Influence of Temperature and Ultrasonic Treatment on Preparation of Titanium Phosphates and Their Powder Properties
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
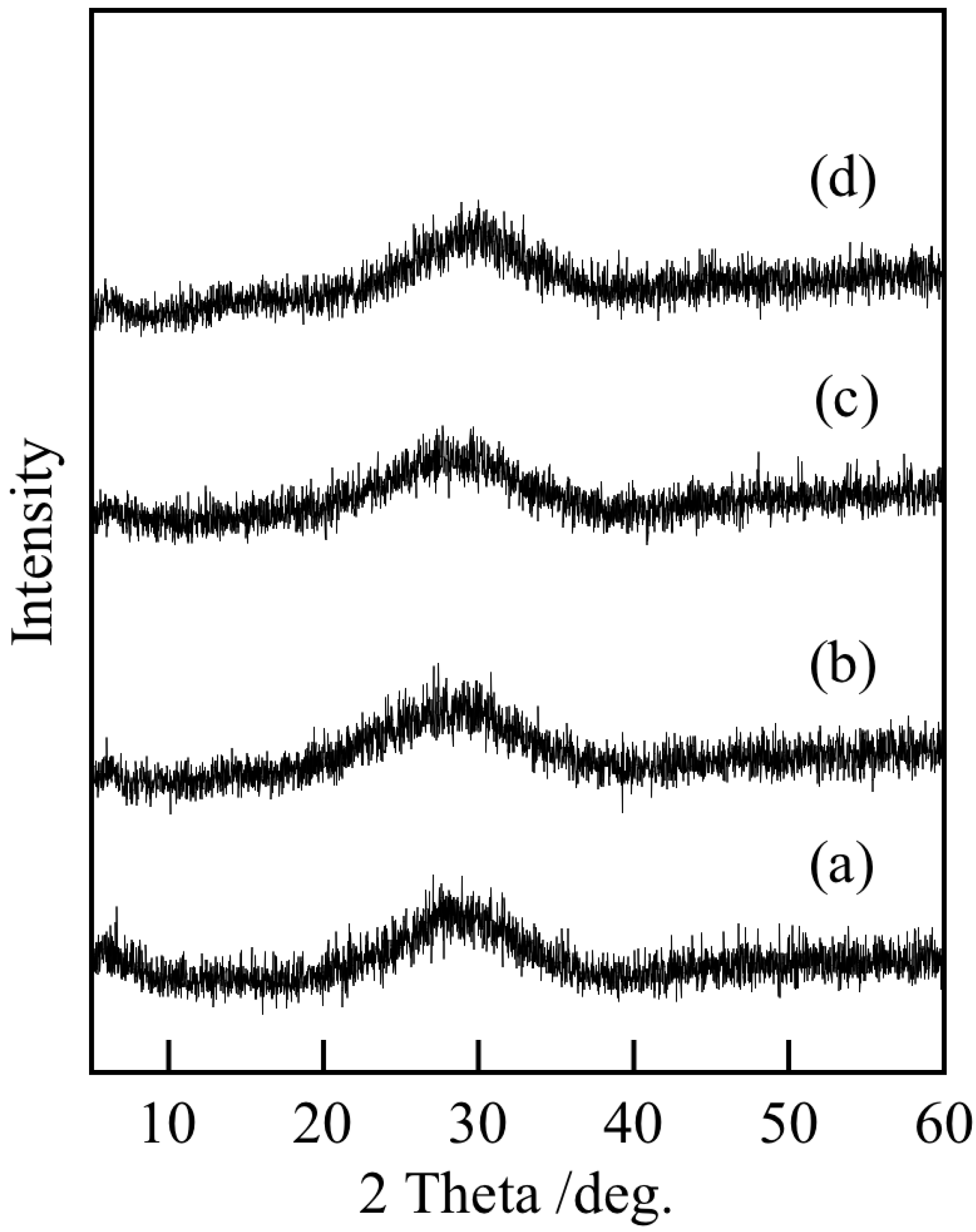
3.1. Chemical Composition and Powder Properties of Precipitates
| Sample | Temperature/°C | Ultrasound/min | Ti/P ratio |
|---|---|---|---|
| A | 7 | 0 | 1.07 |
| B | 7 | 10 | 1.09 |
| C | 40 | 0 | 1.19 |
| D | 40 | 10 | 1.12 |


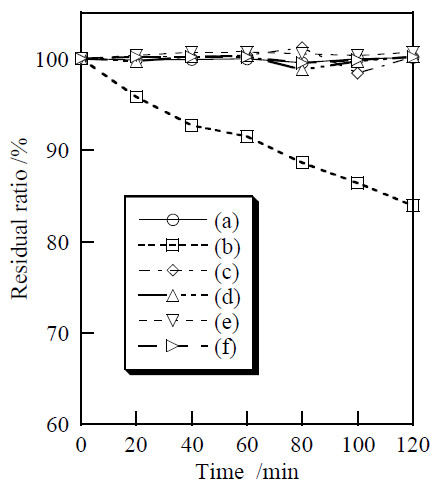
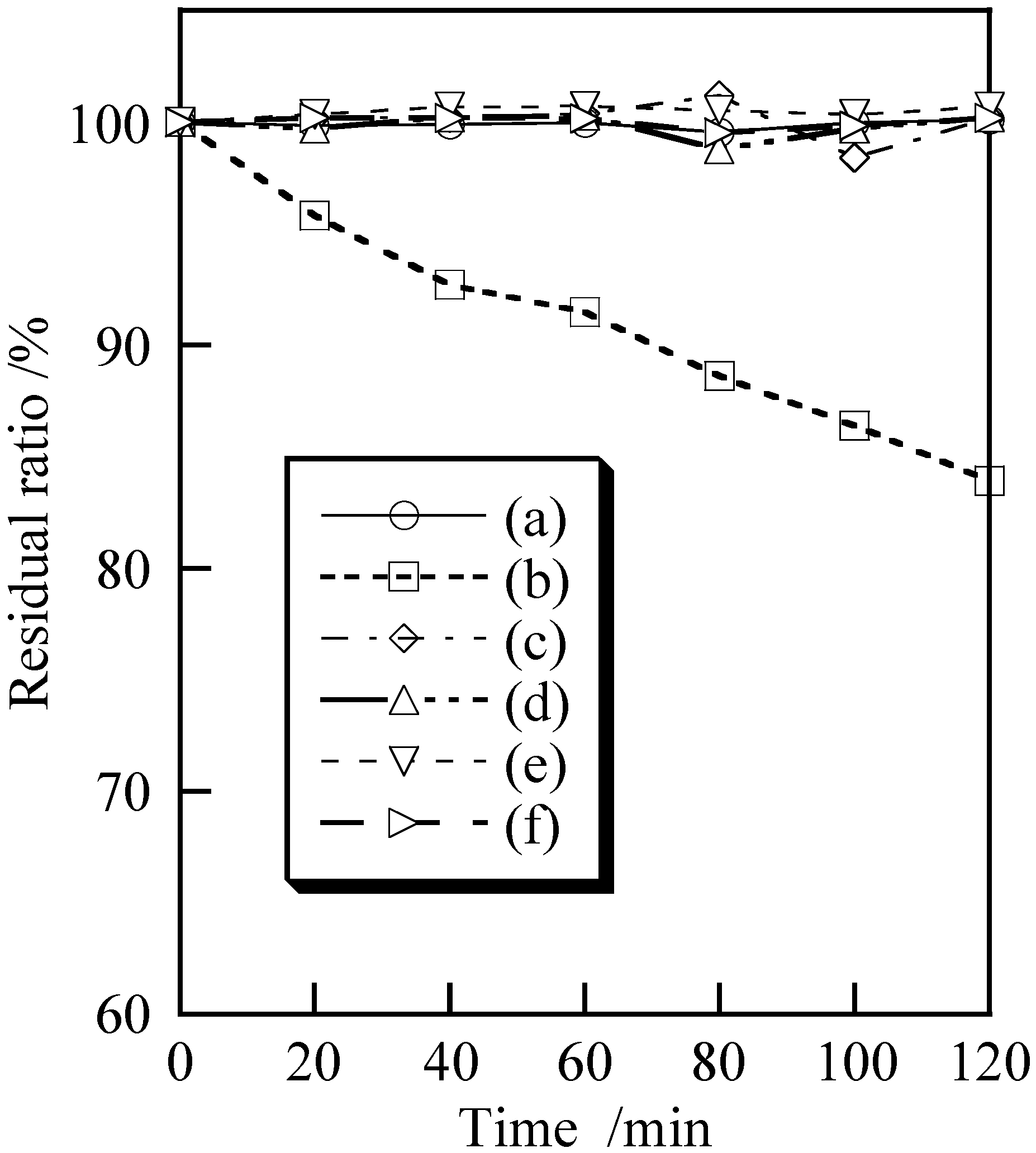
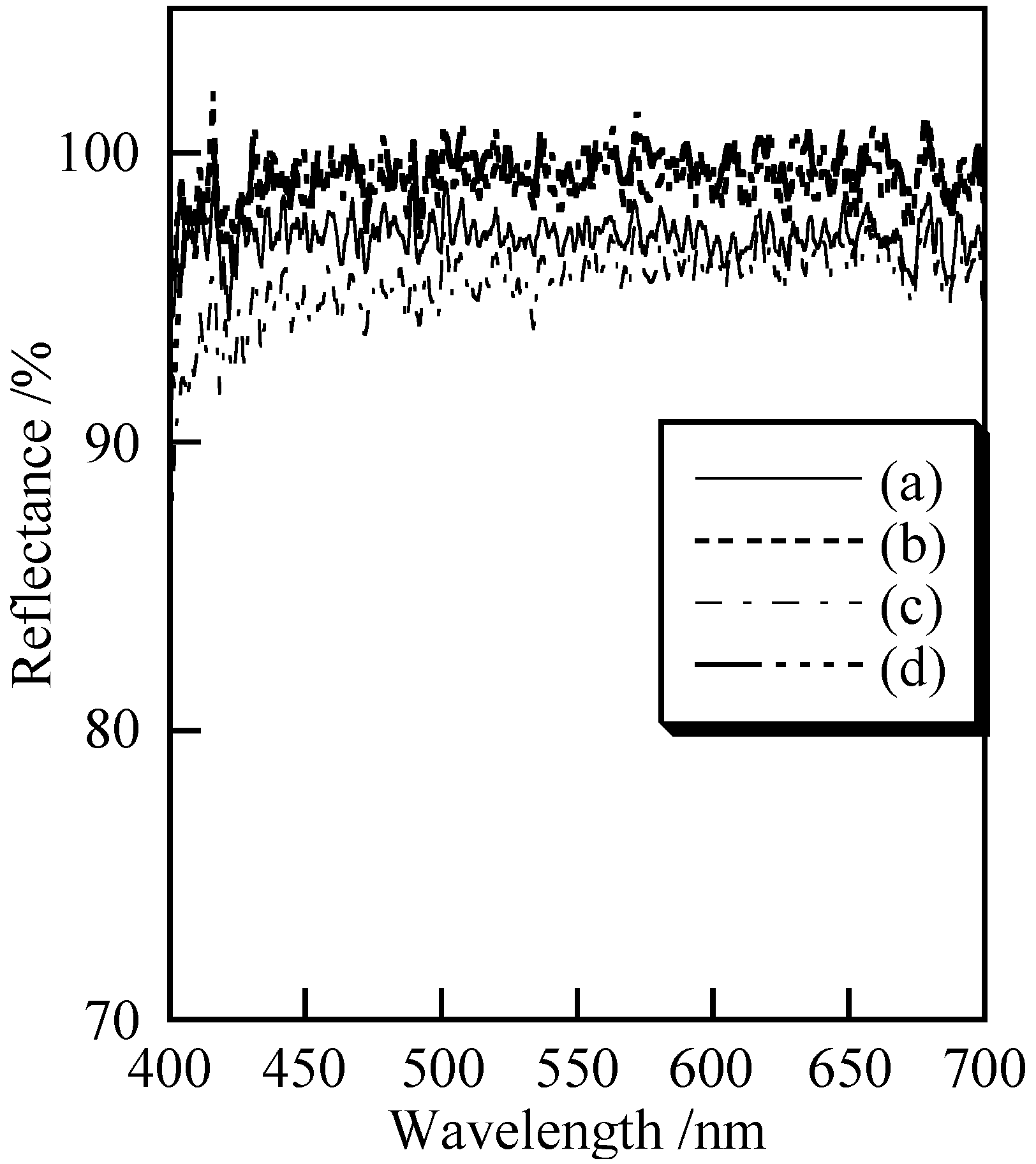
3.2. Cosmetic Properties of Titanium Phosphates
| Sample | Temperature/°C | Ultrasound/min | L* Value | |
|---|---|---|---|---|
| R.T. | 100 °C | |||
| A | 7 | 0 | 95.0 | 89.8 |
| B | 7 | 10 | 98.5 | 91.0 |
| C | 40 | 0 | 93.2 | 86.1 |
| D | 40 | 10 | 99.6 | 88.9 |
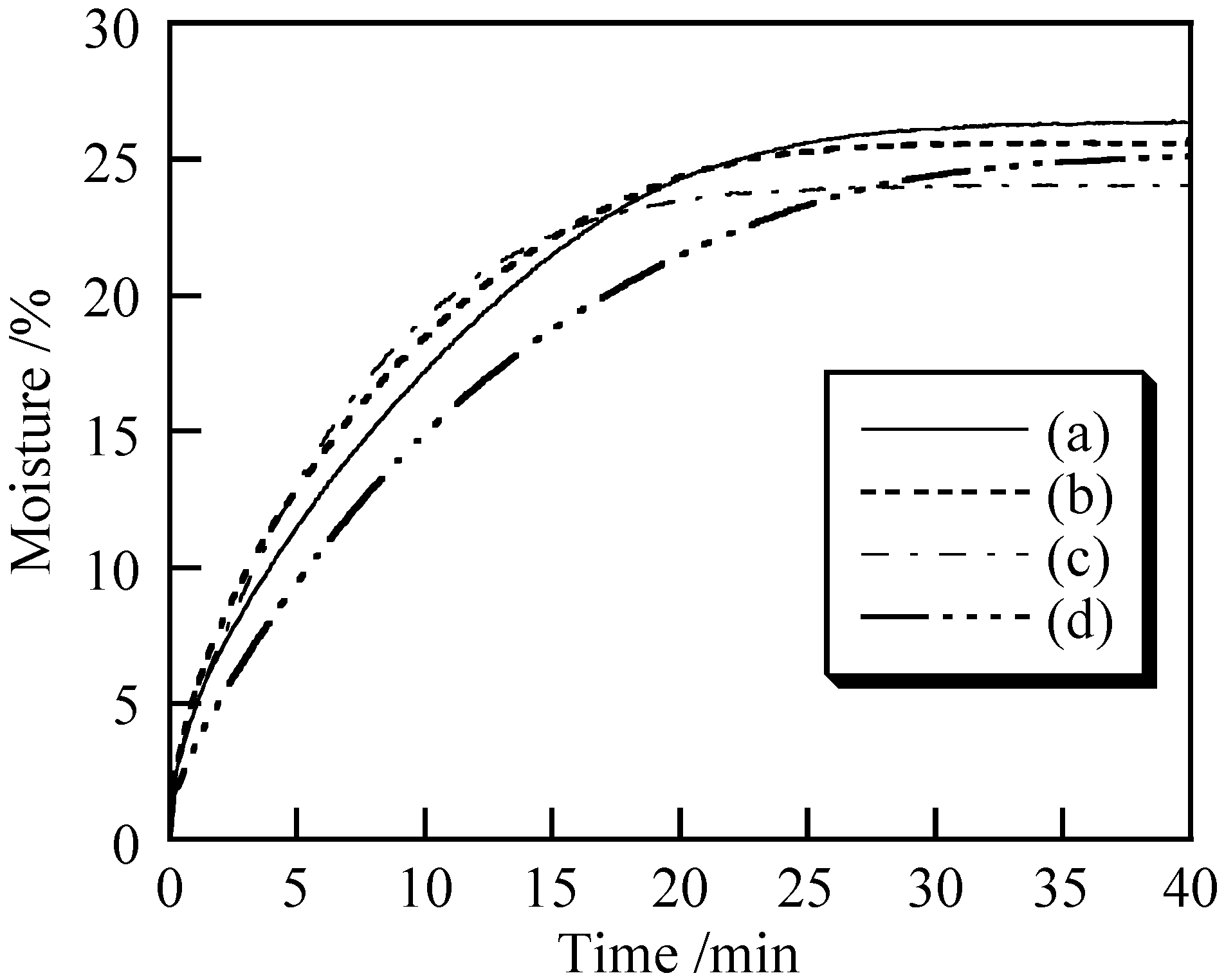
| Sample | Temperature/°C | Ultrasound/min | MIU | MMD |
|---|---|---|---|---|
| A | 7 | 0 | 0.340 | 0.008 |
| B | 7 | 10 | 0.358 | 0.010 |
| C | 40 | 0 | 0.290 | 0.010 |
| D | 40 | 10 | 0.300 | 0.009 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Diebold, U. The surface science of titanium dioxide. Surf. Sci. Rep. 2003, 48, 53–229. [Google Scholar] [CrossRef]
- Senzui, M.; Tamura, T.; Miura, K.; Ikarashi, Y.; Watanabe, Y.; Fujii, M. Study on penetration of titanium dioxide (TiO2) nanoparticles into intact and damaged skin in vitro. J. Toxicol. Sci. 2010, 35, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Gamer, A.O.; Leibold, E.; van Ravenzwaay, B. The in vitro absorption of microfine zinc oxide and titanium dioxide through porcine skin. Toxicol. Vitro 2006, 20, 301–307. [Google Scholar] [CrossRef]
- Jones, D.J.; Aptel, G.; Brandhorst, M.; Jacquin, M.; Jimenez-Jimenez, J.; Jimenez-Lopez, A.; Maireles-Torres, P.; Piwonski, I.; Rodrigues-Castellon, E.; Zajac, J.; et al. High surface area mesoporous titanium phosphate: Synthesis and surface acidity determination. J. Mater. Chem. 2000, 10, 1957–1963. [Google Scholar]
- Bhaumik, A.; Inagaki, S. Titanium Phosphate Molecular Sieves with Ion-Exchange Capacity. J. Am. Chem. Soc. 2001, 123, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Onoda, H.; Yamaguchi, T. Influence of ultrasonic treatment on preparation and powder properties of titanium phosphates. J. Mater. Chem. 2012, 22, 19826–19830. [Google Scholar] [CrossRef]
- Onoda, H.; Yamaguchi, T.; Takenaka, A. Synthesis and pigmental properties of titanium phosphates with the addition of urea. Int. J. Cosmet. Sci. 2012, 34, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Izaki, M.; Sasaki, S.; Mohamad, F.B.; Shinagawa, T.; Ohta, T.; Watase, S.; Sasano, J. Effects of preparation temperature on optical and electrical characteristics of (111)-oriented Cu2O films electrodeposited on (111)-Au film. Thin Solid Films 2012, 520, 1779–1783. [Google Scholar] [CrossRef]
- Cai, Y.; Pan, H.; Xu, X.; Hu, Q.; Li, L.; Tang, R. Ultrasonic Controlled Morphology Transformation of Hollow Calcium Phosphate Nanospheres: A Smart and Biocompatible Drug Release System. Chem. Mater. 2007, 19, 3081–3083. [Google Scholar] [CrossRef]
- Jung, S.H.; Oh, E.; Lim, H.; Shim, D.S.; Cho, S.; Lee, K.H.; Jeong, S.H. Shape-Selective Fabrication of Zinc Phosphate Hexagonal Bipyramids via a Disodium Phosphate-Assisted Sonochemical Route. Cryst. Growth Des. 2009, 9, 3544–3547. [Google Scholar] [CrossRef]
- Ramaswamy, V.; Jagtap, N.B.; Vijayanand, S.; Bhange, D.S.; Awati, P.S. Photocatalytic decomposition of methylene blue on nanocrystalline titania prepared by different methods. Mater. Res. Bull. 2008, 43, 1145–1152. [Google Scholar] [CrossRef]
- Du, P.; Bueno-Lopez, A.; Verbaas, M.; Almeida, A.R.; Makkee, M.; Moulijn, J.A.; Mui, G. The effect of surface OH-population on the photocatalytic activity of rare earth-doped P25-TiO2 in methylene blue degradation. J. Catal. 2008, 260, 75–80. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Yuen, C.W.M.; Kan, C.W.; Cheuk, K.K.L.; Tang, J.C.O.; Li, S.Y. A comprehensive study of silicone-based cosmetic textile agent. Fibers Polym. 2009, 10, 132–140. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onoda, H.; Fujikado, S. Influence of Temperature and Ultrasonic Treatment on Preparation of Titanium Phosphates and Their Powder Properties. Cosmetics 2014, 1, 222-231. https://doi.org/10.3390/cosmetics1040222
Onoda H, Fujikado S. Influence of Temperature and Ultrasonic Treatment on Preparation of Titanium Phosphates and Their Powder Properties. Cosmetics. 2014; 1(4):222-231. https://doi.org/10.3390/cosmetics1040222
Chicago/Turabian StyleOnoda, Hiroaki, and Syohei Fujikado. 2014. "Influence of Temperature and Ultrasonic Treatment on Preparation of Titanium Phosphates and Their Powder Properties" Cosmetics 1, no. 4: 222-231. https://doi.org/10.3390/cosmetics1040222
APA StyleOnoda, H., & Fujikado, S. (2014). Influence of Temperature and Ultrasonic Treatment on Preparation of Titanium Phosphates and Their Powder Properties. Cosmetics, 1(4), 222-231. https://doi.org/10.3390/cosmetics1040222