On the Extraction of Rare Earth Elements from Geothermal Brines
Abstract
:1. Introduction
- Dry steam plants use steam from geothermal wells to directly spin a turbine, which drives a generator that produces electricity. The Geysers in northern California, the world’s largest single source of geothermal power, uses steam technology.
- Flash plants bring hot water to the surface where it boils to produce steam. The hot water (above about 440 °F) “flashes” to steam when pressure is reduced in the surface facility. The steam is then sent directly to a turbine to drive the generator. The remaining liquid water is reinjected.
- Binary cycle plants use hot water to boil an organic fluid similar to the fluid used in air conditioners (a working fluid). The water is never directly in contact with the working fluid—heat is exchanged however. The expanding gas produced by boiling this working fluid is used to spin the turbine and drive the generator. All of the water used in the binary plant is injected into the subsurface, where it is naturally reheated and eventually used again.
1.1. Background
1.2. Technological Importance
1.3. Characteristics, Occurrence, Abundance, and Processing
- Wide range of coordination numbers (generally 6–12, but two, three or four are known).
- Coordination geometries are determined by ligand steric factors rather than crystal field effects.
- They form labile ‘ionic’ complexes that undergo facile exchange of ligand.
- The 4f orbits of Ln3+ ions do not participate directly in bonding. Their spectroscopic and magnetic properties are thus largely uninfluenced by the ligand.
- Small crystal field splitting and sharp electronic spectra in comparison with d-block metals.
- They prefer anionic ligands with donor atoms of high electronegativity (e.g., O, F).
- They readily form hydrated complexes.
- Insoluble hydroxides precipitate at neutral pH unless complexing agents are present.
- The chemistry is largely that of one (3+) oxidation state.
- They do not form multiple bonds (e.g., Ln=O or Ln≡N) of the type known for many transition metals and certain actinides.
- Unlike transition metals, they do not form stable carbonyls and have virtually no chemistry in the 0 oxidation state.
1.3.1. Occurrences
1.3.2. Abundance
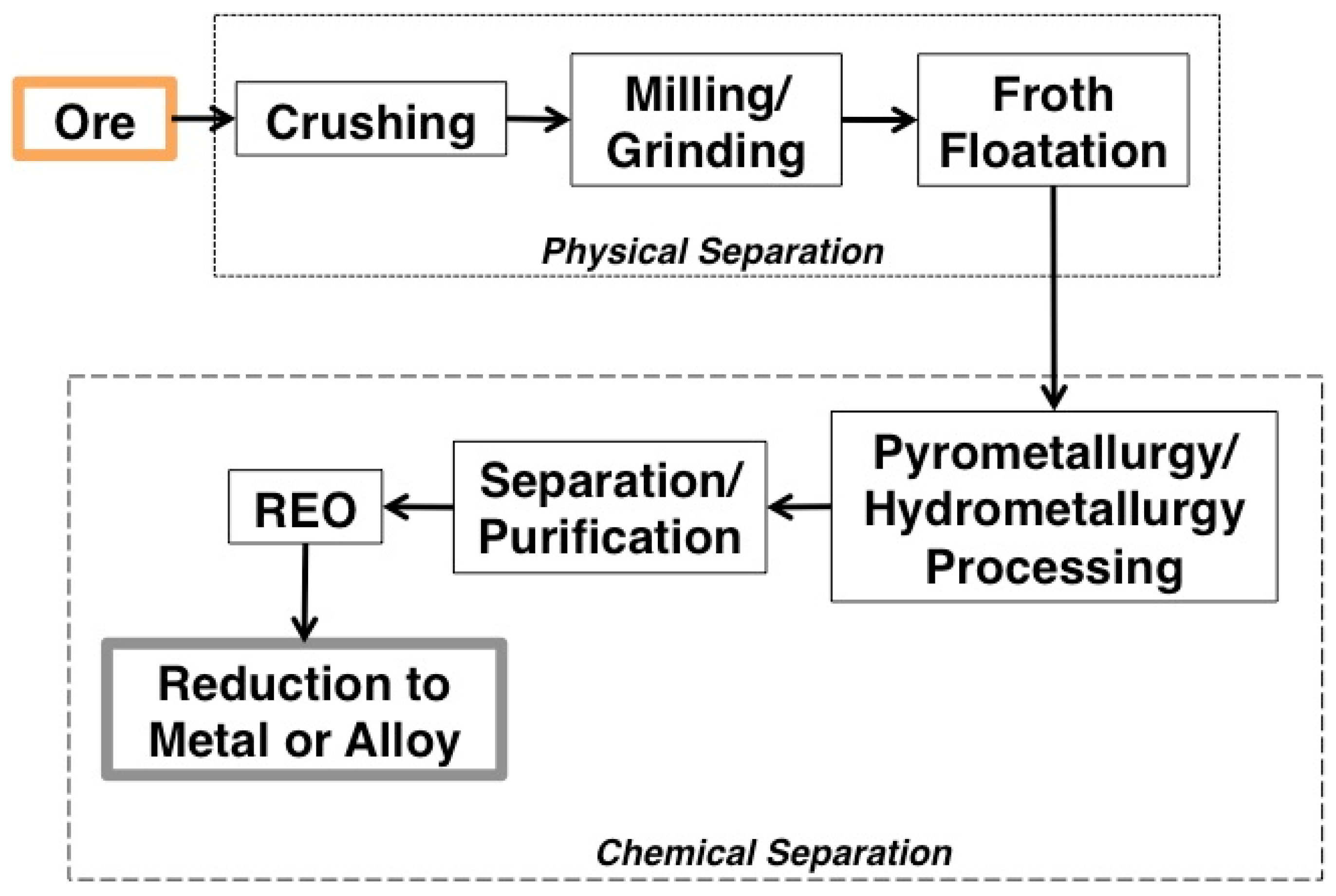
1.3.3. Processing
- Mineral mining and comminution;
- Physical beneficiation, chemical separation, and concentration from the host material in acidic or alkaline solutions;
- Separation and purification using solvent extraction or ion exchange, and;
- Reduction of the individual REOs into pure metals [1].
1.4. Environmental Issues in REE Mining and Processing
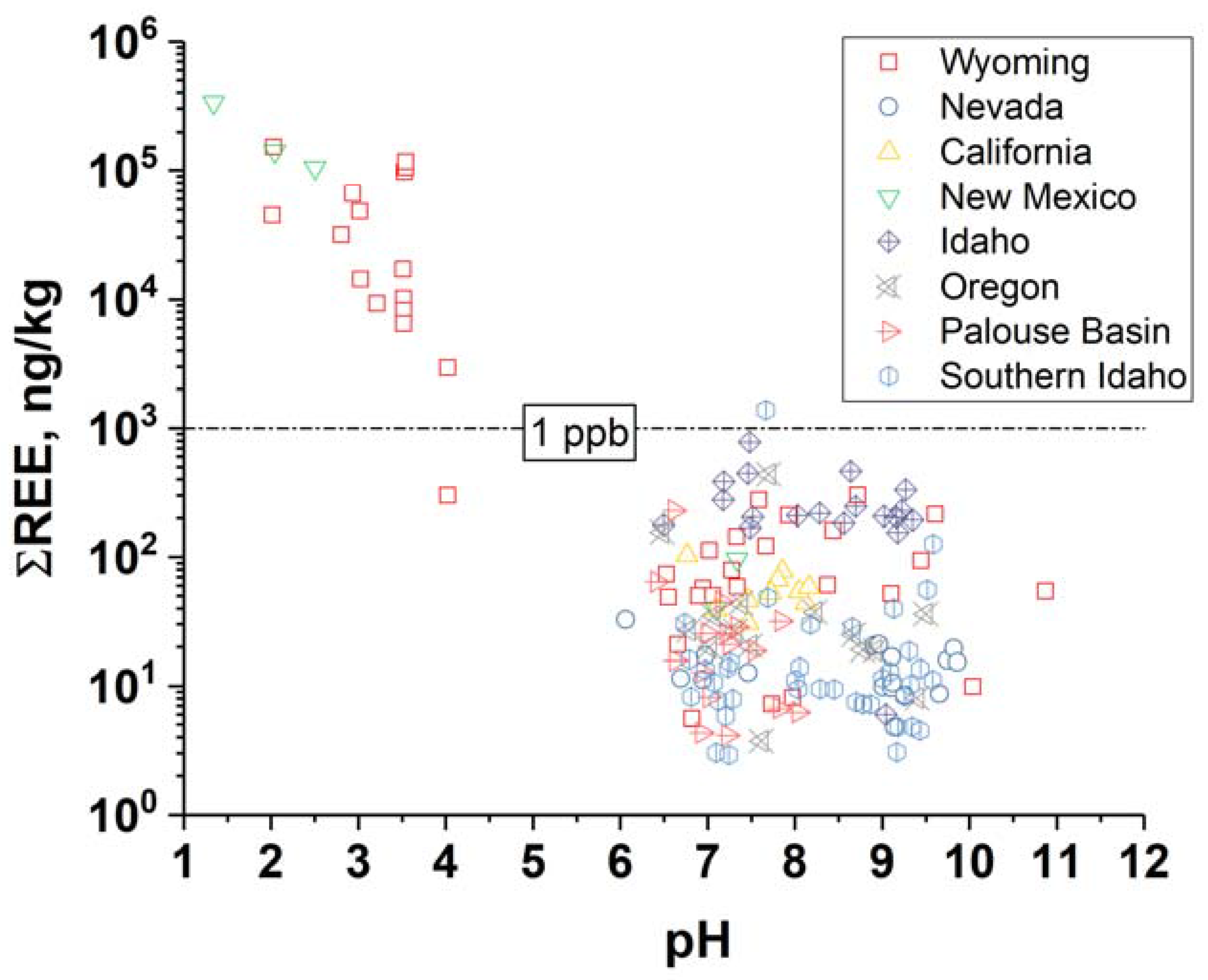
2. REEs in Geothermal Brines
3. Potential Methods of REE Extraction from Geothermal Brines
3.1. Adsorption
3.2. Ion-Exchange
3.3. Solvent Extraction (SX)
3.4. MRT—Molecular Recognition Technology
3.5. Extraction Using Engineered Microbes
3.6. Magnetic Segregation
4. Discussion
5. Conclusions
- REEs are present in measureable quantities in geothermal fluids using modern analytical techniques
- A number of technologies exist for extraction of REEs from geothermal fluids
- Due to the low concentration of REEs in the fluid, REEs recovered from geothermal fluids does not present itself as a resource able to meet current domestic demand
- Economic constraints hinders the viability for REE extraction alone
- Co-recovery with other valuable metals and minerals will improve the overall economic feasibility.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gupta, C.; Krishnamurthy, N. Extractive metallurgy of rare earths. Int. Mater. Rev. 1992, 37, 197–248. [Google Scholar] [CrossRef]
- Reisman, D.J.; Weber, R.J. Rare Earth Elements: A Review of Production, Processing, Recycling, and Associated Environmental Issues; U.S. Environmental Protection Agency: Cincinnati, OH, USA, 2012.
- Johannesson, K.H. Rare Earth Elements in Groundwater Flow Systems; Springer Science & Business Media: Dordrecht, The Netherlands, 2006. [Google Scholar]
- DiPippo, R. Geothermal Power Generation: Developments and Innovation; Woodhead Publishing: Cambridge, UK, 2016. [Google Scholar]
- Moore, J.N.; Simmons, S.F. More power from below. Science 2013, 340, 933–934. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.J.; Komninou, A.; Yardly, B.W.D.; Palmer, M.R. Rare earth element speciation in geothermal fluids from Yellowstone National Park, Wyoming, USA. Geochim. Cosmochim. Acta 1988, 62, 657–663. [Google Scholar] [CrossRef]
- Fowler, A.P.; Zierenberg, R.A. Rare earth element concentrations in geothermal fluids and epidote from the Reykjanes geothermal system, Iceland. In Proceedings of the World Geothermal Congress 2015, Melbourne, Australia, 19–25 April 2015. [Google Scholar]
- Bakane, P. Overview of extraction of minerals/metals with the help of geothermal fluid. In Proceedings of the 38th Workshop on Geothermal Reservoir Engineering Stanford University, Stanford, CA, USA, 11–13 February 2013. [Google Scholar]
- Segneri, B.; Deprizio, J.; Reinhardt, T. Geologic provenance of rare earth elements in the united states, and their potential collocation with geothermal resources. In Proceedings of the Thirty-Ninth Workshop on Geothermal Reservoir Engineering Stanford University, Stanford, CA, USA, 24–26 February 2014. [Google Scholar]
- Gallup, D.L. Geochemistry of geothermal fluids and well scales, and potential for mineral recovery. Ore Geol. Rev. 1998, 12, 225–236. [Google Scholar] [CrossRef]
- Jacoby, M.; Jiang, J. Securing the supply of rare earths. Chem. Eng. News 2010, 88, 9–12. [Google Scholar] [CrossRef]
- Gschneidner, K.A., Jr. The rare earth crisis—The supply/demand situation for 2010–2015. Mater. Matters 2011, 6, 32–37. [Google Scholar]
- Voncken, J.H.L. The Rare Earth Elements: An Introduction; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- USGS. National Minerals Information Center. Available online: http://minerals.usgs.gov/minerals/ (accessed on 5 October 2014).
- Bauer, D.; Diamond, D.; Li, J.; Sandalow, D.; Telleen, P.; Wanner, B. 2010 Critical Materials Strategy; U.S. Department of Energy: Washington, DC, USA, 2010.
- Grasso, V.B. Rare Earth Elements in National Defense: Background, Oversight Issues, and Options for Congress; U.S. Environmental Protection Agency: Cincinnati, OH, USA, 2013.
- Office of the Under Secretary of Defense for Acquisition, Technology and Logistics. Strategic and Critical Materials 2013 Report on Stockpile Requirements; U.S. Department of Defense: Arlington, VA, USA, 2013.
- Jordens, A.; Cheng, Y.P.; Waters, K.E. A review of the beneficiation of rare earth element bearing minerals. Miner. Eng. 2013, 41, 97–114. [Google Scholar] [CrossRef]
- Cotton, S. Lanthanide and Actinide Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Long, K.R.; Van Gosen, B.S.; Foley, N.K.; Cordier, D. The Principal Rare Earth Elements Deposits of the United States: A Summary of Domestic Deposits and A Global Perspective; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Ober, J.A. Mineral Commodity Summaries 2017; U.S. Geological Survey: Reston, VA, USA, 2017.
- Weng, Z.; Jowitt, S.M.; Mudd, G.M.; Haque, N. A detailed assessment of global rare earth element resources: Opportunities and challenges. Econ. Geol. 2015, 110, 1925–1952. [Google Scholar] [CrossRef]
- Weng, Z.; Jowitt, S.; Mudd, G.; Haque, N. Assessing rare earth element mineral deposit types and links to environmental impacts. Appl. Earth Sci. 2013, 122, 83–96. [Google Scholar] [CrossRef]
- Neupane, G.; Wendt, D.S. Assessment of mineral resources in geothermal brines in the us. In Proceedings of the 42nd Workshop on Geothermal Reservoir Engineering, Stanford University, Stanford, CA, USA, 13–15 February 2017. [Google Scholar]
- Elderfield, H.; Greaves, M.J. The rare earth elements in seawater. Nature 1982, 296, 214–219. [Google Scholar] [CrossRef]
- Haque, N.; Hughes, A.; Lim, S.; Vernon, C. Rare earth elements: Overview of mining, mineralogy, uses, sustainability and environmental impact. Resources 2014, 3, 614–635. [Google Scholar] [CrossRef]
- Humphries, M. Rare earth elements: The global supply chain. Congr. Res. Serv. 2012, 2011, 7–5700. [Google Scholar]
- Anderson, C.; Anderson, C.; Taylor, P. Survey of recycled rare earths metallurgical processing. Can. Metall. Q. 2013, 52, 249–256. [Google Scholar] [CrossRef]
- Anderson, C.D.; Taylor, P.R.; Anderson, C.G. Rare Earth Floatation Fundamentals: A Review. In Proceedings of the XXVIII International Mineral Processing Congress 2016, Quebec City, QC, Canada, 11–15 September 2016. [Google Scholar]
- Kronholm, B.; Anderson, C.G.; Taylor, P.R. A primer on hydrometallurgical rare earth separations. JOM 2013, 65, 1321–1326. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, T.A.; Dreisinger, D.; Doyle, F. A critical review on solvent extraction of rare earths from aqueous solutions. Miner. Eng. 2014, 56, 10–28. [Google Scholar] [CrossRef]
- Faris, N.; Ram, R.; Tardio, J.; Bhargava, S.; McMaster, S.; Pownceby, M.I. Application of ferrous pyrometallurgy to the beneficiation of rare earth bearing iron ores—A review. Miner. Eng. 2017, 110, 20–30. [Google Scholar] [CrossRef]
- Guo, L.; Chen, J.; Shen, L.; Zhang, J.; Zhang, D.; Deng, Y. Highly selective extraction and separation of rare earths(III) using bifunctional ionic liquid extractant. ACS Sustain. Chem. Eng. 2014, 2, 1968–1975. [Google Scholar] [CrossRef]
- Sun, X.; Waters, K.E. Development of industrial extractants into functional ionic liquids for environmentally friendly rare earth separation. ACS Sustain. Chem. Eng. 2014, 2, 1910–1917. [Google Scholar] [CrossRef]
- Krishnamurthy, N.; Gupta, C.K. Extractive Metallurgy of Rare Earths; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Eyring, L. Progress in the Science and Technology of the Rare Earths; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Lucas, J.; Lucas, P.; Le Mercier, T.; Rollat, A.; Davenport, W.G. Rare Earths: Science, Technology, Production and Use; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Ali, S. Social and environmental impact of the rare earth industries. Resources 2014, 3, 123–134. [Google Scholar] [CrossRef]
- Tharumarajah, R.; Koltun, P. Cradle to gate assessment of environmental impact of rare earth metals. In Proceedings of the 7th Australian Conference on Life Cycle Assessment, Melbourne, Australia, 9–10 March 2011. [Google Scholar]
- Norgate, T.; Haque, N. Energy and greenhouse gas impacts of mining and mineral processing operations. J. Clean. Prod. 2010, 18, 266–274. [Google Scholar] [CrossRef]
- Northey, S.; Haque, N.; Mudd, G. Using sustainability reporting to assess the environmental footprint of copper mining. J. Clean. Prod. 2013, 40, 118–128. [Google Scholar] [CrossRef]
- Koltun, P.; Tharumarajah, A. Life cycle impact of rare earth elements. ISRN Metall. 2014, 2014. [Google Scholar] [CrossRef]
- Gammons, C.H.; Wood, S.A.; Jonas, J.P.; Madison, J.P. Geochemistry of the rare-earth elements and uranium in the acidic Berkeley Pit lake, Butte, Montana. Chem. Geol. 2003, 198, 269–288. [Google Scholar] [CrossRef]
- Van Middlesworth, P.E.; Wood, S.A. The aqueous geochemistry of the rare earth elements and yttrium. Part 7. REE, Th and U contents in thermal springs associated with the idaho batholith. Appl. Geochem. 1998, 13, 861–884. [Google Scholar] [CrossRef]
- Nelson, B.J.; Wood, S.A.; Osiensky, J.L. Rare earth element geochemistry of groundwater in the Palouse Basin, northern Idaho–eastern Washington. Geochem. Explor. Environ. Anal. 2004, 4, 227–241. [Google Scholar] [CrossRef]
- Wood, S.A. The aqueous geochemistry of the rare-earth elements and yttrium: 1. Review of available low-temperature data for inorganic complexes and the inorganic ree speciation of natural waters. Chem. Geol. 1990, 82, 159–186. [Google Scholar] [CrossRef]
- Wood, S.A.; Shannon, W.M. Rare-earth elements in geothermal waters from Oregon, Nevada, and California. J. Solid State Chem. 2003, 171, 246–253. [Google Scholar] [CrossRef]
- Araki, K.; Yoshida, M.; Uezu, K.; Goto, M.; Furusaki, S. Lanthanide-imprinted resins prepared by surface template polymerization. J. Chem. Eng. Jpn. 2000, 33, 665–668. [Google Scholar] [CrossRef]
- Alakhras, F.A.; Dari, K.A.; Mubarak, M.S. Synthesis and chelating properties of some poly(amidoxime-hydroxamic acid) resins toward some trivalent lanthanide metal ions. J. Appl. Polym. Sci. 2005, 97, 691–696. [Google Scholar] [CrossRef]
- Bou-Maroun, E.; Goetz-Grandmont, G.J.; Boos, A. Sorption of europium(III) and copper(II) by a mesostructured silica doped with acyl-hydroxypyrazole derivatives—Extraction, kinetic and capacity studies. Colloids Surf. A 2006, 287, 1–9. [Google Scholar] [CrossRef]
- Chen, Y.G.; Zhu, B.H.; Wu, D.B.; Wang, Q.G.; Yang, Y.H.; Ye, W.M.; Guo, J.F. Eu(III) adsorption using di(2-ethylhexyl) phosphoric acid-immobilized magnetic GMZ bentonite. Chem. Eng. J. 2012, 181, 387–396. [Google Scholar] [CrossRef]
- Choi, S.H.; Lee, K.P.; Sohn, S.H. Graft copolymer-lanthanide complexes obtained by radiation grafting on polyethylene film. J. Appl. Polym. Sci. 2003, 87, 328–336. [Google Scholar] [CrossRef]
- Das, N.; Das, D. Recovery of rare earth metals through biosorption: An overview. J. Rare Earths 2013, 31, 933–943. [Google Scholar] [CrossRef]
- Dupont, D.; Brullot, W.; Bloemen, M.; Verbiest, T.; Binnemans, K. Selective uptake of rare earths from aqueous solutions by edta-functionalized magnetic and nonmagnetic nanoparticles. ACS Appl. Mater. Interfaces 2014, 6, 4980–4988. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Wang, Z.H.; Li, D.Q.; Niu, C.J. Adsorption of heavy rare earth(III) with extraction resin containing bis(2,4,4-trimethylpentyl) monothiophosphinic acid. J. Alloys Compd. 2004, 374, 434–437. [Google Scholar] [CrossRef]
- Ramakrishnan, K.; Rao, T.P. Ion imprinted polymer solid phase extraction (IIP-SPE) for preconcentrative separation of erbium(III) from adjacent lanthanides and yttrium. Sep. Sci. Technol. 2006, 41, 233–246. [Google Scholar] [CrossRef]
- Shibata, J.; Matsumoto, S.; Yamamoto, H. A novel separation technology for a heavy rare earth residue using a solvent impregnated resin. Solvent Extr. Res. Dev. Jpn. 2000, 7, 167–175. [Google Scholar]
- Wang, F.; Zhao, J.; Zhou, H.; Li, W.; Sui, N.; Liu, H. O-carboxymethyl chitosan entrapped by silica: Preparation and adsorption behaviour toward neodymium (III) ions. J. Chem. Technol. Biotechnol. 2013, 88, 317–325. [Google Scholar] [CrossRef]
- Wu, D.; Sun, Y.; Wang, Q. Adsorption of lanthanum (III) from aqueous solution using 2-ethylhexyl phosphonic acid mono-2-ethylhexyl ester-grafted magnetic silica nanocomposites. J. Hazard. Mater. 2013, 260, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Ogata, T.; Narita, H.; Tanaka, M. Adsorption behavior of rare earth elements on silica gel modified with diglycol amic acid. Hydrometallurgy 2015, 152, 178–182. [Google Scholar] [CrossRef]
- Koeppenkastrop, D.; De Carlo, E.H. Uptake of rare earth elements from solution by metal oxides. Environ. Sci. Technol. 1993, 27, 1796–1802. [Google Scholar] [CrossRef]
- Ashour, R.M.; El-sayed, R.; Abdel-Magied, A.F.; Abdel-khalek, A.A.; Ali, M.; Forsberg, K.; Uheida, A.; Muhammed, M.; Dutta, J. Selective separation of rare earth ions from aqueous solution using functionalized magnetite nanoparticles: Kinetic and thermodynamic studies. Chem. Eng. J. 2017, 327, 286–296. [Google Scholar] [CrossRef]
- Thomas, H.; Reinhardt, T.P.; Segneri, B. Low temperature geothermal mineral recovery program. In Proceedings of the 40th Workshop on Geothermal Reservoir Engineering, Stanford University, Stanford, CA, USA, 26–28 January 2015. [Google Scholar]
- Thomas, H.P.; Reinhardt, T.P.; Andersen, A.; Segneri, B. Critical and strategic materials and potential importance for geothermal projects. In Proceedings of the 41st Workshop on Geothermal Reservoir Engineering, Stanford University, Stanford, CA, USA, 22–24 February 2016. [Google Scholar]
- Smith, Y.R.; Bhattacharyya, D.; Willhard, T.; Misra, M. Adsorption of aqueous rare earth elements using carbon black derived from recycled tires. Chem. Eng. J. 2016, 296, 102–111. [Google Scholar] [CrossRef]
- Izatt, R.M.; Izatt, S.R.; Bruening, R.L.; Izatt, N.E.; Moyer, B.A. Challenges to achievement of metal sustainability in our high-tech society. Chem. Soc. Rev. 2014, 43, 2451–2475. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.R. Resource Recovery and Recycling from Metallurgical Wastes; Elsevier: Amsterdam, The Netherlands, 2011; Volume 7. [Google Scholar]
- Sohn, H.Y. Hydrometallurgical principles. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Izatt, N.E.; Bruening, R.L.; Krakowiak, K.E.; Izatt, S.R. Contributions of professor Reed M. Izatt to molecular recognition technology: From laboratory to commercial application. Ind. Eng. Chem. Res. 2000, 39, 3405–3411. [Google Scholar] [CrossRef]
- Izatt, S.R.; Bruening, R.L.; Izatt, N.E. Metal separations and recovery in the mining industry. JOM 2012, 64, 1279–1284. [Google Scholar] [CrossRef]
- Izatt, R.M. Charles J. Pedersen: Innovator in macrocyclic chemistry and co-recipient of the 1987 Nobel Prize in chemistry. Chem. Soc. Rev. 2007, 36, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, C.J. Cyclic polyethers and their complexes with metal salts. J. Am. Chem. Soc. 1967, 89, 7017–7036. [Google Scholar] [CrossRef]
- Izatt, R.M.; Nelson, D.; Rytting, J.; Haymore, B.; Christensen, J.J. Calorimetric study of the interaction in aqueous solution of several uni-and bivalent metal ions with the cyclic polyether dicyclohexyl-18-crown-6 at 10,25, and 40.deg. J. Am. Chem. Soc. 1971, 93, 1619–1623. [Google Scholar] [CrossRef]
- Lamb, J.; Izatt, R.; Swain, C.; Christensen, J. A systematic study of the effect of macrocycle ring size and donor atom type on the log K, .DELTA.H, and T.DELTA.S of reactions at 25.degree.C in methanol of mono-and divalent cations with crown ethers. J. Am. Chem. Soc. 1980, 102, 475–479. [Google Scholar] [CrossRef]
- Christensen, J.J.; Eatough, D.J.; Izatt, R.M. The synthesis and ion bindings of synthetic multidentate macrocyclic compounds. Chem. Rev. 1974, 74, 351–384. [Google Scholar] [CrossRef] [PubMed]
- Izatt, R.; Lamb, J.; Bruening, R. Comparison of bulk, emulsion, thin sheet supported, and hollo fiber supported liquid membranes in macrocycle-mediated cation separations. Sep. Sci. Technol. 1988, 23, 1645–1658. [Google Scholar] [CrossRef]
- Izatt, R.M.; Bruening, R.L.; Bruening, M.L.; Tarbet, B.J.; Krakowiak, K.E.; Bradshaw, J.S.; Christensen, J.J. Removal and separation of metal ions from aqueous solutions using a silica-gel-bonded macrocycle system. Anal. Chem. 1988, 60, 1825–1826. [Google Scholar] [CrossRef]
- Izatt, R.M.; Izatt, S.R.; Izatt, N.E.; Krakowiak, K.E.; Bruening, R.L.; Navarro, L. Industrial applications of molecular recognition technology to separations of platinum group metals and selective removal of metal impurities from process streams. Green Chem. 2015, 17, 2236–2245. [Google Scholar] [CrossRef]
- Gadd, G.M. Biosorption: Critical review of scientific rationale, environmental importance and significance for pollution treatment. J. Chem. Technol. Biotechnol. 2009, 84, 13–28. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Yun, Y.-S. Bacterial biosorbents and biosorption. Biotechnol. Adv. 2008, 26, 266–291. [Google Scholar] [CrossRef] [PubMed]
- Valls, M.; Atrian, S.; de Lorenzo, V.; Fernández, L.A. Engineering a mouse metallothionein on the cell surface of Ralstonia eutropha CH34 for immobilization of heavy metals in soil. Nat. Biotechnol. 2000, 18, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, K.; Ueda, M. Bioadsorption of cadmium ion by cell surface-engineered yeasts displaying metallothionein and hexa-His. Appl. Microbiol. Biotechnol. 2003, 63, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Nishitani, T.; Shimada, M.; Kuroda, K.; Ueda, M. Molecular design of yeast cell surface for adsorption and recovery of molybdenum, one of rare metals. Appl. Microbiol. Biotechnol. 2010, 86, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Pazirandeh, M.; Chrisey, L.; Mauro, J.; Campbell, J.; Gaber, B. Expression of the neurospora crassa metallothionein gene in escherichia coli and its effect on heavy-metal uptake. Appl. Microbiol. Biotechnol. 1995, 43, 1112–1117. [Google Scholar] [CrossRef] [PubMed]
- Park, D.M.; Reed, D.W.; Yung, M.C.; Eslamimanesh, A.; Lencka, M.M.; Anderko, A.; Fujita, Y.; Riman, R.E.; Navrotsky, A.; Jiao, Y. Bioadsorption of rare earth elements through cell surface display of lanthanide binding tags. Environ. Sci. Technol. 2016, 50, 2735–2742. [Google Scholar] [CrossRef] [PubMed]
- Daughtry, K.D.; Martin, L.J.; Sarraju, A.; Imperiali, B.; Allen, K.N. Tailoring encodable lanthanide-binding tags as MRI contrast agents. Chem. Biol. Chem. 2012, 13, 2567–2574. [Google Scholar] [CrossRef] [PubMed]
- Charrier, M.; Ajo-Franklin, C.M. Engineering thermophilic microorganisms to selectively extract strategic minerals. In Proceedings of the 42nd Workshop on Geothermal Reservoir Engineering, Stanford University, Stanford, CA, USA, 13–15 February 2017. [Google Scholar]
- Lo, Y.C.; Cheng, C.L.; Han, Y.L.; Chen, B.Y.; Chang, J.S. Recovery of high-value metals from geothermal sites by biosorption and bioaccumulation. Bioresour. Technol. 2014, 160, 182–190. [Google Scholar] [CrossRef] [PubMed]
- O’brien, T.B. Recovery of Elements from Hydrothermal Products. U.S. Patent 8377165, 19 February 2013. [Google Scholar]
- Werner, H. Contribution to the mineral extraction from supersaturated geothermal brines salton sea area, California. Geothermics 1970, 2, 1651–1655. [Google Scholar] [CrossRef]
- Kennedy, A. The recovery of lithium and other minerals from geothermal water at Wairakei. In Proceedings of the UN Conference on New Sources of Energy, Rome, Italy, 21–31 August 1961. [Google Scholar]
- Maimoni, A. Minerals recovery from salton sea geothermal brines—A literature review and proposed cementation process. Geothermics 1982, 11, 239–258. [Google Scholar] [CrossRef]
- Harrison, S. Technologies for Extracting Valuable Metals and Compounds from Geothermal Fluids; California Energy Commission: Sacramento, CA, USA, 2014. [Google Scholar]
- Wood, S.A. Behavior of Rare Earth Element in Geothermal Systems; A New Exploration/Exploitation Tool; University of Idaho (US): Moscow, ID, USA, 2002. [Google Scholar]
- Bourcier, W.; Bruton, C.; Roberts, S.; Viani, B.; Conley, S.; Martin, S. Pilot-Scale Geothermal Silica Recovery at Mammoth Lakes; California Energy Commission: Sacramento, CA, USA, 2009. [Google Scholar]
- Farley, E.; Watson, E.; MacDonald, D.; Bartlett, R.; Krishnan, G. Recovery of Heavy Metals from High Salinity Geothermal Brine; SRI International: Menlo Park, CA, USA, 1980. [Google Scholar]
- Brown, K.L.; Roberts, P.J.C. Extraction of gold and silver from geothermal fluid. In Proceedings of the 10th New Zealand Geothermal Workshop, Auckland, New Zealand, 2–4 November 1988. [Google Scholar]
- Brown, K.L. Gold deposition from geothermal discharges in New Zealand. Econ. Geol. 1986, 81, 979–983. [Google Scholar] [CrossRef]
- Premuzic, E.T.; Lin, M.S.; Bohenek, M.; Bajsarowicz, V.; McCloud, M. Advanced Biochemical Processes for Geothermal Brines: Current Developments; Brookhaven National Lab.: Upton, NY, USA, 1997. [Google Scholar]
- Gallup, D.L.; Ririe, G.T. Platinum Recovery. U.S. Patent 5290339, 1 March 1994. [Google Scholar]
- Christopher, D.; Stewart, M.; Rice, J. The Recovery and Separation of Mineral Values from Geothermal Brines. Research Report, 1974–1975; Hazen Research, Inc.: Golden, CO, USA, 1975. [Google Scholar]
- Gambogi, J. Rare earths. In 2014 Minerals Yearbook; U.S. Gological Survey: Reston, VA, USA, 2016. [Google Scholar]
- Gambogi, J. Gold. In 2014 Minerals Yearbook; U.S. Gological Survey: Reston, VA, USA, 2016. [Google Scholar]
- Moore, J. Personal communication, Energy & Geoscience Institute: Salt Lake City, UT, USA, 2017.
| Mineral | Mineral Chemistry | REO wt % |
|---|---|---|
| Aeschynite | (Ce,Ca,Fe,Th)(Ti,Nb)2(O,OH)6 | 36 |
| Allanite (orthite) | (Ce,Ca,Y)2(Al,Fe)3(SiO4)3(OH) | 3–51 |
| Ancylite–(Ce) | SrCe(CO3)2(OH)·H2O | 46–53 |
| Bastnäsite–(Ce) | (Ce,La)(CO3)F | 70–74 |
| Britholite–(Ce) | (Ce,Ca)5(SiO4,PO4)3(OH,F) | 56 |
| Brockite | (Ca,Th,Ce)(PO4)·H2O | |
| Calcio–ancylite–(Ce) | (Ca,Sr)Ce3(CO3)4(OH)3·H2O | 60 |
| Cerianite–(Ce) | (Ce4+,Th)O2 | 81 |
| Cerite–(Ce) | Ce93+Fe3+(SiO4)6[SiO3(OH)](OH)3 | 60 |
| Churchite–(Y) | YPO4·2H2O | 44 |
| Euxenite–(Y) | (Y,Ca,Ce,U,Th)(Nb,Ta,Ti)2O6 | <40 |
| Fergusonite–(Ce) | (Ce,La,Y)NbO4 | 47 |
| Fergusonite–(Y) | YNbO4 | |
| Florencite–(Ce) | CeAl3(PO4)2(OH)6 | 32 |
| Fluocerite | (Ce,La)F3 | |
| Fluorapatite–(Ce) | (Ca,Ce)5(PO4)3F | 0–21 |
| Gadolinite | (Ce,La,Nd,Y)2Fe2+Be2Si2O10 | 40 |
| Hingganite–(Y) | (Y,Yb,Er)2Be2Si2O8(OH)2 | |
| Huanghoite–(Ce) | BaCe(CO3)2F | 38 |
| Hydroxylbastnäsite–(Ce) | (Ce,La)(CO3)(OH,F) | 75 |
| Iimoriite–(Y) | Y2(SiO4)(CO3) | |
| Kainosite–(Y) | Ca2(Y,Ce)2Si4O12(CO3)·H2O | 38 |
| Loparite–(Ce) | (Ce,Na,Ca)(Ti,Nb)O3 | 32–34 |
| Monazite–(Ce) | (Ce,La,Nd,Th)PO4 | 35–71 |
| Mosandrite | (Na,Ca,Ce)3Ti(SiO4)2F | <65 |
| Parisite–(Ce) | Ca(Ce,La)2(CO3)3F2 | 59 |
| Rhabdophane | (Ce,La)PO4·H2O | |
| Samarskite–(Y) | (Y,Ce,U,Fe3+)3(Nb,Ta,Ti)5O16 | 12 |
| Synchysite–(Ce) | Ca(Ce,La)(CO3)2F | 49–52 |
| Thalénite–(Y) | Y3Si3O10(OH) | 63 |
| Uraninite | (U,Th,Ce)O2 | |
| Vitusite–(Ce) | Na3(Ce,La,Nd)(PO4)2 | |
| Xenotime–(Y) | YPO4 | 52–67 |
| Yttrofluorite | (Ca,Y)F2 | |
| Yttrotantalite–(Y) | (Y,U,Fe2+)(Ta,Nb)O4 | <24 |
| Element | Crustal Abundance (ppm) | Resource Tons | Production Tons/Annum | Years of Reserves |
|---|---|---|---|---|
| La | 32 | 22,600,000 | 12,500 | 1800 |
| Ce | 68 | 317,000,000 | 24,000 | 1300 |
| Pr | 9.5 | 4,800,000 | 2400 | 2000 |
| Nd | 38 | 16,700,000 | 7300 | 2300 |
| Pm | NA | NA | NA | NA |
| Sm | 7.9 | 2,900,000 | 700 | 4100 |
| Eu | 2.1 | 244,333 | 400 | 610 |
| Gd | 7.7 | 3,622,143 | 400 | 9100 |
| Tb | 1.1 | 566,104 | 10 | 57,000 |
| Dy | 6 | 2,980,000 | 100 | 29,800 |
| Ho | 1.4 | NA | 10 | NA |
| Er | 3.8 | 1,850,000 | 500 | 3700 |
| Tm | 0.48 | 334,255 | 50 | 6700 |
| Yb | 3.3 | 1,900,000 | 50 | 38,000 |
| Lu | 0.4 | 395,000 | NA | NA |
| Y | 30 | 9,000,000 | 8900 | 1011 |
| Sc | 22 | NA | NA | NA |
| Activity | Emission Source(s) | Primary Pollutants of Concern |
|---|---|---|
| Mining | Overburden | Radiological contaminates |
| Waste rock | Metals | |
| Sub-ore stockpile | Mine influenced waters (e.g., acid/alkaline drainage) | |
| Ore stockpile | Dust and associated pollutants (e.g., PM 2.5) | |
| Processing | Crushing/Grinding | Dust |
| Tailings | Radiological contaminates | |
| Tailings impoundment | Metals | |
| Separation and Purification | Turbidity | |
| Liquid waste | Organics | |
| Dust and associated pollutants | ||
| Recycling | Collection | Transportation pollutants |
| Dismantling and separation | Dust and associated pollutants | |
| Scrap waste | Volatile Organic Compounds | |
| Landfill | Metals | |
| Processing | Dust and associated pollutants | |
| Volatile Organic Compounds | ||
| Dioxins | ||
| Metals | ||
| Organics |
| Minerals | Extraction Technology | Reference |
|---|---|---|
| Ag | Sulphidization | [97] |
| Precipitation by metallic iron | [93] | |
| Deposition in steel vessel | [98] | |
| Au | Scale deposition | [99] |
| Deposition in extraction vessel | [98] | |
| Bioleaching | [100] | |
| Pt | Carbon interaction with brine | [100] |
| Sr | Evaporative extraction | [101] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, Y.R.; Kumar, P.; McLennan, J.D. On the Extraction of Rare Earth Elements from Geothermal Brines. Resources 2017, 6, 39. https://doi.org/10.3390/resources6030039
Smith YR, Kumar P, McLennan JD. On the Extraction of Rare Earth Elements from Geothermal Brines. Resources. 2017; 6(3):39. https://doi.org/10.3390/resources6030039
Chicago/Turabian StyleSmith, York R., Pankaj Kumar, and John D. McLennan. 2017. "On the Extraction of Rare Earth Elements from Geothermal Brines" Resources 6, no. 3: 39. https://doi.org/10.3390/resources6030039
APA StyleSmith, Y. R., Kumar, P., & McLennan, J. D. (2017). On the Extraction of Rare Earth Elements from Geothermal Brines. Resources, 6(3), 39. https://doi.org/10.3390/resources6030039






