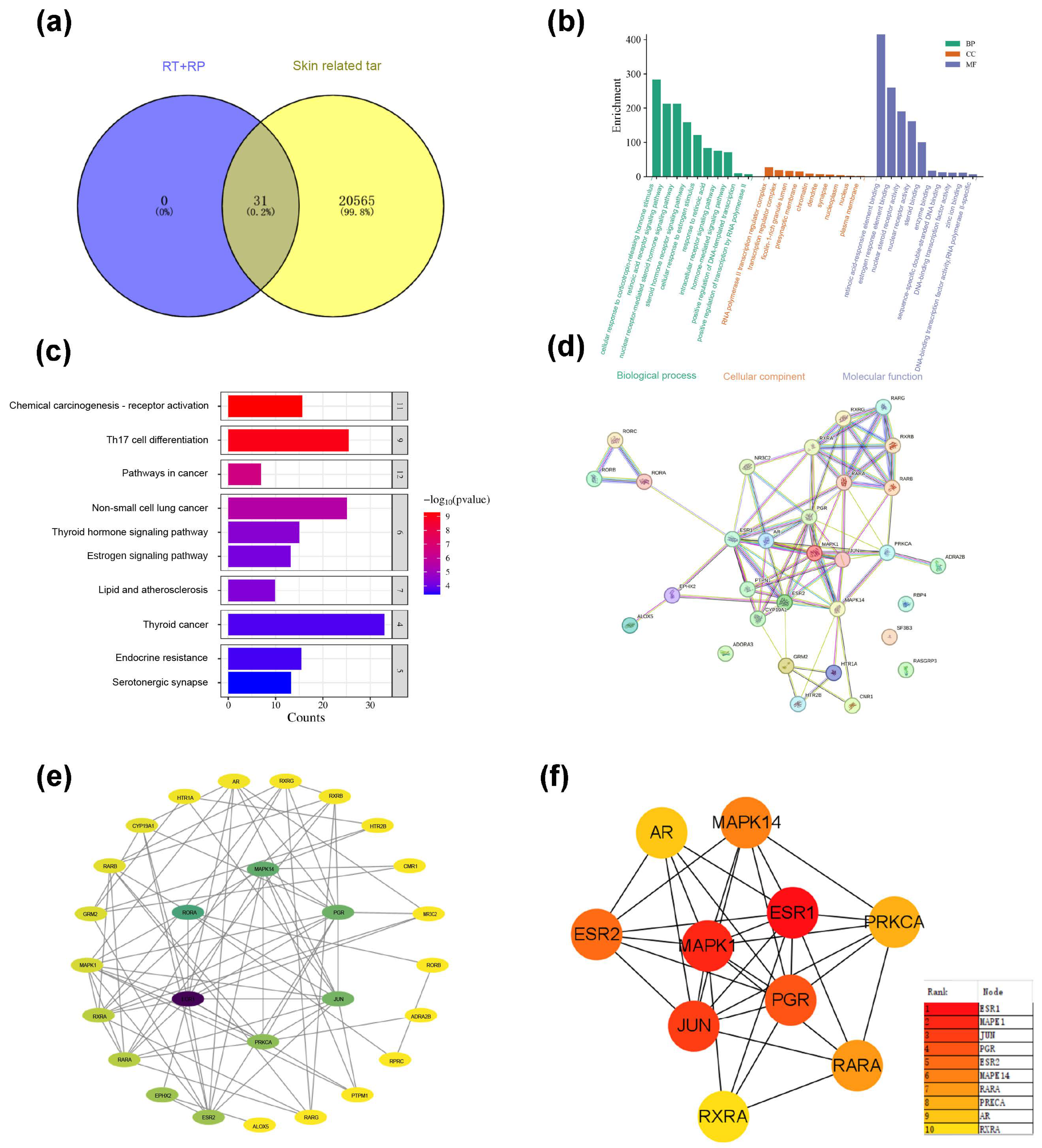
Query the CAS of RT and RP (RP: 78-81-2; RT: 68-26-8) will be input to the Pubchem website (https://pubchem.ncbi.nlm.nih.gov/ (accessed on 10 February 2024)) to obtain the Isomeric SMILES structural formulas of RP and RT. Then, upload it to the SwissTargetPrediction database (http://www.swisstargetprediction.ch/) (accessed on 10 February 2024), select the species as “Homo sapiens”, filter with Proba ability > 0.1, and output in CSV format, and a total of 31 targets were predicted; with the keywords “skin dry; skin allergies; skin ageing; photography; skin soothing”. Then use gene cards (GeneCards—Human Genes) Gene Database|(Gene Search) website searched for relevant targets for skin repair, exported the CSV format, and enriched a total of 20,596 targets. The targets of RP and RT and the target genes of skin repair were introduced into the Venny 2.1.0 (https://bioinfogp.cnb.csic.es/tools/venny/ (accessed on 10 February 2024)) website, and the intersection was taken. After removing the duplication value, 31 core gene targets were obtained, as shown in Figure 8a.
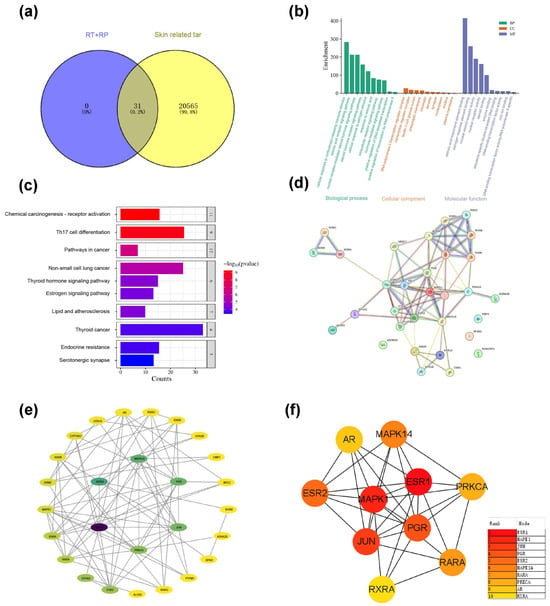
Figure 8.
(a) Enrich intersection targets of RP&RT and skin ageing-related. (b) GO enrichment diagram of RP&RT related to skin ageing. (c) KEGG enrichment diagram of RP&RT to skin ageing-related. (d) Protein–molecule interactions (PPI). (e) The network diagram of component-target-pathway. (f) TOP10 core target enrichment.
3.10.2. GO Enrichment and KEGG Enrichment
Each gene’s essential function is based on its protein domain and the literature studied. GO and KEGG are databases of gene-related functions stored based on different classification ideas. GO enrichment analysis was carried out with the help of the DAVID website (DAVID: Functional AnnotationTools (https://david.ncifcrf.gov) (accessed on 12 February 2024)), and data with p < 0.01 were screened, and a total of 30 GO entries were obtained, including 102 biological processes (BP), 15 cellular components (CC) and 37 molecular functions (MF). To more intuitively understand the gene function, the p-value and Count were used as a reference to order, and a histogram (Figure 8b) was generated with the help of the Microinformatics website (https://www.bioinformatics.com.cn/) (accessed on 10 February 2024). Biological process (BP) analysis found that key target genes are mainly involved in the cell’s response to estrogen stimulation, cell response to corticotropin-releasing hormone stimulation, retinoic acid receptor signalling pathway, nuclear receptor-mediated steroid hormone signalling pathway; Analysis of cellular components (CC) suggests that it interacts with RNA polymerase II transcription regulatory complex, rich ficolin-1 particle cavity, presynaptic membrane, chromatin related; related items on molecular function (MF) mainly include retinoic acid responsive element binding retinoic acid-responsive element binding, estrogen-responsive element binding, nuclear receptor activity, steroid binding, atomic steroid receptor activity, etc.
A total of 104 signalling pathways were screened out based on KEGG analysis based on the DAVID database (Figure 8c). According to the sequencing of Log P and each pathway’s enrichment genes, the potential pathways that RT&RP mainly participates in skin repair include Th17 cell differentiation, non-small cell lung cancer pathway, chemical carcinogenesis-receptor activation, pathways in cancer, lipid, and atherosclerosis, and thyroid hormone signalling pathway. The targets involved include the MAPK family, the JUN family, the PGR family, etc.
3.10.3. Construction of Potential Target PPI Network
A preliminary protein interaction information map was obtained after uploading the shared protein gene to the STRING website (Figure 8d). Further, a compound-target network was constructed based on Cytoscape 3.7.2 software to screen core nodes based on network topology characteristics such as node degree values, and the network diagram was drawn, as shown in Figure 8e,f.
As shown by the enriched pathways, MAPK, or mitogen-activated protein kinase, plays an important role in cell proliferation, differentiation, apoptosis, autophagy, and cell cycle arrest. MAPKs have three subgroups: MAPK, MAP2K, and MAP3K. Activation of MAPK is a cascade process in which MAP3Ks first phosphorylate and activate MAP2K, then MAP2K phosphorylates MAPK, and finally activates downstream effector molecules. On the one hand, the MAPK signalling pathway slows down the process of skin photoageing by regulating the expression of transcription factor AP-1 (activator protein 1). Collagen is the main component of the extracellular matrix. MMPs (matrix metalloproteinases) can specifically degrade almost all ECM components, resulting in reduced collagen synthesis and degradation of elastic fibres, accelerating skin photoageing. ERK and JNK bind to the MMP-1 promoter by activating AP-1, participating in the expression of MMP-1, causing the phenotype of skin ageing. On the other hand, studies have shown that the continuous activation of p38MAPK induces the production of ROS (reactive oxygen species) in mitochondria, which serves as a cellular stress compensation mechanism, further promotes the autophagy behaviour of cells, helps cells clear damaged components, and maintains cell homeostasis. JUN: c-Jun. The JUN signalling pathway is important in cell proliferation, differentiation, apoptosis, autophagy, and cellular stress response. JUN is a component of the AP transcription factor, and its activation is mainly achieved through the MAP signalling pathway. The upstream signal of JUN initiates the transcription of downstream genes, mainly through the phosphorylation cascade of ERK, JNK, and p38 MAPK. On the one hand, the JUN pathway regulates the expression of MMP, promotes the degradation of ECM (extracellular matrix), and accelerates the process of skin photoageing. Collagen is the main component of ECM, and MMPs specifically degrade collagen in ECM, resulting in reduced collagen synthesis and degradation of elastic fibres, thereby exacerbating skin photoageing. Activation of JUN enhances the expression of MMPs, disrupts the stability of ECM, and leads to skin ageing. On the other hand, studies have shown that over-activation of JUN can increase ROS production and further damage cells by regulating mitochondrial function and stress response. JUN can help clear damaged cell components, relieve cell stress responses, and promote cell repair and renewal by inducing the autophagy mechanism. However, continued ROS accumulation and excessive activation of autophagy may lead to increased cellular ageing and skin damage. PGR: Progesterone receptor. The PGR signalling pathway plays a vital role in cell proliferation, differentiation, apoptosis, autophagy, and cell cycle regulation. PGR contains two main isoforms: PGR-A and PGR-B. PGR is activated by binding to its ligand progesterone and then by forming a heterodimer with RXR (retinol X receptor) to initiate transcription of downstream genes. On the one hand, the PGR pathway slows down the process of skin photoageing by regulating the expression of antioxidant genes and MMPs. Collagen is the main component of ECM, and MMPs specifically degrade collagen in ECM, resulting in reduced collagen synthesis and degradation of elastic fibres, thus triggering skin photoageing. Activation of PGR can inhibit the expression of MMPs, thereby promoting the stability of ECM and delaying the occurrence of skin ageing. On the other hand, studies have shown that over-activation of PGR can reduce RO production by regulating mitochondrial function and the expression of antioxidant genes, thereby reducing oxidative stress damage in cells. In addition, PGR also alleviates the stress response of cells by inducing autophagy, promotes cell repair and renewal, and helps further delay the photoageing process of the skin.
3.11. Molecular Docking
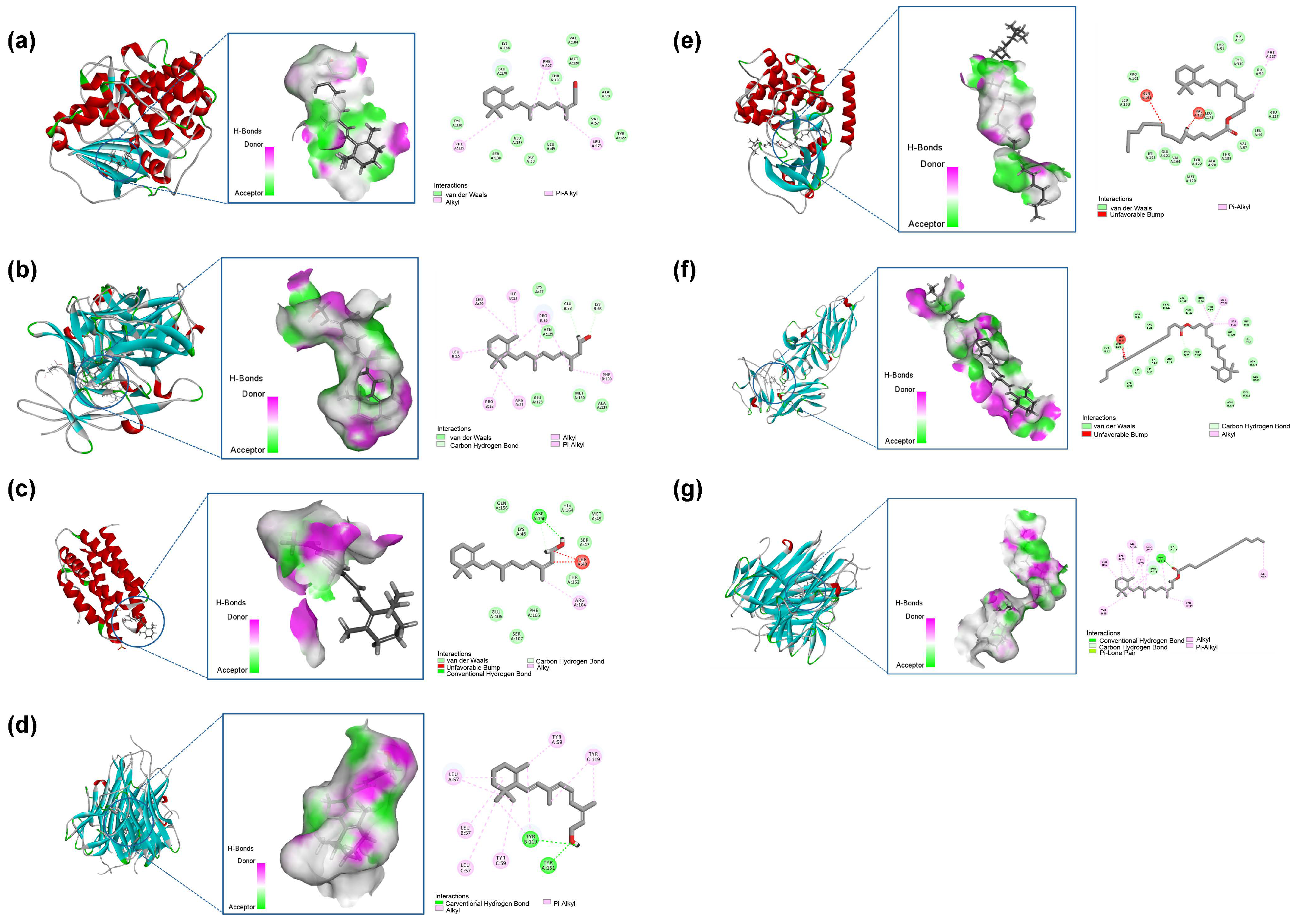
The binding stability of ligands and receptors is generally believed to be negatively correlated with the binding energy values of ligands and receptors—typically, the lower the binding energy value, the better the docking ability (Figure 9). The binding energy of less than 0.7 kcal/mol indicates that the small drug molecule has strong binding activity with the target protein. When the binding energy is less than 0.5 kcal/mol, it indicates good binding [34,35]. The results showed that the binding energy of retinol to the core target was less than −0.5 kcal/mol, the docking binding energy of retinyl palmitate to TNF was less than −0.5 kcal/mol, the binding energy of retinyl palmitate to TNF was more significant than 0, and there was no docking with IL-6. Table 3 for details. Retinol forms one hydrogen bond with each of the TRY-119 and TYR-151 residues of the TNF gene; Retinol forms one hydrogen bond with ASP-26 of the IL-6 gene; Retinol forms one hydrogen bond with each of GLU-33 and LYS-63 residues of the IL-1β gene. Retinyl palmitate forms 2 bonds with the TYR-119 residue of the TNF gene and 1 hydrogen bond with the ILE-118 residue. Hydrogen bonds are one of the main driving forces for the interaction between proteins and ligands and play an important role in stabilising protein–ligand complexes [36]. The results showed that Retinol repaired skin ageing by regulating COL, IL-6, IL-1β, and TNF-α genes. Retinyl palmitate mainly played a role in repairing skin ageing by regulating TNF-α genes.
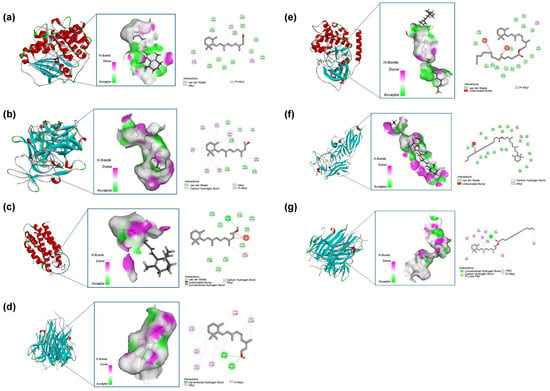
Figure 9.
(a) Retinol-COL. (b) Retinol-IL-6. (c) Retinol-IL-1β. (d) Retinol-TNF-α. (e) Retinyl palmitate-COL. (f) Retinyl palmitate-IL-1β. (g) Retinyl palmitate-TNF-α.

Table 3.
Binding energy of RT and RP docking with key target molecules.
4. Discussion
As the outermost organ of the human body, the skin serves not only as the first line of defence against external threats but is also highly susceptible to accelerated ageing due to environmental factors [37]. Ultraviolet (UV) radiation interacts directly with DNA, inducing the production of pro-inflammatory mediators and reactive oxygen species (ROS), which disrupt the skin’s self-regulatory mechanisms. This leads to photoageing effects such as wrinkle formation, pigmentation, and loss of skin elasticity. Both retinol and retinyl palmitate, when used individually, have been shown to effectively enhance the synthesis of key extracellular matrix components, such as collagen, elastin, and hyaluronic acid [38,39]. Furthermore, studies on the combined treatment of retinol and asiaticoside for skin photoageing suggest the potential for effects between retinol and retinyl palmitate [40,41].
In the field of skincare, retinol is frequently utilised as an active ingredient in cosmetics. It influences the epidermal keratinisation process, improves the structure of the stratum corneum, and reduces transepidermal water loss. Furthermore, it significantly enhances skin appearance, brightens pigmentation, and reduces signs of photoageing. However, despite its crucial role in combating photoageing, excessive use of retinol can lead to skin irritation or allergic reactions in some cases. Additionally, the instability of retinol limits its development as a cosmetic ingredient. Retinyl palmitate, a derivative of vitamin A, is commonly used in skincare products. It is similar to retinol but relatively more stable, gradually converting into retinol within the skin, thereby exhibiting antioxidant properties and promoting cellular renewal.
This study explored the effects of combined treatment. The advantage of the cell scratch wound healing assay lies in its ability to observe and quantitatively analyse cell migration speed and direction visually. PPAR-α is primarily expressed in tissues such as the liver, heart, and kidneys, regulating processes such as lipid metabolism, cholesterol metabolism, and glucose metabolism. Our research demonstrated that after treatment with retinol and retinyl palmitate, cell migration activity significantly increased, and the expression of PPAR-α protein, related to skin ageing, was markedly restored compared to the UV model group. The haemoglobin denaturation test is used to assess the impact of compounds on haemoglobin structure, detecting structural changes due to denaturation that may lead to functional loss. This test is commonly employed to evaluate the effects of compounds on protein structure, particularly in assessing skin irritation caused by drugs, cosmetics, or other compounds. The hemolysis test for the combined use of retinol and retinyl palmitate indicated that their joint application could effectively reduce skin irritation or allergic reactions caused by retinol, providing better biocompatibility and potentially leading to more comprehensive and effective therapeutic outcomes. Immunohistochemical results revealed that retinol could directly stimulate collagen production and improve skin structure, while retinyl palmitate gradually converts into retinol within the skin, exerting similar effects. When used, they may jointly promote skin cell renewal and repair, accelerating the recovery process of skin damage. Metabolomics research findings showed that retinol and retinyl palmitate jointly regulate the secretion of various metabolites, such as DL-arginine, L-glutathione (reduced form), and 5-(3′-hydroxyphenyl)-γ-valerolactone-3′-O-glucuronide, which play crucial roles in skin health. When used in combination, they may promote skin health and enhance biosafety by regulating metabolite secretion. Transcriptomics studies revealed that the combined treatment of retinol and retinyl palmitate may combat photoageing through multiple pathways, including allograft rejection, drug metabolism, extracellular ma-trix-receptor interaction, vitamin B6 metabolism, and retinol metabolism. This multi-pathway effect not only enhances drug efficacy but also helps reduce potential side effects associated with single-pathway treatment, improving biosafety.
In summary, the use of retinol and retinyl palmitate enhances biosafety by reducing skin irritation and allergic reactions, enhancing antioxidant properties, promoting cell renewal and repair, improving drug stability and bioavailability, combating photoageing through multi-pathway, and regulating metabolite secretion.
5. Conclusions
This study found that the combined use of RP (retinyl palmitate) and RT (retinol) can mitigate UVB-induced skin ageing by promoting cell migration, reducing oxidative stress and inflammation through the PPAR-α pathway, and enhancing biocompatibility. Results from multi-omics analyses showed that the combined therapy can inhibit pro-inflammatory factors, enhance anti-inflammatory responses, effectively alleviate UVB-induced skin damage, and exert anti-ageing effects. Molecular docking techniques were also employed to predict the potential impact of combined RT and RP treatment on anti-inflammatory factors. In summary, the RP&RT combination demonstrates potential as a therapeutic approach for treating UVB-induced skin photoageing with reduced irritability and alleviated onset delay issues; however, further preclinical studies are required to understand its efficacy and mechanisms of action fully.
Author Contributions
Conceptualisation: L.Z.; experiment execution: Y.W.; data curation, formal analysis, investigation, and methodology: J.Z., L.Z., J.W. and Y.W.; funding acquisition: P.S.; project administration, resources, and supervision: Y.W.; software: X.N.; validation: L.Z. and J.W.; visualisation and writing—original draft: Y.W.; writing—review and editing: P.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
All animal experiments were performed under the Guidelines for the Care and Use of Laboratory Animals of Guangdong University of Technology (Guangzhou, China), and the experiments were approved by the Animal Ethics Committee of Guangdong University of Technology (Guangzhou, China; SCXK/20231101).
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets supporting this article’s conclusions are available from the corresponding author upon reasonable request.
Acknowledgments
We thank all members of the laboratory for their technical support and academic discussions.
Conflicts of Interest
Authors Yuan Wang, Xin Nie, Jiangming Zhong, Jing Wang and Peng Shu were employed by the company Shenzhen Hujia Technology Co., Ltd. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Liu, E.; Xue, Z.; Li, Y.; Liao, Y. Photoaging Decoded: Extracellular Matrix Alterations and Mechanisms via Mitogen-Activated Protein Kinase/Matrix Metalloproteinase, Transforming Growth Factor-β Pathways, and Glycosaminoglycan Metabolism. Tissue Eng. Part B Rev. 2024, 2024, 0274. [Google Scholar]
- Weihermann, A.C.; Lorencini, M.; Brohem, C.A.; De Carvalho, C.M. Elastin structure and its involvement in skin photoageing. Int. J. Cosmet. Sci. 2017, 39, 241–247. [Google Scholar] [PubMed]
- Moutraji, R.; Taylor, S.C. Skin aging exposome in the skin of colour populations: A review of the literature. Dermatol. Surg. 2023, 49, 272–277. [Google Scholar] [PubMed]
- Waldman, R.A.; Grant-Kels, J.M. Sunscreen may prevent the development of basal cell carcinoma in individuals with basal cell carcinoma nevus syndrome: A retrospective survey study. J. Am. Acad. Dermatol. 2019, 81, 1028–1030. [Google Scholar]
- Fisher, G.J.; Kang, S.; Varani, J.; Bata-Csorgo, Z.; Wan, Y.; Datta, S.; Voorhees, J.J. Mechanisms of photoaging and chronological skin aging. Arch. Dermatol. Res. 2002, 138, 1462–1470. [Google Scholar]
- Zhang, Y.; Zhou, J. Overview of patent technologies for the application of retinol and its derivatives in cosmetics. Henan Sci. Technol. 2018, 18, 46–48. [Google Scholar]
- Zasada, M.; Budzisz, E.; Erkiert-Polguj, A. A clinical anti-ageing comparative study of 0.3 and 0.5% retinol serums: A clinically controlled trial. Ski. Pharmacol. Physiol. 2020, 33, 102–116. [Google Scholar]
- Kenney, M.C.; Shih, L.M.; Labermeir, U.; Satterfield, D. Modulation of rabbit keratocyte production of collagen, sulfated glycosaminoglycans and fibronectin by retinol and retinoic acid. Biochim. Et Biophys. Acta Mol. Cell Res. 1986, 889, 156–162. [Google Scholar]
- Kim, J.E.; Kim, W.H.; Kim, S.; Na, Y.; Choi, J.; Hong, Y.D.; Shim, S.M. Bioconversion of retinol and its cell barrier function in human immortalised keratinocytes cells and artificial epidermis–dermis skin. Exp. Dermatol. 2023, 32, 822–830. [Google Scholar]
- Park, E.Y.; Wilder, E.T.; Lane, M.A. Retinol Inhibits the Invasion of Retinoic Acid–Resistant Colon Cancer Cells In Vitro and Decreases Matrix Metalloproteinase mRNA, Protein, and Activity Levels. Nutr. Cancer 2007, 57, 66–77. [Google Scholar]
- Quan, T. Human skin aging and the anti-aging properties of retinol. Biomolecules 2023, 13, 1614. [Google Scholar] [CrossRef] [PubMed]
- Chien, A.L.; Kim, D.J.; Cheng, N.; Shin, J.; Leung, S.G.; Nelson, A.M.; Kang, S. Biomarkers of tretinoin precursors and tretinoin efficacy in patients with moderate to severe facial photodamage: A randomised clinical trial. JAMA Dermatol. 2022, 1588, 879–886. [Google Scholar]
- Xu, H.; Song, Q.; Zhou, Q.; Yang, B.; Zhang, J.; Wang, Z.; Zhang, J. Retinol-loaded deep eutectic solvent emulsion: Improved stability and therapeutic efficiency. J. Drug Deliv. Sci. Technol. 2024, 102, 106364. [Google Scholar]
- Mellody, K.T.; Bradley, E.J.; Mambwe, B.; Cotterell, L.F.; Kiss, O.; Halai, P.; Watson, R. Multifaceted amelioration of cutaneous photoageing by (0.3%) retinol. Int. J. Cosmet. Sci. 2022, 44, 625–635. [Google Scholar]
- Fu, P.P.; Xia, Q.; Boudreau, M.D.; Howard, P.C.; Tolleson, W.H.; Wamer, W.G. Physiological role of retinyl palmitate in the skin. Vitam. Horm. 2007, 75, 223–256. [Google Scholar]
- Schiltz, J.R.; Lanigan, J.; Nabial, W.; Petty, B.; Birnbaum, J.E. Retinoic acid induces cyclic changes in epidermal thickness and dermal collagen and glycosaminoglycan biosynthesis rates. J. Investig. Dermatol. 1986, 87, 663–667. [Google Scholar]
- Shu, P.; Jiang, L.; Li, M.; Li, Y.; Yuan, Z.; Lin, L.; Wen, J.; Aisa, H.A.; Du, Z. Comparison of five retinoids for anti-photoaging therapy: Evaluation of anti-inflammatory and anti-oxidative activities in vitro and therapeutic efficacy in vivo. Photochem. Photobiol. 2024, 100, 633–645. [Google Scholar]
- Tomas, M.; Günal-Köroğlu, D.; Kamiloglu, S.; Ozdal, T.; Capanoglu, E. The state of the art in anti-aging: Plant-based phytochemicals for skin care. Immun. Ageing 2025, 22, 5. [Google Scholar]
- Astuti, I.Y.; Yupitawati, A.; Nurulita, N.A. Anti-aging activity of tetrahydrocurcumin, Centella asiatica extract, and its mixture. Adv. Tradit. Med. 2021, 21, 57–63. [Google Scholar]
- Psotova, J.; Svobodova, A.; Kolarova, H.; Walterova, D. Photoprotective properties of Prunella vulgaris and rosmarinic acid on human keratinocytes. J. Photochem. Photobiol. B Biol. 2006, 84, 167–174. [Google Scholar]
- Haftek, M.; Mac-Mary, S.; Bitoux, M.A.-L.; Creidi, P.; Seité, S.; Rougier, A.; Humbert, P. Clinical, biometric and structural evaluation of the long-term effects of a topical treatment with ascorbic acid and madecassoside in photoaged human skin. Exp. Dermatol. 2008, 17, 946–952. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.H.; Zhou, Q.; Xu, Y.; Xu, B.N.; Shu, P.; Peng, L.H. Casting New Light on the Retinol and Retinyl Palmitate Functions as Chemical Enhancers for Transdermal/Topical Drug Delivery. Adv. Healthc. Mater. 2025, 14, 2402836. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Chen, J.; Huang, K.; Fang, S.; Wei, B.; Chen, A. Establishment of skin photoaging SD rat model and discussion of evaluation criteria. J. Chongqing Med. Univ. 2016, 41, 379–383. [Google Scholar]
- Williams, W.R. Phytohormones: Structural and functional relationship to purine nucleotides and some pharmacologic agents. Plant Signal. Behav. 2021, 16, 1837544. [Google Scholar]
- Yang, M.; Jaaks, P.; Dry, J.; Garnett, M.; Menden, M.P.; Saez-Rodriguez, J. Stratification and prediction of drug synergy based on target functional similarity. NPJ Syst. Biol. Appl. 2020, 6, 16. [Google Scholar]
- Himmat, M.; Salim, N.; Al-Dabbagh, M.M.; Saeed, F.; Ahmed, A. Adapting document similarity measures for ligand-based virtual screening. Molecules 2016, 21, 476. [Google Scholar] [CrossRef]
- Cui, B.; Liu, Q.; Tong, L.; Feng, X. The effects of the metformin on inhibition of UVA-induced expression of MMPs and COL-I in human skin fibroblasts. Eur. J. Inflamm. 2019, 17, 1–5. [Google Scholar] [CrossRef]
- Khare, R.; Upmanyu, N.; Jha, M. Exploring the potential effect of methanolic extract of Salvia officinalis against UV exposed skin aging: In vivo and in vitro model. Curr. Aging Sci. 2021, 14, 46–55. [Google Scholar] [CrossRef]
- Pu, X.; Qu, Y. A study on the delayed effect of tilapia skin collagen on skin aging for mice and its possible mechanism. J. Cosmet. Dermatol. 2023, 22, 3436–3444. [Google Scholar] [CrossRef]
- Titisari, R.S.; Herawati, E.; Astirin, O.P. Oral intake of collagen hydrolysate from mackerel scad (Decapterus macarellus) attenuates skin photoaging by suppressing the UVB-induced expression of MMP-1 and IL-6. J. Complement. Integr. Med. 2024, 21, 71–79. [Google Scholar]
- Misawa, E.; Tanaka, M.; Saito, M.; Nabeshima, K.; Yao, R.; Yamauchi, K.; Furukawa, F. Protective effects of Aloe sterols against UVB-induced photoaging in hairless mice. Photodermatol. Photoimmunol. Photomed. 2017, 33, 101–111. [Google Scholar] [PubMed]
- McKay, T.B.; Priyadarsini, S.; Rowsey, T.; Karamichos, D. Arginine Supplementation Promotes Extracellular Matrix and Metabolic Changes in Keratoconus. Cells 2021, 10, 2076. [Google Scholar] [CrossRef]
- Phang, J.M. Perspectives, Past, Present and Future: The Proline Cycle/Proline-Collagen Regulatory Axis. Amino Acids 2021, 53, 1967–1975. [Google Scholar] [PubMed]
- Liao, F.; Yousif, M.; Huang, R.; Qiao, Y.; Hu, Y. Network pharmacology- and molecular docking-based analyses of the antihypertensive mechanism of Ilex kudingcha. Front. Endocrinol. 2023, 14, 1216086. [Google Scholar]
- Zhang, Y.; Li, Z.; Wei, J.; Kong, L.; Song, M.; Zhang, Y.; Jin, Y. Network pharmacology and molecular docking reveal the mechanism of Angelica dahurica against Osteosarcoma. Medicine 2022, 101, e31055. [Google Scholar]
- Du, X.; Li, Y.; Xia, Y.L.; Ai, S.M.; Liang, J.; Sang, P.; Liu, S.Q. Insights into protein–ligand interactions: Mechanisms, models, and methods. Int. J. Mol. Sci. 2016, 17, 144. [Google Scholar] [CrossRef]
- Chung, J.H. Photoaging in asians. Photodermatol. Photoimmunol. Photomed. 2003, 19, 109–121. [Google Scholar]
- Dhaliwal, S.; Rybak, I.; Ellis, S.R.; Notay, M.; Trivedi, M.; Burney, W.; Sivamani, R.K. Prospective, randomized, double-blind assessment of topical bakuchiol and retinol for facial photo Oxford comma. Br. J. Dermatol. 2019, 180, 289–296. [Google Scholar]
- Mambwe, B.; Mellody, K.T.; Kiss, O.; O’Connor, C.; Bell, M.; Watson, R.E.; Langton, A.K. Cosmetic retinoid use in photoaged skin: A review of the compounds, their use and mechanisms of action. Int. J. Cosmet. Sci. 2025, 47, 45–57. [Google Scholar]
- Cook, B.; Riggs, M.; Holley, K.C.; Knaggs, H.; Diwakar, G.; Lephart, E.D. Effects of Retinol, Natural Pea Peptide and Antioxidant Blend in a Topical Formulation: In Vitro and Clinical Evidence. Dermatol. Ther. 2025, 15, 189–200. [Google Scholar]
- Liu, Q.; Shu, P.; Song, Q.; Huang, Z.; Weng, J.; Zhang, L.; Liu, Q. Retinol and Hydroxyasiaticoside Synergistically Relieve Histamine-Induced Atopic Dermatitis Activity by Repressing TRPV1, L1R1, and CD130 Targets. Cosmetics 2024, 11, 203. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).