Geochemistry of Flood Waters from the Tar River, North Carolina Associated with Hurricane Matthew
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Location
2.2. Sampling Protocol
2.3. Nutrient and E. coli Analyses
2.4. Physiochemical Characterization of River Water
2.5. Statistical Analyses
3. Results and Discussion
3.1. Nutrient and E. coli Concentrations
3.2. Nitrogen Dynamics
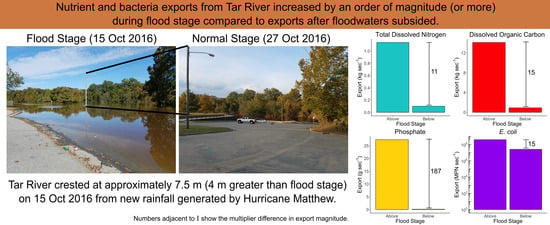
3.3. Nutrient and E. coli Exports
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hathaway, J.M.; Hunt, W.F.; Simmons III, O.D. Statistical evaluation of factors affecting indicator bacteria in urban storm-water runoff. J. Environ. Eng. 2010, 136, 1360–1368. [Google Scholar] [CrossRef]
- Janke, B.D.; Finlay, J.C.; Hobbie, S.E.; Baker, L.A.; Sterner, R.W.; Nidzgorski, D.; Wilson, B.N. Contrasting influences of stormflow and baseflow pathways on nitrogen and phosphorus export from an urban watershed. Biogeochemistry 2014, 121, 209–228. [Google Scholar] [CrossRef]
- Humphrey, C.; Anderson-Evans, E.; O’Driscoll, M.; Manda, A.; Iverson, G. Comparison of Phosphorus Concentrations in Coastal Plain Watersheds Served by Onsite Wastewater Treatment Systems and a Municipal Sewer Treatment System. Water Air Soil Pollut. 2015, 226, 1–18. [Google Scholar] [CrossRef]
- Iverson, G.; Humphrey, C.; Postma, M.H.; O’Driscoll, M.A.; Manda, A.K.; Finley, A. Influence of sewered versus septic systems on watershed exports of E. coli. Water Air Soil Pollut. 2017, 228, 237. [Google Scholar] [CrossRef]
- Osmond, D.L.; Neas, K.; Johnson, A.M.; Cahill, S.L. Fertilizer use in regulated river Basins: Is it what we think? J. Contemp. Water Res. Educ. 2013, 151, 20–26. [Google Scholar] [CrossRef]
- Mallin, M.A.; Cahoon, L.B.; Toothman, B.R.; Parsons, D.C.; McIver, M.R.; Ortwine, M.L.; Harrington, R.N. Impacts of a raw sewage spill on water and sediment quality in an urbanized estuary. Mar. Pollut. Bull. 2007, 54, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Pachepsky, Y.A.; Shelton, D.R. Escherichia coli and Fecal Coliforms in Freshwater and Estuarine Sediments. Crit. Rev. Environ. Sci. Technol. 2011, 41, 1067–1110. [Google Scholar] [CrossRef]
- O’Driscoll, M.A.; Humphrey, C.P., Jr.; Deal, N.E.; Lindbo, D.L.; Zarate-Bermudez, M.A. Meteorological influences on nitrogen dynamics of a coastal onsite wastewater treatment system. J. Environ. Qual. 2014, 43, 1873–1885. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, C.P.; Finley, A.J.; O’Driscoll, M.A.; Manda, A.; Iverson, G. Groundwater and stream E. coli concentrations in coastal plain watersheds served by onsite wastewater and a municipal sewer treatment system. Water Sci. Technol. 2015, 72, 1851–1860. [Google Scholar] [CrossRef] [PubMed]
- Cahoon, L.B.; Hales, J.C.; Carey, E.S.; Loucaides, S.; Rowland, K.R.; Toothman, B.R. Multiple modes of water quality impairment by fecal contamination in a rapidly developing coastal area: Southwest Brunswick County, North Carolina. Environ. Monit. Assess. 2016, 188, 89. [Google Scholar] [CrossRef] [PubMed]
- Iverson, G.; Humphrey, C.P., Jr.; O’Driscoll, M.A.; Sanderford, C.; Jernigan, J.; Serozi, B. Nutrient exports from watersheds with varying septic system densities in the North Carolina Piedmont. J. Environ. Manag. 2018, 211, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, C.; Sanderford, C.; Iverson, G. Concentrations and Exports of Fecal Indicator Bacteria in Watersheds with Varying Densities of Onsite Wastewater Systems. Water Air Soil Pollut. 2018, 229, 277. [Google Scholar] [CrossRef]
- Liwimbi, L.; Graves, A.K.; Israel, D.W.; van Heugten, E.; Robinson, B.; Cahoon, C.W.; Lubbers, J.F. Microbial source tracking in a watershed dominated by swine. Water 2010, 2, 587–604. [Google Scholar] [CrossRef]
- Jokinen, C.C.; Edge, T.A.; Koning, W.; Laing, C.R.; Lapen, D.R.; Miller, J. Spatial and temporal drivers of zoonotic pathogen contamination of an agricultural watershed. J. Environ. Qual. 2012, 41, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Ratnapradipa, D.; Cardinal, C.; Ratnapradipa, K.L.; Scarbrough, A.; Xie, Y. Implications of Hurricane Harvey on public health in Harris County, Texas. J. Environ. Health 2018, 81, 24–32. [Google Scholar]
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Pachauri, R.K., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Paerl, H.W.; Crosswell, J.R.; Van Dam, B.; Hall, N.S.; Rossignol, K.L.; Osburn, C.L.; Hounshell, A.G.; Sloup, R.S.; Harding, L.W., Jr. Two decades of tropical cyclone impacts on North Carolina’s estuarine carbon, nutrient and phytoplankton dynamics: Implications for biogeochemical cycling and water quality in a stormier world. Biogeochemistry 2018, 141, 307–332. [Google Scholar] [CrossRef]
- United States Geological Survey. National Water Information System: Web Interface. USGS 02084000 Tar River at Greenville, NC. 2019. Available online: https://waterdata.usgs.gov/nc/nwis/uv?site_no=02084000 (accessed on 10 January 2019).
- United States Environmental Protection Agency. National Summary of State Information. USEPA, 2017. Available online: https://ofmpub.epa.gov/waters10/attains_nation_cy.control (accessed on 15 December 2017).
- North Carolina Department of Environmental Quality. Nutrient Sensitive Waters and Special Watersheds. 2018. Available online: https://deq.nc.gov/about/divisions/energy-mineral-land-resources/nsw-special-watersheds (accessed on 8 December 2018).
- United States Environmental Protection Agency. Ambient Water Quality Criteria Recommendations. Information Supporting the Development of State and Tribal Nutrient Criteria for Rivers and Streams in Nutrient Ecoregion IX; Report EPA-822-B-00-022; Environmental Protection Agency, Office of Water, Science and Technology, Health and Ecological Criteria Division: Washington, DC, USA, 2000.
- United States Environmental Protection Agency. Recreational Water Quality Criteria; EPA-820-F-12-061; Office of Water: Washington, DC, USA, 2012.
- United States Climate Data. Climate Greenville, NC. Available online: https://www.usclimatedata.com/climate/greenville/north-carolina/united-states/usnc0281/2018/1 (accessed on 8 December 2018).
- North Carolina Division of Water Resources. Tar-Pamlico River Basin Water Resources Plan. 2014. Available online: https://www.ncwater.org/basins/Tar-Pamlico/index.php (accessed on 5 December 2018).
- Harden, S.L. Surface-Water Quality in Agricultural Watersheds of the North Carolina Coastal Plain Associated with Concentrated Animal Feeding Operations; U.S. Geological Survey Scientific Investigations Report 2015-5080; U.S. Geological Survey Scientific: Reston, VA, USA, 2015; 55p, 7 apps. [CrossRef]
- Kendall, C.; McDonnell, J.J. Isotope Tracers in Catchment Hydrology, 1st ed.; Elsevier Science B.V.: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Silva, S.R.; Ging, P.B.; Lee, R.W.; Ebbert, J.C.; Tesoriero, A.J.; Inkpen, E.L. Forensic applications of nitrogen and oxygen isotopes of nitrate in an urban environment. Environ. Forensics 2002, 3, 125–130. [Google Scholar] [CrossRef]
- University of California, Davis. Nitrate (NO3) Analysis by Bacteria Denitrification. Stable Isotope Facility, University of California at Davis. Available online: http://stableisotopefacility.ucdavis.edu/no3.html (accessed on 10 December 2018).
- North Carolina Department of Environmental Quality. Tar-Pamlico River Basin. Ambient Data Summary Sheets. 2019. Available online: https://deq.nc.gov/map-page/tar-pamlico-river-basin (accessed on 9 February 2019).
- Paerl, H.W.; Bales, J.D.; Ausley, L.W.; Buzzel, C.P.; Crowder, L.B.; Eby, L.A.; Fear, J.M.; Go, M.; Peierls, B.L.; Richardson, T.L.; et al. Ecosystem impacts of three sequential hurricanes (Dennis, Floyd, and Irene) on the United States’ Largest lagoonal estuary, Pamlico Sound, NC. Proc. Natl. Acad. Sci. USA 2001, 98, 5655–5660. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Valdes, L.M.; Joyner, A.R.; Peierls, B.L.; Piehler, M.F.; Riggs, S.R.; Christian, R.R.; Eby, L.A.; Crowder, L.B.; Ramus, J.S.; et al. Ecological response to hurricane events in the Pamlico Sound system, North Carolina, and implications for assessment and management in a regime of increased frequency. Estuar. Coasts 2006, 29, 1033–1045. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Inamadar, S. Extreme storms and changes in particulate and dissolved organic carbon in runoff: Entering uncharted waters? Geophys. Rese. Lett. 2013, 40, 1322–1327. [Google Scholar] [CrossRef] [Green Version]
- Del Rosario, K.L.; Humphrey, C.P.; Mitra, S.; O’Driscoll, M. Nitrogen and carbon dynamics beneath on-site wastewater treatment systems in Pitt County, North Carolina. Water Sci. Technol. 2014, 69, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Hathaway, J.M.; Tucker, R.S.; Spooner, J.M.; Hunt, W.F. A traditional analysis of the first flush effect for nutrients in stormwater runoff from two small urban catchments. Water Air Soil Pollut. 2012, 223, 5903–5915. [Google Scholar] [CrossRef]
- Mitsch, W.J.; Gosselink, J.G. Wetlands, 3rd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2000; 172p. [Google Scholar]
- Humphrey, C.; Iverson, G.; O’Driscoll, M. Nitrogen Treatment Efficiency of a Large Onsite Wastewater System in Relation to Water Table Dynamics. Clean-Soil Air Water 2017, 45, 1700551. [Google Scholar] [CrossRef]
- Wallace, J.; Stewart, L.; Hwwdon, A.; Keen, R.; Karim, F.; Kemei, J. Flood water quality and marine sediment and nutrient loads from the Tully and Murray catchments in north Queensland, Australia. Mar. Freshw. Res. 2009, 60, 1123–1131. [Google Scholar] [CrossRef] [Green Version]
- Harris, C.W.; Rees, G.N.; Stoffels, R.J.; Pengelly, J.; Barlow, K.; Silvester, E. Longitudinal trends in concentration and composition of dissolved organic nitrogen (DON) in a largely unregulated river system. Biogoechemistry 2018, 139, 139–153. [Google Scholar] [CrossRef]
- Wiegner, T.N.; Seitzinger, P. Photochemical and microbial degradation of external dissolved organic matter in- puts to rivers. Aquat. Microb. Ecol. 2001, 24, 27–40. [Google Scholar] [CrossRef]
- Seitzinger, S.P.; Sanders, R.W.; Styles, R. Bioavailability of DON from Natural and Anthropogenic Sources to Estuarine Plankton. Limnol. Oceanogr. 2002, 47, 353–366. [Google Scholar] [CrossRef]
- Lebo, M.E.; Paerl, H.W.; Peierls, B.L. Evaluation of progress in achieving the TMDL mandated nitrogen reductions in the Neuse River Basin, North Carolina. Environ. Manag. 2012, 49, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Christian, R.C.; Thomas, C.R. Network analysis of nitrogen inputs and cycling in the Neuse River Estuary, North Carolina, USA. Estuaries 2003, 26, 815–828. [Google Scholar] [CrossRef]
- Siders, A.R. Social justice implications of US managed retreat buyout programs. Clim. Chang. 2019, 152, 239–257. [Google Scholar] [CrossRef]
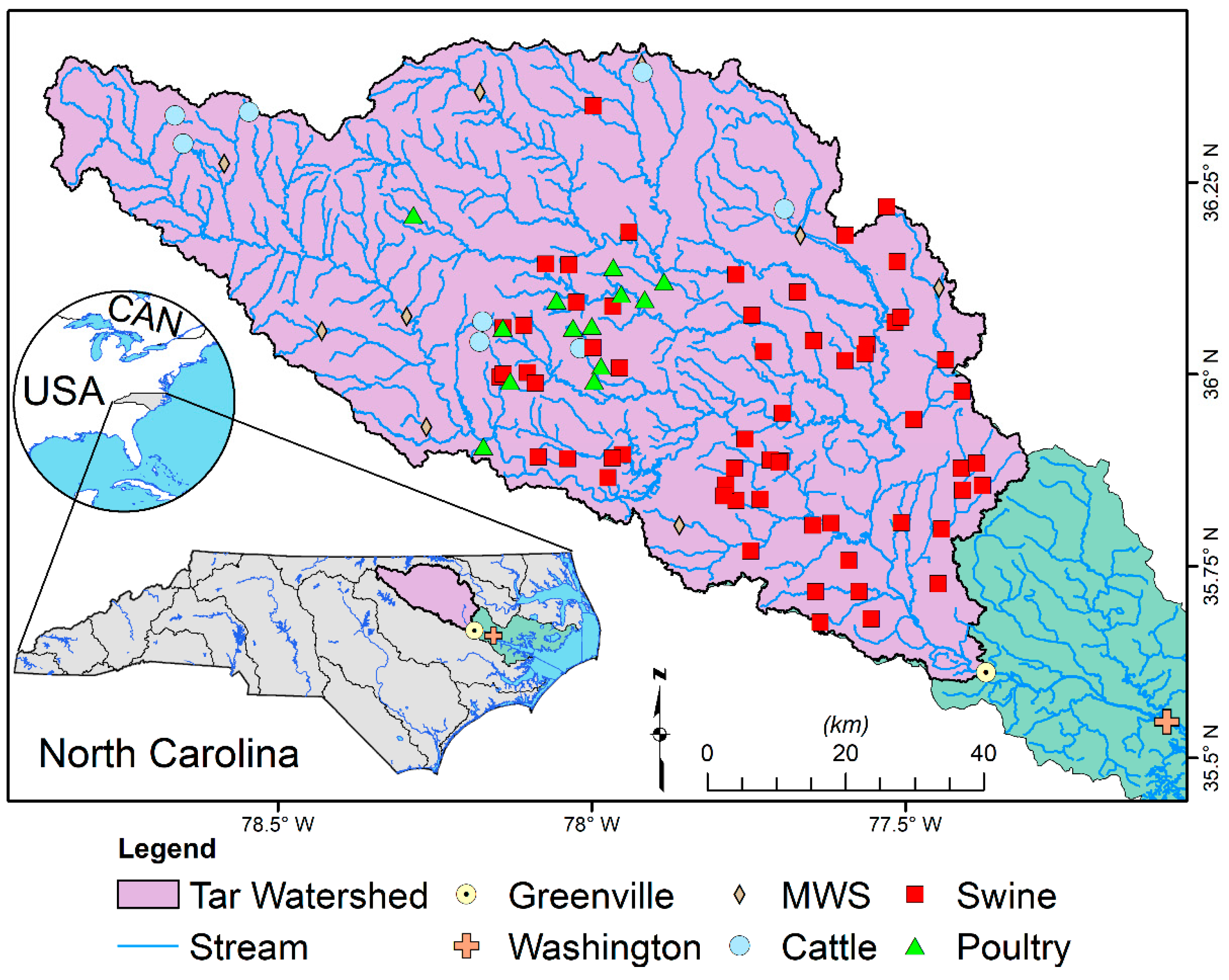
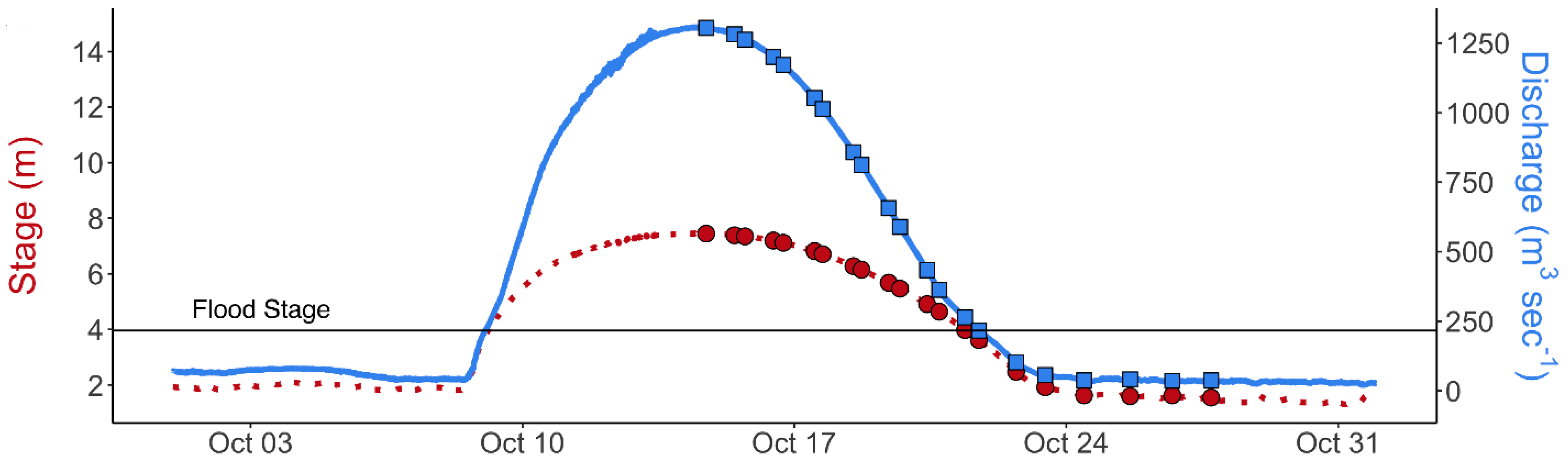
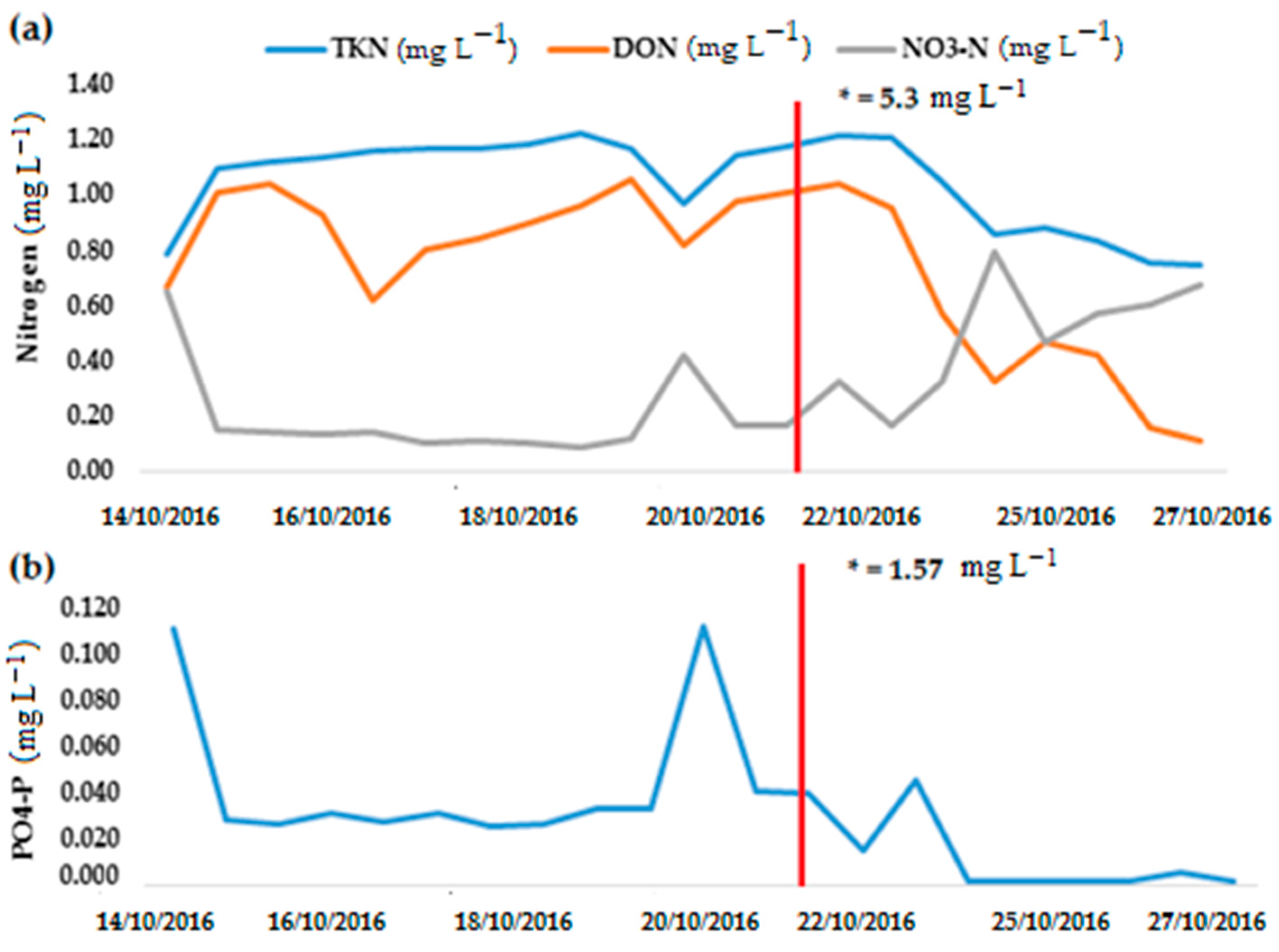

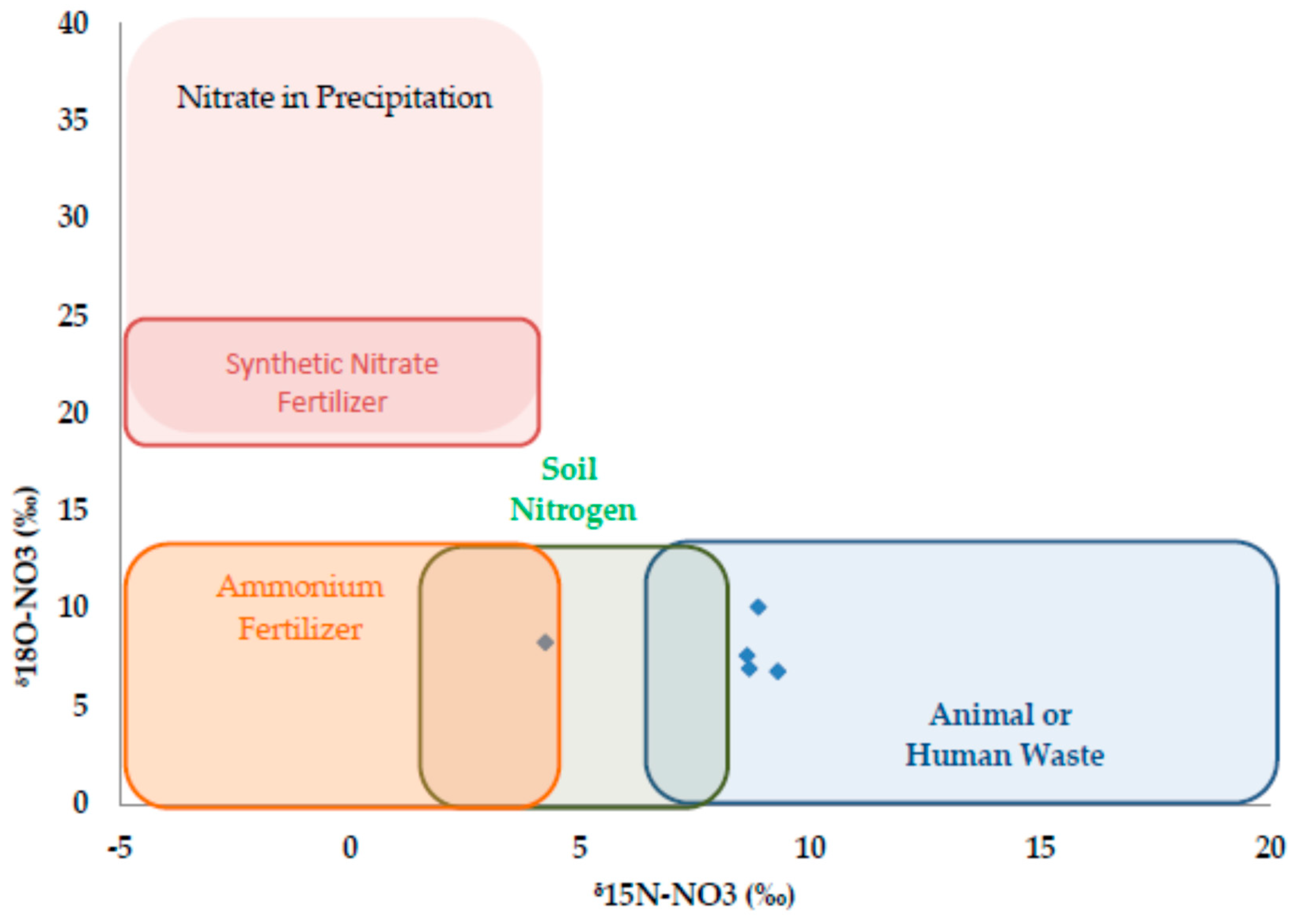
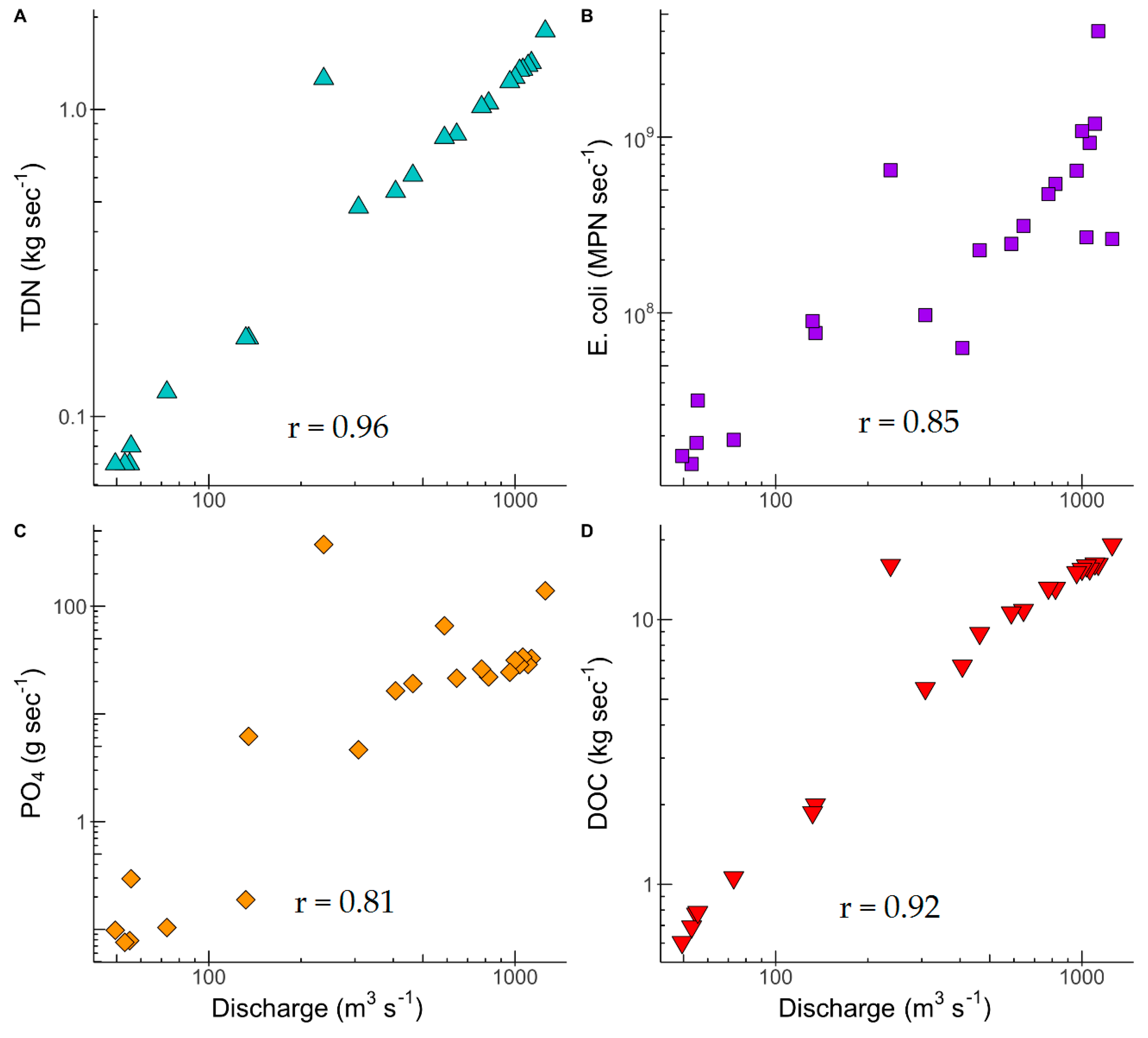
| Sampling Period | Temp (°C) | SC (µS cm−1) | DO (mg L−1) | pH | E. coli (MPN 100 mL−1) | TKN (mg L−1) |
| Above FS | 19.4 (18.4–20.6) | 41.0 (36.0–83.0) | 4.9 (3.3–6.7) | 6.7 (6.1–8.0) | 55 (16–354) | 1.16 (0.79–1.22) |
| Below FS | 18.2 (17.0–20.6) | 90.0 (57.0–105.0) | 7.5 (6.5–8.2) | 6.7 (6.4–7.3) | 45 (26–273) | 0.86 (0.75–5.14) |
| Sampling Period | DON (mg L−1) | NH4-N (mg L−1) | NO3-N (mg L−1) | TDN (mg L−1) | DOC (mg L−1) | PO4-P (mg L−1) |
| Above FS | 0.95 (0.62–1.06) | 0.18 (0.08–0.54) | 0.14 (0.08–0.65) | 1.29 (1.25–1.54) | 15.87 (14.32–17.84) | 0.032 (0.026–0.111) |
| Below FS | 0.44 (0.11–2.08) | 0.51 (0.25–3.06) | 0.52 (0.16–0.79) | 1.39 (1.35–5.30) | 14.13 (12.21–67.67) | 0.002 (0.001–1.577) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Humphrey, C., Jr.; Iverson, G.; Skibiel, C.; Sanderford, C.; Blackmon, J. Geochemistry of Flood Waters from the Tar River, North Carolina Associated with Hurricane Matthew. Resources 2019, 8, 48. https://doi.org/10.3390/resources8010048
Humphrey C Jr., Iverson G, Skibiel C, Sanderford C, Blackmon J. Geochemistry of Flood Waters from the Tar River, North Carolina Associated with Hurricane Matthew. Resources. 2019; 8(1):48. https://doi.org/10.3390/resources8010048
Chicago/Turabian StyleHumphrey, Charles, Jr., Guy Iverson, Caitlin Skibiel, Christa Sanderford, and Jamil Blackmon. 2019. "Geochemistry of Flood Waters from the Tar River, North Carolina Associated with Hurricane Matthew" Resources 8, no. 1: 48. https://doi.org/10.3390/resources8010048
APA StyleHumphrey, C., Jr., Iverson, G., Skibiel, C., Sanderford, C., & Blackmon, J. (2019). Geochemistry of Flood Waters from the Tar River, North Carolina Associated with Hurricane Matthew. Resources, 8(1), 48. https://doi.org/10.3390/resources8010048