Abstract
The physicochemical properties of nonwoven wet wipe fabrics have a strong influence on the ability of microorganisms to attach and multiply, until a biofilm is formed. Cellulose-based fabrics, being biodegradable, represent a major contamination risk. In addition, having a hydrophilic nature, they provide a good platform for microorganisms attachment. To optimize biodegradable wet wipes antimicrobial quality, it is crucial to assess the impact of physicochemical properties, e.g., density, pore size, fiber diameter, contact angle and surface charge. Here, we investigated the physical characteristics of commonly used nonwoven fabrics from both synthetic (Polyethylene terephthalate, PET) and natural components (wood pulp and viscose), to evaluate their effect on microbial contamination. We found that the hydrophobicity of the fabric had varying influence on attachment, depending on the microbial strain. However, the geometry, as well as the fabric pore size greatly affected attachment regardless of the microbial strain, in which a larger pore size resulted in lower accumulation of microbial biomass. Our study gives insight into the characteristics of wet wipes that can affect the preservation efficacy and microbial contamination risk, in one of the biggest segments in the personal care industry.
1. Introduction
Due to recent market demands for sustainable, natural ingredients to be used in personal care products, wet-wipes manufacturers are more and more using biodegradable cellulose-based fibers to replace the synthetic ones. Indeed, our societies are experiencing increasing issues in sewage waste management, microfiber contamination in the oceans and land, due to the plastic pollution derived from the synthetic fibers found in most wet wipes [1].
Wet wipes represent a multiparametric system, comprised of a substrate (the fabric) and of a lotion including antimicrobial agents. Having an extremely large surface area, the fabric bears the responsibility for increasing the risk of microbial contamination issues. Recently, in our previous study, we have explored the inherent difficulty of preserving wet wipes from microbial contamination [2]. Common wet wipes substrates are usually comprised of a blend of two types of fibers. The synthetic fibers, made of either polypropylene, polyethylene terephthalate (PET), and nylon, are suitable for the mass market due to their cost effectiveness, while the biologically biodegradable cellulose-based fibers, such as wood pulp, viscose, cotton and bamboo, contribute to the softness and better touch feeling of the wipes. Latest trends in the cosmetic industry, plastic regulations, and customers demand for natural, biodegradable materials, have been pushing the industry to manufacture 100% cellulose based wet wipes.
Wet wipe preservation is extremely challenging, due to in-use habits, high surface area of the fabric and the lotion which usually contains more than 95% of water. Microorganisms can easily contaminate the final product, leading to multiple product recalls and health risk issues. Cellulose (e.g., viscose) is a polymer, made of glucose units, that microorganisms can degrade using specific enzymes. The cellulase enzyme complex, which catalyzes cellulose decomposition, occurs in a large number of cellulolytic bacterial species of Bacillus, Pseudomonas, Streptomyces, and Clostridium, as well as in fungi [3,4]. In our previous study, we showed that microorganisms prefer to colonize the viscose fibers, creating a large volume of biofilm, in comparison to the synthetic PET fibers, where there was a low volume of microorganisms [2].
Microorganism attachment to surfaces is multifactorial and could be complex to measure. It is conducted by a variety of proteins and non-proteinaceous components, secreted from, or expressed on the microbial cell surface [5]. In addition, the adhesion process is also directly affected by the different physicochemical parameters of the environment and the growth state of the bacteria. As a result, the microorganism may secrete non-specific proteins that adhere to the surface with Van der Waals forces or more specific proteins that chemically bind to certain surface molecules. For example, in S. aureus, proteinaceous surface adhesins can either be covalently linked to the cell wall peptidoglycan, or surface-associated by different means, such as ionic or hydrophobic interactions [6].
We suspected that an additional reason for the larger volume of microorganisms colonizing the viscose fibers, apart from the viscose being a biodegradable material, was due to surface polarity, with viscose being more polar than PET. Multiple studies have shown that the physicochemical properties of fabrics and surfaces have a pronounced effect on microbial adhesion and proliferation, until reaching irreversible attachment and biofilm formation [7,8]. Surface charge and hydrophobicity level were shown to have an influence on microbial adhesion, with superhydrophobic (water contact angle > 150°) having significantly reduced microbial adhesion [9,10,11,12,13]. Hemmatian et al. examined the adhesion tendency of Escherichia coli and Staphylococcus aureus on polystyrene and poly lactic acid, both as films and fibers substrates, with modification of wettability by the plasma process using either O2 or C4F8 gas [10]. Fiber wettability was shown to be the primary factor influencing the cell adhesion, where the hydrophilic fibers resulted in considerably higher adhesion compared to films and hydrophobic surfaces. Geometric properties such as pore volume and the pore size, rather than the porosity itself, were found to be additional factors impacting the bacteria adherence and retention, although to a lesser extent [10].
Kargar M. et al. studied the adhesion of P. aeruginosa on a highly aligned polystyrene nanofibers on a flat polystyrene substrate [14]. Their results were analyzed according to the microorganism position (crossing or aligned to the spacing and fibers) and the ratio between the diameter of the bacteria and the spacing or fibers diameter. It was demonstrated that the total adhesion of P. aeruginosa depended on both the topography size (fiber diameter) and spacing and had a minimum occurrence when the fibers spacing was smaller than the bacterial diameter. Larger fiber spacing compared to the bacteria size led to increased adhesion of the bacteria aligned in the spacing between two fibers. In general, minimal adhesion density was observed on the fiber, where most of the adhesion occurred within the spacing between the fibers, either aligned or crossed the fibers [14].
Although antimicrobial studies of healthcare surfaces and their linked physicochemical characteristics have been reported in several papers [8,10,15,16,17,18,19,20] to our knowledge, the interplay between the textile chemical and geometrical characteristics, and the effect on microbial attachment is far from being conclusive and only very limited data are available in the literature on nonwoven wet wipe behavior in this regard [20].
In this work, we discuss in detail physicochemical aspects of selected nonwoven wet wipes such as the density, pore size, fiber diameter, contact angle and surface charge, and how their interplay affects microbial biomass accumulation. We aimed to close the gap in knowledge for this specific, fast-growing market sector, by investigating the physical properties of personal and home care wet wipes.
2. Materials and Methods
2.1. Fabrics Information
Substrates were sourced from various nonwoven manufacturing companies (Table 1). All fabrics were manufactured using Spunlace technology. Tested fabrics were cut with a roller knife (RTY-2/G OLFA 5 mm). Fabric density information, expressed in grams per square meter (gr/m2; gsm), was determined by the providing company and verified in-house. Fabrics were sterilized using UV exposure for 20 min for each side of the fabric.

Table 1.
Fabric description and properties.
2.2. Contact Angle
The static contact angle (CA) of PET was measured using a contact angle analyzer (KRÜSS Drop Shape Analyzer—DSA30, Hamburg, Germany) [21,22]. The CA of two liquids, water and 1-Bromonaphthalene, representing polar and apolar liquids, respectively, was measured upon deposition of a drop, with the volume of 2–7 µL, on the substrates. The sorption behavior of viscose and wood pulp fabrics was investigated according to the Washburn sorption method [23], since the CA is expected to be smaller than 90 degrees, with two liquids, water and 1-Bromonaphthalene. Washburn measurements were performed with the KRÜSS Force Tensiometer—K100 (Hamburg, Germany). The average value of at least 4 measurements was recorded. Measurements were performed at RT (23 ± 1 °C).
2.3. Surface Zeta Potential
The surface zeta potential of the tested fabrics was determined from streaming potential measurements (EKA Electro Kinetic Analyzer, Anton Paar, Graz, Austria) at Prof. Razi Epstein’s lab, Technion, Israel. A fabric sample was mounted in the gap cell. The reproducible packing density of the fabric was maintained by monitoring the sample size and weight and controlling the sample compression in the measuring cell. A 1 mM NaCl solution was used as the electrolyte and the initial pH was adjusted to pH 4 with 0.005 M HCl, while, during titrations, changes in pH (from about pH 4 to pH 9) were achieved by the addition of 0.005 M NaOH. All were measured at RT.
2.4. pH and Conductivity
Fabrics were wetted at a ratio of 1:4 with double distilled water (ddH2O), containing 0.9% phenoxyethanol (Vadodara, India) and 0.1% ethylhexyl glycerin (Vadodara, India), and placed in polypropylene sealed bags. To ensure homogenous wetting of the fabrics, the liquid was dispersed using a Stomacher bag blender (MRC Laboratory Equipment, Holon, Israel) for 15 min with a paddle speed of 12/s. Following the specified incubation time, the liquid was extracted using a syringe and the pH of the liquid was measured using a SevenEasy pH meter (Schwarzenbach, Switzerland). Electrical conductivity was determined using a single-channel 912 Conductimeter (Metrohm, Herisau, Switzerland). During incubation time, wetted fabrics were maintained at either 25 °C ± 0.1 °C or 40 °C ± 0.1 °C. The conductance range was 0.1 µS–500 mS, with a conductivity accuracy of ±1%, while pH accuracy was determined at ±0.1.
2.5. Fiber Diameter
Tested fabrics were analyzed for their fibers diameter using scanning electron microscopy (SEM) imaging. Briefly, the fabrics were coated with Au using Quorum Q150T ES (Quorum Technology, Lewes, UK), and observed using SEM with a FEG Quanta 250 microscope (Thermo Fisher Scientific, Waltham, MA, USA). Fiber diameters were analyzed using the Xt Microscope Server Software program by manual measurement of the fibers. For the analysis of the results, a minimum of 3 images were taken from each fabric, while in each image there were 15–30 measurements. This part of the work was performed by the Bar Ilan Institute of Nanotechnology and Advanced materials, Bar Ilan University (M.Sc Zoharchen Sofer).
2.6. Pore Size
Fabric through-pore sizes were measured using the capillary flow porometry method. A portion of the sample was punched out of the fabric sheet (using a 24 mm hollow punch). The sample was wetted with a wetting liquid (Porofil, Anton Paar) to fill at least all through pores and sealed into a sample holder. The wetting liquid was then emptied from largest to smallest pores as increasing gas pressure (air or nitrogen) when applied to one side of the sample. The resulting flow of gas was measured until all through pores were emptied. Analysis was then repeated without wetting. The pore size distribution was subsequently calculated from the wet and dry curves according to the Washburn equation [23]. The analyses were run between 20 °C and 25 °C (ambient temperature), using an Anton Paar Porometer 3G zH (Anton Paar, Graz, Austria).
2.7. Surface Area
The surface area of each fabric was determined by Brunauere–Emmette–Teller (BET) surface analysis. A weight of 0.4925–0.5788 g of sample was degassed at 40 °C for 3 h in an external Micromeritics VacPrep 061, then placed into a large bulb cell (with stem 0.9 cm inner diameter, with bulb dimensions of 6 cm × 2.5 cm for total volume of approx. 22 cm³) or a monolith cell (with stem 0.9 cm inner diameter, and sample chamber dimensions of 3.5 cm × 1.7 cm for total volume of approx. 11 cm³). Krypton adsorption was determined at 77.35 K. Surface area was calculated automatically from the adsorption isotherm using the BET equation [24].
2.8. Total Microbial Bioburden Enumeration
Microbial bioburden enumeration test was performed based on the USP <61> Microbial enumeration test. The test provides the total number of bacteria, yeast, and mold that may be present within a sample, and grow under aerobic conditions [25]. The acceptance criteria for the microbial limit in cosmetics products are ≤2 × 102 CFU/g for total aerobic microbial count (TAMC) and ≤2 × 101 CFU/g for total yeast and mold count (TYMC) according to the USP standard, and TAMC + TYMC ≤ 1 × 103 CFU/g according to the ISO 17516 standard [26].
2.9. Microorganism Culture Conditions
Staphylococcus aureus (SA113) [27] was kindly received from Prof. Friedrich Götz, at Tübingen university, and Candida albicans (SC5314) [28] was kindly received from Judith Berman’s lab at Tel-Aviv University. The strains, containing green fluorescence protein (GFP) insert, were streaked from −80 frozen stock onto Tryptic soy agar (TSA, HyLabs, Rehovot, Israel) plate for S. aureus, and Sabouraud dextrose agar (SDA, HyLabs, Israel) plate for C. albicans. S. aureus culture was incubated overnight at 32 °C, and C. albicans was incubated for 48 h at 23 °C. A single colony was picked from each microorganism and resuspended in Tryptic soy broth (TSB) or Sabouraud dextrose broth (SDB), respectively. Broth cultures were incubated overnight with shaking at 200 rpm. The following day, cultures were diluted 1:100 with fresh media and further incubated to reach logarithmic growth phase. Both cultures were then centrifuged at 4000× g and resuspended in HEPES buffer at pH = 7 (Sigma-Aldrich, Jerusalem, Israel) for S. aureus, and HEPES buffer at pH = 5.5 (Sigma-Aldrich, Israel) for C. albicans, to reach a final O.D.600 of 1.5. Tested fabrics were cut into pieces of 2 cm2 and placed in a 6-well plate. A total of 2 mL of microorganisms’ culture were added to the fabrics using a pipette, while making sure the fabric is completely soaked by the media, then incubated for 16 h at with shaking at 200 rpm. Fabrics were then washed using sterile deionized H2O, by pipetting the water gently on top of the fabrics, to remove unattached microorganisms. The washing procedure was standardized throughout the entire experiment. Fabrics were placed back in a sterile 6-well plate with fresh 2 mL HEPES buffer and were then visualized microscopically.
2.10. Confocal Laser Scanning Microscopy (CLSM) and Image Analysis
In order to visualize the attached GFP expressed, microorganisms on the tested fabrics, the fabrics were placed in ibidi (NBT New Biotechnology Ltd., Jerusalem, Israel), a 35 mm imaging dish, with a polymer coverslip bottom. Images were taken using a FV-1200 confocal microscope (Olympus, Tokyo, Japan) equipped with 20×/0.75 objective. For GFP excitation we used a 488 nm laser, emission was collected between 505–550 nm. For each image 60–200 stacks were taken using 2 µm stack size configuration. Images were analyzed using Fiji software version 1.53t. Stacked images were analyzed manually with 3D objects counter plug, to determine the biomass accumulation of the microorganisms. To calculate the relative biomass accumulation, total biomass volume was divided by the total volume of the analyzed images (2 µm stack × number of stacks × image area). For each Fabric type that was tested 2 different fabrics were imaged, 3–5 fields in each fabric.
2.11. Statistical Analysis
Statistical analysis of the data was performed using Prism GraphPad version 9.5.0 (GraphPad Software, 2024). Conductivity and pH change over time, CA and biomass accumulation data were statistically analyzed using 2-way ANOVA with Tukey’s multiple comparisons test. Correlation was analyzed using the Pearson correlation coefficient. All statistical analysis was performed using a p value of 0.05 or 0.1. All experiments were repeated at least 3 times.
3. Results and Discussion
3.1. Fabrics Information
Fabrics were sourced from five different nonwoven substrate manufacturers (Table 1), who provided information regarding the fabric density (gr/m2; gsm). Fabrics were assessed for their total microbial load and the results are summarized in Table 2. Aside from two types of fabrics, viscose 50 gsm #2 and wood pulp 60 gsm, all fabrics were found to have >2 × 102 CFU/g for TAMC, and >2 × 101 CFU/g for TYMC. Although these results do not meet USP nor the ISO 17516 acceptance criteria for a final product, the substrates were found clean from microorganisms following the addition of the formulation containing the preservative.

Table 2.
Total microbial bioburden enumeration.
3.2. Fabrics Contact Angle
To evaluate the hydrophobicity of wet wipes fabrics surfaces, the contact angle (CA) was measured using polar liquid, i.e., water, and an apolar liquid, 1-Bromonaphthalene. The contact angles of the tested fabrics are summarized in Figure 1. PET fabrics exhibit a water CA of approximately 150°, indicating a very hydrophobic character, whereas fabrics made from cellulose-based fibers, viscose and wood pulp displayed a water CA of ~50° or lower, showing a more hydrophilic nature [10,22]. Similar to the preceding results, Hemmatian et al. demonstrated water contact angle of 110–180° for hydrophobic treated surfaces (C4F8 plasma) and less than 55° for hydrophilic treated surfaces (by O2 plasma) [10].
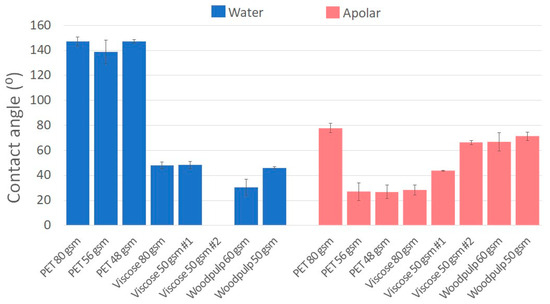
Figure 1.
Water (blue) and 1-Bromonaphthalene (red) contact angles of various fabrics, including PET, viscose and wood pulp, with varying gsm values, which ranged from hydrophobic to hydrophilic behavior.
Analyzing the contact angles of both polar and apolar liquids reveals the complexity of the fiber surfaces. For example, wood pulps and viscose show relatively low water CA, as expected from a hydrophilic surface, however, their apolar CA is in the range of ~40–75°, not as high as can be expected from a pure hydrophilic surface. This suggests that during the manufacturing process, additional coating or treatment is taking place, which may affect the hydrophobicity of the fabrics.
In addition, the nature of the nonwoven fabrics, having rough surface with loosen fibers sticking out along the surface, may locally affects the absorption process of the liquid, either water or apolar one, during a drop shape measurement. As a result, broader range of contact angles values can be observed even within the same fabric with similar chemical structure due to their random surface roughness [10].
3.3. Zeta Potential as an Indicator for Fabrics Polarity:
Figure 2A presents pH-dependent surface zeta potential curves of viscose 50 gsm #1, viscose 50 gsm #2, wood pulp 60 gsm, wood pulp 50 gsm, PET 48 gsm, and PET 56 gsm fabrics. All six fabrics exhibit a negative zeta potential (~−15 to ~−83 mv) throughout the entire pH region (pH 4.5–9), and it becomes more negative as the pH increases (Figure 2A). However, a clear difference in zeta potential between the cellulose-based fabrics, i.e., viscose and wood pulp, and the PET material can be seen. The hydrophilic cellulose fibers, viscose and wood pulp, display a plateaued negative zeta potential, starting from pH ~5. The hydrophobic polyethylene terephthalate fabrics show higher negative ZP with a significant decrease between pH 4.5–7 (higher absolute value). Examination of the zeta potential around pH 7.2 indicated that viscose and wood pulp fibers were approximately −24–−29 mV. In contrast, the zeta potentials of PET fabrics were equivalent to approximately −70 and −81 mV.
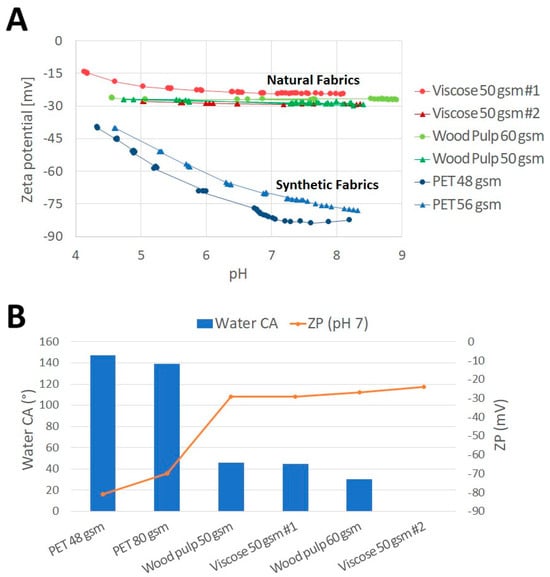
Figure 2.
Fabric hydrophobicity expressed by zeta potential and contact angle. Fabric surface zeta potential (A). Fabric surface zeta potential (ZP) as a function of their water contact angle measurements (CA) (B).
Analyzing the fabrics surface zeta potential as a function of their water contact angle reveals a significant, negative correlation (R = −0.975, p value < 0.01) between these findings (Figure 2B). The zeta potential charge of the wet wipe fabrics is more negative as the hydrophobicity of the surface increases, indicated by the increasing water CA. A linear relationship between zeta potential and water CA was previously observed by Jacobasch and Schurz, who examined various of polymer surfaces [29].
The surface charge characteristics of fabrics is known to depend on the chemical properties of the fibers, including dissociation (ionization) of surface functional groups and/or adsorption of ions from the solution [29,30,31]. In the absence of ionizable functional groups, as in the case of viscose and wood pulp, the fabric surfaces can acquire a surface charge through the adsorption of anions from solution, e.g., hydroxyls ions [29,30,31]. As a result, a moderate negative zeta potential will appear. However, in the case of PET, following titration with alkaline solution, which may lead to PET surface hydrolysis and dissociation of carboxyl functional groups, the zeta potential becomes more negative (higher absolute value).
3.4. pH and Conductivity Change over Time
The pH of the tested fabrics at T = 0 was in the range of 4.7–8.0, and changed over time, reaching a plateau following around 24 h incubation at both at 25 °C (Figure 3A) and at 40 °C. All fabrics had an increase in pH ranging at 0.35–1.5 units, except for both wood pulp fabrics with 50 and 60 gsm, which had a decrease in pH from −0.66 to −1.8 units. The change in pH in comparison to time 0, was not significant. The ionic strength associated with the pH change was assessed by measuring the solution conductivity (Figure 3B). In this case, the conductivity of the fabrics changed significantly over time (p < 0.05) in each fabric at each time point. Aside for the wood pulp fabrics having a significantly higher conductivity of 1030–1400 µS following 4 weeks of measurements (p < 0.0001), all other fabric types had a rather low conductivity ranging from 100–490 µS. The conductivity results of the wood pulp fabrics may suggest residual electrolytes from the manufacturing process specifically for this type of material [32,33].
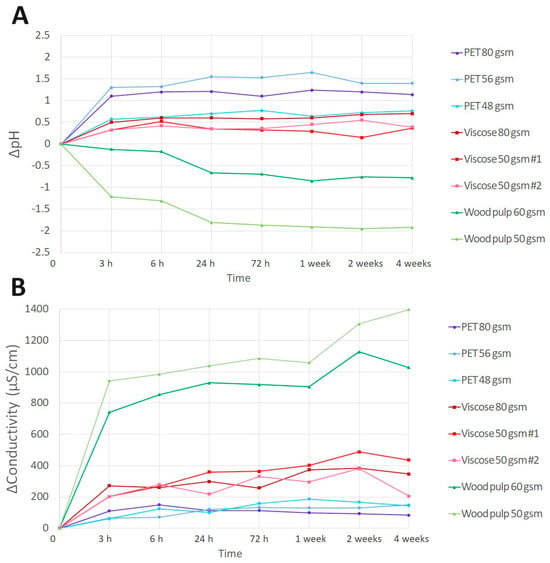
Figure 3.
Fabrics pH and conductivity change over time. Wetted fabrics, at a ratio of 1:4 with deionized H2O, values of pH (A) and conductivity (µS/cm) (B) change (∆) measurements over time (4 weeks).
In this experiment, the solution in which the fabrics were wetted in was deionized H2O, containing a preservative with a low ionic strength (0.9% phenoxyethanol and 0.1% ethylhexyl glycerin). In the final wet wipe product, the lotion usually contains a surfactant, therefore, the ionic strength recorded by the conductivity change in all fabrics, did not result in pH change in the final product lotion (not shown). However, in cases where the lotion contains >98% water, while the substrate is manufactured mainly from wood pulp, such conductivity could result in a pH change of the final product.
3.5. Fabrics Hydrophilicity and Microbial Biomass Accumulation
Surface charge and hydrophobicity are known as major influencing factors for microbial–surface interaction. Generally, positive to neutral charged surfaces are more prone to microbial attachment in comparison to negatively charged surfaces, this is due to the microbial cell envelope charge, being usually negative [34,35] Intuitively, hydrophilic fabrics, made of cellulose-based fibers, such as viscose and wood pulp, are more vulnerable to microbial adherence and contamination of the fabrics, in comparison to hydrophobic, polyethylene-based fabrics [2,9,10,36]. However, some theories and studies suggest that bacteria will adhere more strongly to more hydrophobic fibers [37]. The latter assumption is based on the physics of bacterial adhesion to surfaces [38]. These conflicting theories and data arise by the test method, and often do not consider the multiparametric system in which the experiment was conducted in [6,39].
When investigating the adhesion of the two tested microorganisms to fabrics with various polarities, we found that S. aureus generally had higher adhesion to all tested fabrics, in comparison to C. albicans (1.25–5 times more), apart from PET 48 gsm which had low adhesion by both microorganisms. Most notable, S. aureus biomass was significantly higher than C. albicans in viscose and wood pulp fabrics (Figure 4A,B). The adhesion to wood pulp was the highest for both microorganisms. In S. aureus, highest adhesion was followed by viscose, and then PET having the lowest attached biomass (Figure 4A). In C. albicans the adhesion to PET and viscose was similar (Figure 4B). Finally, we did not find a significant correlation between the hydrophilicity of the fabrics and microbial attachment, using the available tools known in the literature. These results correlate with the literature showing that the adhesion of S. aureus decreased when textile CA increased [10], however, the literature regarding C. albicans adhesion on fabrics based on their polarity is lacking.
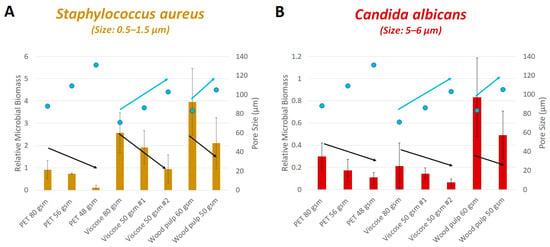
Figure 4.
Relative microbial biomass accumulation on fabrics as a function of the fabrics pore size. Relative microbial biomass accumulation of GFP-expressed S. aureus (A) and C. albicans (B) on tested fabrics with varying densities (PET, viscose and wood pulp), as a function of fabrics pore size (Blue dots). Black arrows represent a visible trend, as the density of the fabrics decreases so is the relative biomass accumulation. Blue arrows represent a visible trend in which the pore size increases as the microbial biomass decreases.
3.6. Fabrics Geometry and Its Correlation with Microbial Biomass Accumulation
Fabrics physical–geometrical characteristics were measured (pore size, fiber diameter and surface area), and results are summarized in Table 1 and Figure 5 and Figure 6. The relationship between the fabric pore size and the relative microbial biomass accumulation, using CLSM, is summarized in Figure 4. Due to the relatively high microbial bioburden on the tested dry substrates (Table 2), GFP-expressed microorganisms, S. aureus and C. albicans, were selected to visualize only the tested microorganisms, using fluorescence imaging.
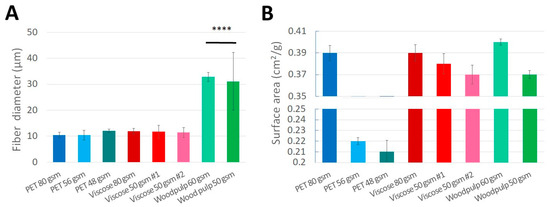
Figure 5.
Fibers diameter and fabrics surface area. Fibers diameter (A) and Fabrics surface area (B) of the tested fabrics with varying densities (PET, viscose and wood pulp). (**** = p value < 0.0001).
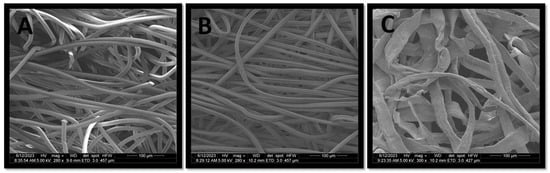
Figure 6.
Scanning electron microscopy (SEM) images of selected wet wipes fabrics. (A) PET, (B) viscose and (C) wood pulp.
In our experimental design, we chose fabrics with various densities, to evaluate the effect of the physical properties on the microbial ability to adhere. As expected, as the fabrics density (gsm) decreases, the pore diameter size increases (Figure 4). Viscose 50 gsm #1 and #2, have similar density, however, their pore size is different. We found that larger pore size resulted in lower microbial biomass. Although there is a clear trend within fabric type (Figure 4A,B, black and blue arrows), in which the microbial biomass is decreasing as the pore diameter size of the fabric is increasing, when we include all fabric types in a single statistical analysis, there was no significant correlation between the microbial biomass and pore size. However, when we analyze only the viscose fabrics separately from the other fabrics, we found a significant, negative correlation between the fabric pore size and the biomass accumulation of both S. aureus and C. albicans (R = −0.9967, p value < 0.1 and R = −1, p value < 0.005, respectively). The literature regarding the effect of the surface pore size on microbial adhesion and biofilm formation exist mainly in surfaces with much smaller pore size (up to 10 µm), [40], while the tested fabrics in this study examined pore sizes ranging from 70–130 µm, in which the available literature for such pore size is limited.
When measuring the fiber diameter (Figure 5A), all fabrics had similar diameter of 10.5–12.1 µm, aside from the wood pulp fibers which were significantly larger (p value < 0.0001), 31–33 µm in diameter. This larger fiber diameter is due to the shape of the wood pulp fiber, resembling a ribbon-like shape (Figure 6C) in comparison to the rounded shape of the PET and viscose fibers (Figure 6A,B). Considering that both S. aureus and C. albicans had significantly higher biomass accumulation on wood pulp fabrics, in comparison to most of the viscose and PET fabrics (C. albicans biomass accumulation on wood pulp 60 gsm was significantly higher than all fabrics, p value < 0.05. Wood pulp 50 gsm was significantly higher than viscose 50 gsm #1 and #2 and PET 48 gsm, p value < 0.05), suggesting that the shape of the wood pulp fibers enables a larger area for the microorganisms to attach and accumulate. Indeed, the previous literature showed that fiber diameter is an important influencing factor for microbial adhesion, while fiber diameter size which is similar and higher to the size of the microorganism, will allow better attachment [14,15]. Similar to the literature regarding pore size, fiber diameter used in previous studies examined much smaller sizes in comparison to the tested fibers in our study.
The surface area of the fabrics was measured as well (Figure 5B), and as expected, fabrics with smaller densities had smaller surface area. We found a significant positive correlation between the microbial biomass accumulation of both S. aureus and C. albicans and the viscose fabrics surface area (R = 0.9931, p value < 0.0373 and R = 0.9992, p value < 0.0252, respectively).
3.7. Microorganisms Penetration into the Fabrics
By analyzing the microbial biomass accumulation on the fabrics as a function of the fabric depth, we found that within the same fabric type, the larger the pore size, the easier it is for the microorganism to penetrate deeper (Figure 7). In S. aureus for example, we found a significant positive correlation between the maximal penetration depth and the PET fabric pore size (R= −0.94, p value < 6.6 × 10−6) (Figure 7A–C). Although in other cases (e.g., Figure 7D–F) the correlation was not significant, the same trend was observed. This analysis showed that the microorganisms size has an effect on its ability to penetrate the fabric (Figure 7). Fabrics with lower pore size will be harder to penetrate by C. albicans, having a cell size of 5–6 µm, rather than S. aureus with cell size of 0.5–1.5 µm. Specifically in wood pulp 60 gsm, S. aureus was able to penetrate to a maximal depth of 80 µm while C. albicans only to a depth of 60 µm (Figure 8A,C). Similar behavior was observed in wood pulp 50 gsm (Figure 8B,D).
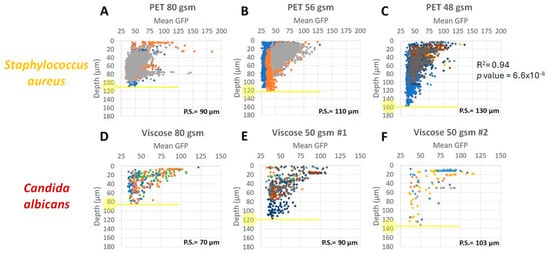
Figure 7.
Microorganisms penetration depth based on fabric pore size. GFP-expressed microorganisms, S. aureus ((A): PET 80 gsm, (B): PET 56 gsm, and (C): PET 48 gsm) and C. albicans ((D): viscose 80 gsm, (E): viscose 50 gsm#1, and (F): viscose 50 gsm #2) were visualized on selected fabrics, using CLSM. Fabrics pore size (P.S.) are indicated on each individual graph. Penetration depth is marked in yellow.
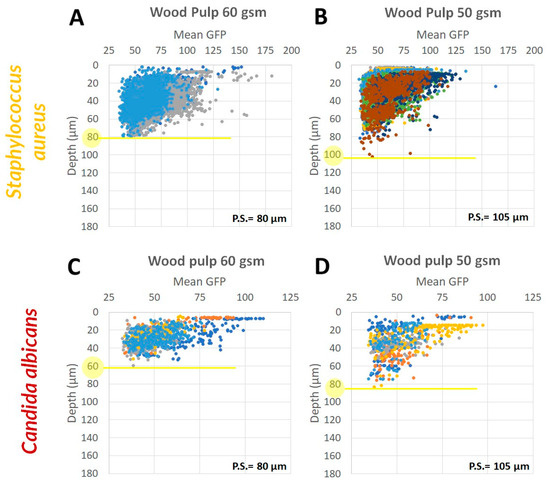
Figure 8.
Microorganisms penetration depth based on fabric pore size and microbial cell size. GFP-expressed microorganisms, S. aureus ((A): wood pulp 60 gsm, (B): wood pulp 50 gsm) and C. albicans ((C): wood pulp 60 gsm, (D): wood pulp 50 gsm) were visualized on wood pulp 60 and 50 gsm fabrics, using CLSM. Fabrics pore size (P.S.) is indicated on each individual graph. Penetration depth is marked in yellow.
The fabrics geometry and fibers shape may also affect the microorganisms penetration ability. The penetration depth of both microorganisms to the wood pulp fabric was rather low (60–100 µm, Figure 8) in comparison to PET and viscose (80–160 µm, Figure 7), suggesting that is the fibers ribbon shape is restricting the ability of the microorganisms to penetrate deeper, in comparison to the viscose and PET tubular shape (Figure 6).
From our data, it appears that the ability to penetrate deeper to the fabric does not cause a total higher biomass accumulation, rather the opposite. When the fabrics pore sizes were smaller, microorganisms accumulated on the surface of the fabric, resulting in higher attachment (Figure 4, blue dots). This was in correlation with the study by Hemmatian et al., where denser structures with smaller pore size substrates limited the penetration of the microorganisms [10], however, once again, the pore sizes of the substrates were much smaller (2–26 µm), than the sizes in our study (70–130 µm), therefore, smaller pore size in their study resulted in lower total adhesion.
4. Conclusions
The physicochemical properties of commercially available nonwoven wet wipes fabrics were investigated, as a function of microorganisms ability to attach to the substrate, consequently contaminating the final cosmetic product. In our study, the intuitive link between fabric hydrophilicity and microbial biomass accumulation did not find experimental substantiation. Wood pulp fabrics had the highest microbial biomass, while the reason for this finding was attributed to the ribbon shape of the fibers, allowing a larger area for the microorganisms to attach [10,14]. In S. aureus, biomass attached to the viscose fabrics was generally higher than PET, while in C. albicans, PET and viscose had similar accumulated biomass. The pore size of tested fabrics was in the range of 70–130 µm, and along with the fabrics density, show a significant factor affecting microbial biomass and ability of the microorganisms to penetrate deeper into the substrate. Our study shed light on various characteristics of wet wipes substrates, that can influence the preservation strength and contamination risk, while the mitigation of such risks can help avoid product recall and infection cases in users.
Author Contributions
Conceptualization, P.S. and N.Z.; methodology, P.S., I.Y. and N.Z.; software, M.B.; validation, N.Z., P.S. and I.Y.; formal analysis, N.Z. and I.Y.; investigation, N.Z. and I.Y.; resources, P.S.; data curation, M.B. and I.Y.; writing—original draft preparation, N.Z. and I.Y.; writing—review and editing, N.Z. and P.S.; visualization, M.B.; supervision, P.S.; project administration, N.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
We would like to acknowledge Oran Shkalo who carried out some of the experiments and Yana Gurianov for the establishment of the CLSM preliminary methodology administration and experiments.
Conflicts of Interest
The authors declare no conflicts of interest. The authors are employees of Sharon Personal Care. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Hadley, T.; Hickey, K.; Lix, K.; Sharma, S.; Berretta, T.; Navessin, T. Flushed but Not Forgotten: The Rising Costs and Opportunities of Disposable Wet Wipes. Bioresources 2023, 18, 2271–2287. [Google Scholar] [CrossRef]
- Salama, P.; Gliksberg, A.; Cohen, M.; Tzafrir, I.; Ziklo, N. Why Are Wet Wipes So Difficult to Preserve? Understanding the Intrinsic Causes. Cosmetics 2021, 8, 73. [Google Scholar] [CrossRef]
- Dworkin, M.; Falkow, S.; Rosenberg, E.; Schleifer, K.-H.; Stackebrandt, E. (Eds.) The Prokaryotes; Springer: New York, NY, USA, 2006; ISBN 978-0-387-25492-0. [Google Scholar]
- Killham, K.; Prosser, J.I. The Bacteria and Archaea. In Soil Microbiology, Ecology and Biochemistry; Elsevier: Amsterdam, The Netherlands, 2015; pp. 41–76. [Google Scholar]
- Heilmann, C. Adhesion Mechanisms of Staphylococci. Adv. Exp. Med. Biol. 2011, 715, 105–123. [Google Scholar] [CrossRef] [PubMed]
- Goulter, R.M.; Gentle, I.R.; Dykes, G.A. Issues in Determining Factors Influencing Bacterial Attachment: A Review Using the Attachment of Escherichia Coli to Abiotic Surfaces as an Example. Lett. Appl. Microbiol. 2009, 49, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Ghosh, S.; Bhowmick, N.; Roy Choudhury, P.K. Role of Physicochemical Factors on Bacterial Attachment in Textile Fibrous Media. Water Environ. J. 2019, 33, 21–30. [Google Scholar] [CrossRef]
- Song, F.; Koo, H.; Ren, D. Effects of Material Properties on Bacterial Adhesion and Biofilm Formation. J. Dent. Res. 2015, 94, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Berne, C.; Ellison, C.K.; Ducret, A.; Brun, Y.V. Bacterial Adhesion at the Single-Cell Level. Nat. Rev. Microbiol. 2018, 16, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Hemmatian, T.; Lee, H.; Kim, J. Bacteria Adhesion of Textiles Influenced by Wettability and Pore Characteristics of Fibrous Substrates. Polymers 2021, 13, 223. [Google Scholar] [CrossRef] [PubMed]
- Cavitt, T.B.; Pathak, N. Modeling Bacterial Attachment Mechanisms on Superhydrophobic and Superhydrophilic Substrates. Pharmaceuticals 2021, 14, 977. [Google Scholar] [CrossRef]
- Yoon, S.H.; Rungraeng, N.; Song, W.; Jun, S. Superhydrophobic and Superhydrophilic Nanocomposite Coatings for Preventing Escherichia Coli K-12 Adhesion on Food Contact Surface. J. Food Eng. 2014, 131, 135–141. [Google Scholar] [CrossRef]
- Sohn, Y.S.; Jung, S.K.; Lee, S.Y.; Kim, H.T. Antibacterial Effects of a Carbon Nitride (CN) Layer Formed on Non-Woven Polypropylene Fabrics Using the Modified DC-Pulsed Sputtering Method. Polymers 2023, 15, 2641. [Google Scholar] [CrossRef]
- Kargar, M.; Wang, J.; Nain, A.S.; Behkam, B. Controlling Bacterial Adhesion to Surfaces Using Topographical Cues: A Study of the Interaction of Pseudomonas Aeruginosa with Nanofiber-Textured Surfaces. Soft Matter 2012, 8, 10254–10259. [Google Scholar] [CrossRef]
- Abrigo, M.; Kingshott, P.; McArthur, S.L. Electrospun Polystyrene Fiber Diameter Influencing Bacterial Attachment, Proliferation, and Growth. ACS Appl. Mater. Interfaces 2015, 7, 7644–7652. [Google Scholar] [CrossRef]
- Erramilli, S.; Genzer, J. Influence of Surface Topography Attributes on Settlement and Adhesion of Natural and Synthetic Species. Soft Matter 2019, 15, 4045–4067. [Google Scholar] [CrossRef]
- Varshney, S.; Sain, A.; Gupta, D.; Sharma, S. Factors Affecting Bacterial Adhesion on Selected Textile Fibres. Indian J. Microbiol. 2021, 61, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Cheng, Y.; Wang, S.Y.; Borca-Tasciuc, D.A.; Worobo, R.W.; Moraru, C.I. Bacterial Attachment and Biofilm Formation on Surfaces Are Reduced by Small-Diameter Nanoscale Pores: How Small Is Small Enough? NPJ Biofilms Microbiomes 2015, 1, 15022. [Google Scholar] [CrossRef] [PubMed]
- Todros, S.; Pavan, P.G.; Natali, A.N. Synthetic Surgical Meshes Used in Abdominal Wall Surgery: Part I—Materials and Structural Conformation. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 689–699. [Google Scholar] [CrossRef]
- Pascoe, M.J.; Mandal, S.; Williams, O.A.; Maillard, J.Y. Impact of Material Properties in Determining Quaternary Ammonium Compound Adsorption and Wipe Product Efficacy against Biofilms. J. Hosp. Infect. 2022, 126, 37–43. [Google Scholar] [CrossRef]
- Kaelble, D.H. Dispersion-Polar Surface Tension Properties of Organic Solids. J. Adhes. 1970, 2, 66–81. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the Surface Free Energy of Polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Washburn, E.W. The Dynamics of Capillary Flow. Phys. Rev. 1921, 17, 374. [Google Scholar] [CrossRef]
- George, A.; Stephen Brunauer, B. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Yapar, E.A.; Cengiz, G. International Standards of Microbiological Quality in Cosmetic Products. Curr. Pers. MAPs 2018, 2, 120–122. [Google Scholar]
- Wirotesangthong, M. Microbiological Limits in Cosmetics. Isan J. Pharm. Sci. 2021, 17, 1–12. [Google Scholar] [CrossRef]
- Biswas, R.; Voggu, L.; Simon, U.K.; Hentschel, P.; Thumm, G.; Götz, F. Activity of the Major Staphylococcal Autolysin Atl. FEMS Microbiol. Lett. 2006, 259, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Gerami-Nejad, M.; Zacchi, L.F.; McClellan, M.; Matter, K.; Berman, J. Shuttle Vectors for Facile Gap Repair Cloning and Integration into a Neutral Locus in Candida Albicans. Microbiology 2013, 159, 565–579. [Google Scholar] [CrossRef]
- Jacobasch, H.-J.; Schurz, J. Characterization of Polymer Surfaces by Means of Electrokinetic Measurements. Prog. Colloid. Polym. Sci. 1988, 77, 40–48. [Google Scholar]
- Ripoll, L.; Bordes, C.; Marote, P.; Etheve, S.; Elaissari, A.; Fessi, H. Electrokinetic Properties of Bare or Nanoparticle-Functionalized Textile Fabrics. Colloids Surf. A Physicochem. Eng. Asp. 2012, 397, 24–32. [Google Scholar] [CrossRef]
- Elimelech, M.; Chen, W.H.; Waypa, J.J. Measuring the Zeta (Electrokinetic) Potential of Reverse Osmosis Membranes by a Streaming Potential Analyzer. Desalination 1994, 95, 269–286. [Google Scholar] [CrossRef]
- Quintana, E.; Valls, C.; Roncero, M.B. Dissolving-Grade Pulp: A Sustainable Source for Fiber Production. Wood Sci. Technol. 2024, 58, 23–85. [Google Scholar] [CrossRef]
- Kosan, B.; Thüringisches, F.M. Suitable Dissolving Pulps and Their Impacts on Solution Spinning of Cellulose Man-Made Fibers. Cellulose 2024, 31, 1941–1955. [Google Scholar] [CrossRef]
- Wilson, W.W.; Wade, M.M.; Holman, S.C.; Champlin, F.R. Status of Methods for Assessing Bacterial Cell Surface Charge. J. Microbiol. Methods 2001, 43, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Bawazir, M.; Dhall, A.; Kim, H.E.; He, L.; Heo, J.; Hwang, G. Implication of Surface Properties, Bacterial Motility, and Hydrodynamic Conditions on Bacterial Surface Sensing and Their Initial Adhesion. Front. Bioeng. Biotechnol. 2021, 9, 643722. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Li, Q.; Zhang, Z.; Wang, X.; Niu, H. Recent Advances in Superhydrophobic and Antibacterial Cellulose-Based Fibers and Fabrics: Bio-Inspiration, Strategies, and Applications. Adv. Fiber Mater. 2023, 5, 1555–1591. [Google Scholar] [CrossRef] [PubMed]
- Møllebjerg, A.; Palmén, L.G.; Gori, K.; Meyer, R.L. The Bacterial Life Cycle in Textiles Is Governed by Fiber Hydrophobicity. Microbiol. Spectr. 2021, 9, e01185-21. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Newby, B.Z. Applicability of the Extended Derjaguin–Landau–Verwey–Overbeek Theory on the Adsorption of Bovine Serum Albumin on Solid Surfaces. Biointerphases 2014, 9, 041006. [Google Scholar] [CrossRef] [PubMed]
- Salas-Tovar, J.A.; Escobedo-García, S.; Olivas, G.I.; Acosta-Muñiz, C.H.; Harte, F.; Sepulveda, D.R. Method-Induced Variation in the Bacterial Cell Surface Hydrophobicity MATH Test. J. Microbiol. Methods 2021, 185, 106234. [Google Scholar] [CrossRef]
- Chong, P.; Erable, B.; Bergel, A. Effect of Pore Size on the Current Produced by 3-Dimensional Porous Microbial Anodes: A Critical Review. Bioresour. Technol. 2019, 289, 121641. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).