Simulations and Laboratory Tests for Assessing Phosphorus Recovery Efficiency from Sewage Sludge
Abstract
1. Introduction
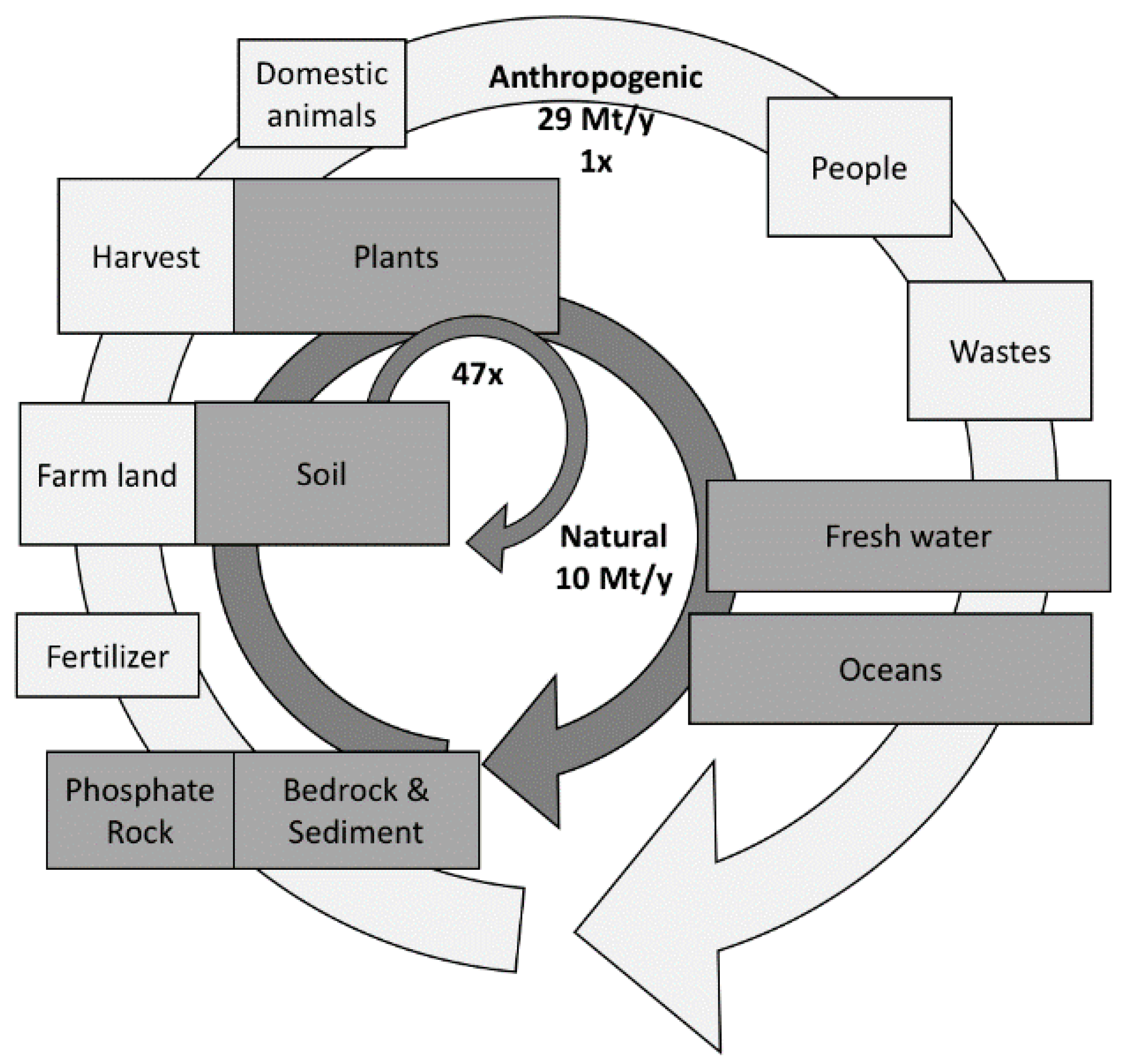
2. The Phosphorus Resources Issue
Phosphorous Waste Reduction and Recovery
3. Phosphorus Recovery Technologies
Struvite Crystallization
4. Materials and Methods
4.1. Model Description
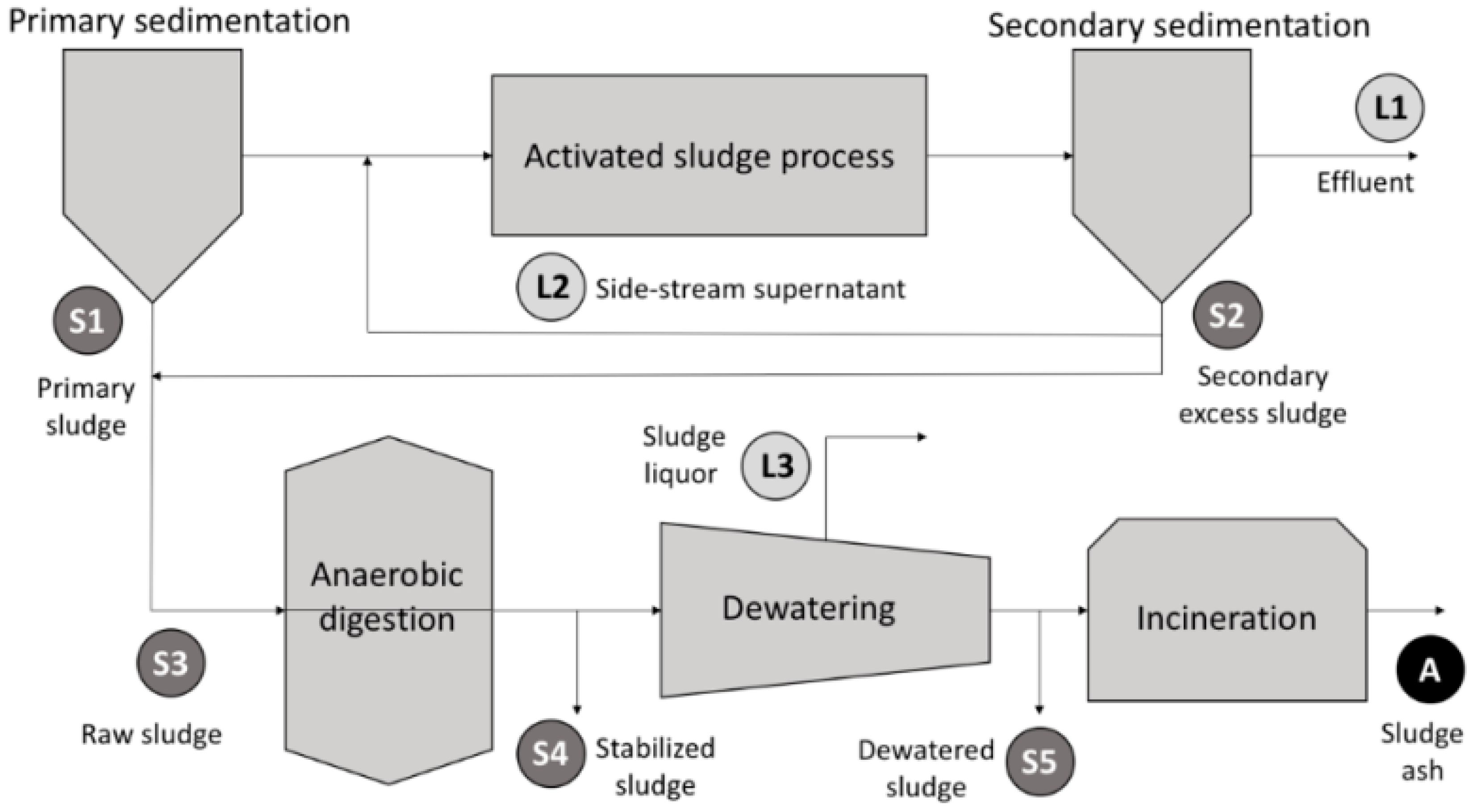
4.2. Experimental Setup
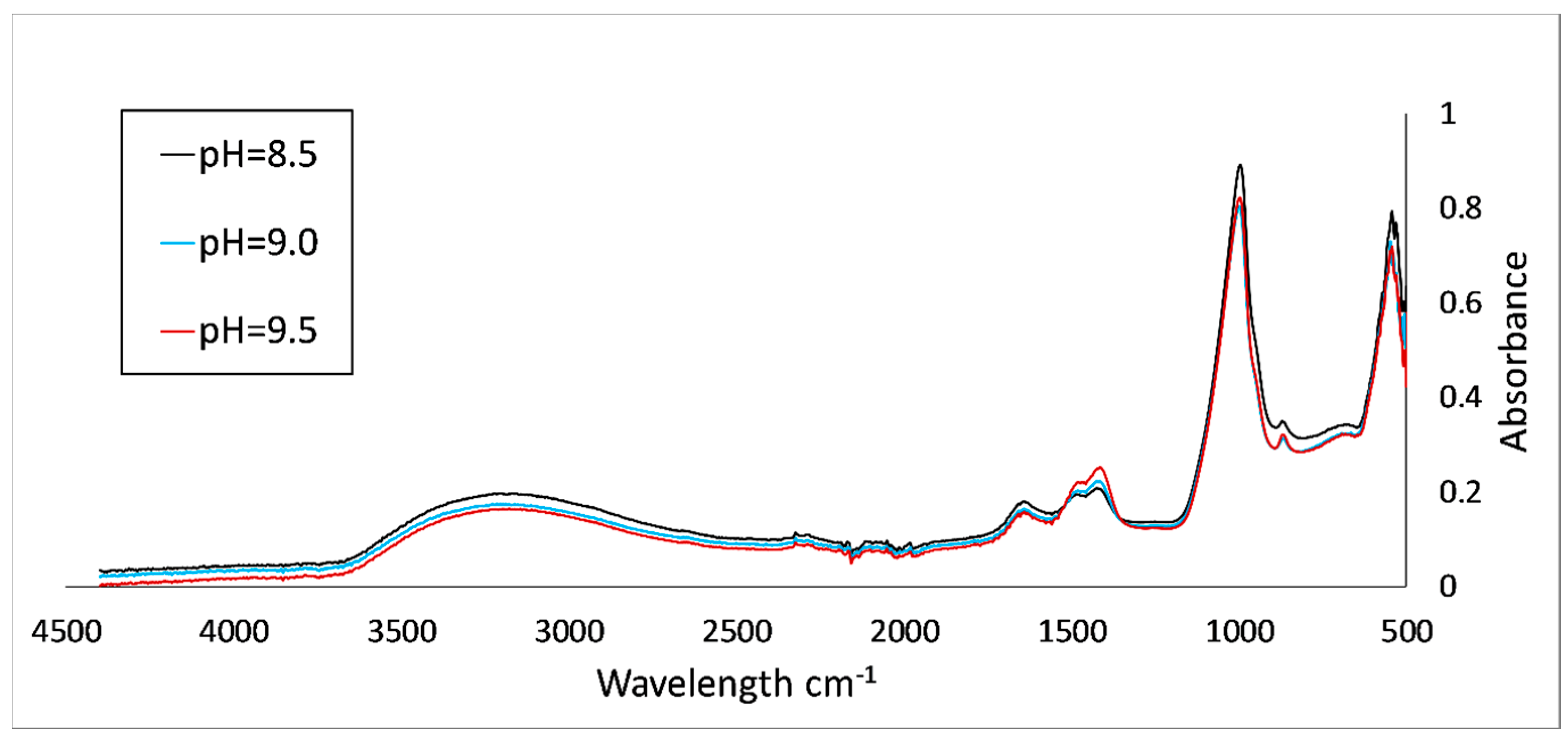
5. Results and Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Callegari, A.; Boguniewicz-Zablocka, J.; Capodaglio, A.G. Experimental Application of an Advanced Separation Process for NOM Removal from Surface Drinking Water Supply. Separations 2017, 4, 32. [Google Scholar] [CrossRef]
- Søndergaard, M.; Jensen, J.P.; Jeppesen, E. Role of sediment and internal loading of phosphorus in shallow lakes. Hydrobiologia 2003, 506–509, 135–145. [Google Scholar] [CrossRef]
- Copetti, D.; Valsecchi, L.; Capodaglio, A.G.; Tartari, G. Direct measurement of nutrient concentrations in freshwaters with a miniaturized analytical probe: Evaluation and validation. Environ. Monit. Assess. 2017, 189, 144. [Google Scholar] [CrossRef] [PubMed]
- Viviano, G.; Valsecchi, S.; Polesello, S.; Capodaglio, A.G.; Tartari, G.; Salerno, F. Combined Use of Caffeine and Turbidity to Evaluate the Impact of CSOs on River Water Quality. Water Air Soil Pollut. 2017, 228, 330. [Google Scholar] [CrossRef]
- Bendoricchio, G.; Di Luzio, M.; Baschieri, P.; Capodaglio, A.G. Diffuse pollution in the Lagoon of Venice. Water Sci. Technol. 1993, 28, 69–78. [Google Scholar] [CrossRef]
- Capodaglio, A.G.; Muraca, A.; Becchi, G. Accounting for water quality effects of future urbanization: Diffuse pollution loads estimates and control in Mantua’s Lakes (Italy). Water Sci. Technol. 2003, 47, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Capodaglio, A.G.; Boguniewicz, J.; Llorens, E.; Salerno, F.; Copetti, D.; Legnani, E.; Buraschi, E.; Tartari, G. Integrated lake/catchment approach as a basis for the implementation of the WFD in the Lake Pusiano watershed. In River Basin Management—Progress towards Implementation of the European Water Framework Directive; CRC Press: Boca Raton, FL, USA, 2005; pp. 77–86. [Google Scholar]
- Copetti, D.; Marziali, L.; Viviano, G.; Valsecchi, L.; Guzzella, L.; Capodaglio, A.G.; Tartari, G.; Polesello, S.; Valsecchi, S.; Mezzanotte, V.; et al. Intensive monitoring of conventional and surrogate quality parameters in a highly urbanized river affected by multiple combined sewer overflows. Water Sci. Technol. Water Supply 2018, in press. [Google Scholar]
- Capodaglio, A.G.; Hlavínek, P.; Raboni, M. Physico-chemical technologies for nitrogen removal from wastewaters: A review. Revista Ambiente Agua 2015, 10, 481–498. [Google Scholar] [CrossRef]
- Capodaglio, A.G.; Hlavínek, P.; Raboni, M. Advances in wastewater nitrogen removal by biological processes: State of the art review. Revista Ambiente Água 2016, 11, 250–267. [Google Scholar] [CrossRef]
- Capodaglio, A.G.; Ghilardi, P.; Boguniewicz-Zablocka, J. New paradigms in urban water management for conservation and sustainability. Water Pract. Technol. 2016, 11, 176–186. [Google Scholar] [CrossRef]
- Capodaglio, A.G. Integrated, Decentralized Wastewater Management for Resource Recovery in Rural and Peri-Urban Areas. Resources 2017, 6, 22. [Google Scholar] [CrossRef]
- Capodaglio, A.G.; Callegari, A.; Cecconet, D.; Molognoni, D. Sustainability of decentralized wastewater treatment technologies. Water Pract. Technol. 2017, 12, 463–477. [Google Scholar] [CrossRef]
- Daneshgar, S.; Callegari, A.; Capodaglio, A.G.; Vaccari, D. The Potential Phosphorus Crisis: Resource Conservation and Possible Escape Technologies: A Review. Resources 2018, 7, 37. [Google Scholar] [CrossRef]
- Van Kauwenbergh, S.J. World Phosphate Rock Reserves and Resources; International Fertilizer Development Center: Muscle Shoals, AL, USA, 2010; ISBN 978-9-88999-167-3. [Google Scholar]
- Jasinski, S.M. Phosphate Rock, USGS Mineral Commodities Summary. Available online: http://minerals.usgs.gov/minerals/pubs/commodity/phosphate_rock/ (accessed on 25 March 2016).
- IFA. International Fertilizer Association Production and International Trade Report. 2016. Available online: https://fertilizer.org/Statistics (accessed on 18 April 2018).
- Cooper, J.; Lombardi, R.; Boardman, D.; Carliell-Marquet, C. The future distribution and production of global phosphate rock reserves. Resour. Conserv. Recycl. 2011, 57, 78–86. [Google Scholar] [CrossRef]
- Smil, V. Phosphorus: Global Transfers, Causes and consequences of global environmental change. In Encyclopedia of Global Environmental Change; Douglas, I., Munn, T., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2002; Volume 3, pp. 536–542. ISBN 0-471-97796-9. [Google Scholar]
- Yang, L.; Zhou, H.; Moccia, R. Membrane Filtration Coupled with Chemical Precipitation to Treat Recirculating Aquaculture System Effluent. J. Environ. Qual. 2006, 35, 2419–2424. [Google Scholar] [CrossRef] [PubMed]
- Cornel, P.; Schaum, C. Phosphorus recovery from wastewater: Needs, techniques and costs. Water Sci. Technol. 2009, 59, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Schoumans, O.F.; Rulkens, W.H.; Oenema, O.; Ehlert, P.A.I. Phosphorus Recovery from Animal Manure: Technical Opportunities and Agro-Economical Perspectives; Alterra Report No. 2158; Alterra: Denver, CO, USA, 2010. [Google Scholar]
- Desmidt, E.; Ghyselbrecht, K.; Zhang, Y.; Pinoy, P.; Van der Bruggen, B.; Verstraete, W.; Rabaey, K.; Meesschaert, B. Global Phosphorus Scarcity and Full-Scale P-Recovery Techniques: A Review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 336–384. [Google Scholar] [CrossRef]
- Donatello, S.; Cheeseman, C.R. Recycling and recovery routes for Incinerated Sewage Sludge Ash (ISSA): A review. Waste Manag. 2013, 33, 2328–2340. [Google Scholar] [CrossRef] [PubMed]
- Romero-Güiza, M.S.; Astals, S.; Mata-Alvarez, J.; Chimenos, J.M. Feasibility of coupling anaerobic digestion and struvite precipitation in the same reactor: Evaluation of different magnesium sources. Chem. Eng. J. 2015, 270, 542–548. [Google Scholar] [CrossRef]
- Jaffer, Y.; Clark, T.A.; Pearce, P.; Parsons, S.A. Potential phosphorus recovery by struvite formation. Water Res. 2002, 36, 1834–1842. [Google Scholar] [CrossRef]
- Gell, K.; Ruijter, F.J.; Kuntke, P.; Graaff, M.; Smit, A.L. Saftey and effectiveness of struvite from black water and urine as phosphorus fertilizer. J. Agric. Sci. 2011, 3, 67. [Google Scholar]
- Ronteltap, M.; Maurer, M.; Gujer, W. The behaviour of pharmaceuticals and heavy metals during struvite precipitation in urine. Water Res. 2007, 41, 1859–1868. [Google Scholar] [CrossRef] [PubMed]
- Winker, M.; Vinnerås, B.; Muskolus, A.; Arnold, U.; Clemens, J. Fertiliser products from new sanitation systems: Their potential values and risks. Bioresour. Technol. 2009, 100, 4090–4096. [Google Scholar] [CrossRef] [PubMed]
- El Hamouri, B. Rethinking natural, extensive systems for tertiary treatment purposes: The high-rate algae pond as an example. Desalination Water Treat. 2009, 4, 128–134. [Google Scholar] [CrossRef]
- Tarayre, C.; De, C.L.; Charlier, R.; Michels, E.; Meers, E.; Camargo-Valero, M.; Delvigne, F. New perspectives for the design of sustainable bioprocesses for phosphorus recovery from waste. Bioresour. Technol. 2016, 206, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Dai, H.; Wu, Y.; Peng, Y.; Lu, X. A comprehensive review of the available media and approaches for phosphorus recovery from wastewater. Water Air Soil Pollut. 2018, 229, 115. [Google Scholar] [CrossRef]
- Qiu, G.; Ting, Y.P. Direct phosphorus recovery from municipal wastewater via osmotic membrane bioreactor (OMBR) for wastewater treatment. Bioresour. Technol. 2014, 170, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chang, W.C.; Tang, C.Y. Osmotic membrane bioreactor (OMBR) technology for wastewater treatment and reclamation: Advances, challenges and prospects for the future. J. Membr. Sci. 2016, 504, 113–132. [Google Scholar] [CrossRef]
- Borgerding, J. Phosphate Deposits in Digestion Systems. J. Water Pollut. Control Fed. 1972, 44, 813–819. [Google Scholar] [CrossRef]
- Doyle, J.D.; Parsons, S.A. Struvite formation, control and recovery. Water Res. 2002, 36, 3925–3940. [Google Scholar] [CrossRef]
- Hao, X.D.; Wang, C.C.; Lan, L.; Van Loosdrecht, M.C.M. Struvite formation, analytical methods and effects of pH and Ca2+. Water Sci. Technol. 2008, 58, 1687–1692. [Google Scholar] [CrossRef] [PubMed]
- Galbraith, S.C.; Schneider, P.A.; Flood, A.E. Model-driven experimental evaluation of struvite nucleation, growth and aggregation kinetics. Water Res. 2014, 56, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Parkhurst, D.L.; Appelo, C.A.J. Description of Input and Examples for PHREEQC Version 3—A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations; U.S. Geological Survey Techniques and Methods, Book 6, No. 6–43A; U.S. Geological Survey: Reston, VA, USA, 2013; Chapter A43; 497p.
- Le Corre, K.S.; ValsamieJones, E.; Hobbs, P.; Parsons, S.A. Phosphorus recovery from wastewater by struvite crystallization: A review. Crit. Rev. Environ. Sci. Technol. 2009, 39, 433–477. [Google Scholar] [CrossRef]
- Parsons, S.A. Recent scientific and technical developments: Struvite precipitation. CEEP Scope Newsl. 2001, 41, 15–22. [Google Scholar]
- US Geological Survey. PHREEQC (Version 3)—A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations. Available online: https://wwwbrr.cr.usgs.gov/projects/GWC_coupled/phreeqc/ (accessed on 30 October 2017).
- Türker, M.; Çelen, I. Removal of ammonia as struvite from anaerobic digester effluents and recycling of magnesium and phosphate. Bioresour. Technol. 2007, 98, 1529–1534. [Google Scholar] [CrossRef] [PubMed]
- Musvoto, E.V.; Wentzel, M.C.M.; Ekama, G.A.M. Integrated chemical–physical processes modelling—II. Simulating aeration treatment of anaerobic digester supernatants. Water Res. 2000, 34, 1868–1880. [Google Scholar] [CrossRef]
- Fang, C.; Zhang, T.; Jiang, R.; Ohtake, H. Phosphate enhance recovery from wastewater by mechanism analysis and optimization of struvite settleability in fluidized bed reactor. Sci. Rep. 2016, 6, 32215. [Google Scholar] [CrossRef] [PubMed]
- Soptrajanov, B.; Stefov, V.; Lutz, H.D.; Engelen, B. Infrared and Raman Spectra of Magnesium Ammonium Phosphate Hexahydrate (struvite) and its Isomorphous Analogues. In Spectroscopy of Emerging Materials; Springer: Dordrecht, The Netherlands, 2004; pp. 299–308. [Google Scholar]

| Source | Production | Reserves | R/C | ||
|---|---|---|---|---|---|
| (Mt/year) | (%) | (Mt) | (%) | (years) | |
| Morocco & Western Sahara | 30 | 13 | 50,000 | 73 | 1667 |
| China | 100 | 45 | 3700 | 5.4 | 37 |
| United States | 25.3 | 12 | 1100 | 1.6 | 40 |
| MENA-M/WS * | 25.7 | 12 | 8166 | 12 | 318 |
| Rest of the world | 37.2 | 17 | 5810 | 8 | 156 |
| World total | 218 | 68,776 | 315 | ||
| As P ** | 28.6 | 9005 | |||
| Sludge Type | Process Name | Method | Products | Operational Scale |
|---|---|---|---|---|
| Sludge liquor | P-ROC | Adsorption | CaP, CaP on CSH | Semi-industrial |
| RECYPHOS | Adsorption | FeP | Semi-industrial | |
| PHOSIEDI | Adsorption | CaP | Lab scale | |
| PHOSTRIP | Precipitation | CaP | Full scale | |
| PRISA | Precipitation | Struvite | Semi-industrial | |
| CRYSTALACTOR | Pellets | CaP, struvite | Full scale | |
| PEARL | Pellets | Struvite | Full scale | |
| Digested sludge | BERLINER VERFAHREN | Without leaching | Struvite | Full scale |
| FIX-PHOS | Without leaching | CaP on CSH | Lab scale | |
| SEABORNE | With leaching | Struvite | Full scale | |
| STUTTGARTER VERFAHREN | With leaching | Struvite | Full scale | |
| LOPROX/PHOXAN | With leaching | Phosphoric acid | Full scale | |
| CAMBI | With leaching | FeP, AlP, CaP | Lab scale/Full scale | |
| AQUA RECI | With leaching | FeP, AlP, CaP | Lab scale/Full scale | |
| K REPO | With leaching | FeP | - | |
| SEPHOS | With leaching | AlP, CaP | Lab scale | |
| SESAL-PHOS | With leaching | CaP | Lab scale | |
| P ASCH | With leaching | Struvite | Semi-industrial | |
| BIOLEACHING | With leaching | Struvite | Lab scale | |
| BIO CON | With leaching | Phosphoric acid | Semi-industrial | |
| Sludge ash | MEPHREC | Thermal treatment | CaP | Semi-industrial |
| ASH DEC | Thermal treatment | Fertilizer | Semi-industrial | |
| THERMPHOS | Thermal treatment | Elemental phosphorus | Industrial process | |
| PHOSPHORUS INDUSTRY | Thermal treatment | Fertilizer | Industrial process |
| Ion | Concentration (mg/L) |
|---|---|
| Ca2+ | 101 |
| Mg2+ | 26.4 |
| P | 40.3 |
| NH4+ | 32.6 |
| Solid Phase | Representative Reaction | Operating Condition | pKsp at 25 °C |
|---|---|---|---|
| Struvite (A) | Mg2+ + NH4+ + PO43− + 6H2O ↔ MgNH4PO4·6H2O | 7 < pH < 11 | 13.26 |
| Newberyite (A) | Mg2+ + HPO42− + 3H2O ↔ MgHPO4·3H2O | High Mg2+/P, pH < 6 | 5.8 |
| Bobierrite (A) | 3Mg2+ + 2PO43− + 8H2O ↔ Mg3(PO4)2·8H2O | Days to precipitate | 25.2 |
| Hydroxyapatite (HAP) | 10Ca2+ + 6PO43− + 2OH− ↔ Ca10(PO4)6(OH)2 | Slow formation from ACP, DCPD | 44.3 |
| Tricalcium phosphate (TCP) | 3Ca2+ + 2PO43− ↔ Ca3(PO4)2 | Slow formation from ACP, DCPD | 32.63 |
| Octacalcium phosphate (OCP) (A) | 8Ca2+ + 2HPO42− + 4PO43− ↔ Ca8(HPO4)2(PO4)4 | Hydrolysis of DCPD at pH = 5–6 | 36.48 |
| Monetite (DCP) (A) | Ca2+ + HPO42− ↔ CaHPO4 | Fast formation from ACP, DCPD | 6.81 |
| Brushite (DCPD) | Ca2+ + HPO42− + 2H2O ↔ CaHPO4·2H2O | pH < 7 | 6.6 |
| Amorphous calcium phosphate (ACP) (A) | 3Ca2+ + 2PO43− + xH2O ↔ Ca3(PO4)2·xH2O | pH > 6 | 25.46 |
| Calcite | Ca2+ + CO32− ↔ CaCO3 | Stable at 25°C and atmospheric P | 8.42–8.22–8.48 |
| Magnesite | Mg2+ + CO32− ↔ MgCO3 | Stable at pH < 10.7 | 7.46–8.2 |
| Brucite | Mg2+ + 2OH− ↔ Mg(OH)2 | pH > 9.5 | 11.16 |
| Ca(OH)2 | Ca2+ + 2OH− ↔ Ca(OH)2 | pH > 9.5 | 5.2 |
| Run | Ca (mg/L) | Mg (mg/L) | NH4 (mg/L) | P (mg/L) | pH | P removed Predicted (%) | P removed Measured * (%) | P precip. Predicted (mg) | P precip. Measured * (mg) | SI Predicted |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 101 | 84 | 35 | 40 | 9 | 82.46 | 84.27 | 33.23 | 31.24 | 0.48 |
| 2 | 101 | 26 | 35 | 40 | 9 | 72.76 | 82.99 | 29.32 | 30.85 | 0.17 |
| 3 | 101 | 84 | 82 | 40 | 9 | 82.15 | 82.05 | 33.11 | 29.77 | 0.84 |
| 4 | 101 | 26 | 82 | 40 | 9 | 72.20 | 82.21 | 29.10 | 30.07 | 0.54 |
| 5 | 101 | 84 | 59 | 40 | 8.5 | 70.28 | 54.72 | 28.32 | 23.60 | 0.54 |
| 6 | 101 | 26 | 59 | 40 | 8.5 | 58.03 | 61.43 | 23.39 | 24.14 | 0.21 |
| 7 | 101 | 84 | 59 | 40 | 9.5 | 92.08 | 91.93 | 37.11 | 34.66 | 0.60 |
| 8 | 101 | 26 | 59 | 40 | 9.5 | 86.33 | 89.28 | 34.79 | 33.65 | 0.32 |
| 9 | 101 | 40 | 35 | 40 | 8.5 | 62.40 | 68.47 | 25.15 | 25.74 | 0.13 |
| 10 | 101 | 40 | 82 | 40 | 8.5 | 61.60 | 62.36 | 24.83 | 24.74 | 0.49 |
| 11 | 101 | 40 | 35 | 40 | 9.5 | 88.59 | 92.15 | 35.70 | 34.09 | 0.22 |
| 12 | 101 | 40 | 82 | 40 | 9.5 | 88.29 | 87.62 | 35.58 | 33.65 | 0.58 |
| 13 | 101 | 40 | 59 | 40 | 9 | 75.86 | 85.74 | 30.57 | 31.37 | 0.52 |
| 14 | 101 | 40 | 59 | 40 | 9 | 75.89 | 82.31 | 30.58 | 30.84 | 0.52 |
| 15 | 101 | 40 | 59 | 40 | 9 | 75.89 | 84.13 | 30.58 | 30.78 | 0.52 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daneshgar, S.; Buttafava, A.; Callegari, A.; Capodaglio, A.G. Simulations and Laboratory Tests for Assessing Phosphorus Recovery Efficiency from Sewage Sludge. Resources 2018, 7, 54. https://doi.org/10.3390/resources7030054
Daneshgar S, Buttafava A, Callegari A, Capodaglio AG. Simulations and Laboratory Tests for Assessing Phosphorus Recovery Efficiency from Sewage Sludge. Resources. 2018; 7(3):54. https://doi.org/10.3390/resources7030054
Chicago/Turabian StyleDaneshgar, Saba, Armando Buttafava, Arianna Callegari, and Andrea G. Capodaglio. 2018. "Simulations and Laboratory Tests for Assessing Phosphorus Recovery Efficiency from Sewage Sludge" Resources 7, no. 3: 54. https://doi.org/10.3390/resources7030054
APA StyleDaneshgar, S., Buttafava, A., Callegari, A., & Capodaglio, A. G. (2018). Simulations and Laboratory Tests for Assessing Phosphorus Recovery Efficiency from Sewage Sludge. Resources, 7(3), 54. https://doi.org/10.3390/resources7030054







