Development of an Industrial Environmental Index to Assess the Sustainability of Industrial Solvent-Based Processes
Abstract
1. Introduction
2. Materials and Methods
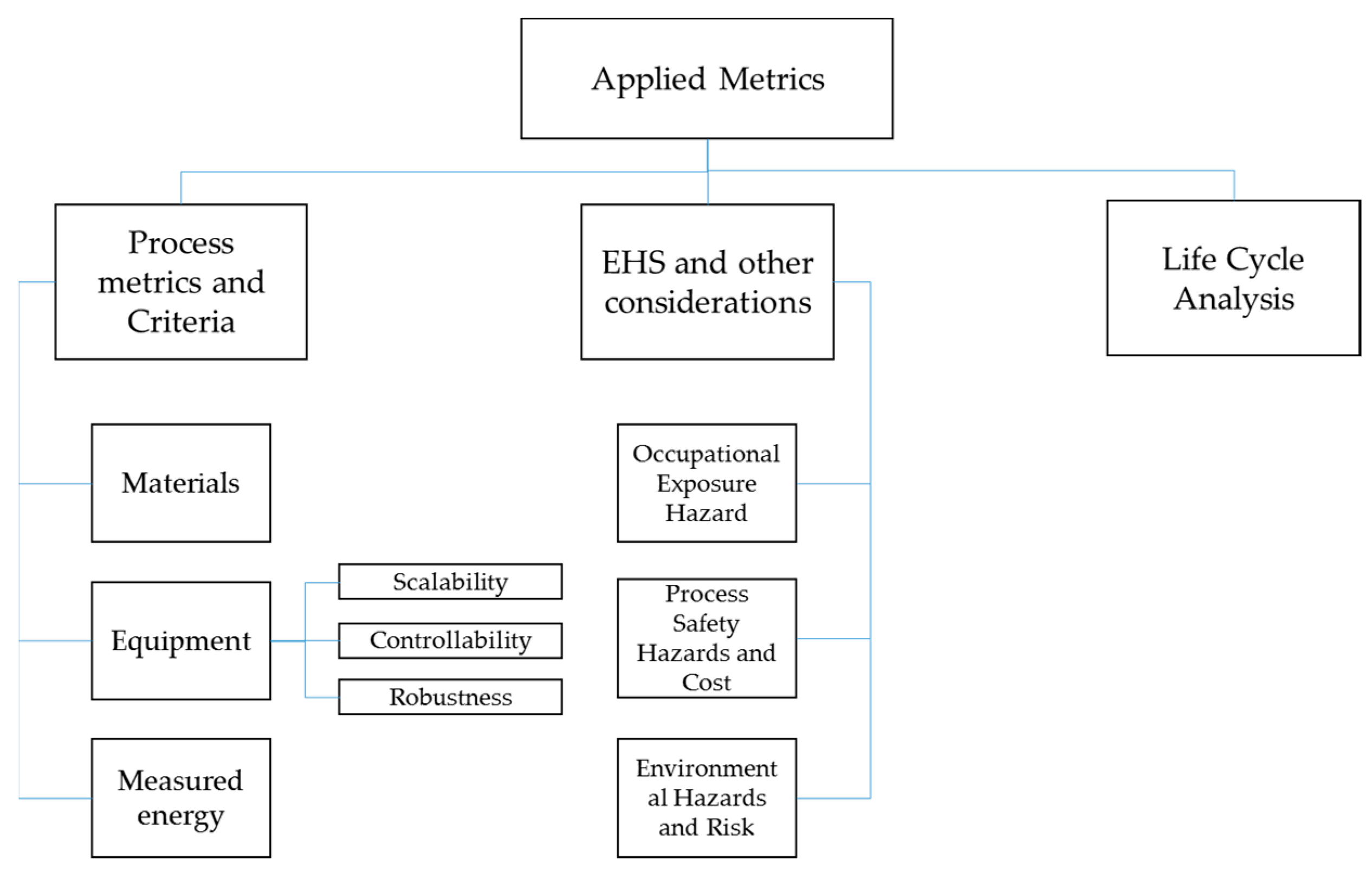
3. Applied Metrics
- Process metrics and criteria: metrics and credentials that relate to the core of the process in terms of inputs (materials, energy), outputs (materials, energy), and equipment.
- Environmental, health and safety metrics: metrics and credentials that deal with the impact of the process on the environment, the process staff, and the process itself.
- LCA, which is briefly covered in this article to conceptually widen the assessment of a process and the aggregation of metrics into an IEI.
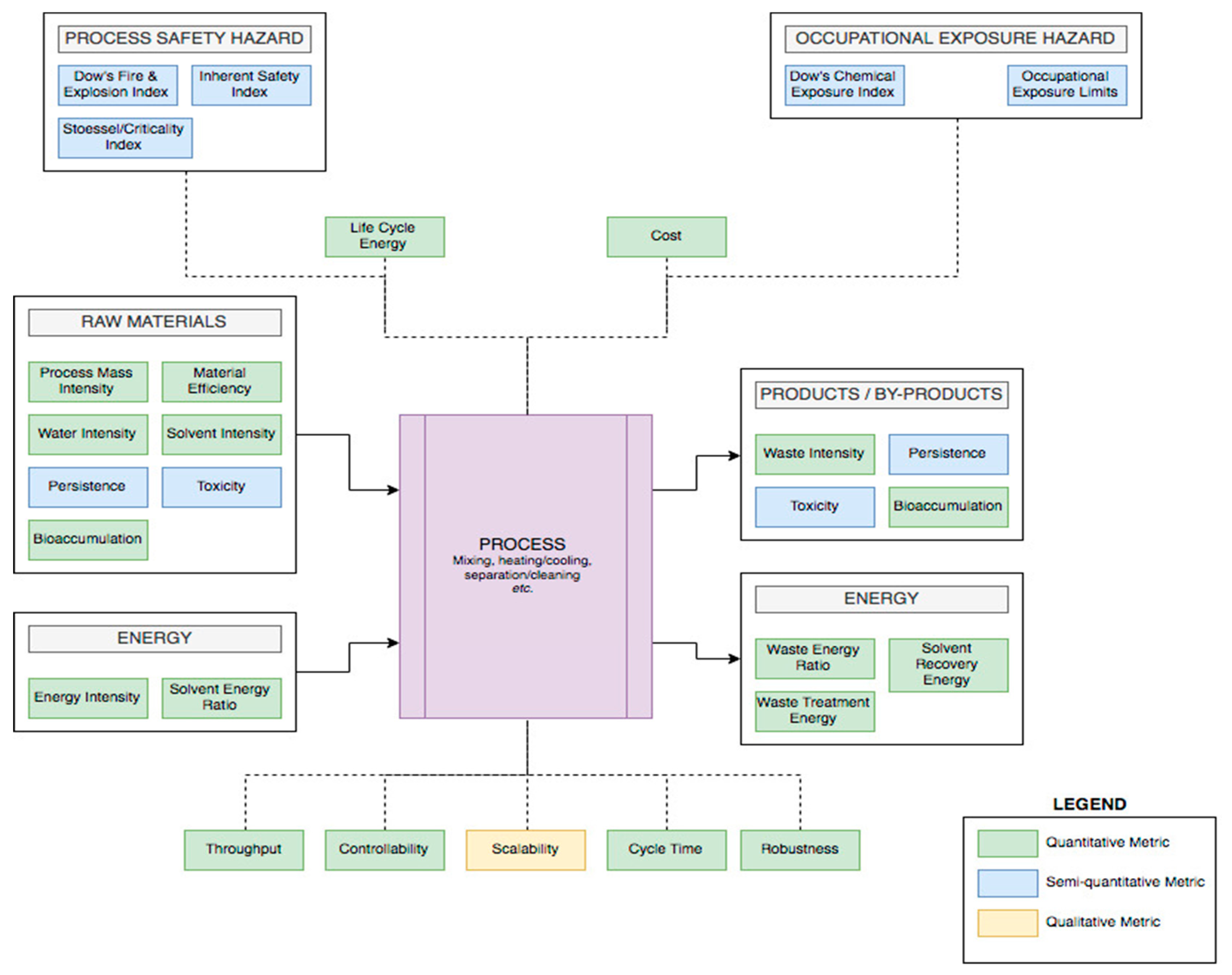
3.1. Process Metrics and Criteria
3.1.1. Materials
3.1.2. Equipment
Scalability
Controllability
Robustness, Throughput, and Cycle Time
3.1.3. Measuring Energy
3.2. EHS and Other Considerations
3.2.1. Occupational Exposure Hazard
- Skin and eye irritants;
- Harmful on single exposure;
- Severely irritating and corrosive;
- Very toxic on single exposure, reproductive hazard.
3.2.2. Process Safety Hazards and Cost
3.2.3. Environmental Hazards and Risk
- Toxicity: It is the most debated and debatable factor because of the various tests available to measure it and assess the risk associated with the use of a substance [33]. Nonetheless, and as mentioned in the introduction of this section, the REACH legislation is facilitating the gathering of toxicity data [5], in addition to the regulatory lists (developed by governments) [9] as well as the hazard data and solvent guides published by chemical companies [17,18,19]. Nowadays, lethal toxicity data are not enough, and eco-toxicity data are slowly being incorporated in the databases [9].
3.3. Life Cycle Analysis
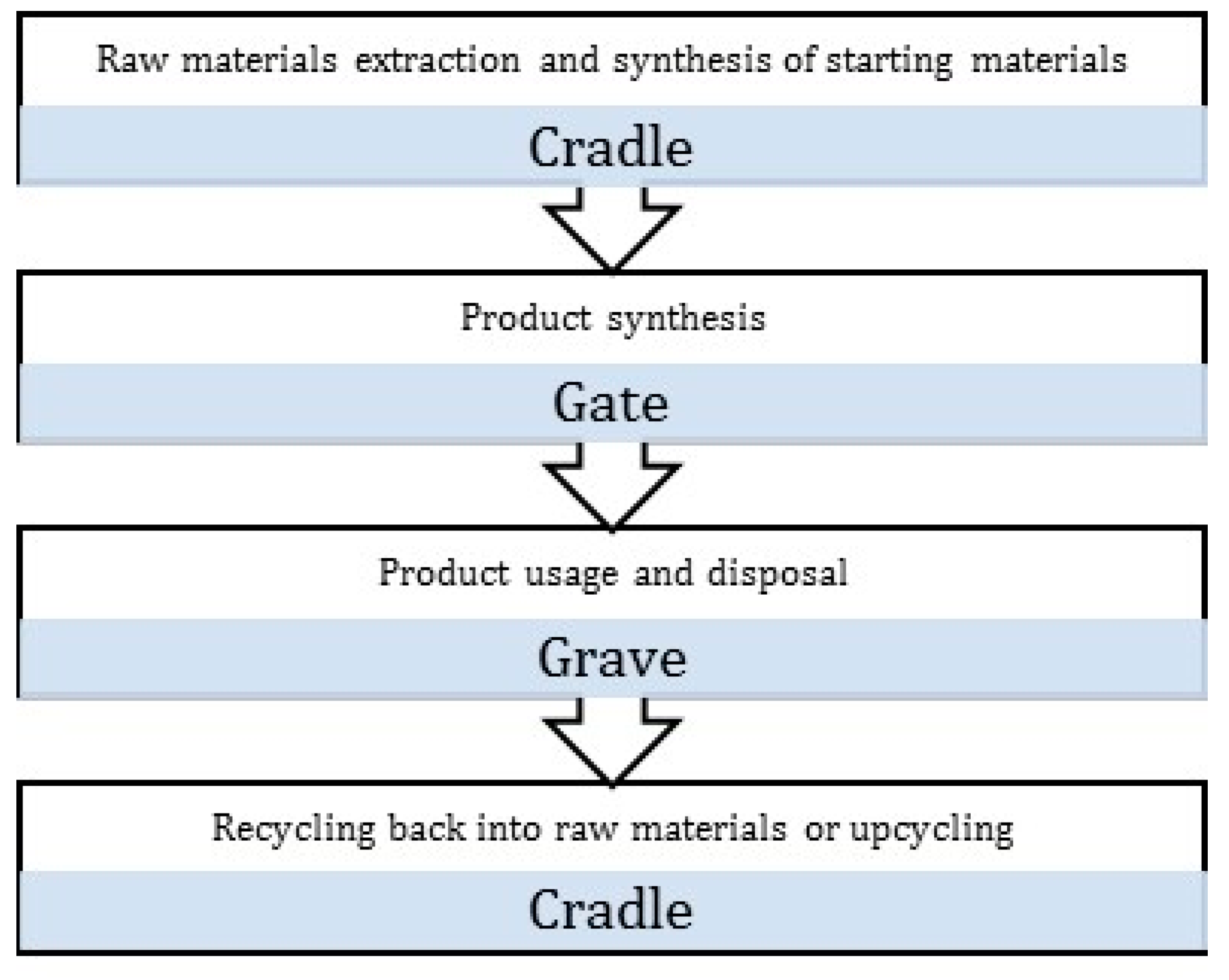
- Goal and scope definition: It is the planning stage of the LCA process where the objectives and the system boundaries are clearly defined. The different system boundaries are detailed in Figure 2. At this stage, the strategy for data collection needs to be developed, and if any comparative studies are to be conducted, it is essential they be carried out on the same functional unit.
- Inventory analysis: This stage mainly consists of data collection. This can be done by direct measurements, literature and database searches (with time, databases are growing and becoming more established), theoretical calculations, and interviews with experts. The data can be either aggregated according to the medium where releases occur (i.e., water, air, or soil), which is known as a midpoint method, or presented as a series of potential environmental impacts, which is known as an endpoint method.
- Impact assessment: The potential environmental impacts discussed above should be put into numbers in order to properly assess the data. The eight most reported impacts are: abiotic depletion (accounting for the depletion of all non-renewable resources), acidification potential, aquatic toxicity, eutrophication potential (potential to cause over-fertilization of water and soil), global warming potential, human toxicity potential, ozone depletion potential, and photochemical oxidants creation potential (potential to generate smog).
- Interpretation: The final stage of LCA consists of identifying both the parts of the life cycle that have the most impacts and the possibilities of improving the total environmental impact of the process.
4. Index Development
- The metrics should be indicative of the process as a whole;
- The metrics should measure performance;
- The metrics should be important in terms of the organization’s short-term and longer-term goals.
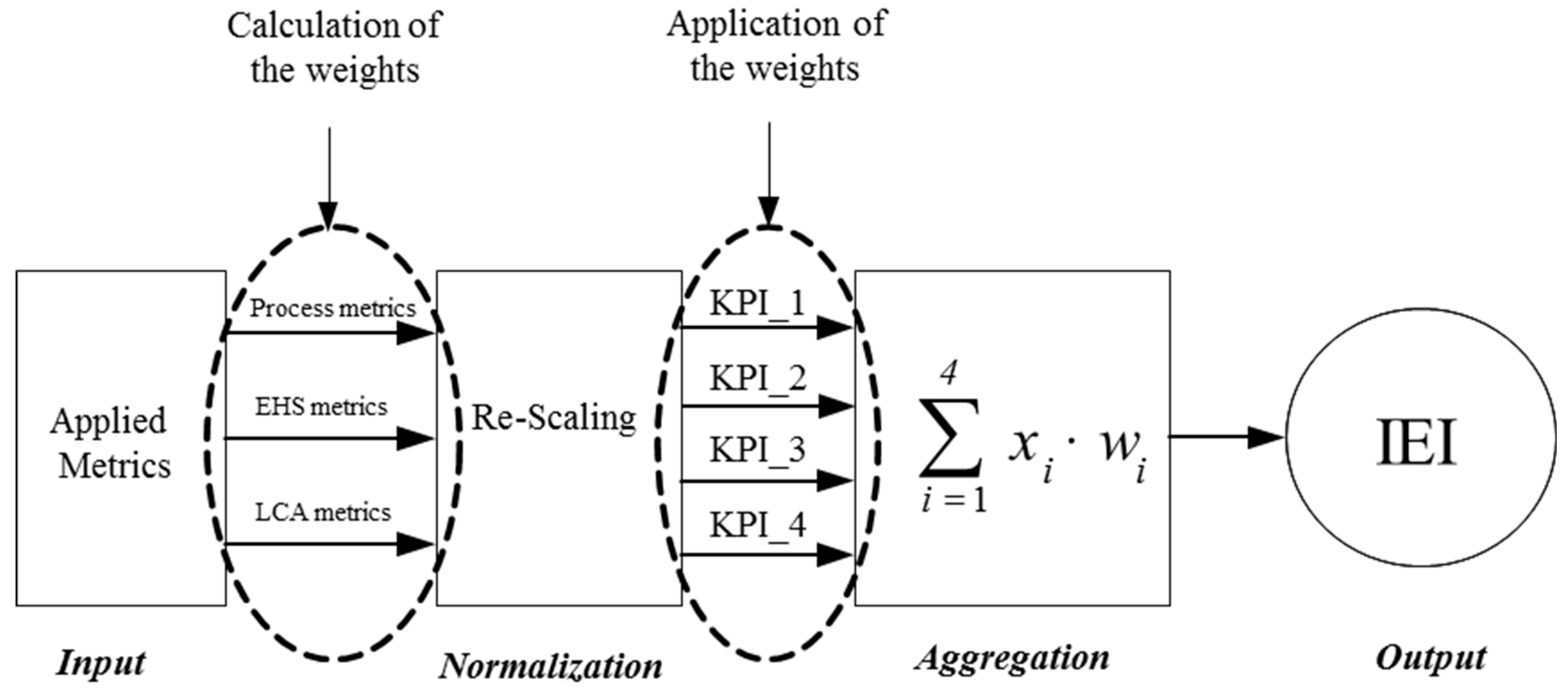
4.1. The Composite Indicator Method as a Means of Metrics Aggregation
4.2. Step-by-Step Approach for the Development of the IEI Index
5. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Sanford, T.; Frumhoff, P.C.; Luers, A.; Gulledge, J. The climate policy narrative for a dangerously warming world. Nat. Clim. Chang. 2014, 4, 164–166. [Google Scholar] [CrossRef]
- Yang, W.; Li, L. Efficiency evaluation of industrial waste gas control in China: A study based on data envelopment analysis (DEA) model. J. Clean. Prod. 2018, 179, 1–11. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, W. Does Whistleblowing Work for Air Pollution Control in China? A Study Based on Three-party Evolutionary Game Model under Incomplete Information. Sustainability 2019, 11, 324. [Google Scholar] [CrossRef]
- United Nations. The Sustainable Development Goals Report 2018; United Nations: New York, NY, USA, 2018. [Google Scholar]
- Lancaster, M. Green Chemistry, an Introductory Text, 2nd ed.; RSC Publishing: Cambridge, UK, 2012. [Google Scholar]
- Sheldon, R.A.; Sanders, J.P.M. Toward concise metrics for the production of chemicals from renewable biomass. Catal. Today 2015, 239, 3–6. [Google Scholar] [CrossRef]
- Lucato, W.; Santos, J.; Pacchini, A. Measuring the sustainability of a manufacturing process: A conceptual framework. Sustainability 2017, 10, 81. [Google Scholar] [CrossRef]
- Curzons, A.D.; Constable, D.C.; Cunningham, V.L. Solvent selection guide: A guide to the integration of environmental, health and safety criteria into the selection of solvents. Clean Prod. Process. 1999, 1, 82–90. [Google Scholar] [CrossRef]
- Jiménez-González, C.; Constable, D.J.C.; Ponder, C.S. Evaluating the “Greenness” of chemical processes and products in the pharmaceutical industry—A green metrics primer. Chem. Soc. Rev. 2012, 41, 1485–1498. [Google Scholar] [CrossRef] [PubMed]
- McDonough, W.; Braungart, M. Cradle to Cradle: Remaking the Way We Make Things; North Point Press: New York, NY, USA, 2010. [Google Scholar]
- Lapkin, A.; Constable, D.J.C. Green Chemistry Metrics: Measuring and Monitoring Sustainable Processes; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2009; pp. 1–337. [Google Scholar]
- Azapagic, A. Developing a framework for sustainable development indicators for the mining and minerals industry. J. Clean. Prod. 2004, 12, 639–662. [Google Scholar] [CrossRef]
- Creencia, E.C.; Nillama, J.A.P.; Librando, I.L. Microwave-Assisted Extraction and Physicochemical Evaluation of Oil from Hevea brasiliensis Seeds. Resources 2018, 7, 28. [Google Scholar] [CrossRef]
- Gaglio, M.; Tamburini, E.; Lucchesi, F.; Aschonitis, V.; Atti, A.; Castaldelli, G.; Fano, E.A. Life Cycle Assessment of Maize-Germ Oil Production and the Use of Bioenergy to Mitigate Environmental Impacts: A Gate-To-Gate Case Study. Resources 2019, 8, 60. [Google Scholar] [CrossRef]
- Constable, D.J.C.; Curzons, A.D.; Freitas dos Santos, L.M.; Geen, G.R.; Kitteringham, J.; Smith, P.; Hannah, R.E.; McGuire, M.A.; Webb, R.L.; Yu, M.; et al. Green chemistry measures for process research and development. Green Chem. 2001, 3, 7–9. [Google Scholar] [CrossRef]
- Schmidt-Bleek, F. Material Flows from a Systematical Point of View. Fresenius Environ. Bull. 1993, 2, 413–418. [Google Scholar]
- Henderson, R.K.; Jiménez-González, C.; Constable, D.J.C.; Alston, S.R.; Inglis, G.G.A.; Fisher, G.; Sherwood, J.; Binks, S.P.; Curzons, A.D. Expanding GSK’s solvent selection guide—Embedding sustainability into solvent selection starting at medicinal chemistry. Green Chem. 2011, 13, 854–862. [Google Scholar] [CrossRef]
- Prat, D.; Pardigon, O.; Flemming, H.-W.; Letestu, S.; Ducandas, V.; Isnard, P.; Guntrum, E.; Senac, T.; Ruisseau, S.; Cruciani, P.; et al. Sanofi’s Solvent Selection Guide: A Step Toward More Sustainable Processes. Org. Process Res. Dev. 2013, 17, 1517–1525. [Google Scholar] [CrossRef]
- Jiménez-González, C.; Curzons, A.D.; Constable, D.J.C.; Cunningham, V.L. Expanding GSK’s Solvent Selection Guide—Application of life cycle assessment to enhance solvent selections. Clean Prod. Process. 2004, 7, 42–50. [Google Scholar]
- Alfonsi, K.; Colberg, J.; Dunn, P.J.; Fevig, T.; Jennings, S.; Johnson, T.A.; Kleine, H.P.; Knight, C.; Nagy, M.A.; Perry, D.A.; et al. Green chemistry tools to influence a medicinal chemistry and research chemistry based organisation. Green Chem. 2008, 10, 31–36. [Google Scholar] [CrossRef]
- Jiménez-González, C.; Ponder, C.S.; Broxterman, Q.; Manley, J. Using the Right Green Yardstick: Why Process Mass Intensity Is Used in the Pharmaceutical Industry to Drive More Sustainable Processes. Org. Process Res. Dev. 2011, 15, 912–917. [Google Scholar] [CrossRef]
- Velasco, M.I.; Kinen, C.O.; de Rossi, R.H.; Rossi, L.I. A green alternative to synthetize azo compounds. Dyes Pigment. 2011, 90, 259–264. [Google Scholar] [CrossRef]
- Jiménez-González, C.; Curzons, A.D.; Constable, D.J.C.; Overcash, M.R.; Cunningham, V.L. How do you select the “greenest” technology? Development of guidance for the pharmaceutical industry. Clean Prod. Process. 2001, 3, 35–41. [Google Scholar] [CrossRef]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Hurme, M.; Rahman, M. Implementing inherent safety throughout process lifecycle. J. Loss Prev. Process Ind. 2005, 18, 238–244. [Google Scholar] [CrossRef]
- Marshall, J.T.; Mundt, A. Dow’s Chemical Exposure Index Guide. Process Saf. Prog. 1995, 14, 163–170. [Google Scholar] [CrossRef]
- AIChE. Dow’s Fire and Explosion Index Hazard Classification Guide; John Wiley & Sons Inc.: Hoboken, NJ, USA, 1994. [Google Scholar]
- Stoessel, F. What is Your Thermal Risk? Chem. Eng. Prog. 1993, 89, 68–75. [Google Scholar]
- Edwards, D.W.; Lawrence, D. Assessing the inherent safety of chemical process routes: Is there a relation between plant costs and inherent safety? Process Saf. Environ. Prot. 1993, 71, 252–258. [Google Scholar]
- Heikkila, A.-M.; Hurme, M.; Jarvelainen, M. Safety Considerations in Process Synthesis. Comput. Chem. Eng. 1996, 20, 115–120. [Google Scholar] [CrossRef]
- Schaltegger, S.; Burritt, R. Contemporary Environmental Accounting; Greenleaf Publishing: Austin, TX, USA, 2000; pp. 1–463. [Google Scholar]
- OECD. Guidance Document for the Development of OECD Guidelines for the Testing of Chemicals; Organisation for Economic Co-operation and Development: Paris, France, 2009; pp. 1–41. [Google Scholar]
- Cronin, M.T.D.; Livingstone, D.J. Predicting Chemical Toxicity and Fate; CRC Press: Washington, DC, USA, 2004; pp. 1–441. [Google Scholar]
- Herrchen, M.; Klein, W. Use of the life-cycle assessment (LCA) toolbox for an environmental evaluation of production processes. Pure Appl. Chem. 2000, 72, 1247–1252. [Google Scholar] [CrossRef]
- Tukker, A. Life Cycle Assessment and the Precautionary Principle. Environ. Sci. Technol. 2000, 36, 70A–75A. [Google Scholar] [CrossRef][Green Version]
- Curzons, A.D.; Jiménez-González, C.; Duncan, A.L.; Constable, D.J.C.; Cunningham, V.L. Fast life cycle assessment of synthetic chemistry (FLASC™) tool. Int. J. Life Cycle. Assess. 2007, 12, 272–280. [Google Scholar] [CrossRef]
- Friedrichs, K. Energy Key Performance Indicators: A European Benchmark and Assessment of Meaningful Indicators for the Use of Energy in Large Corporations. Master’s Thesis, Norges Handelshøyskole (NHH), Bergen, Norway, 2013. [Google Scholar]
- Defra Environmental Key Performance Indicators: Reporting Guidelines for UK Business; Department for Environment, Food & Rural Affairs: London, UK, 2017; pp. 1–76.
- Gensan, E.; Paturi, R. Key Performance Indicators Framework—A Method to Track Business Objectives, Link Business Strategy to Processes and Detail Importance of Key Performance Indicators in Enterprise Business Architecture. In Proceedings of the 15th Americas Conference on Information Systems, San Francisco, CA, USA, 6–9 August 2009; pp. 1–11. [Google Scholar]
- Jollands, N.; Lermit, J.; Patterson, M. The Usefulness of Aggregate Indicators in Policy Making and Evaluation: A Discussion with Application to Eco-Efficiency Indicators in New Zealand; The Australian National University: Canberra, Australia, 2003. [Google Scholar]
- Saisana, M.; Tarantola, S. State-of-the-Art Report on Current Methodologies and Practices for Composite Indicator Development; European Commission-JRC: Ispra, Italy, 2002. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fadel, C.; Tarabieh, K. Development of an Industrial Environmental Index to Assess the Sustainability of Industrial Solvent-Based Processes. Resources 2019, 8, 115. https://doi.org/10.3390/resources8020115
Fadel C, Tarabieh K. Development of an Industrial Environmental Index to Assess the Sustainability of Industrial Solvent-Based Processes. Resources. 2019; 8(2):115. https://doi.org/10.3390/resources8020115
Chicago/Turabian StyleFadel, Chris, and Khaled Tarabieh. 2019. "Development of an Industrial Environmental Index to Assess the Sustainability of Industrial Solvent-Based Processes" Resources 8, no. 2: 115. https://doi.org/10.3390/resources8020115
APA StyleFadel, C., & Tarabieh, K. (2019). Development of an Industrial Environmental Index to Assess the Sustainability of Industrial Solvent-Based Processes. Resources, 8(2), 115. https://doi.org/10.3390/resources8020115





