Net Ecosystem Exchange of CO2 in Deciduous Pine Forest of Lower Western Himalaya, India
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Climate
2.3. Ecophysiological Measurements
2.3.1. Night-Time Canopy Respiration (Rnc)
2.3.2. Soil Respiration (Rs)
2.3.3. Day-Time Net Canopy Photosynthesis (Ac) and Net Primary Productivity (NPP)
2.4. Biophysical Measurements: LAI and Phenology
2.5. Micrometeorological Observations and Data Processing
2.6. Statistical Analysis and Uncertainty Estimate
3. Results
3.1. Environmental Variables and Seasonal Variations
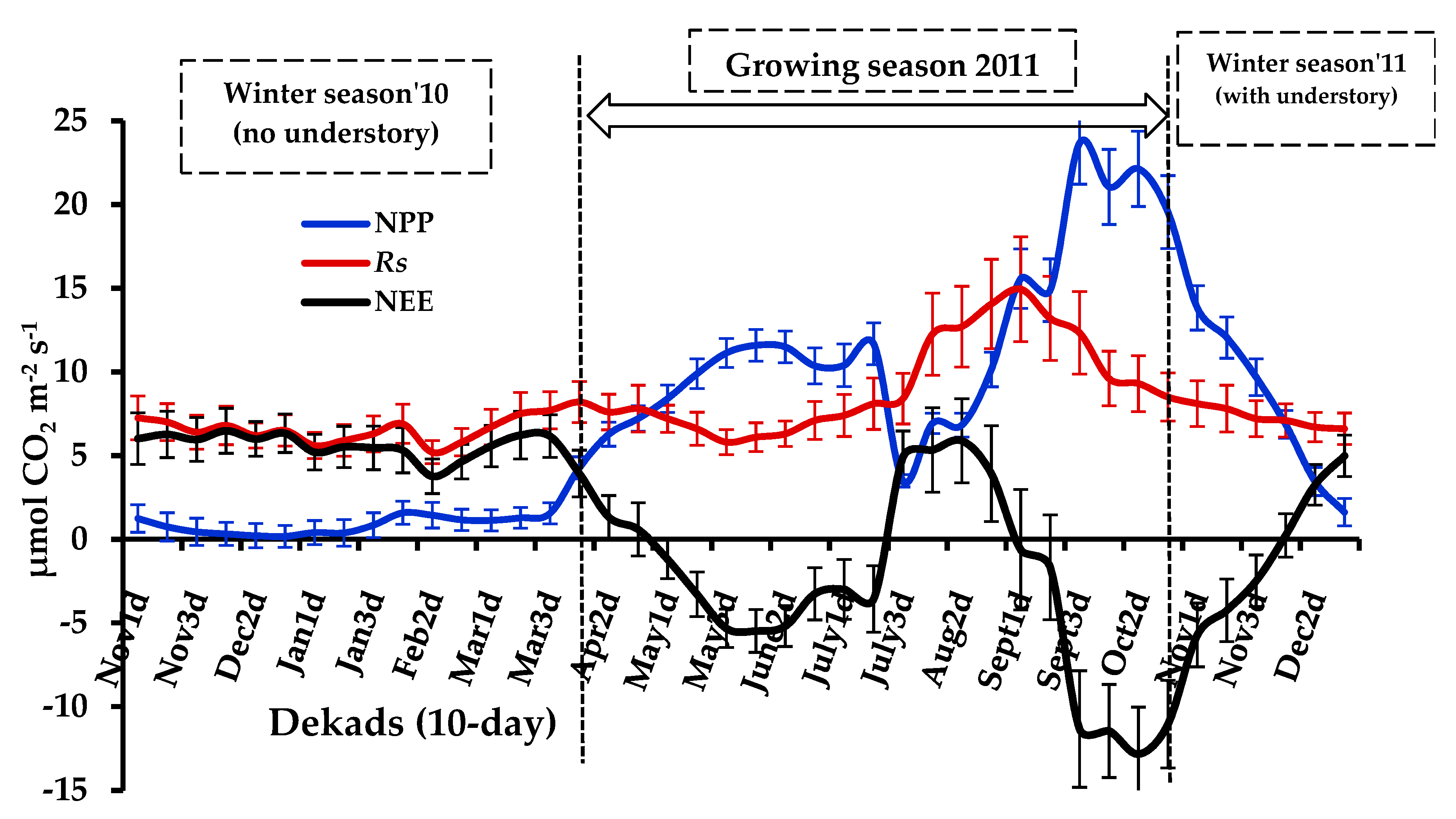
3.2. Seasonal Variations in NPP, Rs, and NEE
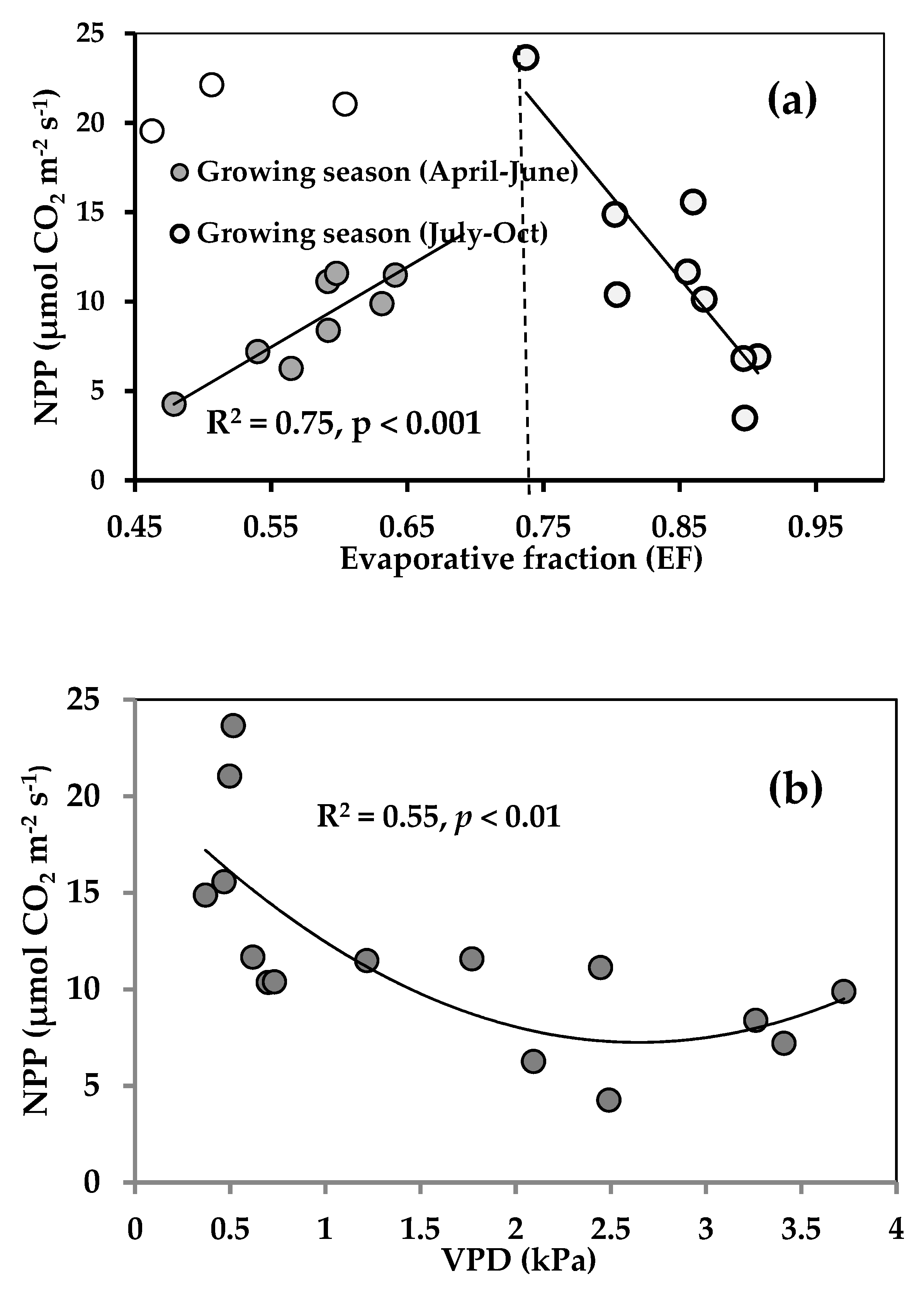
3.3. Seasonal Variations in Factors Controlling NPP and Rs
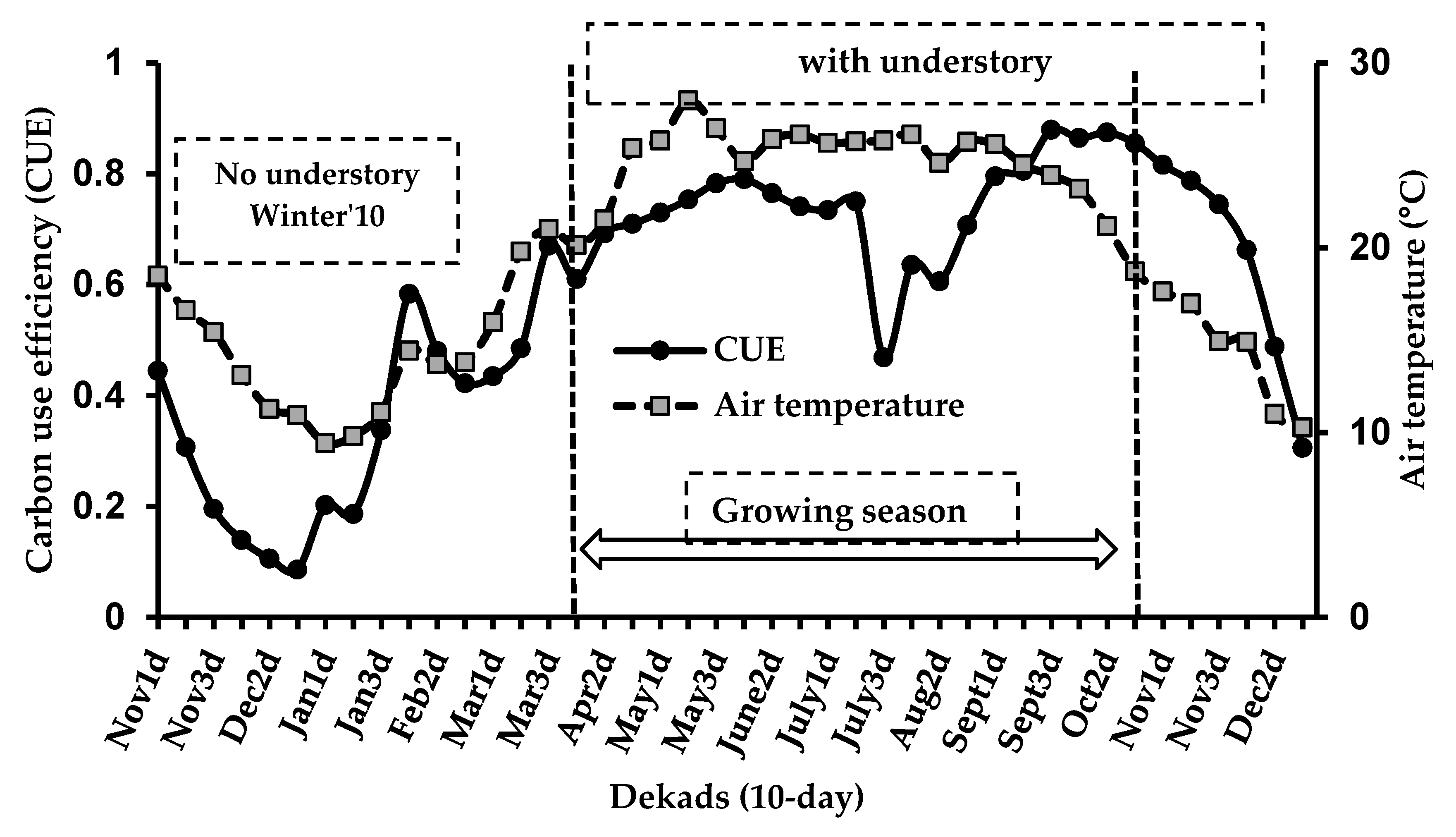
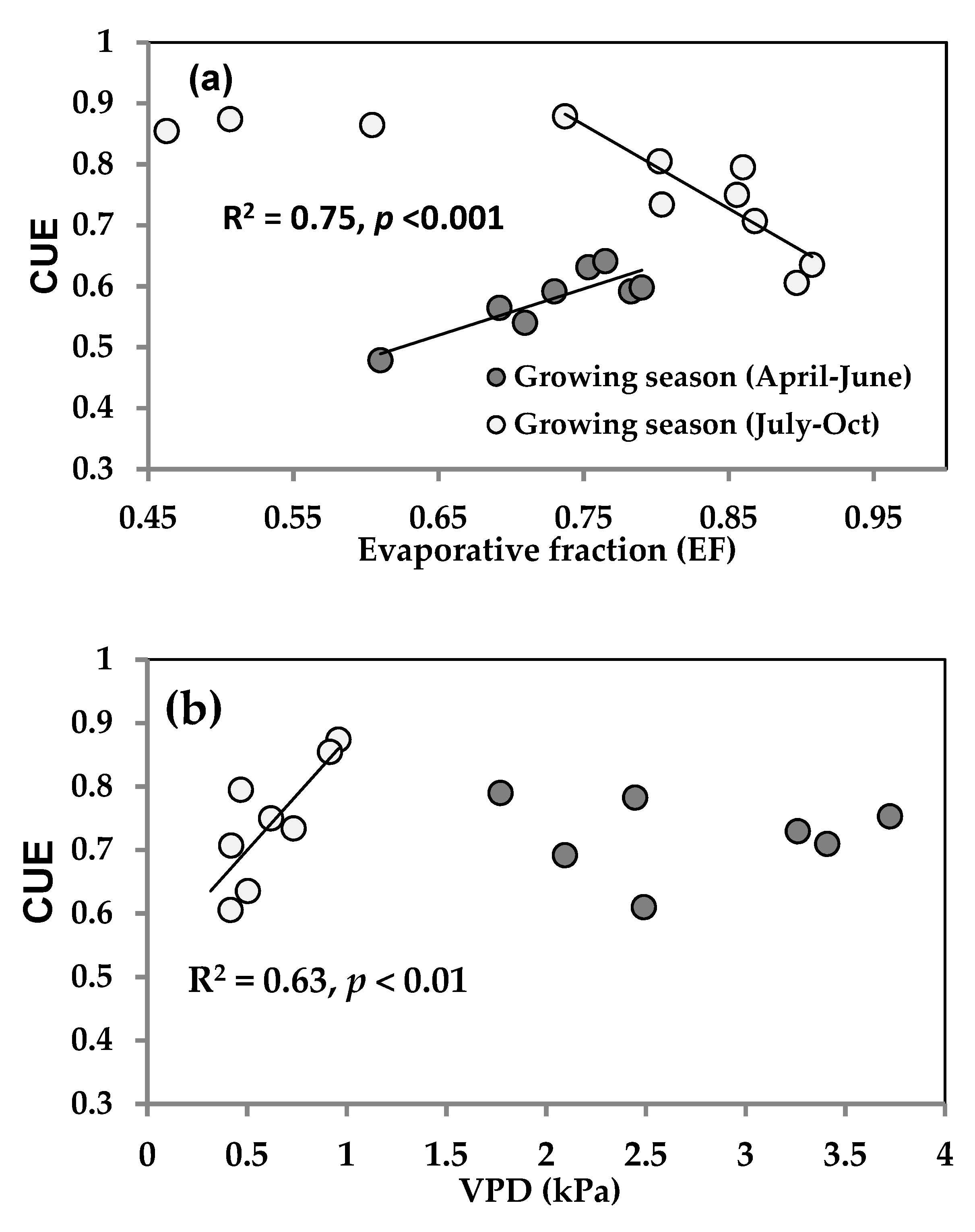
3.4. Seasonality in Carbon Use Efficiency (CUE)
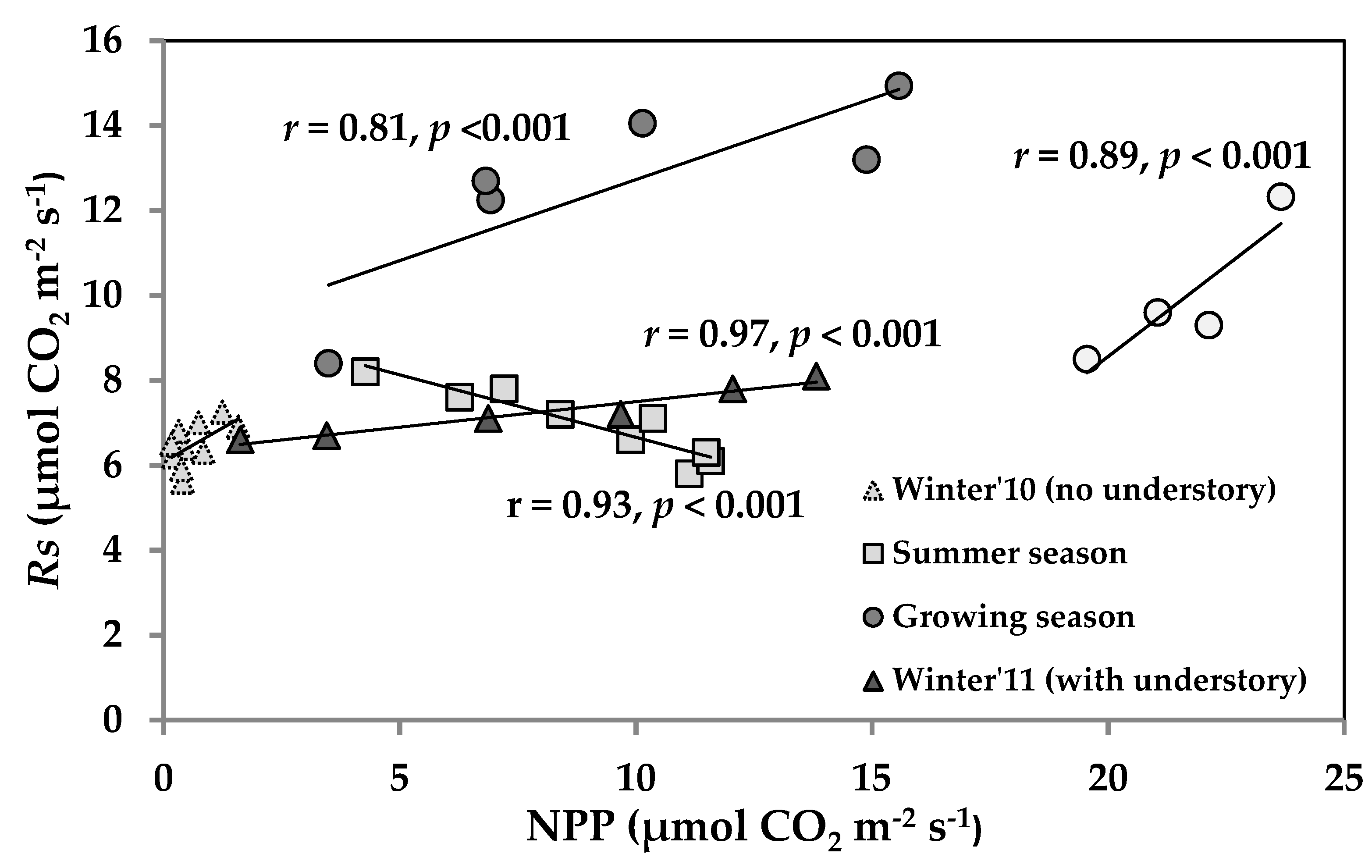
3.5. Temporal Correlation of NPP and Rs across the Seasons and Relation with LAI
4. Discussion
4.1. Seasonal Variability of Ecosystem CO2 Fluxes
4.2. Factors Affecting Ecosystem CO2 Fluxes
4.3. Seasonal Variability of CUE and Associated Factors
4.4. Uncertainty Associated with CO2 Ecosystem Fluxes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
- Net ecosystem productivity (NEP)
- Net primary productivity (NPP)
- Day-time canopy photosynthesis (Ac)
- Gross primary production (GPP)
- Day-time plant respiration (Rdday)
- Soil respiration (Rs)
- Ecosystem respiration (Re)
- Carbon use efficiency (CUE)
- Evaporative fraction (EF)
- Air temperature (AT)
- Vapor pressure deficit (VPD)
- Carbon (C)
- Carbon dioxide (CO2)
- Leaf area index (LAI)
- Dekads (10-days interval)
References
- Le Quéré, C.; Andres, R.J.; Boden, T.; Conway, T.; Houghton, R.A.; House, J.I.; Marland, G.; Peters, G.P.; van der Werf, G.R.; Ahlström, A.; et al. The global carbon budget 1959–2011. Earth Syst. Sci. Data 2013, 5, 165–185. [Google Scholar] [CrossRef]
- Sitch, S.; Friedlingstein, P.; Gruber, N.; Jones, S.D.; Murray-Tortarolo, G.; Ahlström, A.; Doney, S.C.; Graven, H.; Heinze, C.; Huntingford, C.; et al. Recent trends and drivers of regional sources and sinks of carbon dioxide. Biogeosciences 2015, 12, 653–679. [Google Scholar] [CrossRef]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A Large and Persistent Carbon Sink in the World’s Forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Ahlstrom, A.; Raupach, M.R.; Schurgers, G.; Smith, B.; Arneth, A.; Jung, M.; Reichstein, M.; Canadell, J.G.; Friedlingstein, P.; Jain, A.K.; et al. The dominant role of semi-arid ecosystems in the trend and variability of the land CO2 sink. Science 2015, 348, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Baldocchi, D. “Breathing” of the terrestrial biosphere: lessons learned from a global network of carbon dioxide flux measurement systems. Aust. J. Bot. 2008, 56, 1. [Google Scholar]
- Beringer, J.; Hutley, L.B.; McHugh, I.; Arndt, S.K.; Campbell, D.; Cleugh, H.A.; Cleverly, J.; Resco de Dios, V.; Eamus, D.; Evans, B.; et al. An introduction to the Australian and New Zealand flux tower network–OzFlux. Biogeosciences 2016, 13, 5895–5916. [Google Scholar] [CrossRef]
- Singh, N.; Parida, B.R. Environmental factors associated with seasonal variations of night-time plant canopy and soil respiration fluxes in deciduous conifer forest, Western Himalaya, India. Trees 2019, 33, 599–613. [Google Scholar] [CrossRef]
- Keenan, T.F.; Migliavacca, M.; Papale, D.; Baldocchi, D.; Reichstein, M.; Torn, M.; Wutzler, T. Widespread inhibition of daytime ecosystem respiration. Nat. Ecol. Evol. 2019, 3, 407–415. [Google Scholar] [CrossRef]
- Basistha, A.; Arya, D.S.; Goel, N.K. Analysis of historical changes in rainfall in the Indian Himalayas. Int. J. Climatol. 2009, 29, 555–572. [Google Scholar] [CrossRef]
- Shrestha, U.B.; Gautam, S.; Bawa, K.S. Widespread Climate Change in the Himalayas and Associated Changes in Local Ecosystems. PLoS ONE 2012, 7, e36741. [Google Scholar] [CrossRef]
- Forest Survey of India. Indian State of Forest Report 2011; Ministry of Environment and Forests: Dehradun, India, 2011.
- Singh, N.; Bhattacharya, B.K.; Nanda, M.K.; Soni, P.; Parihar, J.S. Radiation and energy balance dynamics over young chir pine (Pinus roxburghii) system in Doon of western Himalayas. J. Earth Syst. Sci. 2014, 123, 1451–1465. [Google Scholar] [CrossRef]
- Singh, N.; Patel, N.R.; Bhattacharya, B.K.; Soni, P.; Parida, B.R.; Parihar, J.S. Analyzing the dynamics and inter-linkages of carbon and water fluxes in subtropical pine (Pinus roxburghii) ecosystem. Agric. For. Meteorol. 2014, 197, 206–218. [Google Scholar] [CrossRef]
- Speckman, H.N.; Frank, J.M.; Bradford, J.B.; Miles, B.L.; Massman, W.J.; Parton, W.J.; Ryan, M.G. Forest ecosystem respiration estimated from eddy covariance and chamber measurements under high turbulence and substantial tree mortality from bark beetles. Glob. Change Biol. 2015, 21, 708–721. [Google Scholar] [CrossRef]
- Hill, T.; Chocholek, M.; Clement, R. The case for increasing the statistical power of eddy covariance ecosystem studies: Why, where and how? Glob. Change Biol. 2017, 23, 2154–2165. [Google Scholar] [CrossRef]
- Berkelhammer, M.; Hu, J.; Bailey, A.; Noone, D.C.; Still, C.J.; Barnard, H.; Gochis, D.; Hsiao, G.S.; Rahn, T.; Turnipseed, A. The nocturnal water cycle in an open-canopy forest. J. Geophys. Res. Atmos. 2013, 118, 10225–10242. [Google Scholar] [CrossRef]
- Collier, S.M.; Ruark, M.D.; Oates, L.G.; Jokela, W.E.; Dell, C.J. Measurement of Greenhouse Gas Flux from Agricultural Soils Using Static Chambers. J. Vis. Exp. 2014. [Google Scholar] [CrossRef] [PubMed]
- Law, B.E.; Kelliher, F.M.; Baldocchi, D.D.; Anthoni, P.M.; Irvine, J.; Moore, D.; Van Tuyl, S. Spatial and temporal variation in respiration in a young ponderosa pine forest during a summer drought. Agric. For. Meteorol. 2001, 110, 27–43. [Google Scholar] [CrossRef]
- Cavaleri, M.A.; Oberbauer, S.F.; Ryan, M.G. Foliar and ecosystem respiration in an old-growth tropical rain forest. Plant Cell Environ. 2008, 31, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Chambers, J.Q.; Tribuzy, E.S.; Toledo, L.C.; Crispim, B.F.; Higuchi, N.; dos Santos, J.; Araújo, A.C.; Kruijt, B.; Nobre, A.D.; Trumbore, S.E. Respiration from a Tropical Forest Ecosystem: Partitioning of sources and low Carbon Use Efficiency. Ecol. Appl. 2004, 14, 72–88. [Google Scholar] [CrossRef]
- Campbell, G.; Norman, J. An Introduction to Environmental Biophysics; Springer-Verlag New York: New York, NY, USA, 1998; p. 71. [Google Scholar]
- Sun, J.; Guan, D.; Wu, J.; Jing, Y.; Yuan, F.; Wang, A.; Jin, C. Day and night respiration of three tree species in a temperate forest of northeastern China. IForest 2015, 8, 25–32. [Google Scholar] [CrossRef]
- Wehr, R.; Munger, J.W.; McManus, J.B.; Nelson, D.D.; Zahniser, M.S.; Davidson, E.A.; Wofsy, S.C.; Saleska, S.R. Seasonality of temperate forest photosynthesis and daytime respiration. Nature 2016, 534, 680–683. [Google Scholar] [CrossRef]
- Amthor, J.S.; Baldocchi, D.D. Terrestrial higher plant respiration and net primary productivity. In Terrestrial Global Productivity; Roy, J., Mooney, H., Saugier, B., Eds.; Elsevier: New York, NY, USA, 2001; pp. 33–59. [Google Scholar]
- Allison, S.D.; Wallenstein, M.D.; Bradford, M.A. Soil-carbon response to warming dependent on microbial physiology. Nat. Geosci. 2010, 3, 336–340. [Google Scholar] [CrossRef]
- Leuning, R.; van Gorsel, E.; Massman, W.J.; Isaac, P.R. Reflections on the surface energy imbalance problem. Agric. For. Meteorol. 2012, 156, 65–74. [Google Scholar] [CrossRef]
- Heusinkveld, B.G.; Jacobs, A.F.G.; Holtslag, A.A.M.; Berkowicz, S.M. Surface energy balance closure in an arid region: Role of soil heat flux. Agric. For. Meteorol. 2004, 122, 21–37. [Google Scholar] [CrossRef]
- Seneviratne, S.I.; Corti, T.; Davin, E.L.; Hirschi, M.; Jaeger, E.B.; Lehner, I.; Orlowsky, B.; Teuling, A.J. Investigating soil moisture–climate interactions in a changing climate: A review. Earth-Sci. Rev. 2010, 99, 125–161. [Google Scholar] [CrossRef]
- Grinsted, A.; Moore, J.C.; Jevrejeva, S. Application of the cross wavelet transform and wavelet coherence to geophysical time series. Nonlinear Process. Geophys. 2004, 11, 561–566. [Google Scholar] [CrossRef]
- Torrence, C.; Compo, G.P. A Practical Guide to Wavelet Analysis. Bull. Am. Meteorol. Soc. 1998, 79, 61–78. [Google Scholar] [CrossRef]
- DeLUCIA, E.H.; Drake, J.E.; Thomas, R.B.; Gonzalez-Meler, M. Forest carbon use efficiency: Is respiration a constant fraction of gross primary production? Glob. Change Biol. 2007, 13, 1157–1167. [Google Scholar] [CrossRef]
- Joshi, M.; Mer, G.S.; Singh, S.P.; Rawat, Y.S. Seasonal pattern of total soil respiration in undisturbed and disturbed ecosystems of Central Himalaya. Biol. Fertil. Soils 1991, 11, 267–272. [Google Scholar] [CrossRef]
- Watham, T.; Kushwaha, S.P.; Patel, N.R.; Dadhwal, V.K. Monitoring of carbon dioxide and water vapour exchange over a young mixed forest plantation using eddy covariance technique. Curr. Sci 2014, 107, 858–867. [Google Scholar]
- Singh, N.; Patel, N.R.; Singh, J.; Raja, P.; Soni, P.; Parihar, J.S. Carbon exchange in some invasive species in the Himalayan foothills. Trop. Ecol. 2016, 57, 263–270. [Google Scholar]
- Patel, N.R.; Padalia, H.; Kushwaha, S.P.S.; Nandy, S.; Watham, T.; Ahongshangbam, J.; Kumar, R.; Dadhwal, V.K.; Senthil, K.A. CO2 Flux Tower and Remote Sensing: Tools for Monitoring Carbon Exchange over Ecosystem Scale in Northwest Himalaya; Navalgund, R., Kumar, A., Nandy, S., Eds.; Springer: Singapore, 2019. [Google Scholar]
- Bahn, M.; Rodeghiero, M.; Anderson-Dunn, M.; Dore, S.; Gimeno, C.; Drösler, M.; Williams, M.; Ammann, C.; Berninger, F.; Flechard, C.; et al. Soil Respiration in European Grasslands in Relation to Climate and Assimilate Supply. Ecosystems 2008, 11, 1352–1367. [Google Scholar] [CrossRef]
- DeForest, J.L.; Noormets, A.; McNulty, S.G.; Sun, G.; Tenney, G.; Chen, J. Phenophases alter the soil respiration–temperature relationship in an oak-dominated forest. Int. J. Biometeorol. 2006, 51, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Luo, Y.; Li, X.; Liu, S.; Yu, G.; Zhou, T.; Bahn, M.; Black, A.; Desai, A.R.; Cescatti, A.; et al. Redefinition and global estimation of basal ecosystem respiration rate. Glob. Biogeochem. Cycles 2011, 25, 1–14. [Google Scholar] [CrossRef]
- Zhang, M.; Yu, G.-R.; Zhang, L.-M.; Sun, X.-M.; Wen, X.-F.; Han, S.-J.; Yan, J.-H. Impact of cloudiness on net ecosystem exchange of carbon dioxide in different types of forest ecosystems in China. Biogeosciences 2010, 7, 711–722. [Google Scholar] [CrossRef]
- Canadell, J.G.; Mooney, H.A.; Baldocchi, D.D.; Berry, J.A.; Ehleringer, J.R.; Field, C.B.; Gower, S.T.; Hollinger, D.Y.; Hunt, J.E.; Jackson, R.B.; et al. Commentary: Carbon Metabolism of the Terrestrial Biosphere: A Multitechnique Approach for Improved Understanding. Ecosystems 2000, 3, 115–130. [Google Scholar] [CrossRef]
- Xue, B.-L.; Guo, Q.; Gong, Y.; Hu, T.; Liu, J.; Ohta, T. The influence of meteorology and phenology on net ecosystem exchange in an eastern Siberian boreal larch forest. J. Plant Ecol. 2016, 9, 520–530. [Google Scholar] [CrossRef]
- Suni, T.; Berninger, F.; Markkanen, T.; Keronen, P.; Rannik, Ü.; Vesala, T. Interannual variability and timing of growing-season CO2 exchange in a boreal forest. J. Geophys. Res. Atmos. 2003, 108. [Google Scholar] [CrossRef]
- McCaughey, J.H.; Lafleur, P.M.; Joiner, D.W.; Bartlett, P.A.; Costello, A.M.; Jelinski, D.E.; Ryan, M.G. Magnitudes and seasonal patterns of energy, water, and carbon exchanges at a boreal young jack pine forest in the BOREAS northern study area. J. Geophys. Res. Atmos. 1997, 102, 28997–29007. [Google Scholar] [CrossRef]
- Wangdi, N.; Mayer, M.; Nirola, M.P.; Zangmo, N.; Orong, K.; Ahmed, I.U.; Darabant, A.; Jandl, R.; Gratzer, G.; Schindlbacher, A. Soil CO2 efflux from two mountain forests in the eastern Himalayas, Bhutan: Components and controls. Biogeosciences 2017, 14, 99–110. [Google Scholar] [CrossRef]
- Lindroth, A.; Verwijst, T.; Halldin, S. Water-use efficiency of willow: Variation with season, humidity and biomass allocation. J. Hydrol. 1994, 156, 1–19. [Google Scholar] [CrossRef]
- Alton, P.B.; North, P.R.; Los, S.O. The impact of diffuse sunlight on canopy light-use efficiency, gross photosynthetic product and net ecosystem exchange in three forest biomes: Impact of Diffuse Sunlight on canopy LUE, GPP and NEE. Glob. Change Biol. 2007, 13, 776–787. [Google Scholar] [CrossRef]
- Kolari, P.; Lappalainen, H.K.; HäNninen, H.; Hari, P. Relationship between temperature and the seasonal course of photosynthesis in Scots pine at northern timberline and in southern boreal zone. Tellus B Chem. Phys. Meteorol. 2007, 59, 542–552. [Google Scholar] [CrossRef]
- Williams, M.; Law, B.E.; Anthoni, P.M.; Unsworth, M.H. Use of a simulation model and ecosystem flux data to examine carbon-water interactions in ponderosa pine. Tree Physiol. 2001, 21, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Stella, P.; Lamaud, E.; Brunet, Y.; Bonnefond, J.-M.; Loustau, D.; Irvine, M. Simultaneous measurements of CO2 and water exchanges over three agroecosystems in South-West France. Biogeosciences 2009, 6, 2957–2971. [Google Scholar] [CrossRef]
- Lambers, H. Cyanide-resistant respiration: A non-phosphorylating electron transport pathway acting as an energy overflow. Physiol. Plant. 1982, 55, 478–485. [Google Scholar] [CrossRef]
- Vicca, S.; Luyssaert, S.; Peñuelas, J.; Campioli, M.; Chapin, F.S.; Ciais, P.; Heinemeyer, A.; Högberg, P.; Kutsch, W.L.; Law, B.E.; et al. Fertile forests produce biomass more efficiently: Forests’ biomass production efficiency. Ecol. Lett. 2012, 15, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Zanotelli, D.; Montagnani, L.; Manca, G.; Tagliavini, M. Net primary productivity, allocation pattern and carbon use efficiency in an apple orchard assessed by integrating eddy covariance, biometric and continuous soil chamber measurements. Biogeosciences 2013, 10, 3089–3108. [Google Scholar] [CrossRef]
- Luo, Y.; Zhou, X. Soil Respiration and the Environment; Academic Press: San Diego, CA, USA, 2010; pp. 1–328. ISBN 9780120887828. [Google Scholar]
- Baldocchi, D.; Sturtevant, C.; Contributors, F. Does day and night sampling reduce spurious correlation between canopy photosynthesis and ecosystem respiration? Agric. For. Meteorol. 2015, 207, 117–126. [Google Scholar] [CrossRef]
- Lee, X.; Massman, W.; Law, B.E. Handbook of Micrometeorology: A Guide for Surface Flux Measurement and Analysis; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004; ISBN 1-402022646. [Google Scholar]
- Aubinet, M.; Feigenwinter, C.; Heinesch, B.; Bernhofer, C.; Canepa, E.; Lindroth, A.; Montagnani, L.; Rebmann, C.; Sedlak, P.; Van Gorsel, E. Direct advection measurements do not help to solve the night-time CO2 closure problem: Evidence from three different forests. Agric. For. Meteorol. 2010, 150, 655–664. [Google Scholar] [CrossRef]
- Billesbach, D.P. Estimating uncertainties in individual eddy covariance flux measurements: A comparison of methods and a proposed new method. Agric. For. Meteorol. 2011, 151, 394–405. [Google Scholar] [CrossRef]
- Singh, J.S. Forests of Himalaya: Structure, Functioning and Impact of Man; Gyanodaya Prakashan: Nainital, India, 1992; pp. 1–294. [Google Scholar]

| Variables | Characteristics | Remarks |
|---|---|---|
| Annual Rainfall | 2020 ± 423 mm | Average over 1940–2010, Peak during July–August |
| Air Temperature | 11.5 °C (January), 27 °C (June) | Peak during May–June |
| Humidity | 52% (April), 85% (August) | Lowest (summer season), Maximum (rainy season) |
| Sunshine | 4.4 h day−1 to 9.3 h day−1 | Minimum (July–August), Maximum (May) |
| Pan Evaporation | 1.2 mm to 7.2 mm | Minimum (December), Maximum (May) |
| Vapor Pressure Deficit (VPD) | 0.37 kPa to 3.7 kPa | Lowest (monsoon), Highest (summer) |
| Soil Moisture | 10% to 24% (mean of the three-soil layer: 0.05 m, 0.2 m, and 0.45 m) | Lowest in May (summer), Maximum (rainy season) |
| Soil type | Mollisols | 3–8 m thickness, Porosity 40–60% |
| Soil Texture | Sandy clay loam | 35% sand, 40% clay, and 25% silt |
| Soil pH | 4.5–6 | Acidic |
| Bulk density | 1030 kg m−3 | 0–20 cm depth |
| Months | NEE (Mean ± SE), CV (%) | Seasons | Phenology |
|---|---|---|---|
| January–February | 310.3 ± 2.8, 2 | Winter | Physiologically dormant stage |
| March–April | 247.0 ± 10.5, 10 | Spring to Summer | 10% to 63% GVF |
| May–July | −314.6 ± 5.4, 4.8 | Summer to Monsoon | 100% GVF |
| August | 208.0 ± 8.6, 4 | Monsoon | 100% GVF |
| September–October | −508.3 ± 23, 11 | Post-monsoon | 100% in September, 92% GVF in October |
| November | −129.2 ± 9.7, 13 | Fall | 80% GVF |
| December | 87.8 ± 14.4, 28 | Winter | Physiologically dormant stage |
| Annual sum | −99 ± 9.9 g C m−2 year−1 (peak carbon sink reached in post-monsoon) | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, N.; Parida, B.R.; Charakborty, J.S.; Patel, N.R. Net Ecosystem Exchange of CO2 in Deciduous Pine Forest of Lower Western Himalaya, India. Resources 2019, 8, 98. https://doi.org/10.3390/resources8020098
Singh N, Parida BR, Charakborty JS, Patel NR. Net Ecosystem Exchange of CO2 in Deciduous Pine Forest of Lower Western Himalaya, India. Resources. 2019; 8(2):98. https://doi.org/10.3390/resources8020098
Chicago/Turabian StyleSingh, Nilendu, Bikash Ranjan Parida, Joyeeta Singh Charakborty, and N.R. Patel. 2019. "Net Ecosystem Exchange of CO2 in Deciduous Pine Forest of Lower Western Himalaya, India" Resources 8, no. 2: 98. https://doi.org/10.3390/resources8020098
APA StyleSingh, N., Parida, B. R., Charakborty, J. S., & Patel, N. R. (2019). Net Ecosystem Exchange of CO2 in Deciduous Pine Forest of Lower Western Himalaya, India. Resources, 8(2), 98. https://doi.org/10.3390/resources8020098







