Loss and Early Recovery of Biomass and Soil Organic Carbon in Restored Mangroves After Paspalum vaginatum Invasion in West Africa
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Field Sampling
2.3. Pore Water Parameters
2.4. Organic Carbon in Vegetation
2.5. Soil Properties and Organic Carbon Content
2.6. Total Ecosystem Carbon Stock and CO2 Equivalent Emissions
2.7. Soil Gas Fluxes
2.8. Statistical Analysis
3. Results
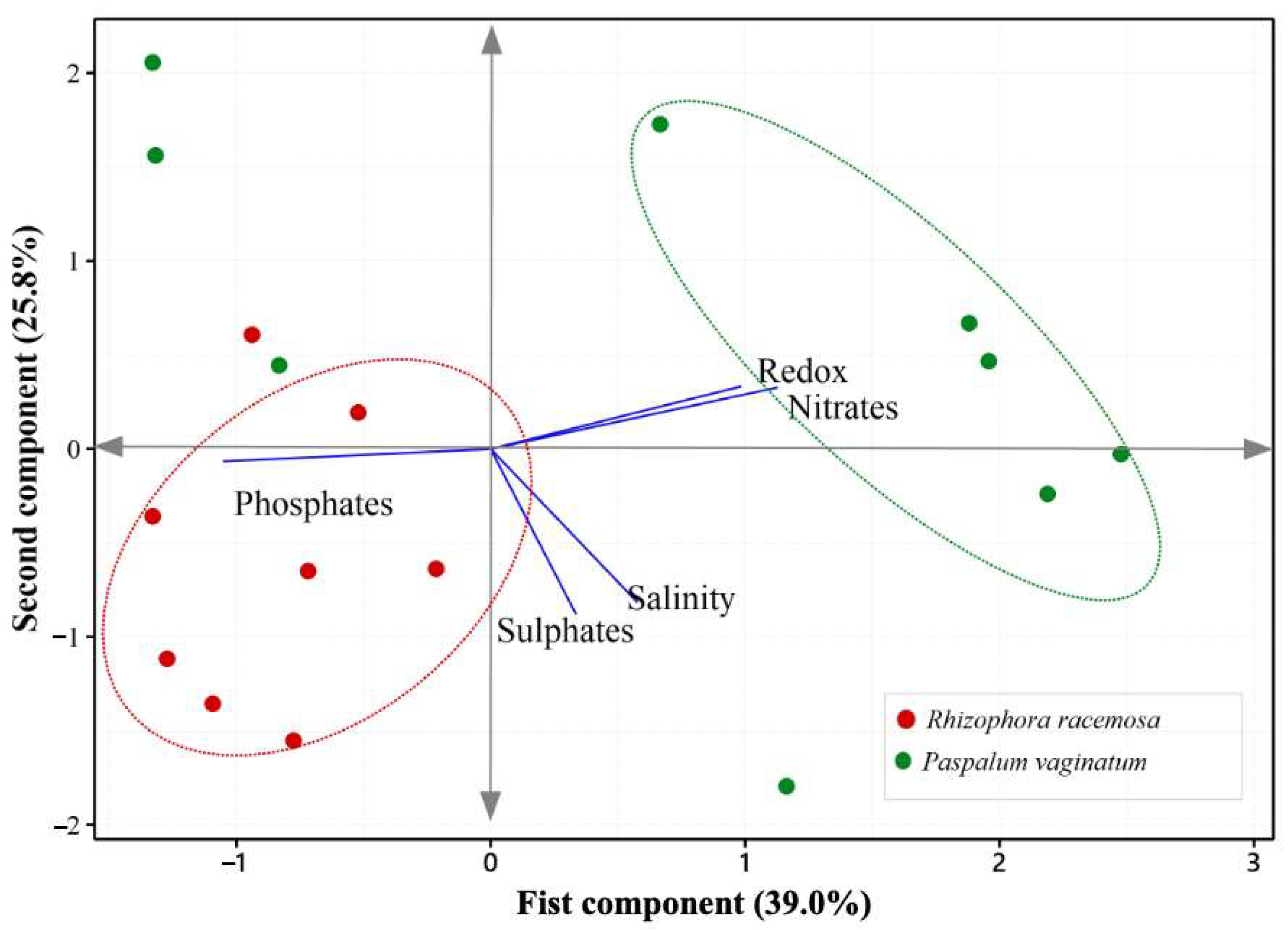
3.1. Environmental Conditions of Pore Water Between the Reference and Degraded Sites
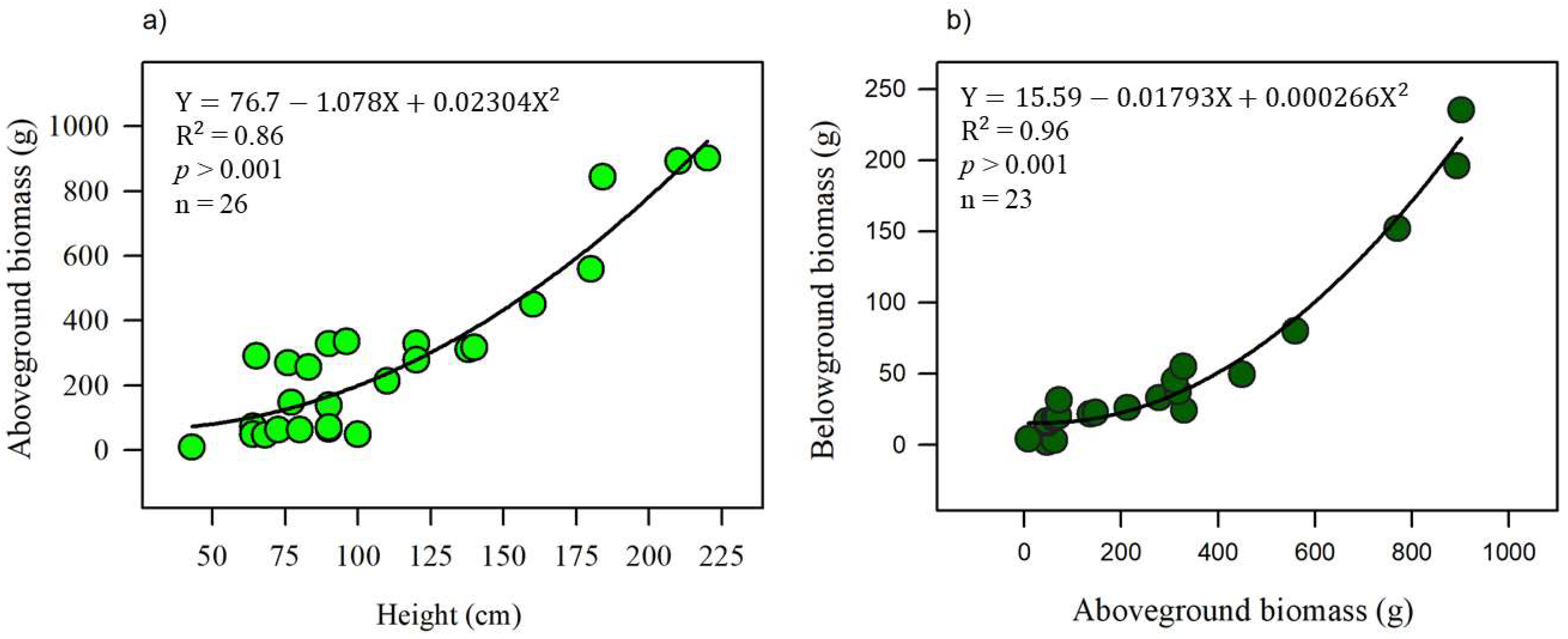
3.2. Biomass Carbon Associated with Invasion and Mangrove Restoration
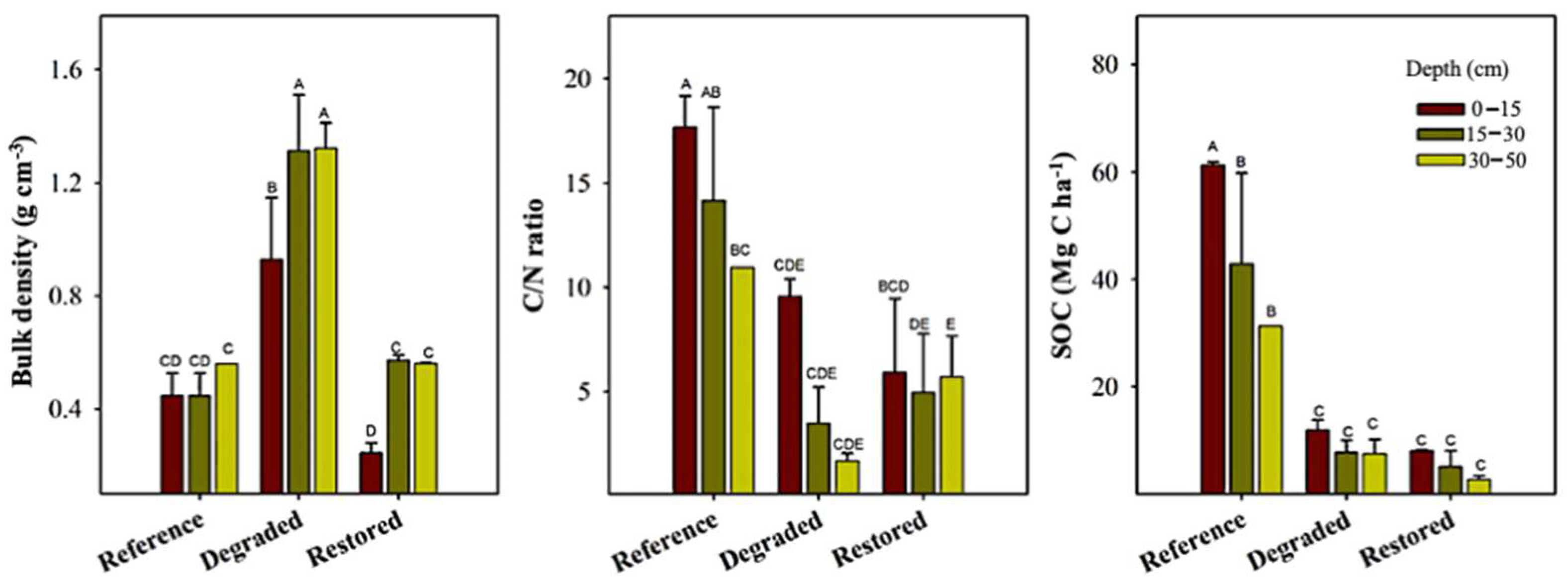
3.3. Soil Properties Following Paspalum vaginatum Invasion and Mangrove Restoration
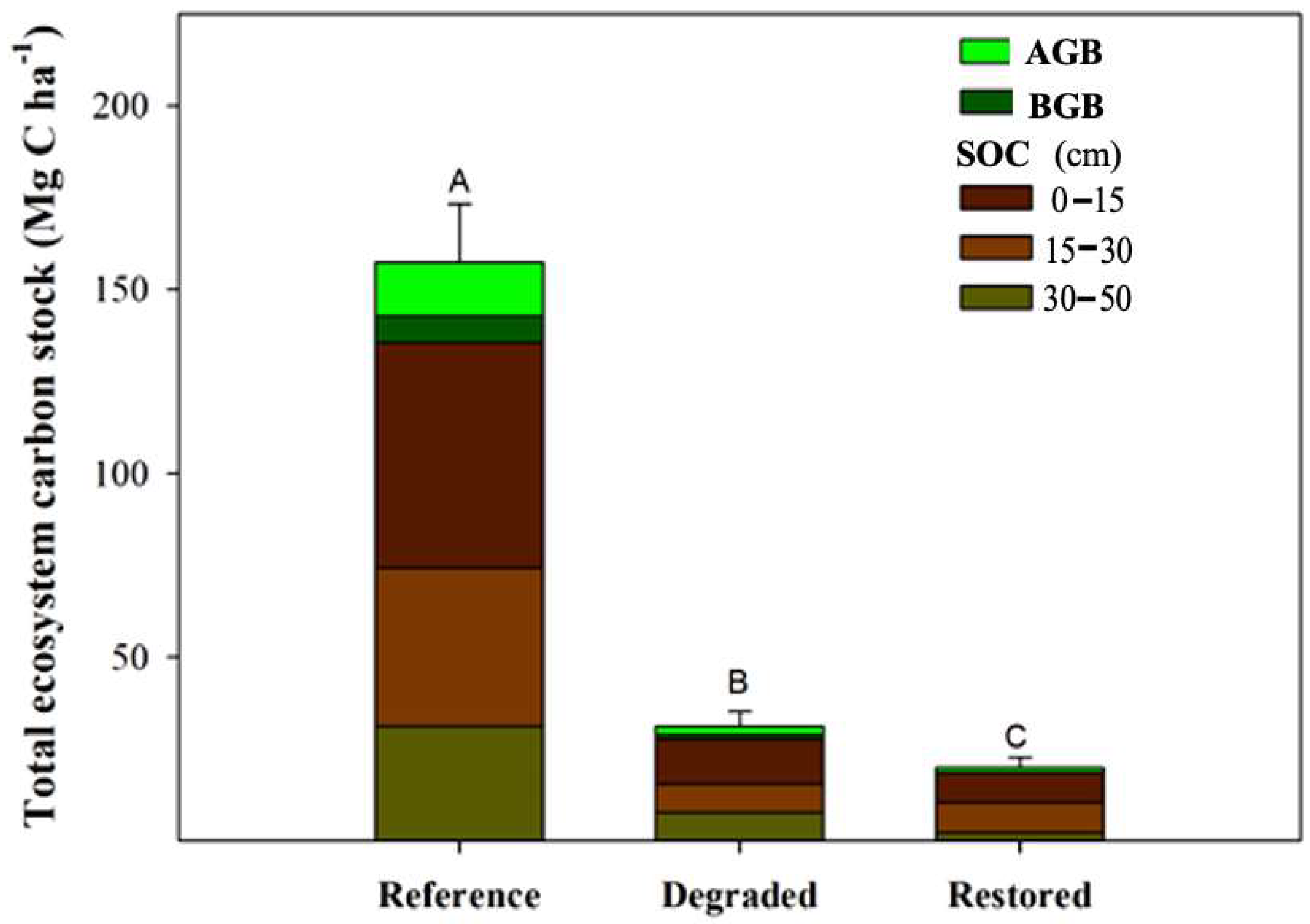
3.4. Total Carbon Stock and CO2 Equivalent Emissions After Invasion and Restoration
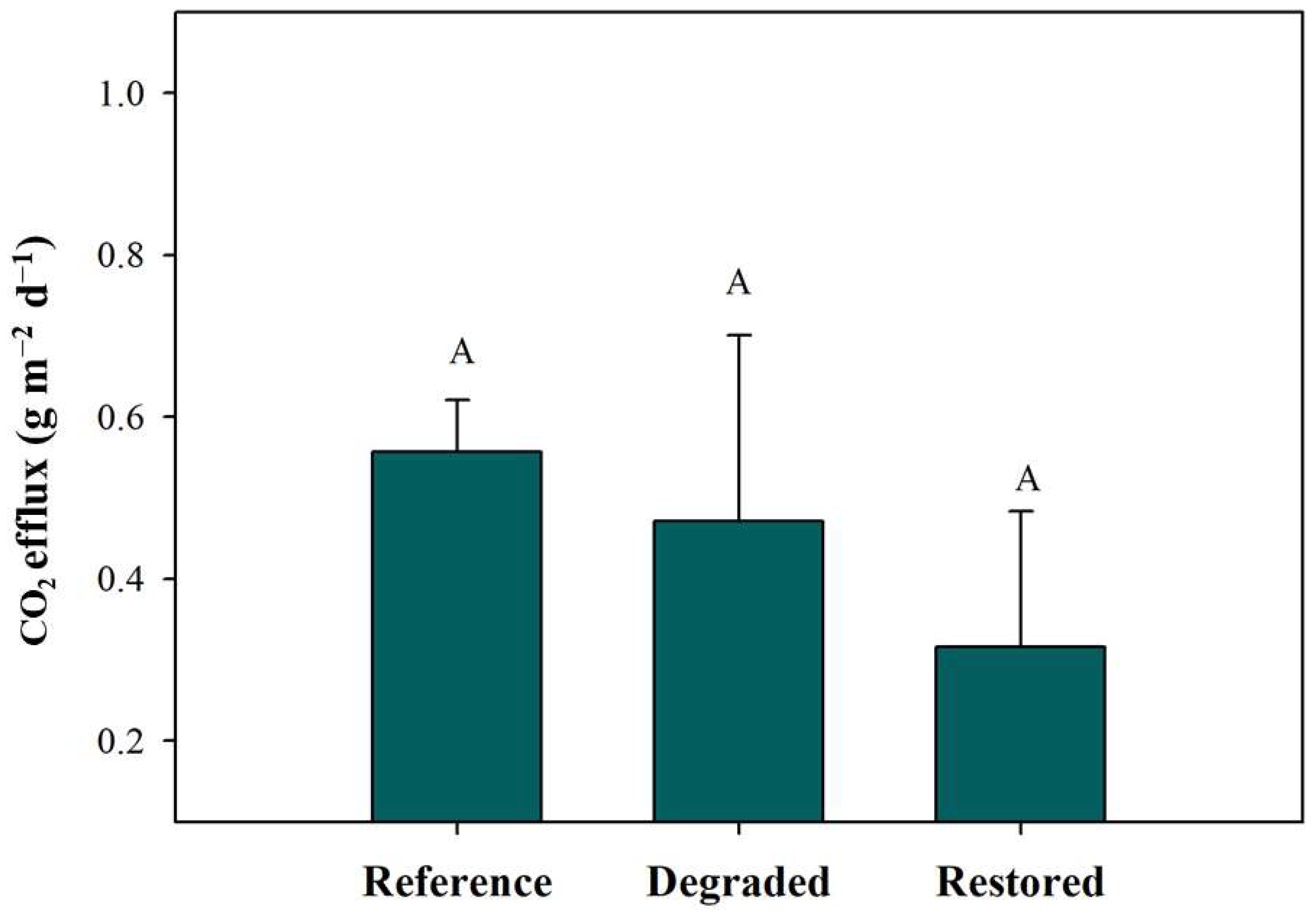
3.5. Changes in Soil Gas Fluxes Following Mangrove Restoration
4. Discussion
4.1. Impact of Paspalum vaginatum Invasion on Carbon Storage in Mangroves
4.2. Soil Effects of Paspalum vaginatum Invasion and Carbon Loss
4.3. Carbon Recovery Following Mangrove Restoration
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


Appendix B
| Component | Parameters | df | t-Value | p-Value | Significance |
|---|---|---|---|---|---|
| Roots | N (%) | 0.53 | 0.624 | NS | |
| C (%) | 4 | 4.24 | 0.013 | S | |
| C/N ratio | 4.55 | 0.010 | S | ||
| Leaves | N (%) | 4 | 36.93 | <0.0001 | S |
| C (%) | 3.83 | 0.019 | S | ||
| C/N ratio | 9.86 | 0.001 | S | ||
| Stems | N (%) | 4 | 3.22 | 0.032 | S |
| C (%) | 13.21 | <0.0001 | S | ||
| C/N ratio | 8.87 | 0.001 | S |
References
- Donato, D.C.; Kauffman, J.B.; Murdiyarso, D.; Kurnianto, S.; Stidham, M.; Kanninen, M. Mangroves among the most carbon-rich forests in the tropics. Nat. Geosci. 2011, 4, 293–297. [Google Scholar] [CrossRef]
- Kida, M.; Fujitake, N. Organic carbon stabilization mechanisms in mangrove soils: A Review. Forests 2020, 11, 981. [Google Scholar] [CrossRef]
- Alongi, D.M. Global significance of mangrove blue carbon in climate change mitigation. Science 2020, 2, 67. [Google Scholar] [CrossRef]
- Lewis, D.B.; Brown, J.A.; Jimenez, K.L. Effects of flooding and warming on soil organic matter mineralization in Avicennia germinans mangrove forests and Juncus roemerianus salt marshes. Estuar. Coast. Shelf Sci. 2014, 139, 11–19. [Google Scholar] [CrossRef]
- Alongi, D.M. Carbon cycling and storage in mangrove forests. Annu. Rev. Mar. Sci. 2014, 6, 195–219. [Google Scholar] [CrossRef]
- Agraz, M.; Keb, C.; Chávez Barrera, J.; Osti-Sáenz, J.; Expósito-Díaz, G.; Alonso-Campos, V.; Muñiz-Salazar, R.; Ruiz-Fernández, A.; Pérez-Bernal, L.; Sánchez-Cabeza, J.-A.; et al. Reserva de carbón en un ecosistema de manglar al norte de México: Cambios ambientales durante 35 años. Rev. Mex. Biodivers. 2020, 91, 912910. [Google Scholar] [CrossRef]
- Lugo, A.E. Mangrove forests: A tough system to invade but an easy one to rehabilitate. Mar. Pollut. Bull. 1999, 37, 427–430. [Google Scholar] [CrossRef]
- Fournier, A.; Penone, C.; Pennino, M.G.; Courchamp, F. Predicting future invaders and future invasions. Proc. Natl. Acad. Sci. USA. 2019, 116, 7905–7910. [Google Scholar] [CrossRef]
- Biswas, S.R.; Biswas, P.L.; Limon, S.H.; Yan, E.-R.; Xu, M.-S.; Khan, M.d.S.I. Plant invasion in mangrove forests worldwide. For. Ecol. Manag. 2018, 429, 480–492. [Google Scholar] [CrossRef]
- Augusthy, S.; Nizam, A.; Kumar, A. The diversity, drivers, consequences and management of plant invasions in the mangrove ecosystems. Sci. Total Environ. 2024, 945, 173851. [Google Scholar] [CrossRef]
- Ehrenfeld, J.G. Effects of exotic plant invasions on soil nutrient cycling processes. Ecosystems 2003, 6, 503–523. [Google Scholar] [CrossRef]
- Bu, N.; Qu, J.; Li, Z.; Li, G.; Zhao, H.; Zhao, B.; Li, B.; Chen, J.; Fang, C. Effects of Spartina alterniflora invasion on soil respiration in the Yangtze River Estuary, China. PLoS ONE 2015, 10, e0121571. [Google Scholar] [CrossRef]
- Xuehui, Z.; Zhongsheng, Z.; Zhe, L.; Min, L.; Haitao, W.; Ming, J. Impacts of Spartina alterniflora invasion on soil carbon contents and stability in the Yellow River Delta, China. Sci. Total Environ. 2021, 775, 145188. [Google Scholar] [CrossRef]
- Xu, X.; Wei, S.; Chen, H.; Li, B.; Nie, M. Effects of Spartina Invasions on Blue Carbon Sequestration in China 2022, 309190 Bytes. Available online: https://figshare.com/articles/dataset/Effects_of_Spartina_invasions_on_blue_carbon_sequestration_in_China/19391063 (accessed on 1 June 2025).
- Li, G.; Xu, S.; Tang, Y.; Wang, Y.; Lou, J.; Zhang, Q.; Zheng, X.; Li, J.; Iqbal, B.; Cheng, P.; et al. Spartina alterniflora invasion altered soil greenhouse gas emissions via affecting labile organic carbon in a coastal wetland. Appl. Soil Ecol. 2024, 203, 105615. [Google Scholar] [CrossRef]
- Agraz Hernández, C.M.; Houndjinou, E.; Reyes Castellanos, J.E.; Chan Keb, C.A.; Osti Saénz, J.; Chávez Barrera, J.C.; Etienne, J.; Glèlè Kakaï, R.L.; Puschendorf, R. Hydrological repair and invasive grass removal restore Rhizophora racemosa mangrove communities in West Africa. Restor. Ecol. 2025, 32, e70084. [Google Scholar] [CrossRef]
- Song, S.; Ding, Y.; Li, W.; Meng, Y.; Zhou, J.; Gou, R.; Zhang, C.; Ye, S.; Saintilan, N.; Krauss, K.W.; et al. Mangrove Reforestation Provides Greater Blue Carbon Benefit than Afforestation for Mitigating Global Climate Change. Nat. Commun. 2023, 14, 756. [Google Scholar] [CrossRef]
- Song, W.; Hou, Y.; Zhu, W.; Fan, Y.; Xu, H.; Cai, C.; Li, G.; Huang, L. Enhancement effects of mangrove restoration on blue carbon storage in Qinzhou Bay. Front. For. Glob. Change 2024, 7, 1328783. [Google Scholar] [CrossRef]
- Sakai, Y.; Kouyama, T.; Kakinuma, K.; Sakaguchi, Y.; Yuasa, N.; Thongkao, S.; Boonming, S.; Chantrapromma, K.; Kato, S. Recovery of mangrove ecosystem carbon stocks through reforestation at abandoned shrimp pond in Southeast Thailand. Ecosyst. Health Sustain. 2023, 9, 0018. [Google Scholar] [CrossRef]
- Yang, F.; Li, R.; Wang, M.; Zhang, L.; Wang, W. Restoration patterns and influencing factors for the vegetation structure and carbon storage in mangroves converted from abandoned ponds. Glob. Ecol. Conserv. 2025, 58, e03430. [Google Scholar] [CrossRef]
- Beselly, S.M.; Van Der Wegen, M.; Reyns, J.; Grueters, U.; Dijkstra, J.T.; Roelvink, D. Strategic mangrove restoration increases carbon stock capacity. Commun. Earth Environ. 2025, 6, 422. [Google Scholar] [CrossRef]
- Jia, P.; Huang, W.; Zhang, Z.; Cheng, J.; Xiao, Y. The carbon sink of mangrove ecological restoration between 1988–2020 in Qinglan Bay, Hainan Island, China. Forests 2022, 13, 1547. [Google Scholar] [CrossRef]
- López-Portillo, J.; Zaldívar-Jiménez, A.; Lara-Domínguez, A.L.; Pérez-Ceballos, R.; Bravo-Mendoza, M.; Núñez Álvarez, N.; Aguirre-Franco, L. Hydrological rehabilitation and sediment elevation as strategies to restore mangroves in terrigenous and calcareous environments in Mexico. In Wetland Carbon and Environmental; Krauss, K.W., Zhu, Z., Stagg, C.L., Eds.; ManagementGeophysical Monograph Series; Wiley: Hoboken, NJ, USA, 2021; pp. 173–190. ISBN 978-1-119-63928-2. [Google Scholar]
- Naidoo, G. The Mangroves of Africa: A review. Mar. Pollut. Bull. 2023, 190, 114859. [Google Scholar] [CrossRef]
- Zanvo, M.G.S.; Barima, Y.S.S.; Salako, K.V.; Koua, K.A.N.; Kolawole, M.A.; Assogbadjo, A.E.; Glèlè Kakaï, R. Mapping spatio-temporal changes in mangroves cover and projection in 2050 of their future state in Benin. Bois for. Trop. 2022, 350, 29–42. [Google Scholar] [CrossRef]
- Kauffman, K.; Donato, D. Protocols for the Measurement, Monitoring and Reporting of Structure, Biomass and Carbon Stocks in Mangrove Forests; Center for International Forestry Research (CIFOR): Bogor, Indonesia, 2012. [Google Scholar]
- Agraz-Hernández, C.M.; Chan-Keb, C.A.; Muñiz-Salazar, R.; Pérez-Balan, R.A.; Vanegas, G.P.; Manzanilla, H.G.; Osti-Sáenz, J.; del Río Rodríguez, R. Pore Water Chemical variability and its effect on phenological production in three mangrove species under drought conditions in southeastern Mexico. Diversity 2022, 14, 668. [Google Scholar] [CrossRef]
- Agraz-Hernández, C.; Del Río-Rodríguez, R.; Chan-Keb, C.; Osti-Saenz, J.; Muñiz-Salazar, R. Nutrient removal efficiency of Rhizophora mangle (L.) Seedlings exposed to experimental dumping of municipal waters. Diversity 2018, 10, 16. [Google Scholar] [CrossRef]
- Chan-Keb, C.A.; Agraz-Hernández, C.M.; Pérez-Balan, R.A.; Expósito-Díaz, G.; Gutiérrez-Alcántara, E.J.; Muñiz-Salazar, R.; Osti-Sáenz, J.; Reyes-Castellano, J.E. Relationship between leaf degradation and pore water chemistry in two mangrove forests of southeastern Mexico. Diversity 2023, 15, 432. [Google Scholar] [CrossRef]
- Zanvo, S.M.G.; Mensah, S.; Salako, K.V.; Glèlè Kakaï, R. Tree height-diameter, aboveground and belowground biomass allometries for two west African mangrove species. Biomass Bioenergy 2023, 176, 106917. [Google Scholar] [CrossRef]
- Jones, A.R.; Raja Segaran, R.; Clarke, K.D.; Waycott, M.; Goh, W.S.H.; Gillanders, B.M. Estimating Mangrove tree biomass and carbon content: A comparison of forest inventory techniques and drone imagery. Front. Mar. Sci. 2020, 6, 784. [Google Scholar] [CrossRef]
- Kauffman, J.B.; Hernandez Trejo, H.; Del Carmen Jesus Garcia, M.; Heider, C.; Contreras, W.M. Carbon stocks of mangroves and losses arising from their conversion to cattle pastures in the Pantanos de Centla, Mexico. Wetl. Ecol. Manag. 2016, 24, 203–216. [Google Scholar] [CrossRef]
- Chan-Keb, C.A.; Agraz-Hernández, C.M.; Pérez-Balan, R.A.; Mas-Qui, O.O.; Osti-Sáenz, J.; Reyes-Castellanos, J.E. Relationship between carbon sequestration and soil physicochemical parameters in northern Campeche, Mexico. Land 2024, 13, 139. [Google Scholar] [CrossRef]
- Nóbrega, G.N.; Ferreira, T.O.; Artur, A.G.; de Mendonça, E.S.; de OLeão, R.A.; Teixeira, A.S.; Otero, X.L. Evaluation of methods for quantifying organic carbon in mangrove soils from semi-arid region. J. Soils Sediments 2015, 15, 282–291. [Google Scholar] [CrossRef]
- Passos, T.R.G.; Artur, A.G.; Nóbrega, G.N.; Otero, X.L.; Ferreira, T.O. Comparison of the quantitative determination of soil organic carbon in coastal wetlands containing reduced forms of Fe and S. Geo-Mar. Lett. 2016, 36, 223–233. [Google Scholar] [CrossRef]
- Agraz-Hernández, C.M.; Chan-Keb, C.A.; Muñiz-Salazar, R.; Pérez-Balan, R.A.; Osti-Sáenz, J.; Gutiérrez-Alcántara, E.J.; Reyes-Castellano, J.E.; May-Colli, L.O.; Conde-Medina, K.P.; Ruiz-Hernández, J. Relationship between blue carbon and methane and the hydrochemistry of mangroves in southeast Mexico. Appl. Ecol. Environ. Res. 2020, 18, 1091–1106. [Google Scholar] [CrossRef]
- Riefner Jr, R.E.; Columbus, J.T. Paspalum Vaginatum (Poaceae), a new threat to wetland diversity in southern California. J. Bot. Res. Inst. Tex. 2008, 2, 743–759. [Google Scholar]
- Saluzzo, H.; Reinoso, P.; Martínez, V. Caracterización y evaluación del crecimiento como césped de Paspalum almum, Paspalum denticulatum y Paspalum vaginatum. Phyton 2015, 84, 51–55. [Google Scholar]
- Elansary, H.O.; Yessoufou, K.; Abdel-Hamid, A.M.E.; El-Esawi, M.A.; Ali, H.M.; Elshikh, M.S. Seaweed extracts enhance salam turfgrass performance during prolonged irrigation intervals and saline shock. Front. Plant Sci. 2017, 8, 830. [Google Scholar] [CrossRef]
- Pérez, A.; Machado, W.; Sanders, C.J. Anthropogenic and environmental influences on nutrient accumulation in mangrove sediments. Mar. Pollut. Bull. 2021, 165, 112174. [Google Scholar] [CrossRef]
- Gu, J.; Wu, J. Blue Carbon Effects of Mangrove Restoration in subtropics where Spartina alterniflora invaded. Ecol. Eng. 2023, 186, 106822. [Google Scholar] [CrossRef]
- Okoye, O.K.; Li, H.; Gong, Z. Retraction of invasive Spartina alterniflora and its effect on the habitat loss of endangered migratory bird species and their decline in YNNR using remote sensing technology. Ecol. Evol. 2020, 10, 13810–13824. [Google Scholar] [CrossRef]
- Rendón-Hernández, E.; Ayala-Pérez, L.A.; Golubov, J.; Torres-Lara, R. Especies exóticas invasoras y sus implicaciones sobre los bosques de manglar en las reservas de La Biosfera Ría Celestún y Ría Lagartos. MYB 2024, 30, e3042627. [Google Scholar] [CrossRef]
- Jacotot, A.; Marchand, C.; Allenbach, M. Increase in growth and alteration of C:N ratios of Avicennia marina and Rhizophora stylosa subject to elevated CO2 concentrations and longer tidal flooding duration. Front. Ecol. Evol. 2019, 7, 98. [Google Scholar] [CrossRef]
- Ahmed, I.U.; Assefa, D.; Godbold, D.L. Land-use change depletes quantity and quality of soil organic matter fractions in Ethiopian Highlands. Forests 2022, 13, 69. [Google Scholar] [CrossRef]
- Thomson, T.; Pilditch, C.A.; Fusi, M.; Prinz, N.; Lundquist, C.J.; Ellis, J.I. Vulnerability of labile organic matter to eutrophication and warming in temperate mangrove ecosystems. Glob. Change Biol. 2025, 31, e70087. [Google Scholar] [CrossRef]
- Palacios, M.M.; Trevathan-Tackett, S.M.; Malerba, M.E.; Macreadie, P.I. Effects of a nutrient enrichment pulse on blue carbon ecosystems. Mar. Pollut. Bull. 2021, 165, 112024. [Google Scholar] [CrossRef]
- López Rosas, H.; Moreno-Casasola, P.; Mendelssohn, I.A. Effects of an African grass invasion on vegetation, soil and interstitial water characteristics in a tropical freshwater marsh in La Mancha, Veracruz (Mexico). J. Plant Interact. 2005, 1, 187–195. [Google Scholar] [CrossRef][Green Version]
- Pendleton, L.; Donato, D.C.; Murray, B.C.; Crooks, S.; Jenkins, W.A.; Sifleet, S.; Craft, C.; Fourqurean, J.W.; Kauffman, J.B.; Marbà, N.; et al. Estimating global “Blue carbon” emissions from conversion and degradation of vegetated coastal ecosystems. PLoS ONE 2012, 7, e43542. [Google Scholar] [CrossRef]
- Grellier, S.; Janeau, J.-L.; Dang Hoai, N.; Nguyen Thi Kim, C.; Le Thi Phuong, Q.; Pham Thi Thu, T.; Tran-Thi, N.-T.; Marchand, C. Changes in soil characteristics and c dynamics after mangrove clearing (Vietnam). Sci. Total Environ. 2017, 593–594, 654–663. [Google Scholar] [CrossRef]
- Nagelkerken, I.; Blaber, S.J.M.; Bouillon, S.; Green, P.; Haywood, M.; Kirton, L.G.; Meynecke, J.-O.; Pawlik, J.; Penrose, H.M.; Sasekumar, A.; et al. The habitat function of mangroves for terrestrial and marine fauna: A Review. Aquat. Bot. 2008, 89, 155–185. [Google Scholar] [CrossRef]
- Bourgeois, C.F.; MacKenzie, R.A.; Sharma, S.; Bhomia, R.K.; Johnson, N.G.; Rovai, A.S.; Worthington, T.A.; Krauss, K.W.; Analuddin, K.; Bukoski, J.J.; et al. Four decades of data indicate that planted mangroves stored up to 75% of the carbon stocks found in intact mature stands. Sci. Adv. 2024, 10, eadk5430. [Google Scholar] [CrossRef]
- Manga, B.A.B.; Ndour, N.; Diatta, A.A.; Dasylva, M. Assessment of carbon sequestration by mangrove plantations in Casamance (Oussouye, Ziguinchor, Senegal). J. Ecol. Nat. Environ. 2022, 14, 109–120. [Google Scholar] [CrossRef]
- Flores-Verdugo, F.; Moreno-Casasola, P.; Agraz-Hernández, C.M.; López-Rosas, H.; Benítez-Pardo, D.; Travieso-Bello, A.C. La topografía y el hidroperíodo: Dos factores que condicionan la restauración de los humedales costeros. Bot. Sci. 2007, 80, S33–S47. [Google Scholar] [CrossRef]
- Krauss, K.W.; Lovelock, C.E.; McKee, K.L.; López-Hoffman, L.; Ewe, S.M.L.; Sousa, W.P. Environmental drivers in mangrove establishment and early development: A Review. Aquat. Bot. 2008, 89, 105–127. [Google Scholar] [CrossRef]
- Carnell, P.E.; Palacios, M.M.; Waryszak, P.; Trevathan-Tackett, S.M.; Masqué, P.; Macreadie, P.I. Blue carbon drawdown by restored mangrove forests improves with Age. J. Environ. Manag. 2022, 306, 114301. [Google Scholar] [CrossRef]
- Jiménez, L.C.Z.; Queiroz, H.M.; Otero, X.L.; Nóbrega, G.N.; Ferreira, T.O. Soil organic matter responses to mangrove restoration: A replanting experience in Northeast Brazil. Int. J. Environ. Res. Public Health 2021, 18, 8981. [Google Scholar] [CrossRef]
- Cameron, C.; Hutley, L.B.; Friess, D.A.; Munksgaard, N.C. Hydroperiod, soil moisture and bioturbation are critical drivers of greenhouse gas fluxes and vary as a function of land use change in mangroves of Sulawesi, Indonesia. Sci. Total Environ. 2019, 654, 365–377. [Google Scholar] [CrossRef]

| Parameters | df | t-Value | p-Value | Significance |
|---|---|---|---|---|
| Redox potential (mV) | 15 | 4.37 | 0.001 | S |
| Salinity (PUS) | 15 | 0.71 | 0.488 | NS |
| Nitrate (mg NO3− L−1) | 15 | 1.44 | 0.171 | NS |
| Phosphate (mg PO43− L−1) | 15 | 1.31 | 0.210 | NS |
| Sulfate (mg SO42− L−1) | 15 | 1.35 | 0.197 | NS |
| Variables | CP1 (39.0%) | CP2 (25.8%) |
|---|---|---|
| Redox potential (mV) | 0.505 | 0.258 |
| Salinity (PUS) | 0.296 | −0.632 |
| Nitrate (mg NO3− L−1) | 0.579 | 0.254 |
| Phosphate (mg PO43− L−1) | −0.540 | −0.051 |
| Sulfate (mg SO42− L−1) | 0.173 | −0.683 |
| Sitio | Height (m) | Density (Ind. ha−1) | Aboveground Biomass (Mg C ha−1) | Belowground Biomass (Mg C ha−1) | Total Biomass (Mg C ha−1) |
|---|---|---|---|---|---|
| Mref | 5.3 ± 1.2 a | 650 ± 71 | 15 ± 1.8 a | 7.3 ± 1.0 a | 22 ± 2.8 a |
| Mdeg | 1.1 ± 0.3 c | N.d | 2.3 ± 0.3 c | 1.4 ± 0.3 c | 3.6 ± 0.5 c |
| Mres | 1.25 ± 0.08 b | 1045 ± 445 | 1.4 ± 0.2 b | 0.30 ± 0.02 b | 1.7 ± 1.0 b |
| Species | Component | N (%) | C (%) | C/N Ratio |
|---|---|---|---|---|
| Rhizophora racemosa | Root | 0.37 ± 0.05 | 38 ± 1.4 | 101 ± 26 |
| Leaves | 1.61 ± 0.01 | 45.64 ± 0.03 | 28 ± 3.1 | |
| Stem | 0.33 ± 0.02 | 45 ± 1.3 | 140 ± 10 | |
| Paspalum vaginatum | Root | 0.40 ± 0.08 | 23 ± 6.7 | 54 ± 7.0 |
| Leave | 0.61 ± 0.04 | 39 ± 2.8 | 64 ± 5.0 | |
| Stem | 0.47 ± 0.04 | 25.8 ± 1.2 | 55.9 ± 7.5 |
| Variable | Factor | df | F-Value | p-Value | Significance |
|---|---|---|---|---|---|
| Bulk density (g cm−3) | Site (A) | 19 | 50.56 | <0.0001 | S |
| Depth (B) | 19 | 7.92 | 0.003 | S | |
| Interaction AxB | 19 | 0.56 | 0.697 | NS | |
| C/N ratio | Site (A) | 19 | 16.43 | <0.0001 | S |
| Depth (B) | 19 | 3.78 | 0.041 | S | |
| Interaction AxB | 19 | 0.90 | 0.486 | NS | |
| SOC (Mg C ha−1) | Site (A) | 19 | 47.10 | <0.000 | S |
| Depth (B) | 19 | 4.30 | 0.029 | S | |
| Interaction AxB | 19 | 1.79 | 0.172 | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chávez Barrera, J.C.; Gallardo Lancho, J.F.; Puschendorf, R.; Agraz Hernández, C.M. Loss and Early Recovery of Biomass and Soil Organic Carbon in Restored Mangroves After Paspalum vaginatum Invasion in West Africa. Resources 2025, 14, 122. https://doi.org/10.3390/resources14080122
Chávez Barrera JC, Gallardo Lancho JF, Puschendorf R, Agraz Hernández CM. Loss and Early Recovery of Biomass and Soil Organic Carbon in Restored Mangroves After Paspalum vaginatum Invasion in West Africa. Resources. 2025; 14(8):122. https://doi.org/10.3390/resources14080122
Chicago/Turabian StyleChávez Barrera, Julio César, Juan Fernando Gallardo Lancho, Robert Puschendorf, and Claudia Maricusa Agraz Hernández. 2025. "Loss and Early Recovery of Biomass and Soil Organic Carbon in Restored Mangroves After Paspalum vaginatum Invasion in West Africa" Resources 14, no. 8: 122. https://doi.org/10.3390/resources14080122
APA StyleChávez Barrera, J. C., Gallardo Lancho, J. F., Puschendorf, R., & Agraz Hernández, C. M. (2025). Loss and Early Recovery of Biomass and Soil Organic Carbon in Restored Mangroves After Paspalum vaginatum Invasion in West Africa. Resources, 14(8), 122. https://doi.org/10.3390/resources14080122









